Abstract
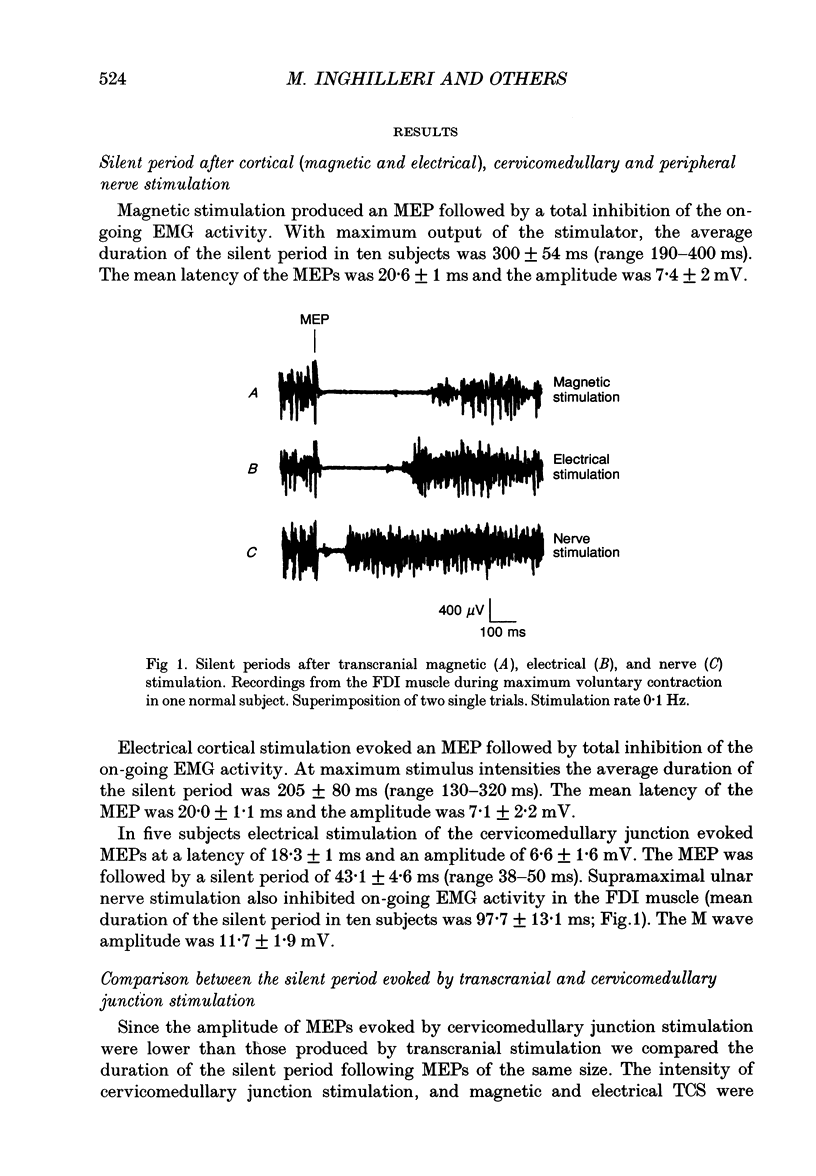
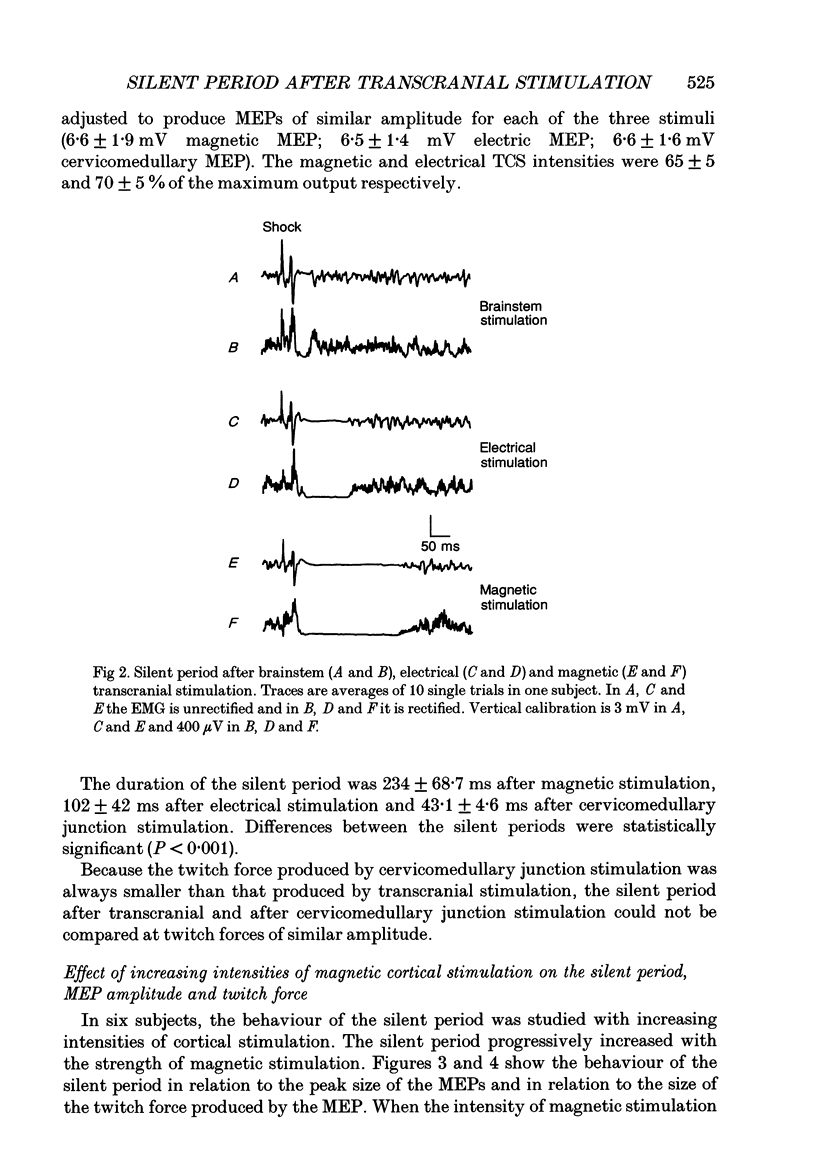
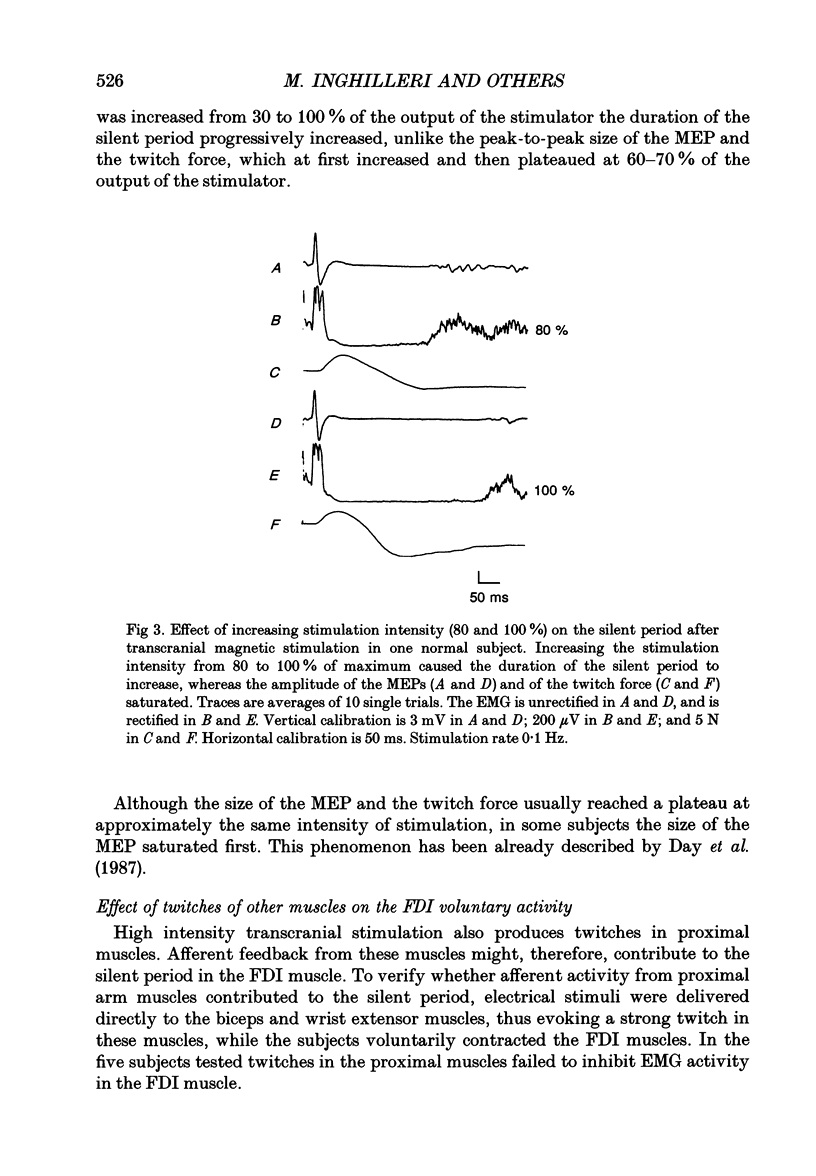
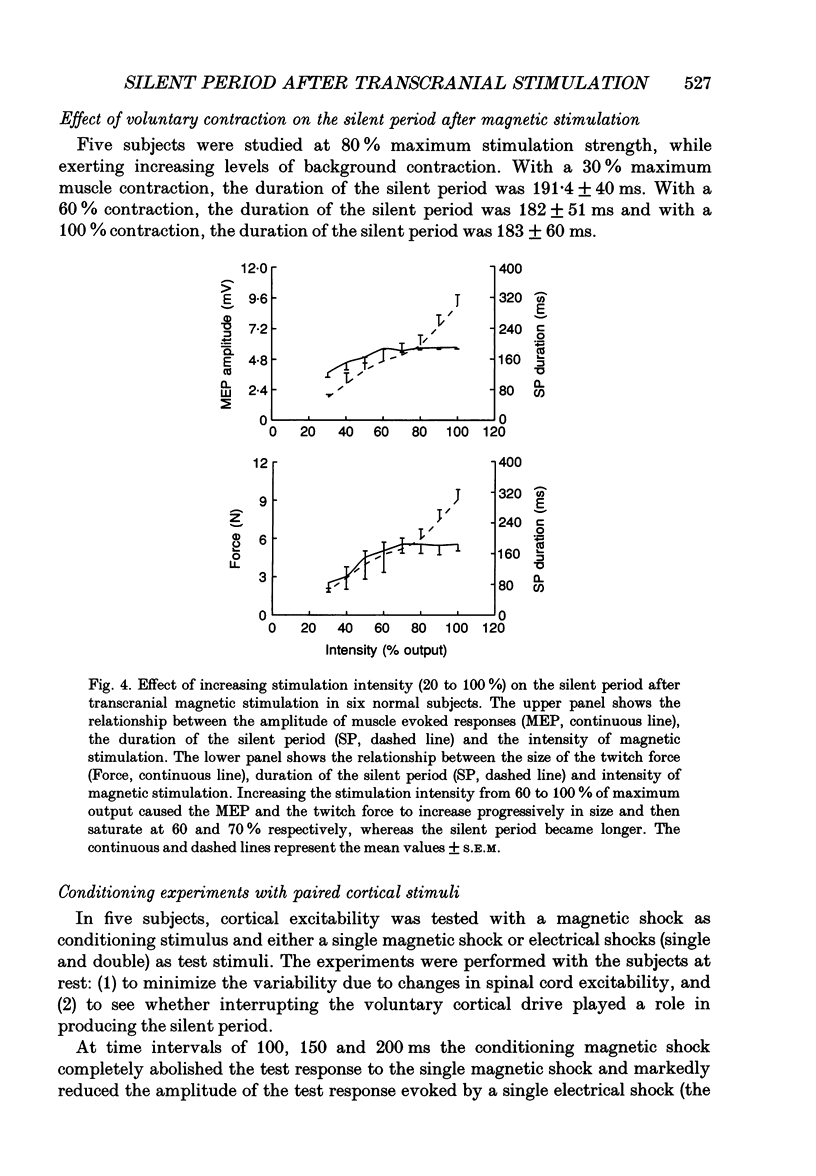
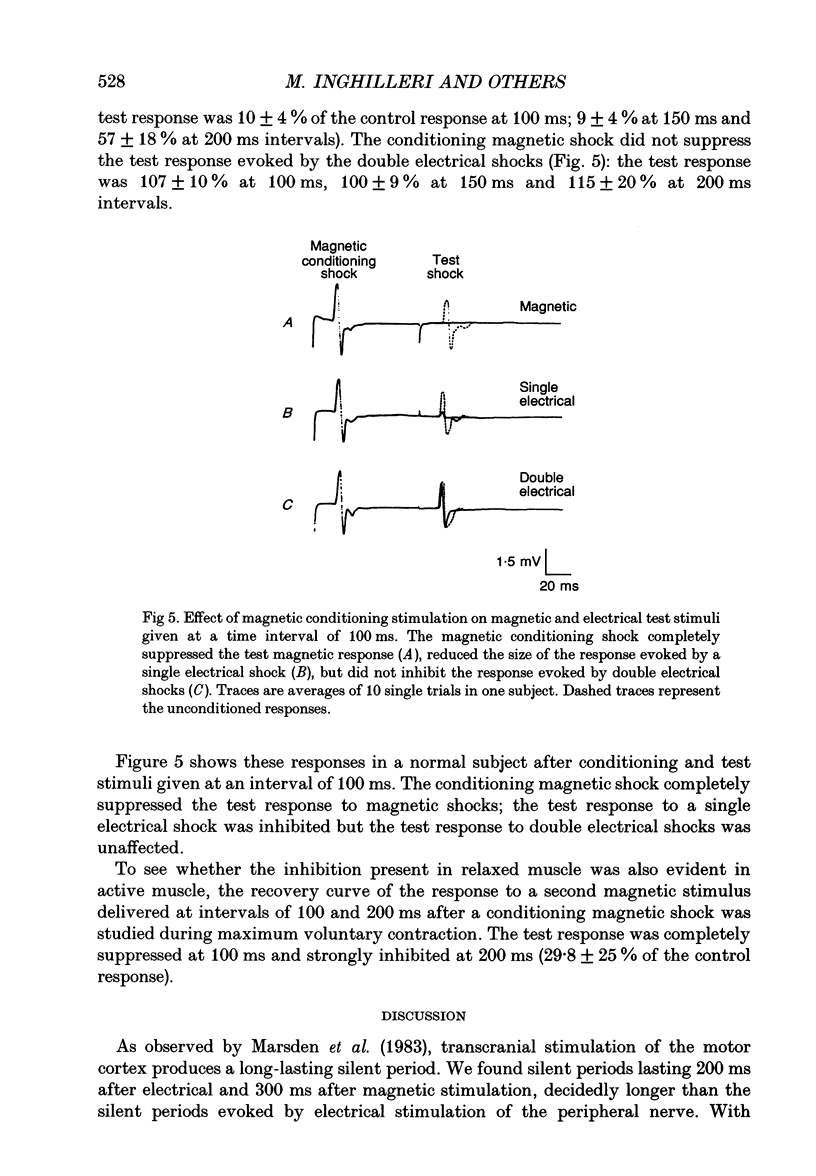
1. The silent period evoked in the first dorsal interosseous (FDI) muscle after electrical and magnetic transcranial stimulation (TCS), electrical stimulation of the cervicomedullary junction and ulnar nerve stimulation was studied in ten healthy subjects. 2. With maximum-intensity shocks, the average duration of the silent period was 200 ms after electrical TCS, 300 ms after magnetic TCS, 43 ms after stimulation at the cervicomedullary junction and 100 ms after peripheral nerve stimulation. 3. The duration of the silent period, the amplitude of the motor-evoked potential, and the twitch force produced in the muscle were compared at increasing intensities of magnetic TCS. When the stimulus strength was increased from 30 to 70% of the stimulator output, the duration of the silent period lengthened as the amplitude of the motor potential and force of the muscle twitch increased. At 70 to 100% of the output, the amplitude of the motor potential and force of the muscle twitch saturated, whereas the duration of the silent period continued to increase. 4. Proximal arm muscle twitches induced by direct electrical stimulation of the biceps and extensor wrist muscles produced no inhibition of voluntary activity in the contracting FDI muscle. 5. The level of background activation had no effect on the duration of the silent period recorded in the FDI muscle after magnetic TCS. 6. Corticomotoneurone excitability after TCS was studied by means of a single magnetic conditioning shock and a test stimulus consisting either of one single magnetic shock or single and double electrical shocks (interstimulus interval 1.8 ms) in the relaxed muscle. A conditioning magnetic shock completely suppressed the response evoked by a second magnetic shock, reduced the size of the response evoked by a single electrical shock but did not affect the response evoked by double electrical shocks. Inhibition of the test magnetic shock was also present during muscle contraction. 7. Our findings indicate that the first 50 ms of the silent period after TCS are produced mainly by spinal mechanisms such as after-hyperpolarization and recurrent inhibition of the spinal motoneurones. If descending inhibitory fibres contribute, their contribution is small. Changes in proprioceptive input probably have a minor influence. From 50 ms onwards the silent period is produced mainly by cortical inhibitory mechanisms.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berardelli A., Inghilleri M., Cruccu G., Manfredi M. Descending volley after electrical and magnetic transcranial stimulation in man. Neurosci Lett. 1990 Apr 20;112(1):54–58. doi: 10.1016/0304-3940(90)90321-y. [DOI] [PubMed] [Google Scholar]
- Berardelli A., Inghilleri M., Rothwell J. C., Cruccu G., Manfredi M. Multiple firing of motoneurones is produced by cortical stimulation but not by direct activation of descending motor tracts. Electroencephalogr Clin Neurophysiol. 1991 Jun;81(3):240–242. doi: 10.1016/0168-5597(91)90078-c. [DOI] [PubMed] [Google Scholar]
- Burke D., Hicks R. G., Stephen J. P. Corticospinal volleys evoked by anodal and cathodal stimulation of the human motor cortex. J Physiol. 1990 Jun;425:283–299. doi: 10.1113/jphysiol.1990.sp018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussel B., Pierrot-Deseilligny E. Inhibition of human motoneurons, probably of Renshaw origin, elicited by an orthodromic motor discharge. J Physiol. 1977 Jul;269(2):319–339. doi: 10.1113/jphysiol.1977.sp011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B., Nordin M., Wallin U., Hagbarth K. E. Motor-unit responses in human wrist flexor and extensor muscles to transcranial cortical stimuli. J Neurophysiol. 1987 Nov;58(5):1168–1185. doi: 10.1152/jn.1987.58.5.1168. [DOI] [PubMed] [Google Scholar]
- Cheney P. D., Fetz E. E., Palmer S. S. Patterns of facilitation and suppression of antagonist forelimb muscles from motor cortex sites in the awake monkey. J Neurophysiol. 1985 Mar;53(3):805–820. doi: 10.1152/jn.1985.53.3.805. [DOI] [PubMed] [Google Scholar]
- Cowan J. M., Day B. L., Marsden C., Rothwell J. C. The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and leg in intact man. J Physiol. 1986 Aug;377:333–347. doi: 10.1113/jphysiol.1986.sp016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Dressler D., Maertens de Noordhout A., Marsden C. D., Nakashima K., Rothwell J. C., Thompson P. D. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989 May;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Rothwell J. C., Thompson P. D., Dick J. P., Cowan J. M., Berardelli A., Marsden C. D. Motor cortex stimulation in intact man. 2. Multiple descending volleys. Brain. 1987 Oct;110(Pt 5):1191–1209. doi: 10.1093/brain/110.5.1191. [DOI] [PubMed] [Google Scholar]
- Day B. L., Rothwell J. C., Thompson P. D., Maertens de Noordhout A., Nakashima K., Shannon K., Marsden C. D. Delay in the execution of voluntary movement by electrical or magnetic brain stimulation in intact man. Evidence for the storage of motor programs in the brain. Brain. 1989 Jun;112(Pt 3):649–663. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- Deuschl G., Michels R., Berardelli A., Schenck E., Inghilleri M., Lücking C. H. Effects of electric and magnetic transcranial stimulation on long latency reflexes. Exp Brain Res. 1991;83(2):403–410. doi: 10.1007/BF00231165. [DOI] [PubMed] [Google Scholar]
- Edgley S. A., Eyre J. A., Lemon R. N., Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. J Physiol. 1990 Jun;425:301–320. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr P., Agostino R., Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991 Aug;81(4):257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Hess C. W., Mills K. R., Murray N. M. Responses in small hand muscles from magnetic stimulation of the human brain. J Physiol. 1987 Jul;388:397–419. doi: 10.1113/jphysiol.1987.sp016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M., Berardelli A., Cruccu G., Priori A., Manfredi M. Corticospinal potentials after transcranial stimulation in humans. J Neurol Neurosurg Psychiatry. 1989 Aug;52(8):970–974. doi: 10.1136/jnnp.52.8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M., Berardelli A., Cruccu G., Priori A., Manfredi M. Motor potentials evoked by paired cortical stimuli. Electroencephalogr Clin Neurophysiol. 1990 Sep-Oct;77(5):382–389. doi: 10.1016/0168-5597(90)90060-q. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., RANDIC M., STRAUGHAN D. W. CORTICAL INHIBITION. Nature. 1964 Mar 28;201:1294–1296. doi: 10.1038/2011294a0. [DOI] [PubMed] [Google Scholar]
- Kernell D. Functional properties of spinal motoneurons and gradation of muscle force. Adv Neurol. 1983;39:213–226. [PubMed] [Google Scholar]
- Krnjević K., Randić M., Straughan D. W. An inhibitory process in the cerebral cortex. J Physiol. 1966 May;184(1):16–48. doi: 10.1113/jphysiol.1966.sp007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Randić M., Straughan D. W. Nature of a cortical inhibitory process. J Physiol. 1966 May;184(1):49–77. doi: 10.1113/jphysiol.1966.sp007903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon R. N., Mantel G. W. The influence of changes in discharge frequency of corticospinal neurones on hand muscles in the monkey. J Physiol. 1989 Jun;413:351–378. doi: 10.1113/jphysiol.1989.sp017658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon R. N., Muir R. B., Mantel G. W. The effects upon the activity of hand and forearm muscles of intracortical stimulation in the vicinity of corticomotor neurones in the conscious monkey. Exp Brain Res. 1987;66(3):621–637. doi: 10.1007/BF00270695. [DOI] [PubMed] [Google Scholar]
- MERTON P. A. The silent period in a muscle of the human hand. J Physiol. 1951 Jun;114(1-2):183–198. doi: 10.1113/jphysiol.1951.sp004610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Direct electrical stimulation of corticospinal pathways through the intact scalp in human subjects. Adv Neurol. 1983;39:387–391. [PubMed] [Google Scholar]
- Morales F. R., Boxer P. A., Fung S. J., Chase M. H. Basic electrophysiological properties of spinal cord motoneurons during old age in the cat. J Neurophysiol. 1987 Jul;58(1):180–194. doi: 10.1152/jn.1987.58.1.180. [DOI] [PubMed] [Google Scholar]
- Rothwell J. C., Thompson P. D., Day B. L., Boyd S., Marsden C. D. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991 Mar;76(2):159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Ugawa Y., Rothwell J. C., Day B. L., Thompson P. D., Marsden C. D. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol. 1991 Apr;29(4):418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]


