Abstract
Excessive iron deposition can lead to ferroptosis, a form of iron-dependent cell death detrimental to neuronal survival. Microglia have been identified as having a high capacity for iron deposition, yet it remains unclear whether microglia undergo ferroptosis while phagocytosing excessive amounts of iron after spinal cord injury (SCI). Here, we observed scattered iron around the epicenter of the injured spinal cord at 7 days post-injury (dpi) in mice, which then accumulated in the lesion core at 14 dpi. Concurrently, microglia exhibited elevated expression of the iron-storage protein ferritin and were found to undergo ferroptosis between 7 and 28 dpi. Additionally, we noted a gradual decrease in glycosylated lysosomal membrane protein (GLMP) which is associated with iron metabolism in microglia undergoing ferroptosis. In situ injection of AAV9-Cx3cr1-shGlmp-eGFP to knock down GLMP specifically in microglia resulted in a significant increase in iron deposition and ferroptosis, leading to an expanded lesion area, aggravated neuronal loss, and subsequent inhibition of functional restoration. Our findings highlight the crucial role of GLMP in mitigating iron overload and ferroptosis in microglia, thereby contributing to axon retention and locomotor recovery after SCI.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-86991-z.
Keywords: Spinal cord injury, Ferroptosis, Iron deposition, Microglia, GLMP
Subject terms: Cell biology, Neuroscience, Molecular medicine, Neurology
Introduction
Spinal cord injury (SCI) leads to bleeding and demyelination, which are primary sources of iron1–3. Excessive iron deposition can trigger ferroptosis, a procedural cell death, which significantly impairs neuronal function3,4. Preventing ferroptosis after SCI is crucial in reducing secondary damage, promoting neuronal survival, and exerting a neuroprotective effect, thereby offering insights into functional recovery2,5–7. Two major pathways associated with ferroptosis include the iron metabolism pathway, which regulates the uptake, storage, utilization, and release of iron within cells to maintain iron homeostasis. Disruption of this pathway can lead to iron overload and subsequent ferroptosis. The second pathway involves the cystine/glutamate antiporter (System Xc−), glutathione peroxidase 4 (GPX4) and glutathione (GSH) (System Xc−-GSH-GPX4 pathway), which involves sustaining GSH synthesis, increasing the activity of system Xc−, or promoting GPX4 to prevent cells from ferroptosis induced by various oxidative stress conditions8,9. Given the pivotal role of iron deposition in triggering ferroptosis and leading to impaired functional recovery, strategies for reducing iron deposition after SCI are highly desirable.
Microglia, the resident macrophages of the central nervous system, are known to accumulate around the lesion site after SCI10,11 and play a crucial role in regulating neuroinflammation and repairing damaged spinal cord tissue10,12,13. Notably, microglia are involved in phagocytosis, and possess strong iron storage capacities, accumulating iron in various central nervous system diseases14,15. Additionally, microglia may scavenge excess iron from degenerative neurons, glia and hematogenous macrophages16. However, it remains uncertain whether microglia experience iron overload and are sensitive to ferroptosis after SCI.
Glycosylated lysosomal membrane protein (GLMP) is markedly expressed in disease-associated microglia17, and plays a vital role in reducing iron deposition in Kupffer cells during liver fibrosis18,19. However, it remains to be clarified whether GLMP is involved in the iron metabolism of microglia and how it affects ferroptosis after SCI.
In this study, we aimed to investigate (i) whether microglia accumulate significant iron and exhibit ferroptosis signatures after SCI, and (ii) whether GLMP can reduce iron deposition and ferroptosis in microglia, thereby contributing to tissue repair in the injured spinal cord.
Materials and methods
Animals
All animal experiments were conducted according to the guidance of the Ethics Committee of Anhui Medical University (Approval No. LLSC20220381)). Female C57BL/6J mice, aged 8–10 weeks and weighing 17–22 g, were obtained from Hangzhou Ziyuan Laboratory Animal Technology Co., Ltd and housed in a controlled environment with a 12:12 h light-dark cycle, appropriate temperature and humidity. The mice were randomly kept in standardized cages with ad libitum access to water and food.
Crush spinal cord injury model
Adult mice were anaesthetized with pentobarbital sodium (2%, 50 mg/kg, P-010, Sigma, USA) by intraperitoneal injection. A skin incision was made at T9–T11, and laminectomy was performed. The T10 spinal cord was compressed for 5 s with calibrated Dumont #5 forceps (11252-20, Fine Science Tools, Germany), resulting in a clear red line at the injured site. The skin and muscles around the wound were then stitched by 4–0 silk thread. The mice were monitored in an incubator (22–24 °C) until completely awakening. After SCI surgery, bladder evacuation was manually performed twice daily.
Tissue processing
Mice were anaesthetized with 2% pentobarbital sodium via an intraperitoneal injection before injury (Pre) or at days 3, 7, 14, 28 after SCI (or at 14 and 28 days after in situ injection of adeno-associated virus (AAV). Blood was removed via cardiac perfusion with 0.1 M phosphate-buffered saline (PBS). For western blot analysis, a 5 mm segment of the spinal cord centered on the lesion core was excised. For histology, transcardiac perfusion was performed using PBS, followed by 4% paraformaldehyde (PFA, G1101, Servicebio, China). A 6 mm segment of the spinal cord containing the injury epicenter was harvested, fixed, dehydrated, and embedded in paraffin. Sagittal sections of 6 μm thickness were obtained for histological procedures20. For transmission electron microscope (TEM) analysis, after perfusion with PBS and 4% PFA, 1 mm segment of the spinal cord centered on the lesion core was excised and fixed in electron microscope fixative (G1102, Servicebio, China) for 2 h at room temperature, followed by overnight at 4 °C.
Prussian blue staining
Staining with a Prussian Blue Iron Stain Kit (G1422, Solarbio, China) was conducted according to standard procedures. First, spinal cord sections were dried, dewaxed, hydrated and washed twice with distilled water. Second, sections were immersed in Perl’s stain for 30 min and thoroughly rinsed with distilled water for 3 min. Next, the nuclei were lightly stained with a nuclear fast red solution for 5 min and then washed twice with distilled water. Conventionally, the sections were dehydrated, transparentized, and sealed with neutral resin. Stained sections were examined and photographed using bright-field microscopy (Axio Scope A1, Zeiss). Hemosiderin or ferric iron appeared blue, while the cytoplasm and cellular nucleus exhibited red and pink.
Immunofluorescence staining
After heat-mediated antigen retrieval, spinal cord sections were blocked with 10% normal donkey serum (DSA, SL050, Solarbio, China) in PBS supplemented with 0.3% Triton X-100 (GC204003, Servicebio) at room temperature for 1 h, followed by overnight incubation at 4 °C with primary antibodies, diluted in 1% DSA containing 0.3% Triton X-100. The primary antibodies were as follows: rabbit anti-C-X3-C motif chemokine receptor 1 (CX3CR1, 1:200, NBP1-76872, Novus, USA), mouse anti- Ferritin heavy chain 1 (FTH1, 1:500, MAB9354JF646, Novus), mouse anti-Cyclooxygenase-2 (COX2, 1:600, 66351-1-Ig, Proteintech, China), rabbit anti-Acyl-CoA synthetase long-chain family member 4 (ACSL4, 1:200, DF12141, Affinity), rabbit anti-GLMP (1:200, bs-15078R, Bioss), goat anti-Ionized Calcium Binding Adaptor Molecule 1 (Iba1, 1:500, 011-27991, wako, Japan), rat anti-glial fibrillary acidic protein (GFAP, 1:200, 13-0300, Thermo Fisher Scientific, USA), rabbit anti-neuronal nuclei (NeuN, 1:500, ab177487, Abcam, UK), and goat anti-5-hydroxytryptamine (5-HT, 1:5000, #20080, Immunostar, USA). Sections were washed 3 times with PBS and then incubated for 1 h at room temperature with relevant fluorescent secondary antibodies, which were diluted in 1% DSA in PBS containing 0.3% Triton X-100. The corresponding secondary antibodies used were as follows: donkey anti-mouse Alexa Fluor 488, donkey anti-rabbit Alexa Fluor 488, donkey anti-goat Alexa Fluor 488, donkey anti-rabbit Alexa Fluor 555, donkey anti-goat Alexa Fluor 555, and donkey anti-rat Alexa Fluor 647 (1:500, A-21202, A-21206, A-11055, A-31572, A-21432, A-78947, Thermo Fisher Scientific). The sections were washed 3 times with PBS and stained with 4′, 6-diamidino-2-phenylindole (DAPI) (1 µg/ml, Thermo Fisher Scientific) to mark the nuclei. Stained sections were examined and photographed using confocal microscope (LSM 900, Zeiss, Germany) and fluorescent microscope (Axio Scope A1, Zeiss).
Transmission electron microscope
Tissue embedding, slicing and TEM image acquisition were conducted by Servicebio. Samples underwent post-fixation, washing, dehydration, embedding, and polymerization. Sagittal ultrathin sections of 70 nm were cut using an ultramicrotome (EM UB7, Leica) and observed in a transmission electron microscope (HT 7800, Hitachi). Images of mitochondria in microglia were acquired around the margin of the lesion core. Under TEM, microglia displayed a rich presence of lysosomal apparatuses, numerous lipofuscin-like structures, lipid droplets and multilaminated bodies. The nuclei exhibited characteristic oval, round, or sometimes triangular shapes with dense and highly clumped heterochromatin23,24. Injured mitochondria became damaged, swollen or shrunken, and mitochondrial protrusions were reduced or absent. The mitochondrial outer membrane showed damage, accompanied by an increase in membrane density25–27. The damaged and total mitochondria in microglia were counted under a 10,000× magnification.
Western blot
Total protein from 293T cells and spinal cords was extracted using radio immunoprecipitation assay buffer (P0013B, Beyotime Biotechnology, China), supplemented with protease inhibitor cocktail (04693124001, Roche, Switzerland). The protein was quantified with a BCA kit (BL521A, Biosharp, China) and diluted to 1 mg/ml. Protein samples (20 µl, 20 µg) were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Germany). The membranes were blocked with 5% skim milk for 3 h and then incubated with primary antibodies overnight at 4 °C. The primary antibodies were rabbit anti-GLMP (1:1000, bs-15078R, Boiss, China), mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:2000, Proteintech, 60004-1-Ig) and mouse anti-β-tubulin (1:10000, T0023, Affinity, China). The membranes were then probed with the corresponding HRP-conjugated secondary antibodies for 1 h at room temperature, including goat anti-mouse (1:10000, A4416, Sigma) or goat anti-rabbit (1:10000, A0545, Sigma, USA) secondary antibodies. The protein band signals were obtained using a Tanon system (UVP Chemstudio 515, Analytik Jena, Germany) and quantified with ImageJ/Fiji.
Selection of shRNA sequence and construction of adeno-associated virus vectors
Plasmids were produced by Genomeditech (China). The optimal shRNA sequence for Glmp knockdown was determined using 293T cells (SCSP-502) transfected by shRNA plasmids, namely shRNA120 (#1), shRNA492 (#2), shRNA675 (#3), and a negative control (Control). GMTrans Liposomal Transfection Reagent (TG-10015-2, Genomeditech) was added for transfection according to the manufacturer’s protocol. After successful transfection, western blotting was performed to identify the most effective shRNA sequence for Glmp knockdown21 (refer to Supplementary Figs. 1 and 2). Then the relevant adeno-associated virus (AAV) vectors AAV9-Cx3cr1-shGlmp-eGFP (AAV-shGlmp) and AAV9-Cx3cr1-Control-eGFP (AAV-Control) were constructed with sequences TTCTCCGAACGTGTCACGT (from #2) and TGCCAACCTGAGTGCCGATTT (from Control), respectively. The AAVs were synthesized by Genomeditech. Primer sequence for Cx3cr1 was as follows: 5′-GGGAACATACGTCATTATTG-3′. The virus titre of AAVs was 1.17E + 12 VG/ml.
In situ injection of AAV
1 µl of AAV-shGlmp or AAV-Control was delivered into the lesion core of the injured spinal cord by a microinjection needle (7634-01, Hamilton, Switzerland) at a rate of 0.5 µl/min, this process was conducted by a stereotaxic injector (KDS LEGATO 130, RWD, China)22. The injection site was located at the lesion core, 0.2 mm right to the posterior midline, and 0.8 mm deep to the dorsal surface of the mouse spinal cord. All mice were injected immediately following SCI and were sacrificed at either 14 days post-injury (dpi) or 28 dpi.
Behavioral assessments
Locomotor function of mice was evaluated using Basso Mouse Scale (BMS) scores and footprint test. Before the behavioral analysis, mice were allowed to practice crawling freely in an open field for 1 h. The behavioral tests and assessments were conducted by two experienced researchers. The BMS scores were evaluated before SCI and at 3, 7, 14, 21 and 28 dpi in a wide field, with 8 mice per group28,29. The final score for each mouse was calculated on the average of the scores given by the two researchers. Footprint test was carried out at 28 dpi. Mice were encouraged to walk along a straight line on a white paper for three times, with their fore and hind paws marked with green and red dye, respectively. Paw rotation, stride length, and stride width were measured and analyzed to assess locomotor function, following established methodologies28,30.
Image acquisition and quantitative analysis
Quantitative analyses focused on three sagittal sections per mouse, each containing lesion core, in accordance with established procedures31. Images were captured with Zen 3.3 software (Blue edition) and quantitative analysis was performed using ImageJ/Fiji software (National Institutes of Health, USA).
To quantify the area of iron deposition, Prussian blue staining area was normalized to the area of the spinal cord segment encompassing the lesion core in either 20× or 10× image. Three images were taken under a ×20 objective lens at the rostral, epicentral, and caudal region of sagittal spinal cord sections, respectively. And the blue-stained area was delineated using ImageJ/Fiji software. The percentage was calculated by dividing the blue-stained area by the total spinal cord area in the image2.
To quantify the numbers of FTH1+CX3CR1+, COX2+CX3CR1+, ACSL4+CX3CR1+, GLMP+CX3CR1+, GLMP+Iba1+ cells, and the number of CX3CR1+, Iba1+ cells, three regions surrounding the margin of the lesion core in each sagittal section were captured at ×40 magnification using the Zeiss Axio Scope A1 or Zeiss LSM 900 confocal microscope. Subsequently, 20 μm square grids were applied, and only DAPI+ cells in every 6th square were counted. For each mouse, three sections containing the lesion site were used for cell counting, with a total of 3 mice per group.
To evaluate the extent of injury, GFAP− area was normalized to the area of the spinal cord segment encompassing the lesion core in a 10× image. To assess the fluorescence intensity of retained 5-HT axon at the lesion core, threshold regions of 5-HT were measured through threshold processing.
Neurons preserved in the injured area was observed and quantified by NeuN staining. The sections were devided into 3 zones according to the distance away from the lesion edge: Z1 (0–250 μm), Z2 (250–500 μm), and Z3 (1000–1250 μm), basing on a previous description32. NeuN+ neurons in Z1-Z3 zones were counted with ImageJ/Fiji and expressed as per mm2.
Statistical analysis
Data were presented as mean ± standard error of the mean (SEM). Differences among multiple groups were analyzed using one-way analysis of variance (ANOVA), or two-way ANOVA followed by Tukey’s post hoc test correction. Unpaired two-tailed Student’s t test were used for comparisons between two groups. When p < 0.05, the analysis was considered as statistically significant. Shapiro–Wilk test was performed to determine the normality of data distribution. All data underwent these normality tests and were found to exhibit a normal/Gaussian distribution. All data were analyzed using GraphPad Prism version 9.5.0 software (USA).
Results
Iron deposition increases significantly in the injured spinal cord, accompanied by iron deposition in most microglia
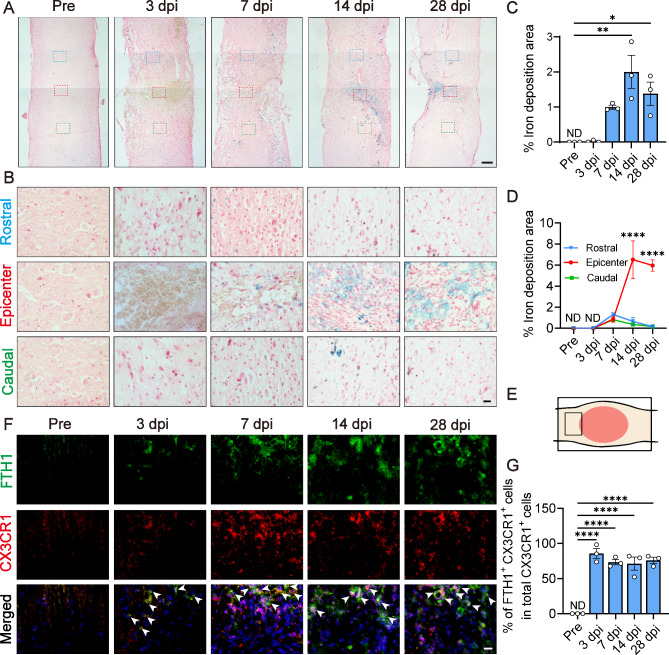
To delineate the temporal and spatial dynamics of iron deposition after SCI, we employed Prussian blue staining across various time points: pre-injury (pre) and at 3, 7, 14 and 28 dpi. Our analysis revealed a progressive accumulation of iron from 7 dpi, with a discernible peak at 14 dpi, where iron concentration reached 2% ± 0.47% within the lesion epicenter. By 28 dpi, iron levels had subsided, particularly in the perilesional areas (Fig. 1A–D). These results confirm the notion of a pronounced iron deposition in the lesion core after SCI.
Fig. 1.
Iron deposition increases in the injured spinal cord, coinciding with an elevated expression of the iron associated protein FTH1 in microglia. (A) Prussian blue stains the iron in sagittal sections pre-injury (pre) and at 3, 7, 14 and 28 days post-injury (dpi). The blue, red and green boxes represent the regions of interest (ROI) in rostral, epicenter and caudal to the lesion site, respectively. Asterisks indicate the lesion core. Scale bar: 200 μm. (B) is the ROI from (A), scale bar: 20 μm. (C) Percentage of iron deposition area relative to the injured spinal cord area pre and at 3, 7, 14 and 28 dpi. ND is short for not detected. (Data in (C) are presented as mean ± SEM, n = 3 independent experiments. *p < 0.05, **p < 0.01, one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test). (D) Proportion of iron deposition area in rostral, epicenter and caudal of the injured spinal cord. (Data in (D) are presented as mean ± SEM, n = 3 independent experiments. ****p < 0.0001, two-way ANOVA followed by Tukey’s post hoc test). (E) Schematic of spinal cord indicates measurement area. The box area is the region shown in (F). (F) Immunofluorescence staining of FTH1 (green), CX3CR1 (red) and nuclei (blue) in sagittal slices pre and at 3, 7, 14 and 28 dpi. Merged shows the merged images of FTH1, CX3CR1, and nuclei. White arrowheads point to the co-staining cells. Scale bar: 20 μm. (G) Quantification of the proportion of FTH1+CX3CR1+ cells in CX3CR1+ cells pre and at 3, 7, 14 and 28 dpi. (Data are presented as mean ± SEM, n = 3 independent experiments. ****p < 0.0001, one-way ANOVA followed by Tukey’s post hoc test).
At 14 dpi, blood-derived macrophages were predominantly localized within the lesion core, whereas microglia were distributed around the margin of lesion site11. Given the well-documented iron-processing capabilities of microglia33, we hypothesized that the observed iron clearance from the lesion periphery could be attributed to the efficient iron handling by microglia. To investigate this, we utilized immunofluorescence staining to mark microglia with CX3CR111,34 and ferritin with FTH1, the heavy chain of the iron-storage protein35,36. The analysis of the co-staining of FTH1 and CX3CR1 at the lesion periphery revealed extensive iron sequestration within microglia from 3 to 28 dpi (Fig. 1E–G), underscoring their substantial role in iron storage.
Microglia exhibit ferroptosis after SCI
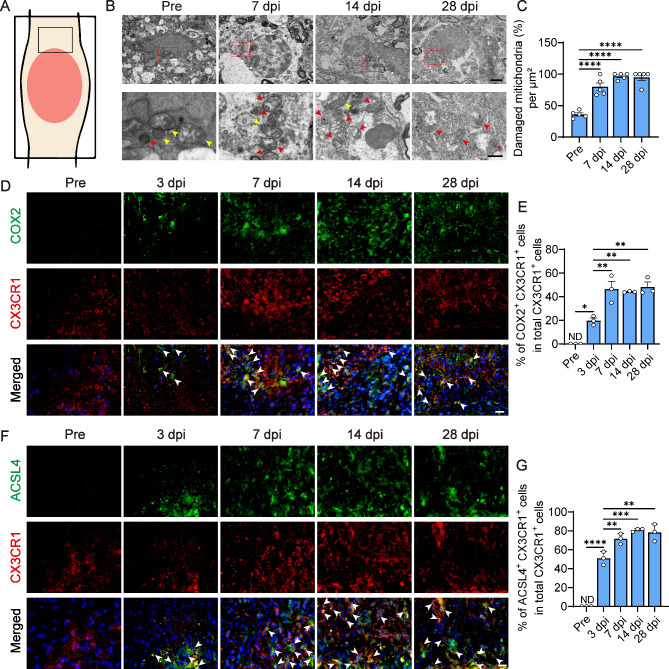
The phenomenon of ferroptosis, an iron-dependent form of cell death, has been documented in vitro within microglia14,37. However, the occurrence of ferroptosis in vivo within microglia after SCI remains unexplored. Ferroptosis is characterized by marked increases in COX2 and ACSL4 expression38–40, alongside morphological changes in mitochondria, including shrinking, swelling, or damage, which are considered definitive indicators of this process2,41. To examine these features, we utilized TEM to assess the ultrastructure of mitochondria and observed that, at 7, 14 and 28 dpi, the majority of microglial mitochondria became swollen and injured, with a paucity of normal mitochondria (Fig. 2B,C). Furthermore, immunofluorescence staining for COX2 and ACSL4 indicators of ferroptosis, and CX3CR1, a microglial marker, revealed the onset of ferroptosis in the injured spinal cord as early as 3 dpi. A significant proportion of microglia exhibited ferroptosis signatures, which increased progressively from 7 to 28 dpi (Fig. 2D–G). Collectively, these data reveal that microglia not only accumulate iron but also undergo ferroptosis following SCI, with this pathological state persisting over an extended period.
Fig. 2.
Ferroptosis occur in microglia after SCI. (A) Schematic of spinal cord indicates that the boxed area is the source region for images in (B,D,F). (B) Transmission electron microscope (TEM) is used to detect the ultrastructure of mitochondria in microglia pre and at 7, 14, 28 dpi. Red boxes represent the regions of interest (ROI) in the low magnification image above. Red arrows point to damaged mitochondria, while yellow arrows point to normal mitochondria. Scale bars: low magnification, 2 μm; higher magnification, 500 nm. (C) Proportion of damaged mitochondria per µm2 in (B). (Data are presented as mean ± SEM, n = 5 regions per group, ****p < 0.0001 by one-way ANOVA followed by Tukey’s post hoc test.) (D) Immunofluorescence staining of COX2 (green), CX3CR1 (red) and nuclei (blue) pre and at 3, 7, 14, 28 dpi. Merged shows the merged images of COX2, CX3CR1, and nuclei. White arrows point to co-stained cells. Scale bar: 20 μm. (E) Quantification of the proportion of COX2+CX3CR1+ cells in CX3CR1+ cells pre and at 3, 7, 14, 28 dpi. (Data are presented as mean ± SEM, n = 3 mice per group, *p < 0.05, **p < 0.01, by one-way ANOVA followed by Tukey’s post hoc test.) (F) Immunofluorescence staining of ACSL4 (green), CX3CR1 (red) and nuclei (blue) pre and at 3, 7, 14, 28 dpi. Merged shows the merged images of ACSL4, CX3CR1, and nuclei. White arrows point to co-stained cells. Scale bar: 20 μm. (G) Quantification of the proportion of ACSL4+CX3CR1+ cells in CX3CR1+ cells pre and at 3, 7, 14, 28 dpi. (All images are from sagittal sections, ND is short for not detected, data are presented as mean ± SEM, n = 3 mice per group, **p < 0.01, ***p < 0.001, ****p < 0.0001 by one-way ANOVA followed by Tukey’s post hoc test.)
GLMP expression dynamics after SCI and its colocalization with microglia
Given the significant expression of GLMP in disease-associated microglia17 and its crucial role in mediating iron deposition18, our investigation aimed to uncover the involvement of GLMP in microglial iron metabolism and its impact on ferroptosis after SCI. Western blotting was employed to monitor changes in GLMP expression pre- and post-SCI. The results indicate a notable decrease in GLMP concentration at 3 dpi, followed by a subsequent increase at 14 dpi, ultimately returning to near-normal levels by 28 dpi (Fig. 3A,B). To determine the extent of GLMP expression in microglia, GLMP was co-stained with CX3CR1 and Iba1 markers. Remarkably, a substantial proportion of microglia expressed GLMP both before and after SCI. However, the expression of GLMP in spinal cord tissue was not completely consistent with the extent of it in microglia, which may be attributed to other cells containing GLMP. The proportion of GLMP-expressing microglia decreased from 3 to 28 dpi (Fig. 3C–G), contrary to the observed gradual increase in microglial ferroptosis during the same period (Fig. 2). These findings suggest a potential involvement of GLMP in microglial iron processing and ferroptosis after SCI.
Fig. 3.
Expression of GLMP in the spinal cord and microglia after SCI. (A) Western blot is utilized for detecting the expression of GLMP pre- and post-SCI. (B) Quantitative analysis of GLMP in (A). (Data are presented as mean ± SEM, n = 4 mice per group, **p < 0.01 by one-way ANOVA followed by Tukey’s post hoc test.) (C) Schematic indicates the source area of pictures in (D,F). (D) Immunofluorescence staining of GLMP (green), CX3CR1 (red) and nuclei (blue) in sagittal sections of the spinal cord pre and at 3, 7, 14, and 28 dpi. White arrows point to co-stained cells. Scale bars: low magnification, 100 μm; higher magnification, 20 μm. (E) Quantification of the proportion of GLMP+CX3CR1+ cells in CX3CR1+ microglia. (Data are presented as mean ± SEM, n = 3 mice per group, *p < 0.05, **p < 0.01 by one-way ANOVA followed by Tukey’s post hoc test.) (F) Immunofluorescence staining of GLMP (green), Iba1 (red) and nuclei (blue) in sagittal sections of the spinal cord pre and at 3, 7, 14, and 28 dpi. White arrows point to co-stained cells. Scale bar: 20 μm. (G) Quantification of the proportion of GLMP+Iba1+ cells in Iba1+ microglia. (Data are presented as mean ± SEM, n = 3 mice per group, **p < 0.01 by one-way ANOVA followed by Tukey’s post hoc test.)
Glmp knockdown exacerbates iron overload and ferroptosis in microglia
To elucidate the role of GLMP in microglia, we employed AAV-Cx3cr1-shGlmp-eGFP (AAV-shGlmp) to specifically knockdown Glmp expression in microglia, following established procedures42,43. The AAV-shGlmp was in situ injected into lesion site immediately after SCI. Immunofluorescence staining performed at 14 days after AAV injection confirmed the successful inhibition of GLMP expression in microglia by AAV-shGlmp (Fig. 4A). Specifically, in situ injection of AAV-shGlmp resulted in a depletion of GLMP+Iba1+ cells, corroborating the efficacy of AAV-shGlmp (Fig. 4B,C).
Fig. 4.
Glmp knockdown results in increased iron deposition and ferroptosis in microglia after SCI. (A) Experimental scheme. AAV-Cx3cr1-shGlmp-eGFP (AAV-shGlmp) or AAV-Cx3Cr1-Control-GFP (AAV-Control) are injected into lesion site immediately after SCI. Immunofluorescence staining is performed at 14 and 28 dpi, and footprint analysis is carried out at 28 dpi. (B) Immunofluorescence staining of GLMP (green), Iba1 (red) and nuclei (blue) in sagittal sections at 14 dpi. Merged shows the merged images of GLMP, Iba1, and nuclei. White arrows point to co-stained cells. Scale bar: 20 μm. (C) Quantification of the proportion of GLMP+Iba1+ cells in Iba1+ microglia in (B). (D) Prussian blue stains the iron in AAV-shGlmp and AAV-Control groups at 14 dpi. Asterisks indicate the lesion core. Red boxes represent the regions of interest (ROI) at the lesion site. Scale bars: low magnification, 100 μm; higher magnification, 20 μm. (E) Percentage of iron deposition area relative to the injured spinal cord area in AAV-shGlmp and AAV-Control groups. (F) Single-plane confocal images of immunofluorescence staining of CX3CR1 (red) and FTH1 (green) in AAV-shGlmp and AAV-Control groups at 14 dpi. Merged shows the merged images of FTH1, CX3CR1, and nuclei. Scale bar: 20 μm. (G) Quantification of microglia expressing FTH1 in (F). (H) TEM detects ultrastructure of mitochondria in microglia in AAV-shGlmp and AAV-Control groups. Red boxes represent the regions of interest (ROI) in the low magnification image above. The red arrows point to damaged mitochondria while the yellow ones to normal mitochondria. Scale bars: low magnification, 2 μm; higher magnification, 500 nm. (I) Proportion of damaged mitochondria in per µm2. (J) Immunofluorescence showing labeling of microglia with CX3CR1 (red) and ferroptosis with COX2 (green) in AAV-shGlmp and AAV-Control groups at 14 dpi. Merged shows the merged images of COX2 and CX3CR1. Asterisks indicate the lesion core. Scale bar: 50 μm. (K) Quantification of percentage of COX2+CX3CR1+ cells in total CX3CR1+ microglia at (J). (All images are from sagittal sections at 14 dpi. White arrows point to co-stained cells. Data are presented as mean ± SEM, n = 3 mice per group, *p < 0.05, **p < 0 0.01 by unpaired two-tailed Student’s t test.)
After treatment with AAV-shGlmp, there was a notable increase in iron deposition within the injured tissue, as evidenced by Prussian blue staining (Fig. 4D,E). Immunofluorescence analysis of FTH1 and CX3CR1 in spinal cord sections at 14 dpi revealed a marked accumulation of iron in microglia within the AAV-shGlmp group (Fig. 4F,G). Notably, microglial mitochondria appeared swollen and damaged to a greater extent in AAV-shGlmp group (Fig. 4H,I). Additionally, co-staining of COX2 and CX3CR1 highlighted a high prevalence of COX2+CX3CR1+ microglia in the AAV-shGlmp group, indicating a pronounced ferroptosis signature (Fig. 4J,K). Taken together, these results support the notion that Glmp knockdown induces iron overload and ferroptosis in microglia, underscoring the vital role of GLMP in microglial ferroptosis.
GLMP knockdown leads to compromised neuronal retention and subsequent impaired functional recovery after SCI
We further examined the impact of GLMP knockdown on neurological outcomes and functional repair following SCI. The GFAP− lesion size was larger in mice treated with AAV-shGlmp compared to those in the AAV-Control group (Fig. 5A,B). In addition, within the AAV-shGlmp group, there was reduced intensity of 5-HT+ axons retention in injured area (Fig. 5A,C), and a modest decrease in retained NeuN+ neurons in the Z1 region (Fig. 5D,E). Moreover, mice with GLMP knockdown in CX3CR1+ microglia displayed lower BMS scores, shorter strides, and larger paw rotation compared to the AAV-Control group, indicative of substantially poorer locomotor functional recovery after SCI (Fig. 5F–J). Collectively, these findings indicate that GLMP plays a critical role in axonal retention, neuronal survival, and locomotor functional recovery after SCI.
Fig. 5.
Glmp knockdown in microglia leads to expansion of damaged area, aggravated neuronal loss, and subsequent inhibition of functional restoration after SCI. (A) Immunofluorescence staining of GFAP (green) and 5-HT (red) in AAV-shGlmp and AAV-Control groups. R is short for rostral, C is short for caudal, D is short for dorsal, and V is short for ventral. Right images show ROI from the box on left images. Scale bars: low magnification, 100 μm; higher magnification, 20 μm. (B) Quantification of percentage of GFAP− area in (A). (Data are presented as mean ± SEM, n = 3 mice per group, *p < 0.05 by unpaired two-tailed Student’s t test.) (C) Quantification of percentage of 5-HT intensity in (A). (Data are presented as mean ± SEM, n = 3 mice per group, *p < 0.05 by unpaired two-tailed Student’s t test.) (D) Representative immunofluorescence images of GFAP (green) and NeuN (red) indicate that NeuN+ neurons in Z1–Z3 zones adjacent to epicenter at 14 dpi. Scale bar: 50 μm. (E) Quantification of the number of NeuN+ neurons in Z1–Z3. (All images are from sagittal sections at 28 dpi. Data are presented as mean ± SEM, n = 3 mice per group, *p < 0.05 by unpaired two-tailed Student’s t test.) (F) BMS scores are counted and analyzed at different time points. *p < 0.05 and **p < 0.01 vs. AAV-Control group by two-way ANOVA, n = 8 animals per group. (G) Representative footprints of uninjured, AAV-Control, and AAV-shGlmp groups at 28 dpi. The green marks are the front paw prints, and the red ones are the hind paws prints. (H,I,J) Quantification of the stride length (H), stride width (I) and paw rotation (J) at 28 dpi. (Data are presented as mean ± SEM, n = 8 mice per group, *p < 0.05, ***p < 0.001, ****p < 0.0001 by one-way ANOVA.)
Discussion
Our findings unveil the dynamic temporal and spatial distribution of iron within injured tissue following SCI. Initially, iron is dispersed at 7 dpi, subsequently accumulating within the lesion site, peaking at 14 dpi. Notably, we observed a significant capacity for iron storage within microglia. The presence of the iron-storage protein ferritin increased in microglia after SCI, coinciding with a period of microglial ferroptosis between 7 and 28 dpi. Furthermore, a considerable proportion of microglia expressed GLMP, a key regulator of iron metabolism. Knockdown of Glmp in microglia resulted in heightened iron deposition and increased susceptibility to ferroptosis, exacerbating tissue iron deposition. These changes led to the expansion of the damaged area, aggravated neuronal loss, impaired axonal retention, and hindered motor function recovery (Fig. 6). Our results indicate that GLMP plays a vital role in tissue repair after SCI by modulating iron metabolism and inhibiting ferroptosis in microglia.
Fig. 6.
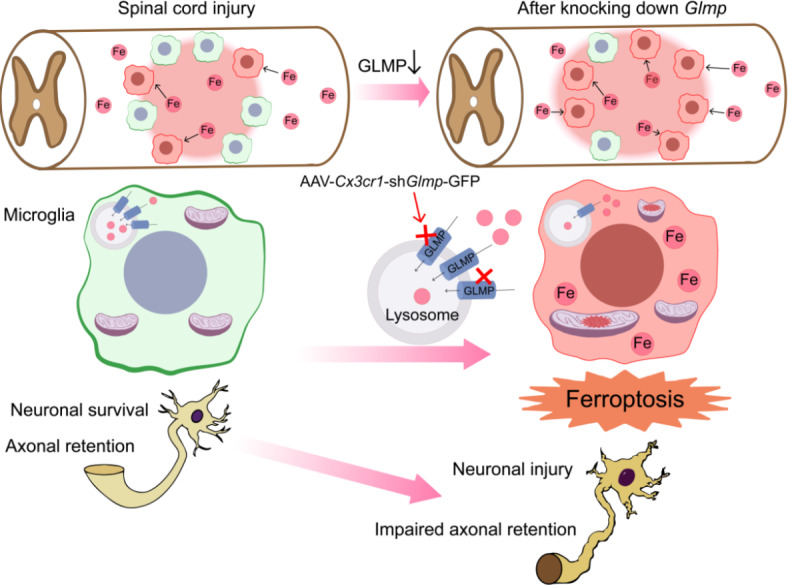
Schematic representation the role of Glmp in iron overload and ferroptosis in microglia after SCI. After SCI, iron accumulates around the lesion site and is taken by microglia. This causes damage to mitochondria, resulting in microglial ferroptosis. GLMP plays a vital role in reducing iron overload and preventing ferroptosis in microglia. Knockdown of GLMP in microglia exacerbates iron overload and ferroptosis, leading to neuronal injury and impaired axonal retention.
SCI leads to hemorrhage and demyelination, both of which are primary sources of iron1. By 14 dpi, the majority of hemorrhage and hemoglobin from the initial injury have been cleared through the coordinated actions of infiltrating macrophages and activated microglia. At this stage, the injury core is predominantly characterized by inflammatory cell infiltration and scar formation, rather than residual hemorrhagic debris1. Importantly, our study identifies FTH1 + CX3CR1 + microglia as the primary cells responsible for iron deposition at 14 dpi44. This suggests that the observed iron accumulation is not non-specific staining of erythrocyte remnants but rather indicative of microglial iron sequestration. These findings are consistent with the known role of microglia in regulating iron homeostasis and mitigating ferroptosis after SCI44. By 28 dpi, the reduced staining intensity reflects the gradual resolution of active inflammation and tissue remodeling processes.
Recent studies have demonstrated that alleviating iron overload and ferroptosis can contribute to the functional recovery after SCI45. Yao et al. discovered that transplantation of mesenchymal stem cells at the injury site attenuates neuronal ferroptosis by promoting mitochondrial transfer46. Ryan et al. constructed a tri-culture system consisting of microglia, neurons and astrocytes, and found that microglia exhibited greater sensitivity to iron dysregulation compared to astrocytes and neurons. They further identified the gene SEC24B as being positively related to microglia ferroptosis and showed that knocking out SEC24B alleviated ferroptosis in human immortalized microglia14. In another study, Feng et al. observed a substantial increase that iron deposition within motor cortex post-SCI. They illustrated that iron metabolism associated proteins such as ferroportin1, transferrin receptor1, divalent metal transporter 1, and iron regulatory protein 1 were upregulated in response to ferroptosis. This indicates that ferroptosis leads to a compensatory increase in iron metabolism in the motor cortex after SCI47. Since iron acts as a trigger for ferroptosis, Yao et al. and Sauerbeck et al. used the iron chelators deferoxamine and deferasirox, respectively, to reduce iron deposition and ameliorate neurological function, underscoring the potential of inhibiting iron deposition and ferroptosis to promote SCI repair7,48. Additionally, studies by Ge at al. revealed that zinc prevented ferroptosis by reducing reactive oxygen species (ROS) production and inflammation, while upregulating the expression of GSH and GPX4 in contusion SCI6. Another research group also unveiled that ferrostatin-1 repressed ROS and attenuated mitochondrial lipid peroxidation after SCI2. Despite these advancements, the specific spatial patterns of iron deposition after SCI remain underexplored, as do the in vivo changes in ferroptosis among astrocytes, microglia, macrophages. Our study contributes to this understanding by mapping the progression of iron deposition and ferroptosis in microglia following SCI.
Rathore et al. assessed the expression of iron deposition in coronal sections using Perl’s histochemistry, finding that iron was dispersed throughout the lesion core at 7 dpi, and restricted to the central core of the lesion epicenter at 14 dpi1. Wang et al. performed Prussian blue staining to quantify temporal changes of iron-positive cells number, observing that iron deposition started at 3 dpi, and elevated at 7 and 14 dpi49, which was consistent with our work. In addition to temporal changes of iron deposition, we used mouse crush SCI model and examined the spatial pattern of iron with Prussian blue staining in sagittal sections of spinal cord pre-injury and at 3, 7, 14, 28 dpi, showing that iron scattered at 7 dpi and spread 3 cm from the lesion to rostral and caudal at 7 dpi, while accumulated within the lesion site after 14 dpi, providing a map for interventing iron deposition.
The role of microglia after SCI has been extensively investigated. These dynamic cells undergo extensive proliferation and exhibit notable changes within the initial 14 dpi, often accumulating around the lesion site. Studies by Bellver-Landete et al. utilized genetic mouse models (Cx3cr1creER::R26-TdT mice received tamoxifen treatment) along with depletion strategies employing the CSF1R inhibitors PLX5622 and PLX73086. Their findings revealed that depleting microglia resulted in poorer recovery outcomes, including a disorganized astroglial scar, reduced neuronal and oligodendrocyte survival, and impaired locomotor performance, suggesting an overall neuroprotective role of microglia after SCI10. Brennan et al. also employed PLX5622 to deplete microglia specifically within the spinal cord, and applied single-cell RNA sequencing and CellChat analysis to systematically reveal the time-dependent and cell-specific changes and predicted intercellular communication post-SCI. Their analysis explored potential mechanisms underlying these interactions, suggesting that microglia coordinate with the recruitment of macrophages and astrocytes to achieve optimal hemostasis, myelin and axon preservation, as well as myelin fragment phagocytosis. Moreover, their research indicated the presence of iron processing microglia at 7 dpi to 28 dpi, and observed an increase in the iron processing genes during that period, implying a relationship between microglia and iron metabolism, ferroptosis after SCI12. In this study, we found that a significant portion of microglia possess a remarkable capacity for iron storage between 3 dpi to 28 dpi and exhibit a ferroptosis signature, emphasizing a novel role of microglia in SCI. The observed clearance of iron outside the injured core may be attributed to the storage capacity of microglia, through the contributions of other cell types to this process cannot be excluded. The influence of microglial ferroptosis on their subtype transformation and neuroprotective function is an avenue requiring to further investigation.
Hakim et al. identified Glmp as unique to disease-associated microglia (DAM) in SCI, noting the presence of DAM from 3 dpi and to 90 dpi17. Disruption of GLMP in gt/gt transgenic mice has been shown to mitigate iron deposition in liver fibrosis18. Additionally, Liu et al. proved GLMP as a lysosomal membrane protein associated with decreasing lipid droplets in hepatocellular carcinoma. Mechanistically, GLMP mRNA ac4C modification can be induced by N-Acetyltransferase 10, triggering the activation of the MAPK/ERK signaling pathway50. Here, we observed a transient decrease in GLMP expression immediately following SCI, with a return to baseline by 28 dpi. By knocking down Glmp in microglia, we demonstrated GLMP’s critical function in diminishing iron overload and preventing microglial ferroptosis after SCI. Furthermore, the presence of GLMP proved essential for promoting tissue repair, enhancing neuronal survival, and facilitating functional recovery after SCI. However, the exact mechanisms by which GLMP modulates iron deposition and ferroptosis in microglia remain to be further explored.
Conclusion
Our finding demonstrates that microglia exhibit a robust capacity for iron accumulation and are susceptible to ferroptosis after SCI. Moreover, GLMP emerges as a pivotal factor in alleviating iron overload and ferroptosis within microglia, concurrently contributing to axon retention and promoting locomotor recovery. This highlights GLMP as a regulatory target for ferroptosis and offers a novel perspective for the treatment of SCI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks for the experimental platform provided by the Scientific Research and Experiment Center of the Second Affiliated Hospital of Anhui Medical University.
Author contributions
J.H.J., L.C. and M.G.Z. designed this study, administered the project and revised the manuscript. F.R.O.Y. carried out the experiments. J.J.L., J.X.H., J.N.Y. F.L.S., Y.Z.Z., and J.W.W. analyzed the data. Z.Y.L., S.S.Y., F.Y. and D.S.T. contributed to writing and revising the manuscript. The authors checked and approved the final version.
Funding
This work was subsidized by the National Natural Science Foundation of China (Nos. 82271413, 82372506), the Clinical Medical Research Transformation Project of Anhui Province (Nos. 202304295107020009, 202304295107020013, 202304295107020014 and 202304295107020011), the Anhui Provincial Natural Science Foundation (Nos. 2208085MH222, 2308085MH257, and 2308085QH266), and the Natural Science Research Project of Anhui Educational Committee (Nos. KJ2021A0310 and 2022AH050713, 2022AH050746).
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All animal experiments were approved by the Institutional Animal Ethical Committee of Anhui Medical University (No. LLSC20160052).
Competing interests
The authors declare no competing interests.
Statement
The study is reported in accordance with ARRIVE guidelines.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fangru Ouyang and Meige Zheng contributed equally to this work.
Contributor Information
Meige Zheng, Email: zhengmg113@126.com.
Li Cheng, Email: chengli7788@163.com.
Juehua Jing, Email: jjhhu@sina.com.
References
- 1.Rathore, K. I. et al. Ceruloplasmin protects injured spinal cord from iron-mediated oxidative damage. J. Neurosci.28, 12736–12747. 10.1523/JNEUROSCI.3649-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge, H. et al. Ferrostatin-1 alleviates white matter injury via decreasing ferroptosis following spinal cord Injury. Mol. Neurobiol.59, 161–176. 10.1007/s12035-021-02571-y (2022). [DOI] [PubMed] [Google Scholar]
- 3.Kang, Y. et al. Erythropoietin inhibits ferroptosis and ameliorates neurological function after spinal cord injury. Neural Regen. Res.18, 881–888. 10.4103/1673-5374.353496 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell149, 1060–1072. 10.1016/j.cell.2012.03.042 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi, Z. et al. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell. Prolif.54, e12992. 10.1111/cpr.12992 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge, M. H. et al. Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci. Ther.27, 1023–1040. 10.1111/cns.13657 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao, X. et al. Deferoxamine promotes recovery of traumatic spinal cord injury by inhibiting ferroptosis. Neural Regen. Res.14, 532–541. 10.4103/1673-5374.245480 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell. Biol.22, 266–282. 10.1038/s41580-020-00324-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David, S., Jhelum, P., Ryan, F., Jeong, S. Y. & Kroner, A. Dysregulation of iron homeostasis in the central nervous system and the role of ferroptosis in neurodegenerative disorders. Antioxid. Redox Signal.37, 150–170. 10.1089/ars.2021.0218 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Bellver-Landete, V. et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun.10, 518. 10.1038/s41467-019-08446-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang, X. et al. Macrophages in spinal cord injury: phenotypic and functional change from exposure to myelin debris. Glia63, 635–651. 10.1002/glia.22774 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan, F. H. et al. Microglia coordinate cellular interactions during spinal cord repair in mice. Nat. Commun.13, 4096. 10.1038/s41467-022-31797-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu, H. et al. Depletion of microglia exacerbates injury and impairs function recovery after spinal cord injury in mice. Cell. Death Dis.11, 528. 10.1038/s41419-020-2733-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan, S. K. et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci.26, 12–26. 10.1038/s41593-022-01221-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song, N., Wang, J., Jiang, H. & Xie, J. Astroglial and microglial contributions to iron metabolism disturbance in Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis.1864, 967–973. 10.1016/j.bbadis.2018.01.008 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Thomsen, M. S. et al. Neurodegeneration with inflammation is accompanied by accumulation of iron and ferritin in microglia and neurons. Neurobiol. Dis.81, 108–118. 10.1016/j.nbd.2015.03.013 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Hakim, R. et al. Spinal cord Injury induces permanent reprogramming of Microglia into a Disease-Associated State which contributes to functional recovery. J. Neurosci.41, 8441–8459. 10.1523/JNEUROSCI.0860-21.2021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong, X. Y. et al. Loss of lysosomal membrane protein NCU-G1 in mice results in spontaneous liver fibrosis with accumulation of lipofuscin and iron in Kupffer cells. Dis. Model. Mech.7, 351–362. 10.1242/dmm.014050 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schieweck, O. et al. NCU-G1 is a highly glycosylated integral membrane protein of the lysosome. Biochem. J.422, 83–90. 10.1042/BJ20090567 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Yu, S. et al. Fascin-1 is highly expressed specifically in microglia after spinal cord injury and regulates microglial migration. Front. Pharmacol.12, 729524. 10.3389/fphar.2021.729524 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, X. et al. Cell proliferation fate mapping reveals regional cardiomyocyte cell-cycle activity in subendocardial muscle of left ventricle. Nat. Commun.12, 5784. 10.1038/s41467-021-25933-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Z. et al. SU16f inhibits fibrotic scar formation and facilitates axon regeneration and locomotor function recovery after spinal cord injury by blocking the PDGFRβ pathway. J. Neuroinflamm.. 19, 95. 10.1186/s12974-022-02449-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobaniec-Lotowska, M. E. A transmission electron microscopic study of microglia/macrophages in the hippocampal cortex and neocortex following chronic exposure to valproate. Int. J. Exp. Pathol.86, 91–96. 10.1111/j.0959-9673.2005.00417.x (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peláez, B., Blázquez, J. L., Pastor, F. E., Sánchez, A. & Amat, P. Lectinhistochemistry and ultrastructure of microglial response to monosodium glutamate-mediated neurotoxicity in the arcuate nucleus. Histol. Histopathol. 14, 165–174. 10.14670/HH-14.165 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Tang, X., Li, X., Zhang, D. & Han, W. Astragaloside-IV alleviates high glucose-induced ferroptosis in retinal pigment epithelial cells by disrupting the expression of miR-138-5p/Sirt1/Nrf2. Bioengineered13, 8240–8254. 10.1080/21655979.2022.2049471 (2022). [DOI] [PMC free article] [PubMed]
- 26.Zhou, H. et al. NCOA4-mediated ferritinophagy is involved in ionizing radiation-induced ferroptosis of intestinal epithelial cells. Redox Biol.55, 102413. 10.1016/j.redox.2022.102413 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, S. et al. TXNRD1: A key regulator involved in the ferroptosis of CML cells induced by cysteine depletion in vitro. Oxid. Med. Cell Longev.2021, 7674565. 10.1155/2021/7674565 (2021). [DOI] [PMC free article] [PubMed]
- 28.Liu, H. et al. SARM1 promotes neuroinflammation and inhibits neural regeneration after spinal cord injury through NF-κB signaling. Theranostics11, 4187–4206. 10.7150/thno.49054 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basso, D. M. et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 23, 635–659. 10.1089/neu.2006.23.635 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Ma, M., Basso, D. M., Walters, P., Stokes, B. T. & Jakeman, L. B. Behavioral and histological outcomes following graded spinal cord contusion injury in the C57Bl/6 mouse. Exp. Neurol.169, 239–254. 10.1006/exnr.2001.7679 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Dias, D. O. et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat. Commun.12, 5501. 10.1038/s41467-021-25585-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanner, I. B. et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J. Neurosci.33, 12870–12886. 10.1523/JNEUROSCI.2121-13.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bishop, G. M., Dang, T. N., Dringen, R. & Robinson, S. R. Accumulation of non-transferrin-bound iron by neurons, astrocytes, and microglia. Neurotox. Res.19, 443–451. 10.1007/s12640-010-9195-x (2011). [DOI] [PubMed] [Google Scholar]
- 34.Mizutani, M. et al. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J. Immunol.188, 29–36. 10.4049/jimmunol.1100421 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, Y. et al. Inhibition of CISD2 promotes ferroptosis through ferritinophagy-mediated ferritin turnover and regulation of p62-Keap1-NRF2 pathway. Cell. Mol. Biol. Lett.27, 81. 10.1186/s11658-022-00383-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang, X. et al. Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ. Res.127, 486–501. 10.1161/CIRCRESAHA.120.316509 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Kapralov, A. A. et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol.16, 278–290. 10.1038/s41589-019-0462-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jhelum, P. et al. Ferroptosis mediates Cuprizone-Induced loss of oligodendrocytes and demyelination. J. Neurosci.40, 9327–9341. 10.1523/JNEUROSCI.1749-20.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu, W. et al. Targeting iNOS alleviates early brain Injury after experimental subarachnoid hemorrhage via promoting ferroptosis of M1 Microglia and reducing Neuroinflammation. Mol. Neurobiol.59, 3124–3139. 10.1007/s12035-022-02788-5 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Gong, F. et al. Trehalose inhibits ferroptosis via NRF2/HO-1 pathway and promotes functional recovery in mice with spinal cord injury. Aging (Albany NY). 14, 3216–3232. 10.18632/aging.204009 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu, T. et al. Induction of ferroptosis in response to graphene quantum dots through mitochondrial oxidative stress in microglia. Part. Fibre Toxicol.1710.1186/s12989-020-00363-1 (2020). [DOI] [PMC free article] [PubMed]
- 42.Griffin, J. M. et al. Astrocyte-selective AAV-ADAMTS4 gene therapy combined with hindlimb rehabilitation promotes functional recovery after spinal cord injury. Exp. Neurol.327, 113232. 10.1016/j.expneurol.2020.113232 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Klaw, M. C., Xu, C., Tom, V. J. & Intraspinal, A. A. V. Injections immediately rostral to a thoracic spinal cord Injury Site efficiently transduces neurons in spinal cord and brain. Mol. Ther. Nucleic Acids. 2, e108. 10.1038/mtna.2013.34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiao, L. et al. Iron metabolism mediates microglia susceptibility in ferroptosis. Front. Cell. Neurosci.16, 995084. 10.3389/fncel.2022.995084 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, Q. S. & Jia, Y. J. Ferroptosis: a critical player and potential therapeutic target in traumatic brain injury and spinal cord injury. Neural Regen. Res.18, 506–512. 10.4103/1673-5374.350187 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao, S. et al. Mesenchymal stem cell attenuates spinal cord injury by inhibiting mitochondrial quality control-associated neuronal ferroptosis. Redox Biol.67, 102871. 10.1016/j.redox.2023.102871 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng, Z. et al. Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol.43, 101984. 10.1016/j.redox.2021.101984 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauerbeck, A., Schonberg, D. L., Laws, J. L. & McTigue, D. M. Systemic iron chelation results in limited functional and histological recovery after traumatic spinal cord injury in rats. Exp. Neurol.248, 53–61. 10.1016/j.expneurol.2013.05.011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, Z., Zhou, W., Zhang, Z., Zhang, L. & Li, M. Metformin alleviates spinal cord injury by inhibiting nerve cell ferroptosis through upregulation of heme oxygenase-1 expression. Neural Regen. Res.19, 2041–2049. 10.4103/1673-5374.390960 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu, Y. et al. N4-acetylcytidine-dependent GLMP mRNA stabilization by NAT10 promotes head and neck squamous cell carcinoma metastasis and remodels tumor microenvironment through MAPK/ERK signaling pathway. Cell. Death Dis.14, 712. 10.1038/s41419-023-06245-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.