Abstract
Determining the impact of forest disturbance and fragmentation on tropical biotas is a central goal of conservation biology. Among tropical forest birds, understory insectivores are particularly sensitive to habitat disturbance and fragmentation, despite their relatively small sizes and freedom from hunting pressure. Why these birds are especially vulnerable to fragmentation is not known. Our data indicate that the best determinant of the persistence of understory insectivorous birds in small fragments is the ability to disperse through deforested countryside habitats. This finding contradicts our initial hypothesis that the decline of insectivorous birds in forest fragments is caused by impoverished invertebrate prey base in fragments. Although we observed significantly fewer insectivorous birds in smaller fragments, extensive sampling of invertebrate communities (106,082 individuals) and avian diets (of 735 birds) revealed no important differences between large and small fragments. Neither habitat specificity nor drier fragment microclimates seemed critical. Bird species that were less affected by forest fragmentation were, in general, those that used the deforested countryside more, and we suggest that the key to their conservation will be found there.
Forest understory insectivores, in general, have high habitat specificity, low mobility, and are more confined to forest interior than other forest passerine guilds, especially in the tropics where forest fragmentation and its consequences are most dramatic (1–5). Although over a dozen hypotheses have been proposed to explain the disappearance of insectivorous bird species from forested habitats around the world (2, 6), four of these are particularly relevant to explaining the decline of understory insectivores. The food scarcity hypothesis states that small fragments are impoverished in prey preferred by understory insectivores (6–8). The microclimate hypothesis proposes that these birds are particularly sensitive physiologically to changes in microclimate associated with forest fragmentation (2, 9). The habitat specificity hypothesis states that the loss of some microhabitat elements (such as army ant swarms, curled leaves, and dead trees) from fragments may affect many understory insectivores negatively (2, 6). Insectivores are more sensitive to such subtle changes because, unlike fruits, flowers, and seeds, invertebrates actively avoid insectivores and, as a result, insectivorous birds have evolved into many specialized niches and seek prey in certain microhabitats. Finally, according to the limited dispersal hypothesis, understory insectivores, because of their relatively sedentary habits and possible psychological avoidance of clearings (1, 10), may be less likely to disperse into more favorable habitats after forest fragmentation and may disappear from fragments as a result of stochastic events and other negative consequences of fragmentation.
Changes in invertebrate communities as a result of forest fragmentation are well documented (11–13). Leaf-litter and soil-dwelling invertebrates decline as a result of desiccation in small forest fragments and generalist edge species that prefer the dense vegetation near fragment edges increase in number (12). Because many understory insectivores forage in the dark and humid leaf litter in relatively open understory and avoid dense vegetation (2), these changes can diminish the birds' prey base. If the food scarcity hypothesis is correct and a reduced resource base is the main reason for the decline of insectivorous understory birds in smaller forest fragments, these fragments should exhibit some combination of lower invertebrate abundance and biomass. In addition, food limitation may be apparent in the quantity and composition of invertebrates in the birds' diets. Given the centrality of food availability to the reproductive success of songbirds in general (14), we decided to test the food scarcity hypothesis in tropical humid forest, where it has received relatively little attention (15).
Methods
Research Site.
We tested our predictions by sampling birds, bird diets, and invertebrates in large and small forest fragments near Las Cruces, southern Costa Rica. Data were collected between 1 July 1999–15 September 2000, in Las Cruces Forest and three smaller forest fragments (8°47′N, 82°57′W). These fragments are Pacific premontane (elevation 1,100 m) humid forest surrounded by pastures, plantations of coffee and other crops, and human settlements. The fragments have been isolated since the mid-1950s. Las Cruces Forest is the largest midelevation fragment in the region [227 hectares (ha)]; in it, we established three study transects (referred to below as “forest transects”) separated from each other by 400-1000 m. The small primary forest fragments (4–5 ha) studied were 400–2,300 m from Las Cruces Forest and there was a study transect in each small fragment (referred to below as “fragment transects”). Each of the six transects was 200-m long and within 150 m of the forest edge, to control for “edge effects” (16).
Sampling Birds.
We mist-netted in each transect with 12 12-m, 36-mm mesh nets, for a total of 82,944 m h divided evenly between forest and fragment transects, following published methodology (17).
Sampling Invertebrates.
We sampled invertebrates with pitfall traps [Bioquip (Gardena, CA) product no. 2838A] filled with equal parts of ethylene glycol and water, sticky traps, 30 cm × 30 cm plastic sheets covered with Tangle-Trap (Bioquip product no. 2870C), and timed searches. With this combination, we were able to sample both passive and actively moving invertebrates on various substrates. The traps were placed randomly along transects. The 1-week pitfall traps were active for 1 week, 2-week sticky traps were active for 2 weeks, and other traps were active for 1 day. For searches, each 200-m transect was divided into 40 5-m sections. Two people spent 5 min searching each section for invertebrates in flight, in the leaf litter, and on vegetation; invertebrates longer than 5 mm were captured with nets and forceps and placed in 70% alcohol. We identified all invertebrates to order and measured their lengths. For 1-week pitfall traps and searches, we measured only invertebrates longer than 5 mm and obtained the cumulative dry weight of all of the specimens in each order for each sample-day.
Bird Diet.
We obtained diet samples from insectivorous and omnivorous birds by using nonlethal 1.5% potassium antimony tartarate, based on established protocol (18). The first author examined each regurgitate under a stereo microscope and estimated the number, length, and weight of prey items eaten based on a reference collection and published regressions of weight on length (19). Invertebrates were identified to order, except Formicidae (ants), which were identified to family. For 14 understory insectivorous species that occurred both in forest and fragment transects, we had at least four diet samples from each treatment. That is the minimum number thought to offer adequate representation of the diet of a species within a given time period (20), and for 11 of these species, we had at least 10 diet samples from each treatment.
Vegetation.
At 30 randomly selected points in each transect, we measured canopy closure by taking photos of the canopy from ground-level with a 17-mm lens and later analyzing them with Adobe Systems (Mountain View, CA) photoshop. We assessed distribution of canopy height by measuring the height of the highest vegetation above these points with a rangefinder.
Results
Bird Community.
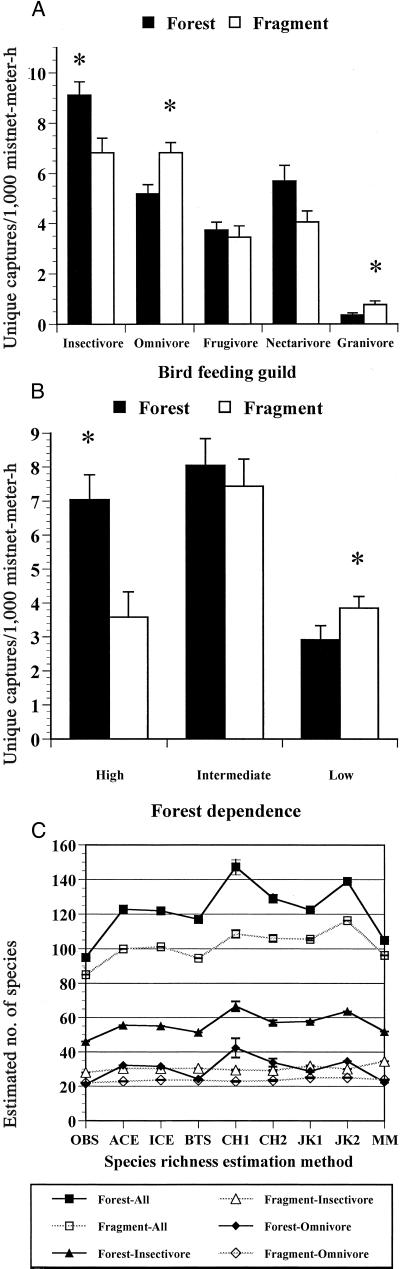
We mist-netted 1,202 birds in forest transects and 1,096 birds in fragment transects, representing 116 species (nomenclature based on ref. 21). Overall daily capture rates did not differ between forest and fragment transects (t = 1.50, P = 0.137), but certain guilds and forest-dependence classes showed pronounced differences (Fig. 1). Most large and specialized insectivorous species, such as black-faced antthrush (Formicarius analis) and ruddy woodcreeper (Dendrocincla homochroa), did not occur along fragment transects and many of the remaining insectivores, such as white-breasted wood-wren (Henicorhina leucosticta) and white-throated spadebill (Platyrinchus mystaceus), occurred there in significantly lower numbers than along forest transects (see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org). Insectivorous species were significantly fewer in the fragments than in Las Cruces Forest (t = 3.08, P = 0.028), especially those species feeding on large invertebrates and small vertebrates (t = 4.01, P = 0.008); insectivores feeding on small invertebrates tended to be fewer as well (t = 2.45, P = 0.058). Both sampled and estimated species richness was higher along forest transects for all species and for insectivores (Fig. 1C). As in previous studies (1, 3, 4), we observed significantly fewer species and individuals of understory insectivores in small fragments and none of the ground-foraging or army ant-following species were observed in those fragments.
Figure 1.
Comparisons of abundance and species richness of birds captured with mist nets. The asterisks indicate a significant difference (P < 0.05). (A) More insectivorous birds were captured along forest transects (t = 2.67, P = 0.0089), and more omnivorous (t = 2.70, P = 0.0081) and granivorous (t = 2.18, P = 0.0315) birds were captured along fragment transects. Guild assignments were based on ref. 22. (B) More high forest-dependent species were captured along forest transects (t = 3.63, P = 0.0005) and more low forest-dependent species (t = 2.40, P = 0.019) were captured along fragment transects. High, intermediate, and low forest-dependent classes correspond to forest-dependent, forest generalist, and nonforest species in ref. 23. (C) Observed and estimated species richness values are based on methods provided in ESTIMATES (ref. 24, http://viceroy.eeb.uconn.edu/EstimateS). OBS, observed number of species; ACE, abundance-based coverage estimator; ICE, incidence-based coverage estimator; BTS, bootstrap; CH, Chao; JK, Jackknife; MM, Michaelis–Menten. SEs for Chao1, Chao2, and Jackknife1 methods are included.
Invertebrate Community.
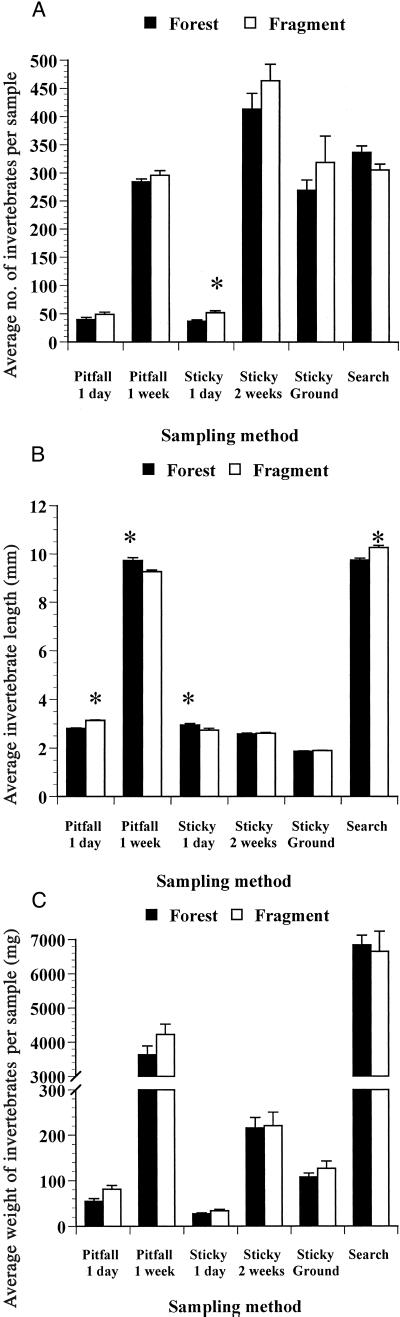
Individuals/sample, average length, and dry biomass/sample of invertebrates varied between forest and fragment transects with no overall pattern (Fig. 2). When we compared these variables for some invertebrate orders and classes, we again found no overall differences between forest and fragment transects (Table 1). Overall, numbers of individuals/sample and dry biomass/sample values were about 15% lower in forest samples, but the differences were not significant. Of 319 independent t tests comparing individuals/sample, average length, and dry biomass/sample values between forest and fragment transects for different invertebrate taxa sampled with various methods, 69 tests were significant and greater values were almost equally distributed between forest (35/69) and fragment transects (34/69). These tests do not pose a multiple comparison problem because the null hypothesis for each test was different (25).
Figure 2.
Comparisons of average number, length, and dry weight of invertebrates captured. For sample sizes, see Table 1. With 1-week pitfall traps and searches, only invertebrates ≥ 5 mm were taken into consideration. The asterisks indicate a significant difference (P < 0.001). (A) Average number of invertebrates captured/sample. (B) Average length of invertebrates captured (mm). (C) Average dry weight of invertebrates/sample (mg). For sticky traps and 1-day pitfall traps, dry weight was estimated by using the appropriate regression equations (19).
Table 1.
The distribution of significantly greater values of invertebrate measures between forest and fragment transects
| Sampling method | No. captured | Individuals/sample
|
Average
invertebrate length
|
Dry
biomass/sample
|
|||
|---|---|---|---|---|---|---|---|
| Forest | Fragment | Forest | Fragment | Forest | Fragment | ||
| 1-day sticky (36) | 9,630 | Diptera Hemiptera Hymenoptera | Arachnida Coleoptera | Diptera Hymenoptera Isopida | Hymenoptera | ||
| 2-week sticky (18) | 47,356 | Collembola | Orthoptera | Coleoptera Collembola Hymenoptera Orthoptera Thysanoptera | Diptera Lepidoptera | Collembola | Isoptera |
| Ground sticky (12) | 19,970 | Diptera | Arachnida Coleoptera Collembola | Diptera Orthoptera | Coleoptera Collembola | Diptera | |
| 1-day pitfall (16) | 4,206 | Collembola | Orthoptera Diptera | Coleoptera | Formicidae Hymenoptera | Coleoptera Collembola | Diptera Hymenoptera Orthoptera |
| 1-week pitfall (36) | 9,801 | Chilopoda Decapoda Dermaptera | Coleoptera Hemiptera | Annelida Blattaria Orthoptera | Coleoptera Formicidae Hymenoptera | Blattaria Decapoda Dermaptera | Coleoptera |
| Search (8) | 15,119 | Coleoptera Hemiptera Hymenoptera Isopoda | Lepidoptera | Coleoptera Formicidae Orthoptera | Arachnida Dermaptera Isopoda Phasmida | Homoptera Lepidoptera | |
Values next to sampling methods indicate number of samples from each transect. Dry biomass values for sticky traps and 1-day pitfall traps were estimated by using appropriate regression equations (19).
There were, however, two discernible trends. First, all eight significantly greater values for Diptera were from fragment transects. Second, a number of mainly forest floor/leaf-litter groups [such as Annelida, Blattaria, Collembola, Decapoda (forest crabs), Dermaptera, Isopoda, Mollusca, and Thysanura], which comprised 7.3% of the individuals and 12.5% of the biomass sampled, were somewhat better represented along forest transects. There were also significantly more army ant (Eciton burchelli) swarms during searches along forest transects (7 of 8 encounters, binomial test, P = 0.0313).
Bird Diet.
In bird diet samples, Coleoptera, Orthoptera, Formicidae, and Arachnida were the most common prey, comprising about 75% of the individuals found in diet samples. The taxonomic distribution of prey items in forest and fragment diet samples did not differ significantly for any bird group (all χ2 < 11.91, all P > 0.99).
Overall, estimated dry weight of invertebrates was not significantly different between forest and fragment diet samples (F = 2.60, P = 0.108; two-factor ANOVA with origin of sample and species as factors).
The average number of prey items/diet sample (all t < 1.64, all P > 0.156) and estimated dry weight (19) of consumed prey (all t < 1.60, all P > 0.111) values, although greater in general in forest samples, did not differ significantly between forest and fragment samples for any of the 14 species with enough diet samples from both treatments (Table 3, which is published as supporting information on the PNAS web site). Even though the average lengths of invertebrates in diet samples were greater in the forest samples of 11 species, the values differed significantly only for Henicorhina leucosticta (P = 0.016) and Sittasomus griseicapillus (P = 0.008).
When we compared the diet samples obtained in Las Cruces Forest of insectivorous species present and absent from small fragments, prey taxonomic distribution did not differ significantly for insectivores with large prey (χ2 = 0.58, P > 0.99) or with small prey (χ2 = 0.36, P > 0.99). Nor did the estimated dry weight of consumed prey of insectivores with large prey (t = 0.005, P = 0.996) or with small prey (t = 0.262, P = 0.793).
Vegetation Structure.
Neither canopy closure (F = 0.414, P > 0.5) nor canopy height distribution (χ2 = 15.15, P > 0.1) differed significantly between forest and fragment transects. Average canopy closure was around 80% for all transects.
Discussion
Although there were significantly fewer understory insectivores in small fragments, invertebrate abundance, average length, and dry biomass values along forest and fragment transects were surprisingly close. We obtained similar results from examination of bird diets, with no significant differences in diet composition, biomass, or prey items per sample, and only 2 of 14 bird species exhibited significant differences in average length of invertebrates eaten. Even though the significant reduction in army ant swarms in small fragments may affect the three army ant-following species negatively, the food scarcity hypothesis does not seem to be supported as the primary cause of the disappearance of understory insectivorous birds from small forest fragments around Las Cruces. This finding differs from the findings of two recent studies in the temperate zone where increased food abundance in larger forest fragments was positively correlated with the abundance and reproductive performance of the two understory insectivorous bird species studied (7, 8).
The microclimate hypothesis, which states that sedentary understory insectivores react more unfavorably to microclimate fluctuations in forest fragments than more mobile species that are frequently exposed to different microclimates, was not tested by our observations. We tried to control for microclimate differences by sampling only near forest edges, but this does not preclude the possibility that understory insectivores in larger fragments may forage near edges and seek shelter in the forest interior when climatic conditions become intolerable.
With respect to the habitat specificity hypothesis, some understory insectivorous species may have disappeared from fragments because of the reduction or disappearance of some critical habitat elements, such as army ant swarms. However, the small fragment where we sometimes observed army ant swarms (although only once during invertebrate sampling) was also missing army ant-following bird species absent from other fragments, and a number of bird species missing from small fragments feed on invertebrate resources that were not significantly different between forest and fragments sites. Thus, this hypothesis is tentatively rejected.
Dispersal, crucial in the colonization of habitat islands (26, 27), may be the key mechanism that makes it more likely that small and short-lived bird species will go extinct as a result of habitat fragmentation compared with large and long-lived species (28, 29). Likewise, the limited dispersal capabilities of understory insectivores (1, 2) may be the most important factor in their sensitivity to fragmentation. At our site, presence of a bird species in the deforested open countryside around forest fragments was the best determinant of its occurrence in smaller fragments, in agreement with the limited dispersal hypothesis. Of the 18 species (15 insectivorous) we caught significantly more times along Las Cruces Forest transects than along fragment transects, only two (one insectivorous) were detected in nonforest habitats in a separate study (30). In that study, the average detection rate in nonforest for these 18 species was 0.0005 individuals/person-hour (3 of 5,007 observations) and none of the six insectivorous species feeding on large prey were found in nonforest habitats. Conversely, of the 12 species we caught significantly more frequently along fragment transects (three insectivorous, all feeding on small prey), 9 were present in nonforest (30), and the average detection rate in nonforest was 0.296 individuals/person-hour (1,172 of 5,007 observations). Only one of these nine species was insectivorous and it accounted for 1.8% of the observations. These detection rates were significantly different (Mann–Whitney U test, P = 0.004), and insectivores, especially those feeding on large prey, were significantly under-represented both in forest fragments and in nonforest habitats.
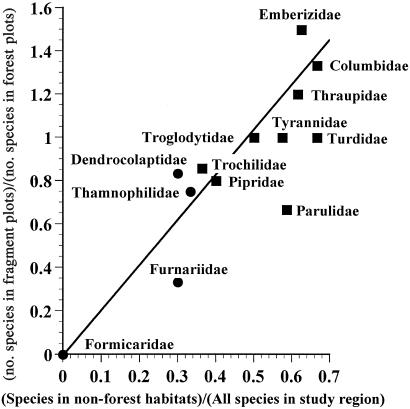
There was also a significant positive correlation (r2 = 0.714, P < 0.0001) between the number of species of a bird family present in nonforest habitats (30) and the number of species of that bird family present in small fragments (Fig. 3). Thus, ability to move through and possibly forage in deforested habitats around forest fragments may link small populations that would otherwise be isolated and vulnerable to edge effects and stochastic events. This mobility may greatly enhance the “rescue effect” (31) and thus improve the survival chances of forest-dependent organisms in forest fragments, as was observed in previous studies (1, 32). In addition, forest bird species frequently detected in the matrix surrounding forest fragments are also likely to be more tolerant of ecological changes in fragments, such as those resulting from edge effects (16, 33), and may be more capable of occasionally foraging and even nesting in some matrix habitats near fragments.
Figure 3.
Increased species diversity of a bird family in the nonforest matrix correlates with increased species diversity in small fragments (r2 = 0.714, P < 0.0001). Families that are not well sampled with mist nets, such as Accipitridae, Strigidae, Trogonidae, and Cotingidae, were not included. The data for all of the species in the study region were provided by James Zook (unpublished data). Note that the four families in the superfamily Furnarioidea, which is particularly sensitive to forest disturbance (2), have the lowest values and are marked with circles.
Thus, the limited dispersal hypothesis is best supported by our study. Inability to use deforested countryside habitats, rather than food scarcity in fragments, seems to be the major cause of the decline of understory insectivores in forest fragments around Las Cruces. The actual mechanism of decline, whether it is increased nest predation, changes in microclimate, negative stochastic effects caused by small population size, or a combination thereof, remains to be elucidated. Although forest fragmentation and other forms of habitat disturbance may reduce the breeding success of most forest bird species equally, possibly as a result of increased nest predation of all species (33), more sedentary species may be less able than other birds to “commute” through nonforest from their breeding territories to small fragments containing sufficient resources. Such regular “commuting” from nesting areas to foraging areas that are unsuitable for nesting has been observed in frugivorous bats in Mexican lowland tropical forest fragments (M. Evelyn, unpublished data). Increased mobility increases the chances of renesting in fragments if conditions become more favorable, making it less likely that more vagile species will become locally extinct. In addition, forest bird species that are likely to move through nonforest habitats are more likely to occasionally use those habitats for foraging and nesting, mitigating the effects of fragmentation. Sedentary behavior can also explain the decline of understory insectivores in unfragmented but otherwise disturbed forests (2, 5).
More research comparing the movement, foraging, and breeding patterns of understory insectivores and other guilds in both forest fragments of various sizes and in open countryside is needed to reveal the actual mechanism(s) of the disappearance of understory insectivores. Meanwhile, better integration of agricultural/human-dominated habitats into conservation strategies, such as linking forest fragments with shade coffee plantations, fence rows, and windbreaks composed of native tree species, may make deforested areas more hospitable to understory insectivores and other fragmentation-sensitive groups by enabling them to disperse between forest fragments and prevent local extinctions.
Supplementary Material
Acknowledgments
We thank the Costa Rican government and the Organization for Tropical Studies for allowing us to work at Las Cruces Biological Research Station (LCBRS), the staff of LCBRS for their support, and Tom Davis, Forrest Fleischmann, Angelina Sanderson, Jason Sandí, Jeisson Sandí, Audrey Tapia, and Parker VanValkenburgh for their help with field work. We thank Walter Loewenstern and Thomas Brokaw for graduate fellowships, the Bing undergraduate research fund, Sigma Xi Grant-in-Aid of Research, the Winslow Foundation, and Peter and Helen Bing for support. We appreciate critical comments from Richard Bierregaard, Carol Boggs, Chris Canaday, Marcus Feldman, Oliver Komar, William Laurance, Gary Luck, Harold Mooney, Phillip Stouffer, and Peter Vitousek.
References
- 1.Stouffer P C, Bierregaard R O. Ecology. 1995;76:2429–2445. [Google Scholar]
- 2.Canaday C. Biol Conserv. 1996;77:63–77. [Google Scholar]
- 3.Stratford J A, Stouffer P C. Conserv Biol. 1999;16:1416–1423. [Google Scholar]
- 4.Kattan G H, Alvarez-Lopez H, Giraldo M. Conserv Biol. 1994;8:138–146. [Google Scholar]
- 5.Thiollay J M. Conserv Biol. 1992;6:47–63. [Google Scholar]
- 6.Ford H A, Barrett G W, Saunders D A, Recher H F. Biol Conserv. 2001;97:71–88. [Google Scholar]
- 7.Burke D M, Nol E. Auk. 1998;115:96–104. [Google Scholar]
- 8.Zanette L, Doyle P, Tremont S M. Ecology. 2000;81:1654–1666. [Google Scholar]
- 9.Karr J R, Freemark K E. Ecology. 1983;64:1481–1494. [Google Scholar]
- 10.Greenberg R. Can J Zool. 1988;67:1194–1199. [Google Scholar]
- 11.Didham R K, Ghazoul J, Stork N E, Davis A J. Trends Ecol Evol. 1996;11:255–260. doi: 10.1016/0169-5347(96)20047-3. [DOI] [PubMed] [Google Scholar]
- 12.Didham R K. In: Tropical Forest Remnants. Laurance W F, Bierregaard R O, editors. Chicago: University of Chicago Press; 1997. pp. 55–70. [Google Scholar]
- 13.Ricketts T H, Daily G C, Ehrlich P R, Fay J P. Conserv Biol. 2001;15:378–388. [Google Scholar]
- 14.Martin T E. Annu Rev Ecol Syst. 1987;18:453–488. [Google Scholar]
- 15.Ahumada J A. Auk. 2001;118:191–210. [Google Scholar]
- 16.Restrepo C, Gómez N. Ecol Appl. 1998;8:170–183. [Google Scholar]
- 17.Karr J R. Inland Bird Banding. 1979;51:1–10. [Google Scholar]
- 18.Poulin B, Lefebvre G, McNeil R. Condor. 1994;96:98–104. [Google Scholar]
- 19.Schoener T W. Ann Entomol Soc Am. 1980;73:106–109. [Google Scholar]
- 20.Cooper R J, Martinat P J, Whitmore R C. In: Avian Foraging: Theory, Methodology and Applications. Ralph M L, Verner C J, Jehl J R Jr, editors. Los Angeles: Cooper Ornithol. Soc.; 1990. pp. 104–109. [Google Scholar]
- 21.Clements J F. Birds of the World: A Checklist. Vista, CA: Ibis; 2000. [Google Scholar]
- 22.Karr J K, Robinson S K, Blake J G, Bierregaard R O., Jr . In: Four Neotropical Rainforests. Gentry A H, editor. New Haven, CT: Yale Univ. Press; 1990. pp. 237–269. [Google Scholar]
- 23.Stiles F G. In: Conservation of Tropical Forest Birds. Diamond A W, Lovejoy T E, editors. Cambridge, U.K.: ICBP Technical Publication; 1985. pp. 141–185. [Google Scholar]
- 24.Colwell R K. ESTIMATES 6.0b1: Statistical Estimation of Species Richness and Shared Species from Samples. Storrs, CT: Univ. of Connecticut; 2001. [Google Scholar]
- 25.Fisher L D, van Belle G. Biostatistics: A Methodology for the Health Sciences. New York: Wiley; 1993. pp. 596–629. [Google Scholar]
- 26.Paradis E, Baillie S R, Sutherland W J, Gregory R D. J Anim Ecol. 1998;67:518–536. [Google Scholar]
- 27.Brown J H, Kodric-Brown A. Ecology. 1977;58:445–449. [Google Scholar]
- 28.Beissinger S. Proc Natl Acad Sci USA. 2000;97:11688–11689. doi: 10.1073/pnas.97.22.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owens I P F, Bennett P M. Proc Natl Acad Sci USA. 2000;97:12144–12148. doi: 10.1073/pnas.200223397. . (First Published September 26, 2000; 10.1073/pnas.200223397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes, J. B., Daily, G. C. & Ehrlich, P. R. Ecol. Lett., in press.
- 31.Invargsson P K. Trends Ecol Evol. 2001;16:62–63. [Google Scholar]
- 32.Laurance W F. Conserv Biol. 1991;5:79–89. [Google Scholar]
- 33.Paton P W C. Conserv Biol. 1994;8:17–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.