Abstract
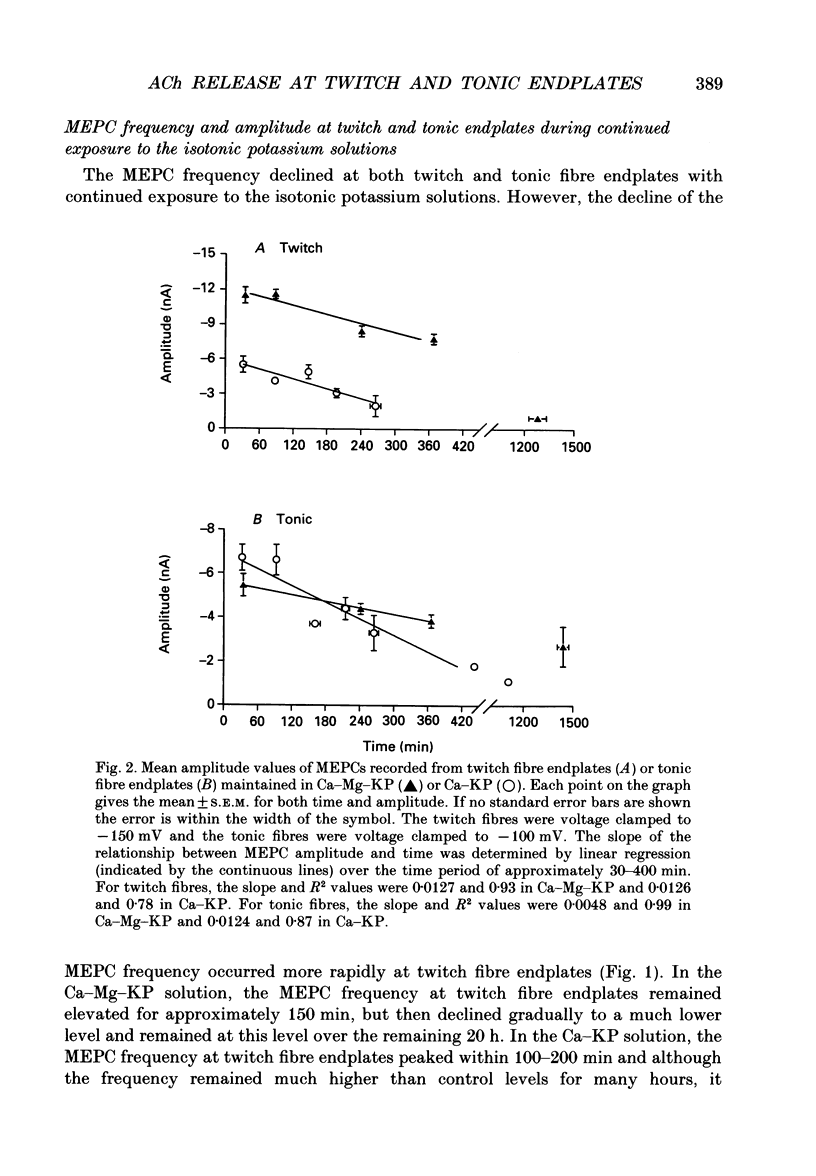
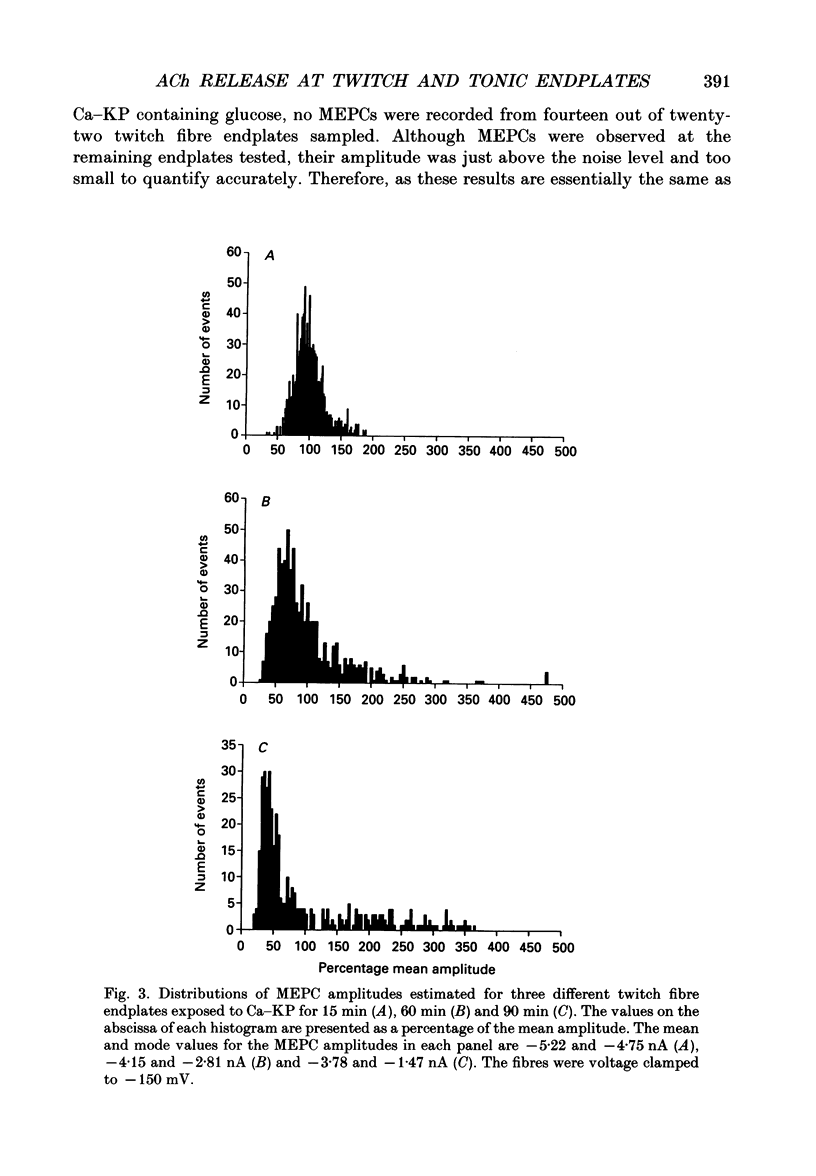
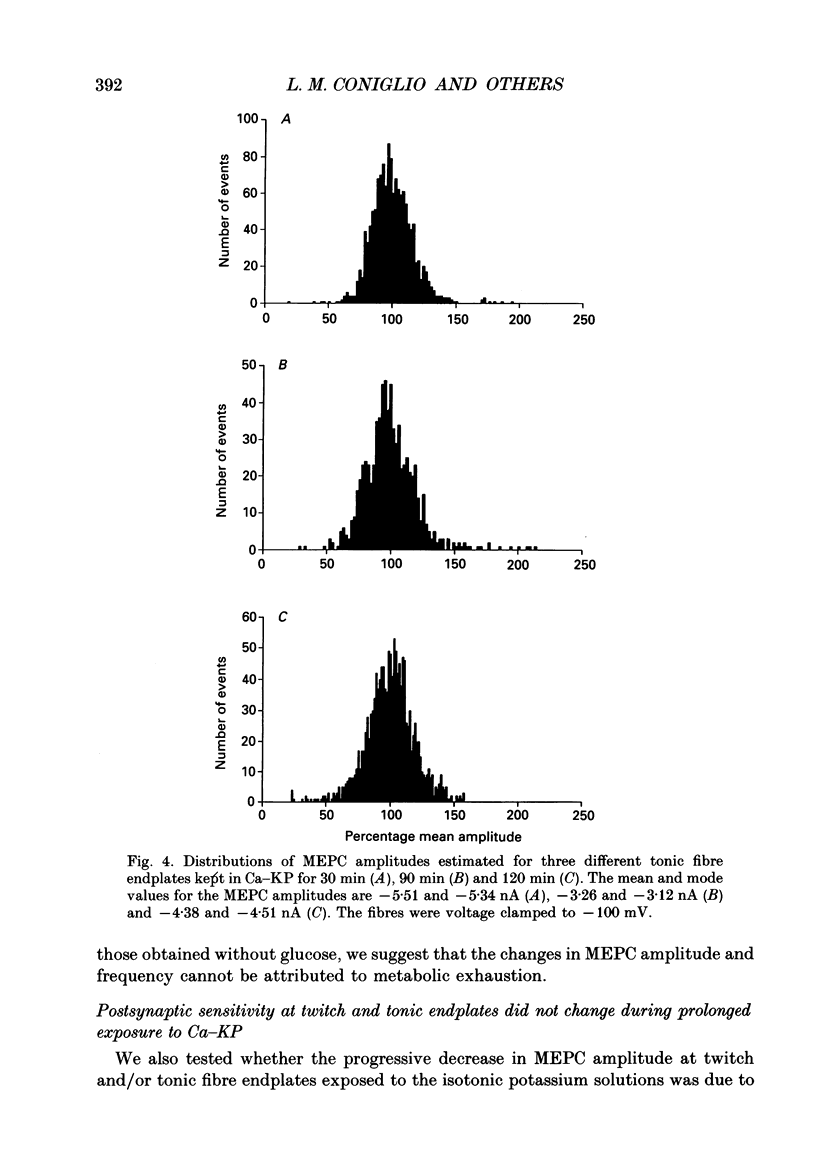
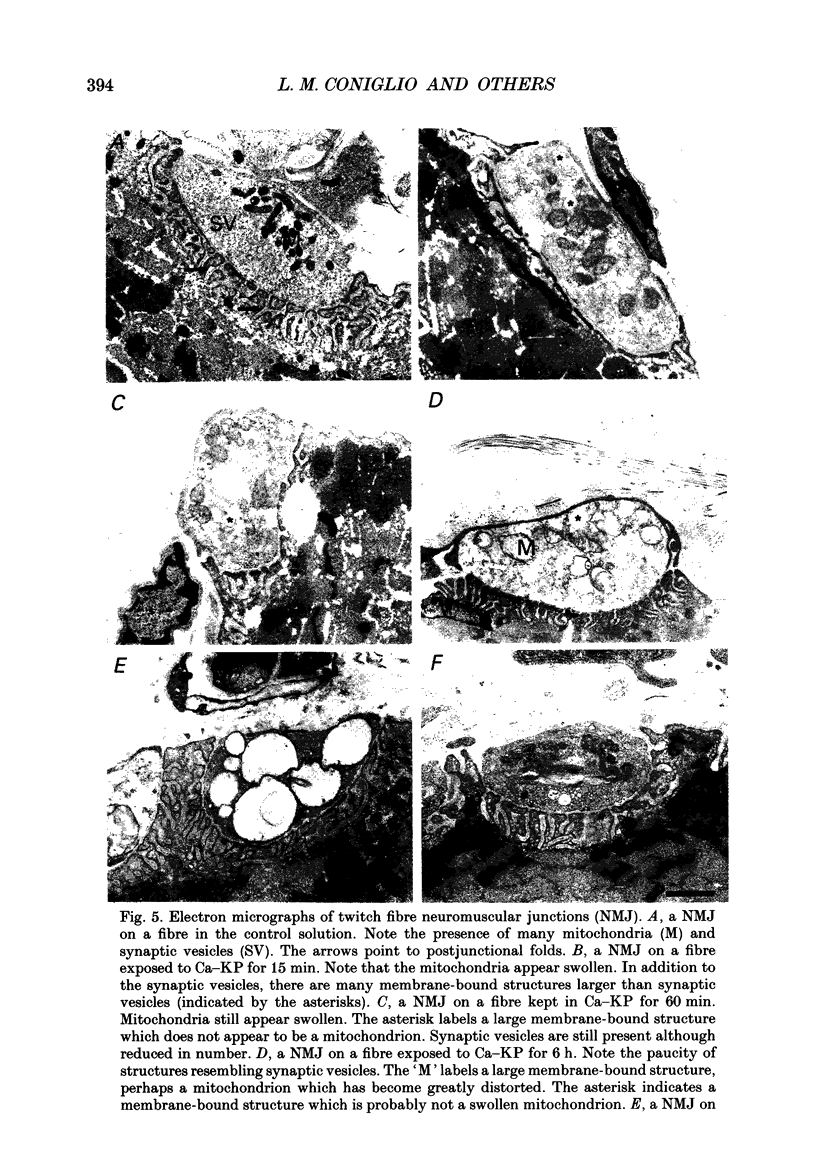
1. Miniature endplate currents (MEPCs) were recorded in vitro from voltage-clamped twitch and tonic muscle fibres in costocutaneous muscles of the garter snake, Thamnophis. Recordings were made from fibres in a control sodium-containing solution and then during exposure to an isotonic potassium solution containing either 1.0 mM calcium and 4.2 mM magnesium or 3.6 mM calcium. The experiments were done at two levels of external calcium in order to demonstrate that the change in MEPC frequency was calcium dependent. During the initial exposure to the isotonic potassium solutions, the MEPC frequency was increased manyfold at both twitch and tonic fibres, but it declined progressively with continued exposure. MEPCs were recorded from both fibre types throughout a 20 h exposure to the isotonic potassium solution with 1 mM calcium, but no MEPCs were recorded at most twitch endplates after approximately 6 h in the isotonic potassium solution containing 3.6 mM calcium. In contrast, MEPCs were still present at tonic fibre endplates after 20 h in the isotonic potassium solution containing 3.6 mM calcium. 2. After 30 min in the isotonic potassium solution with 1 mM calcium, the MEPC amplitude recorded from both fibre types was approximately twice that in the control sodium-containing solution. At tonic endplates, the MEPC amplitude was also twofold greater in the isotonic potassium solution with 3.6 mM calcium than in sodium-containing solution. In contrast, after 30 min in the isotonic potassium solution containing 3.6 mM calcium, the MEPC amplitude at twitch endplates was similar to that in control solution. 3. In both fibre types, MEPC amplitude decreased progressively with continued exposure to the isotonic potassium solutions. The progressive decrease in MEPC amplitude was not due to a gradual decrease in postsynaptic sensitivity to acetylcholine. 4. The effects of high potassium were reversible as MEPCs were recorded at twitch fibre endplates in preparations which were returned to the control sodium-containing solution after a 20 h exposure to the isotonic potassium solution containing 3.6 mM calcium. 5. Ultrastructural examination showed that after a 6 h exposure to the isotonic potassium solutions most nerve terminals innervating twitch fibre endplates were devoid of synaptic vesicles whereas at the same time many synaptic vesicles were present in nerve terminals innervating tonic fibre endplates. Surprisingly, numerous synaptic vesicles were present in nerve terminals innervating either fibre type in muscle preparations exposed to the isotonic potassium solutions for 20 h.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Images in this article
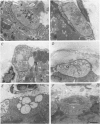
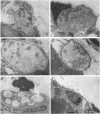
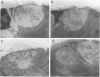
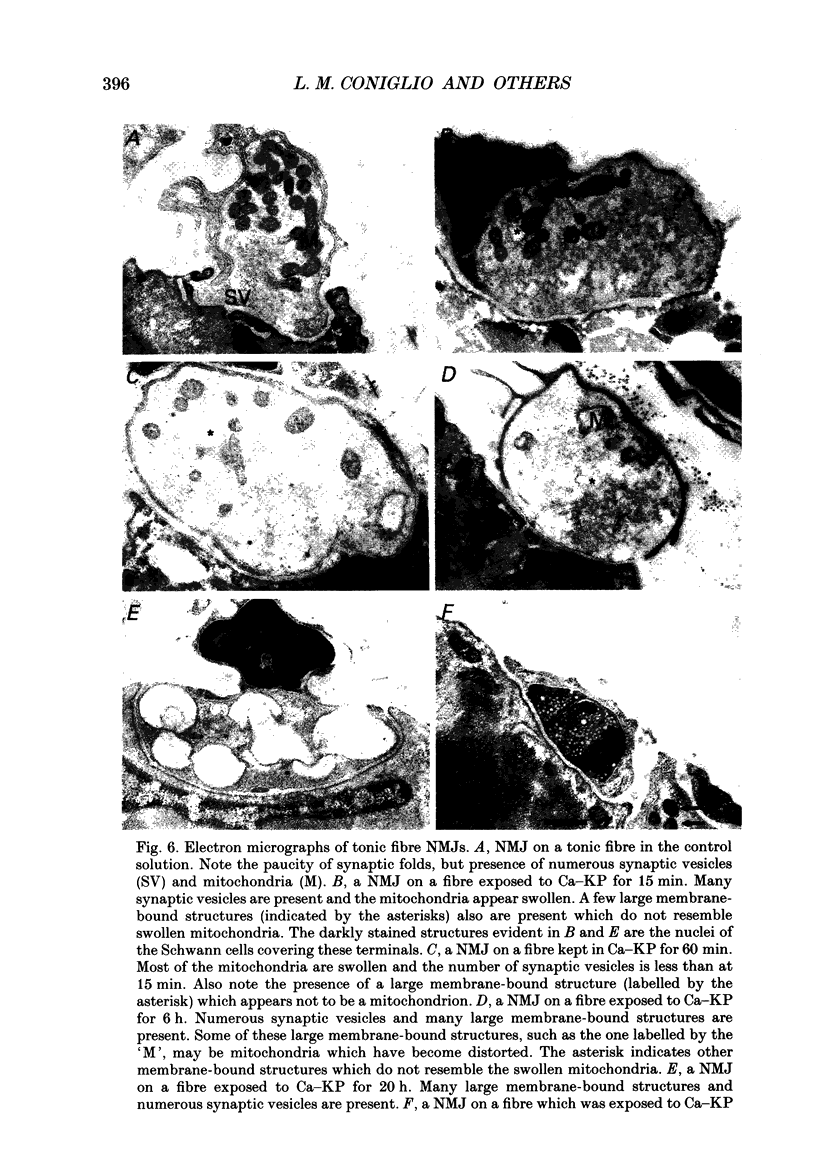
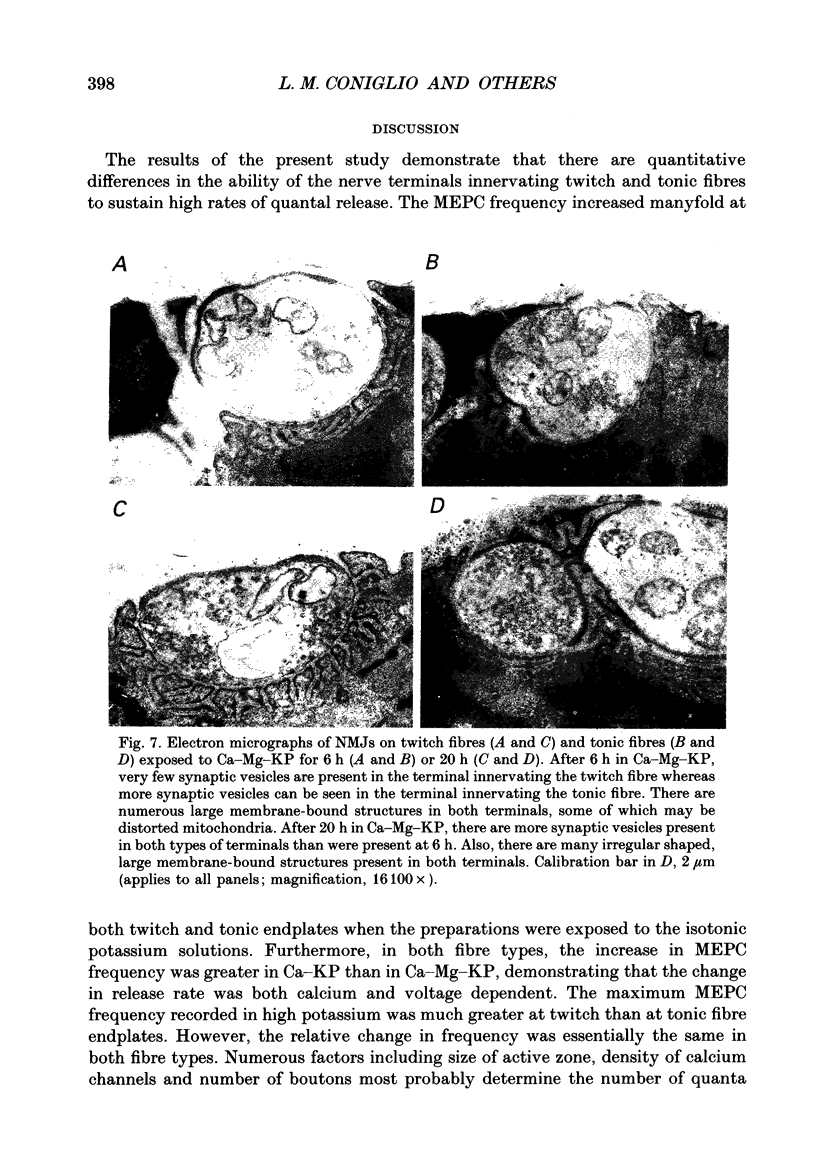
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coniglio L. M., Hendricks G. M., Parsons R. L. Effects of lanthanum at snake twitch and tonic muscle fibre endplates. J Physiol. 1993 Jul;466:405–419. [PMC free article] [PubMed] [Google Scholar]
- Connor E. A., Fiekers J. F., Neel D. S., Parsons R. L., Schnitzler R. M. Comparison of cholinergic activation and desensitization at snake twitch and slow muscle fibre end-plates. J Physiol. 1984 Jun;351:657–674. doi: 10.1113/jphysiol.1984.sp015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L., Podolsky R. J., Tice L. W. Calcium activation of frog slow muscle fibres. J Physiol. 1967 Jan;188(2):261–271. doi: 10.1113/jphysiol.1967.sp008137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Parsons R. L. Characteristics of the acetylcholine-operated channel at twitch and slow fibre neuromuscular junctions of the garter snake. J Physiol. 1981 Jan;310:145–158. doi: 10.1113/jphysiol.1981.sp013541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E. Two types of nicotinic acetylcholine receptor channels at slow fibre end-plates of the garter snake. J Physiol. 1989 Feb;409:313–331. doi: 10.1113/jphysiol.1989.sp017499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatatis A., Holtzclaw L., Payza K., Russell J. T. Secretion from rat neurohypophysial nerve terminals (neurosecretosomes) rapidly inactivates despite continued elevation of intracellular Ca2+. Brain Res. 1992 Mar 6;574(1-2):33–41. doi: 10.1016/0006-8993(92)90796-c. [DOI] [PubMed] [Google Scholar]
- Florey E., Kriebel M. E. Reversible effect of depolarization by K-propionate on sub-miniature endplate potential to bell-miniature endplate potential ratios, on miniature endplate potential frequencies and amplitudes, and on synaptic vesicle diameters and densities in frog neuromuscular junctions. Neuroscience. 1988 Dec;27(3):1055–1072. doi: 10.1016/0306-4522(88)90210-2. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Van Helden D. Effects of permeant monovalent cations on end-plate channels. J Physiol. 1979 Mar;288:509–528. [PMC free article] [PubMed] [Google Scholar]
- Gennaro J. F., Jr, Nastuk W. L., Rutherford D. T. Reversible depletion of synaptic vesicles induced by application of high external potassium to the frog neuromuscular junction. J Physiol. 1978 Jul;280:237–247. doi: 10.1113/jphysiol.1978.sp012382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick J. C., Coniglio L. M., Parsons R. L. Staurosporine inhibits the extent of acetylcholine receptor recovery from carbachol-induced desensitization in snake twitch fibres. Br J Pharmacol. 1991 Dec;104(4):879–886. doi: 10.1111/j.1476-5381.1991.tb12521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick J. C., Parsons R. L. Mechanism of staurosporine-induced decrease in acetylcholine receptor recovery from desensitization. Br J Pharmacol. 1993 Mar;108(3):741–748. doi: 10.1111/j.1476-5381.1993.tb12871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A. Vertebrate slow muscle fibers. Physiol Rev. 1970 Jan;50(1):40–62. doi: 10.1152/physrev.1970.50.1.40. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Structural changes after transmitter release at the frog neuromuscular junction. J Cell Biol. 1981 Mar;88(3):564–580. doi: 10.1083/jcb.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A. C. Frog tonic muscle fibers: extracellular calcium and excitation-contraction coupling. Am J Physiol. 1970 Nov;219(5):1446–1450. doi: 10.1152/ajplegacy.1970.219.5.1446. [DOI] [PubMed] [Google Scholar]
- Lichtman J. W., Wilkinson R. S., Rich M. M. Multiple innervation of tonic endplates revealed by activity-dependent uptake of fluorescent probes. 1985 Mar 28-Apr 3Nature. 314(6009):357–359. doi: 10.1038/314357a0. [DOI] [PubMed] [Google Scholar]
- Molenaar P. C., Polak R. L. Effect of potassium propionate on free and bound acetylcholine in frog muscle. Brain Res. 1989 Jan 16;477(1-2):109–117. doi: 10.1016/0006-8993(89)91398-x. [DOI] [PubMed] [Google Scholar]
- Morgan D. L., Proske U. Vertebrate slow muscle: its structure, pattern of innervation, and mechanical properties. Physiol Rev. 1984 Jan;64(1):103–169. doi: 10.1152/physrev.1984.64.1.103. [DOI] [PubMed] [Google Scholar]
- OGATA T., MORI M. HISTOCHEMICAL STUDY OF OXIDATIVE ENZYMES IN VERTEBRATE MUSCLES. J Histochem Cytochem. 1964 Mar;12:171–182. doi: 10.1177/12.3.171. [DOI] [PubMed] [Google Scholar]
- Proske U., Vaughan P. Histological and electrophysiological investigation of lizard skeletal muscle. J Physiol. 1968 Dec;199(3):495–509. doi: 10.1113/jphysiol.1968.sp008665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash J. E., Walrond J. P., Morita M. Structural and functional correlates of synaptic transmission in the vertebrate neuromuscular junction. J Electron Microsc Tech. 1988 Oct;10(2):153–185. doi: 10.1002/jemt.1060100204. [DOI] [PubMed] [Google Scholar]
- Ridge R. M. Different types of extrafusal muscle fibres in snake costocutaneous muscles. J Physiol. 1971 Sep;217(2):393–418. doi: 10.1113/jphysiol.1971.sp009578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. J., Pappas G. D., Kriebel M. E. The fine structure of identified frog neuromuscular junctions in relation to synaptic activity. Brain Res. 1978 Apr 14;144(2):213–239. doi: 10.1016/0006-8993(78)90151-8. [DOI] [PubMed] [Google Scholar]
- Ruff R. L., Spiegel P. Ca sensitivity and acetylcholine receptor currents of twitch and tonic snake muscle fibers. Am J Physiol. 1990 Dec;259(6 Pt 1):C911–C919. doi: 10.1152/ajpcell.1990.259.6.C911. [DOI] [PubMed] [Google Scholar]
- Searl T., Prior C., Marshall I. G. The effects of L-vesamicol, an inhibitor of vesicular acetylcholine uptake, on two populations of miniature endplate currents at the snake neuromuscular junction. Neuroscience. 1990;35(1):145–156. doi: 10.1016/0306-4522(90)90129-r. [DOI] [PubMed] [Google Scholar]
- Torri-Tarelli F., Haimann C., Ceccarelli B. Coated vesicles and pits during enhanced quantal release of acetylcholine at the neuromuscular junction. J Neurocytol. 1987 Apr;16(2):205–214. doi: 10.1007/BF01795304. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. The regulation of quantal size. Prog Neurobiol. 1991;36(2):93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
- Walrond J. P., Reese T. S. Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. J Neurosci. 1985 May;5(5):1118–1131. doi: 10.1523/JNEUROSCI.05-05-01118.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. S., Lichtman J. W. Regular alternation of fiber types in the transversus abdominis muscle of the garter snake. J Neurosci. 1985 Nov;5(11):2979–2988. doi: 10.1523/JNEUROSCI.05-11-02979.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. S., Nemeth P. M. Metabolic fiber types of snake transversus abdominis muscle. Am J Physiol. 1989 Jun;256(6 Pt 1):C1176–C1183. doi: 10.1152/ajpcell.1989.256.6.C1176. [DOI] [PubMed] [Google Scholar]