Abstract
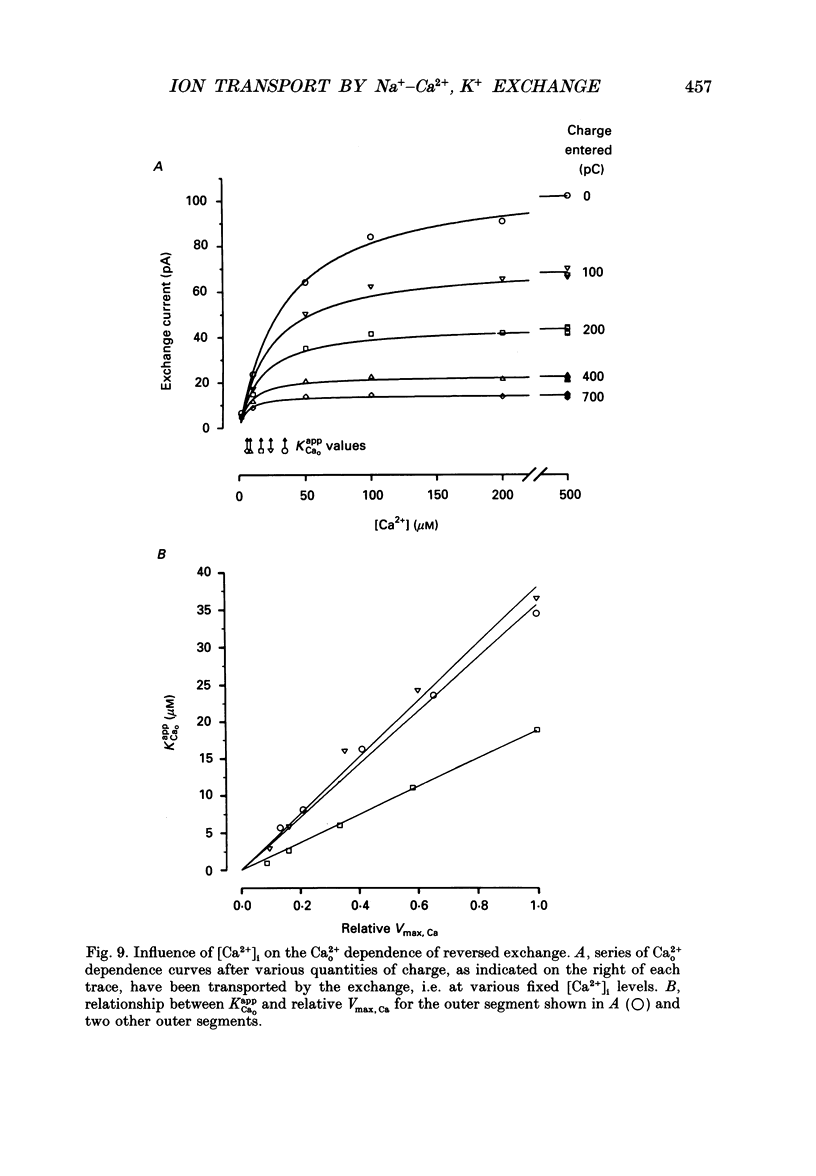
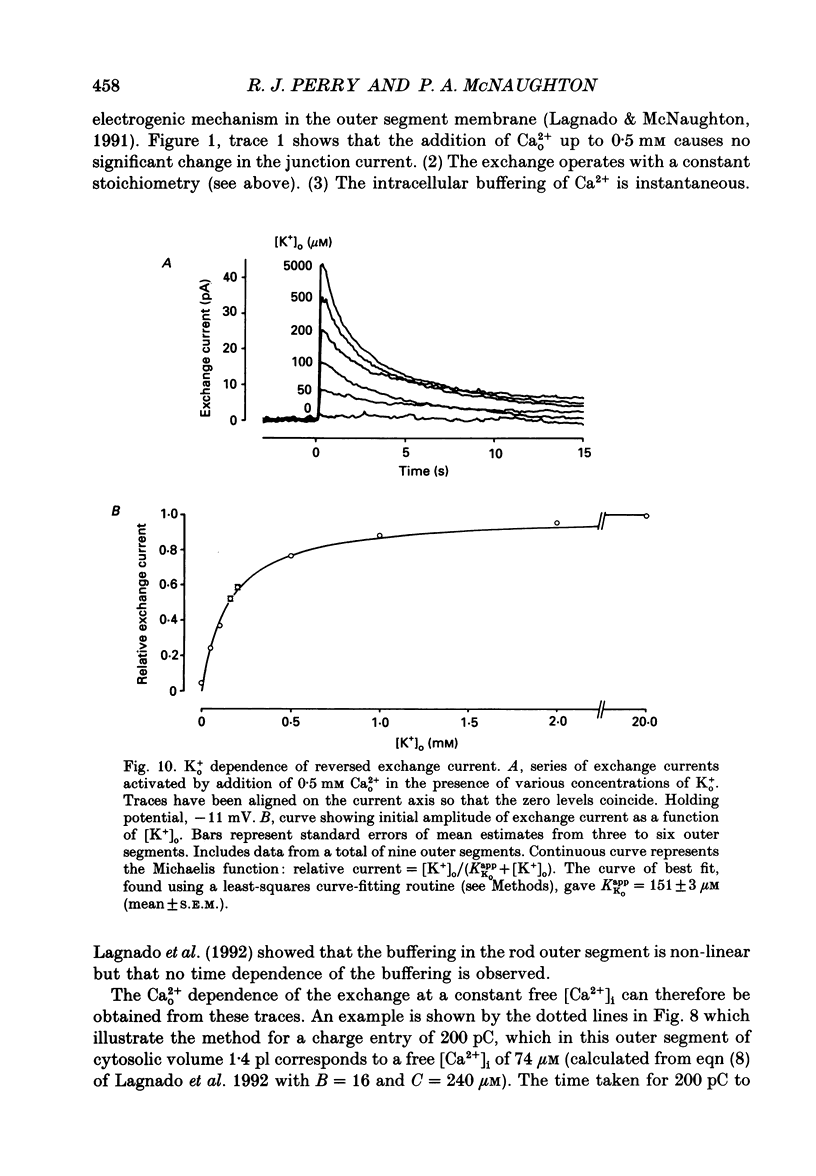
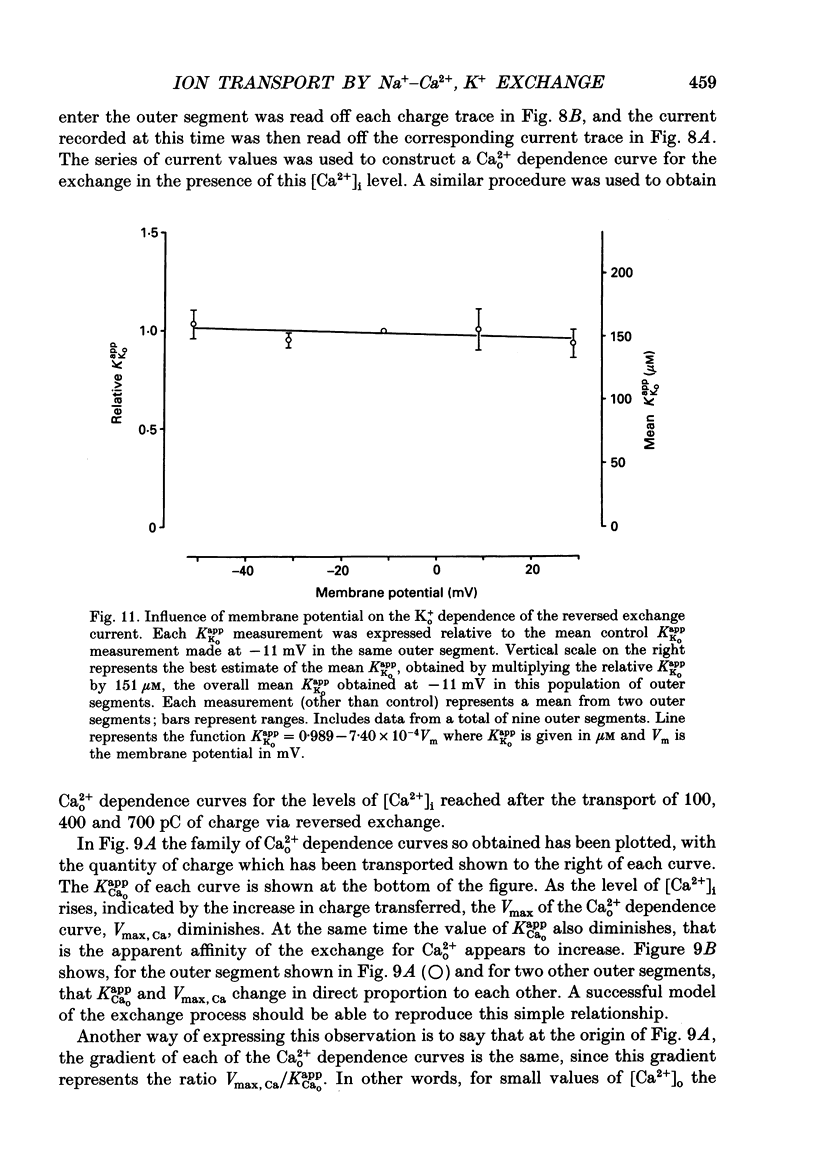
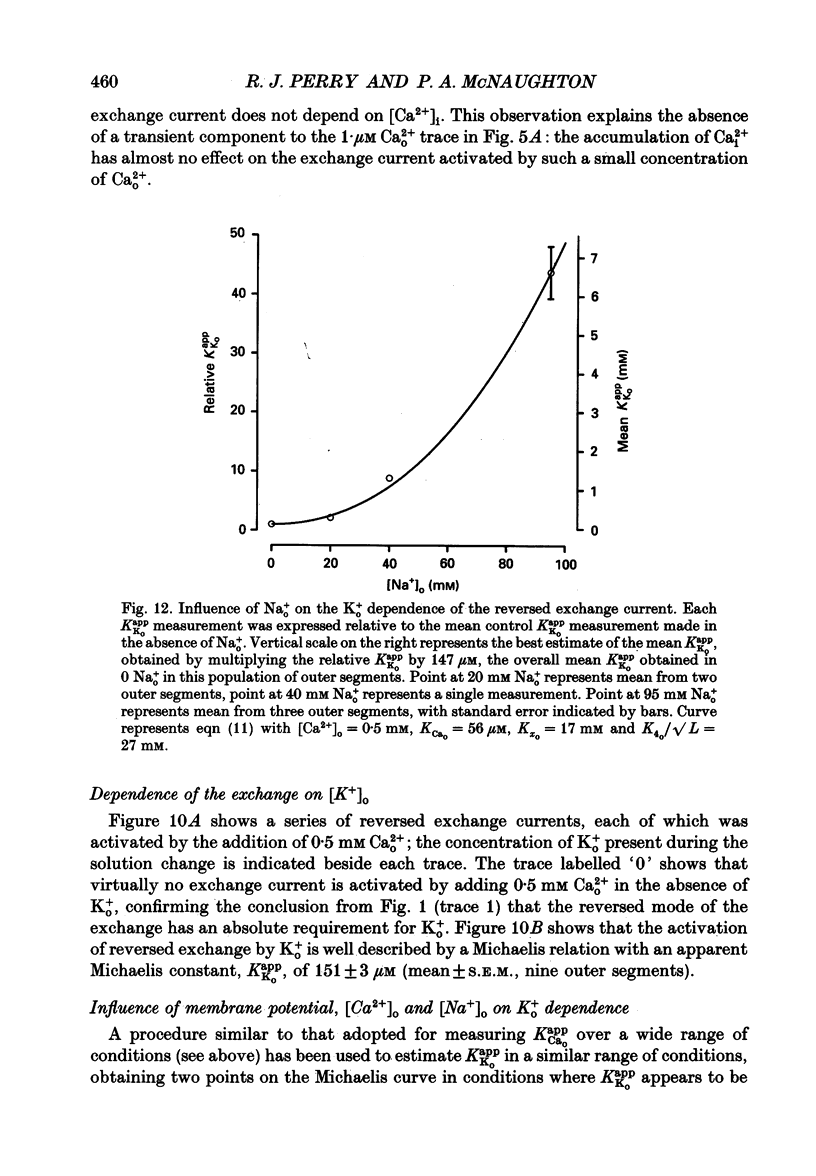
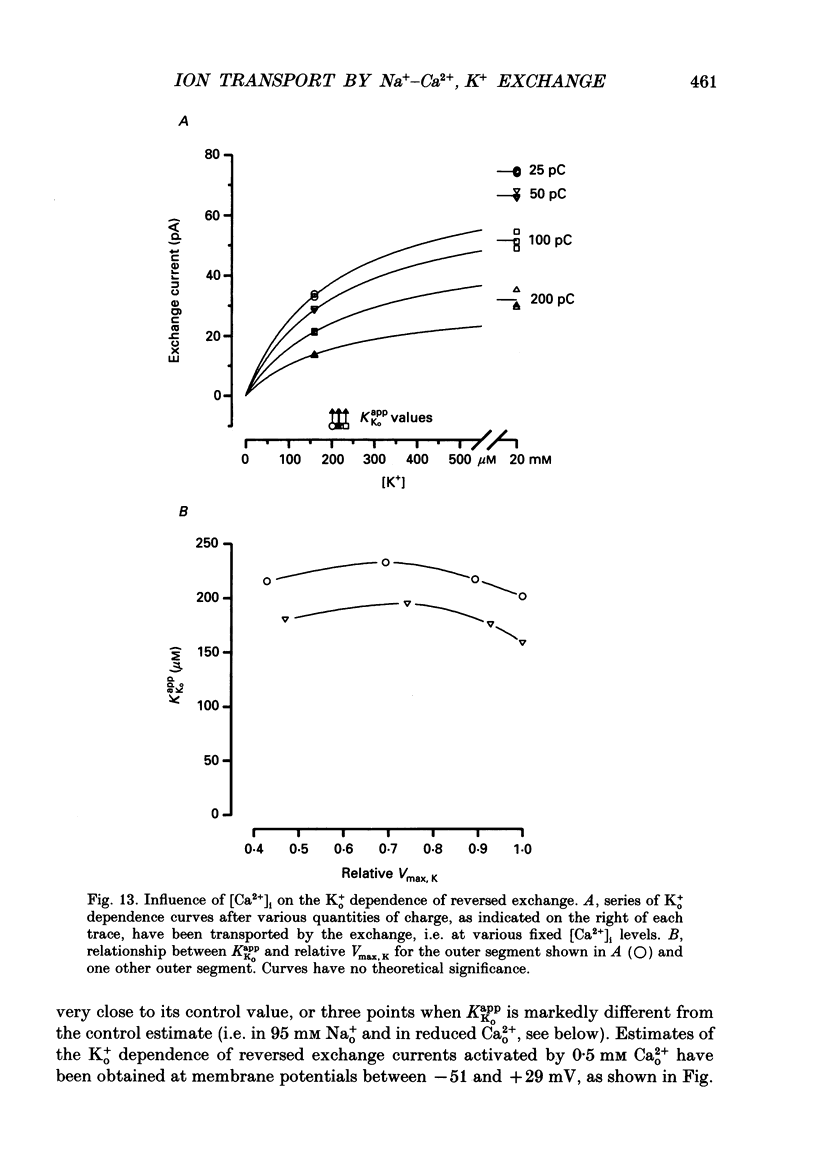
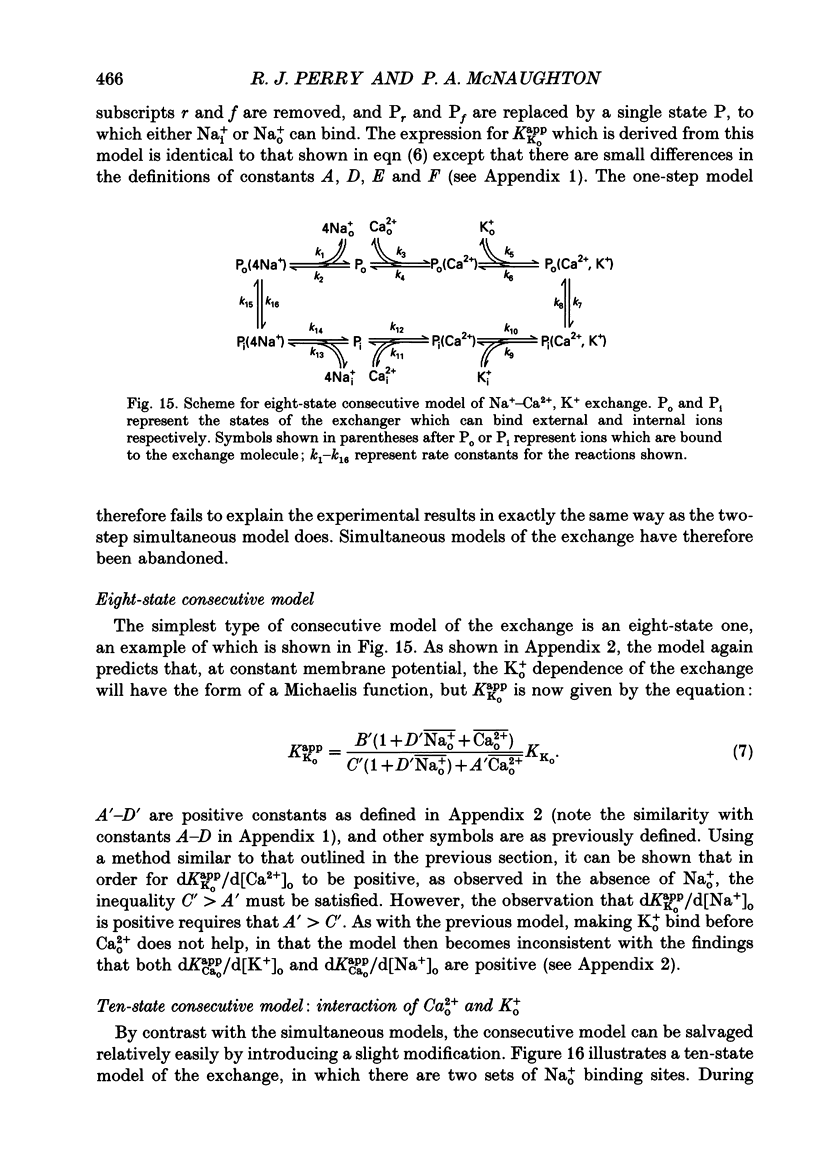
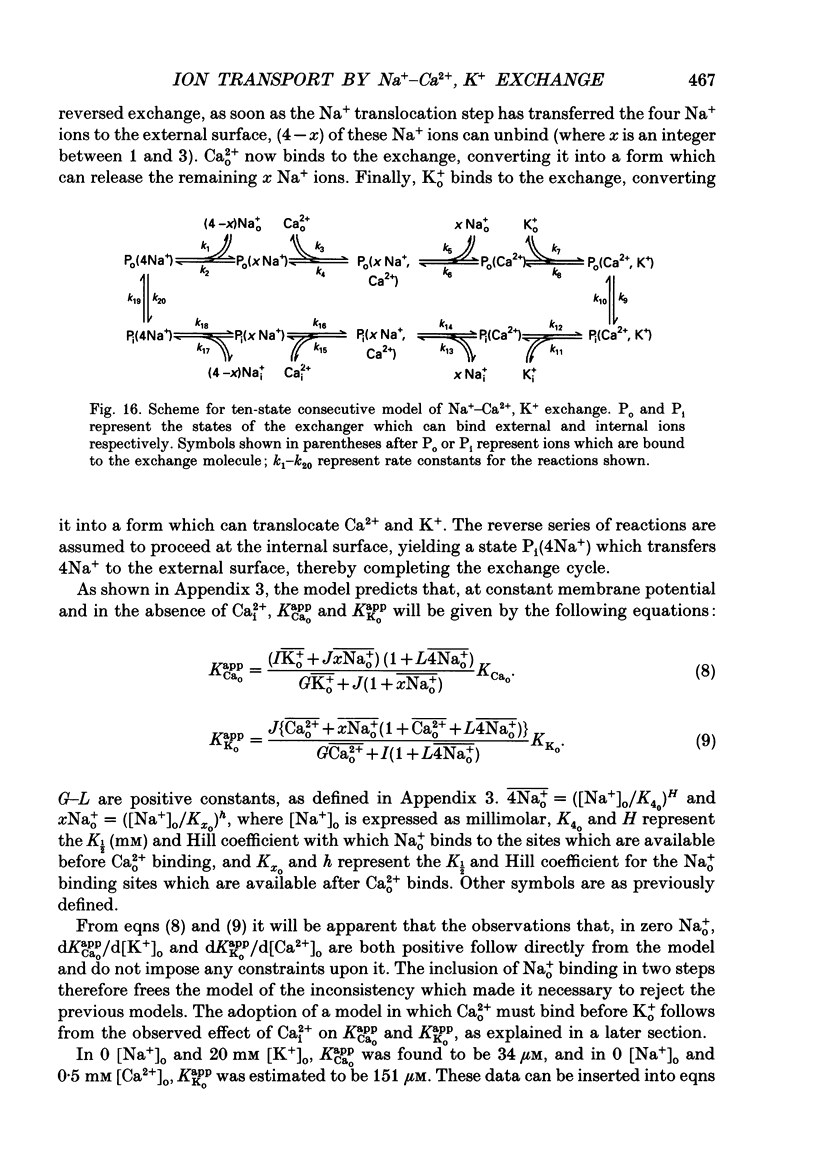
1. Membrane currents caused by the operation of electrogenic Na(+)-Ca2+,K+ exchange were recorded from isolated rod outer segments under voltage-clamp using a whole-cell electrode. 2. Reversed mode exchange currents (Na+i-Ca2+o,K+o) were recorded with a high internal [Na+] and when both Ca2+ and K+ were present in the external solution. Omission of either Ca2+ or K+ completely suppressed both the reversed exchange current and the entry of Ca2+. 3. The charge transferred by the exchange per Ca2+ ion transported was identical in both forward and reversed modes. 4. The reversed exchange current declined as Ca2+ accumulated inside the outer segment, and the form of this decline was consistent with a first-order inhibition by internal Ca2+. 5. The reversed exchange current was increased e-fold by a 230 mV depolarization over the range -51 to +29 mV. 6. The activation of reversed exchange by external Ca2+ was well described by first-order kinetics with a Michaelis constant, KappCao, of 34 microM in the presence of 20 mM external K+. KappCao was reduced by lowering external [K+], was increased by adding external Na+ and was unaffected by membrane potential. 7. External K+ also activated the exchange in a first-order manner with a Michaelis constant, KappKo, of 151 microM in the presence of 0.5 mM external Ca2+. KappKo was reduced by lowering external [Ca2+], increased by adding external Na+ and was unaffected by membrane potential. 8. When the level of internal Ca2+ was increased via reversed exchange, KappCao diminished in proportion to the reduction in the maximum current, but KappKo remained approximately constant. 9. These observations cannot be reconciled with simple models of the exchange in which ions bind simultaneously at opposite faces of the membrane before transport occurs. The results are broadly consistent with a consecutive model of the exchange in which unbinding of Na+ at either the external or the internal membrane surface is followed by binding of Ca2+ and then K+, and are fully reproduced by a model in which Ca2+ binds before all of the Na+ has dissociated from the exchange molecule.
Full text
PDF





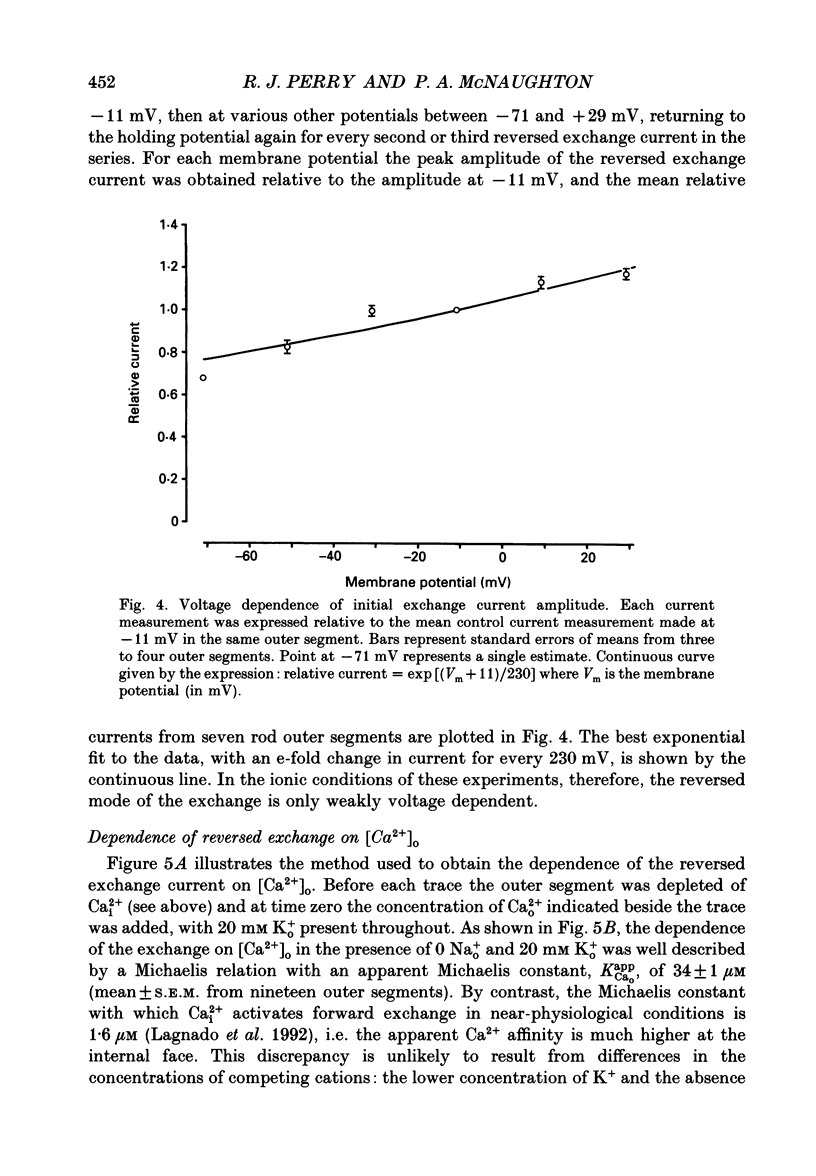
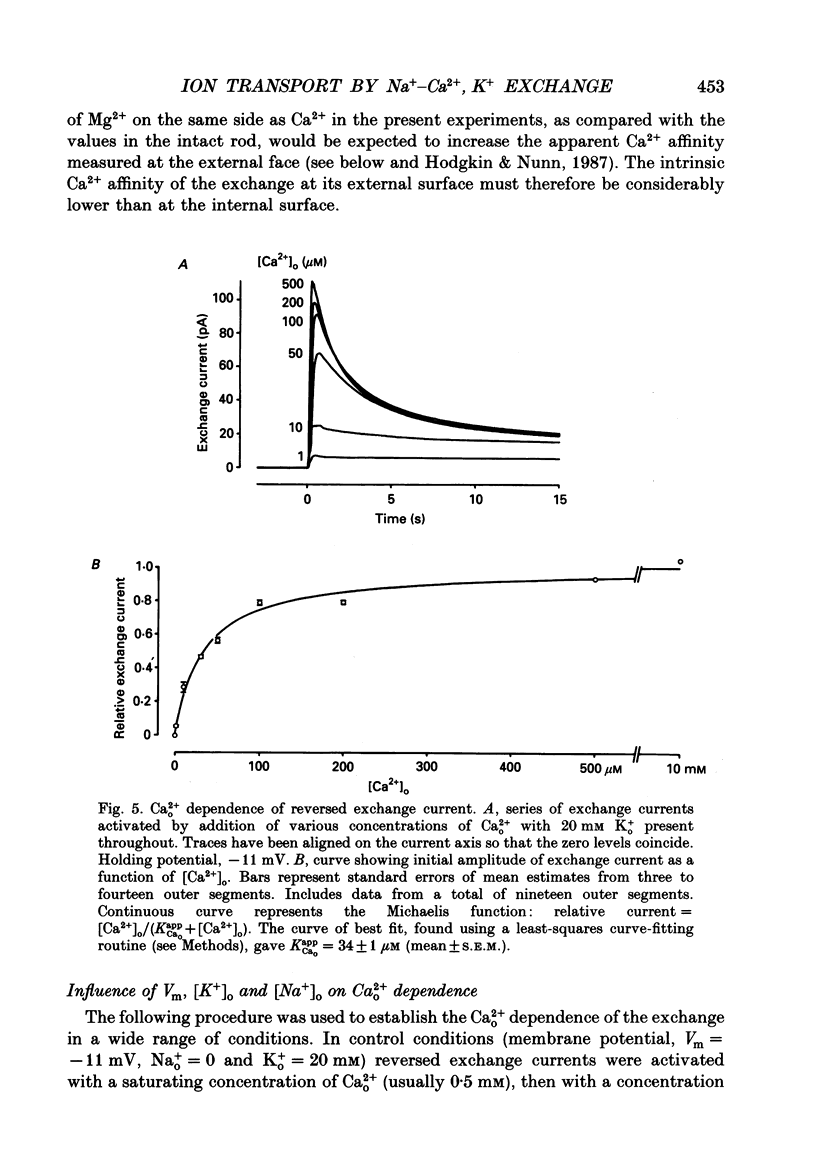

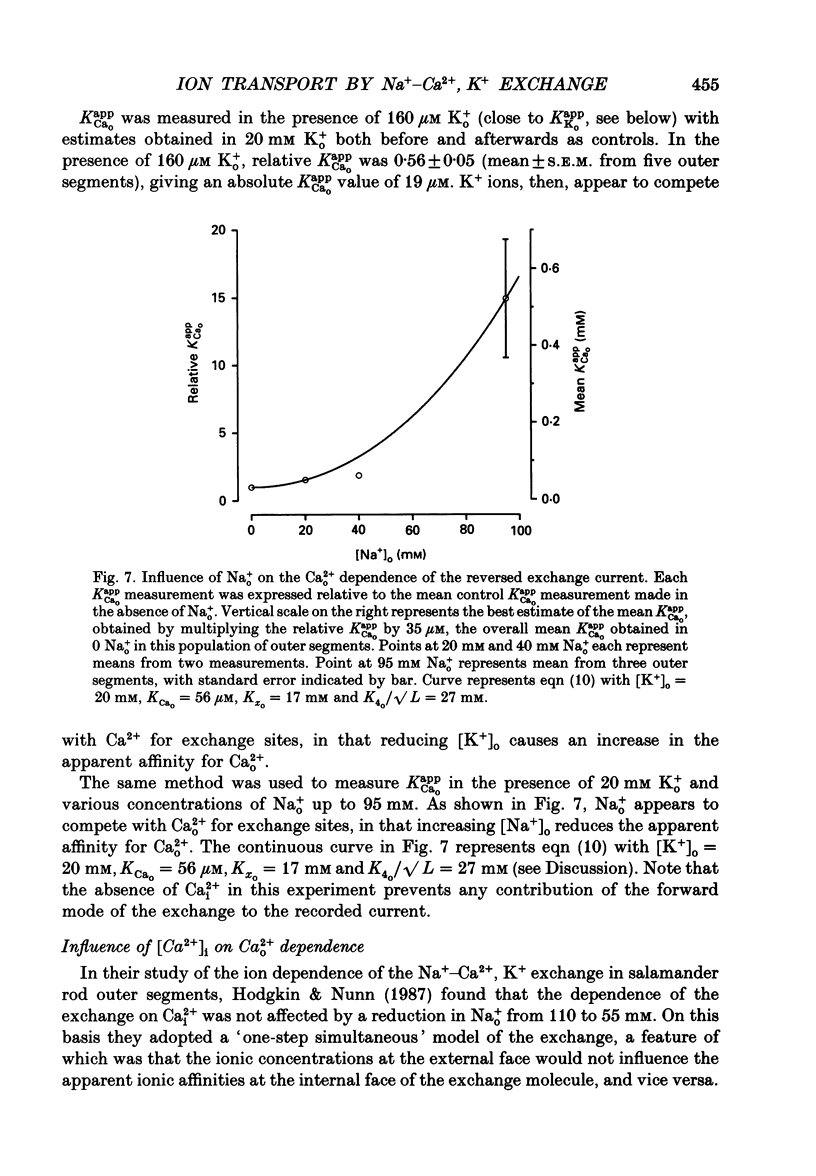
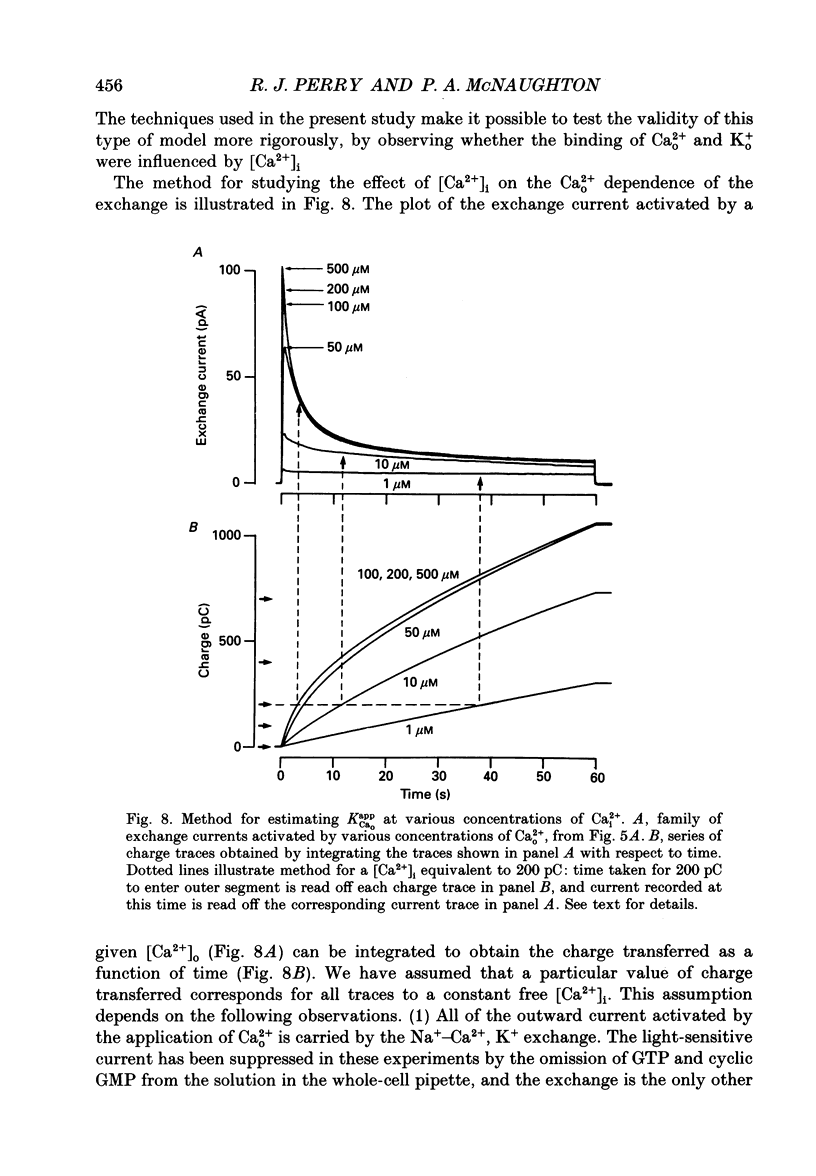















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Lagnado L., Perry R. J., Robinson D. W., McNaughton P. A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989 Feb 23;337(6209):740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel U., Wolbring G., Wohlfart P., Cook N. J. The sodium-calcium exchanger of bovine rod photoreceptors: K(+)-dependence of the purified and reconstituted protein. Biochim Biophys Acta. 1991 Jan 30;1061(2):247–252. doi: 10.1016/0005-2736(91)90290-o. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. Measurement of sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. The effect of ions on sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:371–398. doi: 10.1113/jphysiol.1987.sp016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992 Sep;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Ion transport by the Na-Ca exchange in isolated rod outer segments. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4548–4552. doi: 10.1073/pnas.85.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L., McNaughton P. A. Net charge transport during sodium-dependent calcium extrusion in isolated salamander rod outer segments. J Gen Physiol. 1991 Sep;98(3):479–495. doi: 10.1085/jgp.98.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leser G. P., Nicoll D. A., Applebury M. L. Distinctive properties of the purified Na-Ca exchanger from rod outer segments. Ann N Y Acad Sci. 1991;639:222–233. doi: 10.1111/j.1749-6632.1991.tb17309.x. [DOI] [PubMed] [Google Scholar]
- Sather W. A., Detwiler P. B. Intracellular biochemical manipulation of phototransduction in detached rod outer segments. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9290–9294. doi: 10.1073/pnas.84.24.9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetkamp P. P., Basu D. K., Szerencsei R. T. Na+-Ca2+ exchange in bovine rod outer segments requires and transports K+. Am J Physiol. 1989 Jul;257(1 Pt 1):C153–C157. doi: 10.1152/ajpcell.1989.257.1.C153. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P., Szerencsei R. T., Basu D. K. Unidirectional Na+, Ca2+, and K+ fluxes through the bovine rod outer segment Na-Ca-K exchanger. J Biol Chem. 1991 Jan 5;266(1):198–206. [PubMed] [Google Scholar]
- Schnetkamp P. P., Szerencsei R. T. Effect of potassium ions and membrane potential on the Na-Ca-K exchanger in isolated intact bovine rod outer segments. J Biol Chem. 1991 Jan 5;266(1):189–197. [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]