Abstract
The essential cause of menopause is ovarian failure, which can cause decline in sex hormones (especially estrogen) that can increase the risk of metabolic diseases, such as cardiovascular disease and osteoporosis. This study screened 1511 eligible patients from 2148 perimenopausal and postmenopausal women, measuring various physiological and biochemical indicators to analyze differences among age groups (40–44, 45–49, and 50–54 years) with laboratory techniques. The study found no significant difference in the incidence of cardiovascular disease betweenperimenopausal and postmenopausal women. But the incidence of osteoporosis was higher in postmenopausal women and was associated with age (p < 0.05). Additionally, follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), total cholesterol (TC), lumbar spine bone mineral density (BMD) (T1), right femoral BMD (T2) and femoral neck BMD were significantly correlated in both groups. Significant differences were observed in FSH, LH, E2, TC, low-density lipoprotein (LDL), L2-L4, T1, femoral neck reduction and T2 among women in different age groups. Correlation analysis indicated that age increased the risk of cardiovascular disease and osteoporosis in bothperimenopausal and postmenopausal women. This study contributes to a deeper understanding of the pathogenesis of cardiovascular disease and osteoporosis in perimenopausal and menopausal women.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-86613-8.
Keywords: Perimenopausal, Postmenopausal, Cardiovascular disease, Osteoporosis, Estradiol
Subject terms: Endocrine system and metabolic diseases, Diseases
Introduction
Menopause is an important stage in a woman’s life, which is mainly divided into two stages, namely perimenopause and postmenopause1. Perimenopause refers to the stage when a woman transitions from regular menstruation during the reproductive period to menopause, which usually occurs in women between the ages of 40 and 55 2. Postmenopause is the stage in the female reproductive system when the menstrual cycle stops2. The typical characteristics of perimenopausal and postmenopausal women are decreased ovarian function and disordered sex hormone secretion. These changes can have adverse effects on women’s psychological and physical health3. Common symptoms of menopause and perimenopause include irregular menstruation, back and leg pain, hot flashes, mood swings, insomnia and depression4,5.
Women in perimenopause and menopause for a long time may suffer from metabolic-related diseases such as hypertension, coronary heart disease and osteoporosis6–8. And the risk of other types of diseases can also increase, such as cardiovascular disease, menopausal symptoms, vaginal atrophy and urinary tract infections. The secretion level of sex hormones in perimenopausal and postmenopausal women decreases which affects the blood lipid metabolism, vascular function and cardiovascular health of the female population9–11. Estrogen plays an important role in maintaining lipid metabolism balance. The decrease in estrogen levels may lead to an increase in total cholesterol (TC), triglycerides (TG) and low-density lipoprotein (LDL) levels, while the level of high-density lipoprotein (HDL) decreases12,13. Some studies have shown that the increase in TC and LDL increases the risk of cardiovascular disease in postmenopausal women14,15. Estrogen has a protective effect on vascular endothelial cells and vascular walls, promotes the proliferation and repair of endothelial cells, and maintains the elasticity and stability of vascular walls16. The decrease in estrogen levels further can cause impaired vascular endothelial function, weaken the elasticity and stability of vascular walls and increase the risk of arteriosclerosis and cardiovascular disease17. In addition, the occurrence of postmenopausal osteoporosis is also related to the decrease in estrogen levels which leads to reduced bone strength and increased fracture risk18. As an important biochemical marker of bone turnover and formation, osteocalcin is involved in bone mineralization and calcium homeostasis19. Studies have found that serum total osteocalcin in postmenopausal women is closely related to glucose and lipid metabolism, and is negatively correlated with TC, LDL, fasting blood glucose (FPG) and postprandial blood glucose (PBG)20.
The incidence of cardiovascular disease and osteoporosis inperimenopausal and postmenopausal women is mainly caused by estrogen levels, and conventional treatments have greater side effects on women during this period21,22. Therefore, an increasing number of studies have begun to use hormone replacement therapy (HRT) to treat cardiovascular disease and osteoporosis inperimenopausal and postmenopausal women. Studies have found that estrogen has a positive effect on cardiovascular health. It can improve blood lipid metabolism, reduce LDL levels and increase HDL levels23. This mechanism helps reduce the risk of atherosclerosis and lowers the incidence of cardiovascular disease. Hormone replacement therapy (HRT) is considered an effective treatment for postmenopausal osteoporosis and fractures24. HRT can help balance bone resorption and formation, significantly improve bone density and reduce the incidence of fractures by supplementing estrogen25. Studies have shown that postmenopausal women who receive HRT have a significantly reduced risk of fractures in key areas such as the hip and spine26,27.
Although estrogen has significant benefits in treating cardiovascular disease and osteoporosis in postmenopausal and postmenopausal women, there are certain risks, such as breast cancer, cardiovascular events and thrombosis28,29. In order to further understand the pathogenesis of metabolic diseases in postmenopausal and postmenopausal women and solve the side effects during HRT treatment, this study selected perimenopausal and postmenopausal women aged 40–54 years old in West China Second University Hospital from October 2020 to October 2024 as research subjects. This study explored the impact of different age stages on female menopause and the pathogenesis of perimenopausal women and postmenopausal women by measuring various physiological and biochemical indicators. The results of this study can provide data support for the study of the pathogenesis of cardiovascular disease and bone disease in clinical perimenopausal and postmenopausal women, and provide a scientific basis for solving the health problems of women.
Methods
Study participants
The subjects of this study were 2148perimenopausal and postmenopausal women aged 40 to 54 years who were registered in West China Second Hospital, China from October 2020 to October 2024 (Table S1). Perimenopausal women show irregular menstruation and decreased estrogen levels, while postmenopausal women have been amenorrhea for more than 1 year30,31. The study subjects excluded women who met any of the following conditions: (1) other endocrine diseases, such as hypothyroidism and Cushing’s syndrome; (2) severe debilitating diseases, such as cancer, liver and kidney dysfunction; (3) smoking or drinking habits; (4) history of estrogen replacement therapy; (5) women who could not remember their last menstrual cycle. This study has been approved by the Ethics Committee of West China Second Medical College, Sichuan University (Number: KL059), and all procedures involving human participants were in accordance with ethical standards. All participants signed written informed consent.
Data collection of the metabolic related diseases
Postmenopausal and perimenopausal women are at increased risk of developing metabolic-related diseases, such as cardiovascular disease and skeletal system diseases32,33, especially hypertension and coronary artery disease34. Clinically, systolic blood pressure over 140 mmHg or diastolic blood pressure over 90 mmHg is considered hypertension35. In addition, the diagnosis of coronary heart disease is usually based on its common symptoms (such as chest pain, chest tightness, dyspnea and fatigue) combined with coronary angiography36. Dual-energy X-ray absorptiometry (DEXA) is an instrument widely used to measure bone density and screen for osteoporosis37. According to the standards of the World Health Organization (WHO), a T-score between − 1 and − 2.5 indicates osteopenia, while T-score below − 2.5 is diagnosed as osteoporosis38.
Measurements of anthropometric indexes
The weight and height of all participants who met the screening criteria were measured using a standard stadiometer and scale. The body mass index (BMI) was calculated based on the ratio of weight (kg) to height (m39) squared. The waist circumference (between the 10th rib (lower costal arch) and ilium (iliac crest)) was measured with a measuring tape applied to the subject’s skin while the subject was breathing naturally. The hip circumference was measured with a flexible measuring tape passed horizontally around the widest part of the hips (the distance in the two upper ilium) with the subject’s feet together. The waist-to-hip ratio (WHR) is the ratio of waist circumference to hip circumference. The subject’s body fat fraction (BFR) was measured using the electrical impedance measurement technique40. The subject’s bone mineral density (BMD) was measured using DEXA at the lumbar spine L2 to L4 (L2-L4) BMD and T1 values, and at the femoral neck BMD and T2 values.
Laboratory measurements
The researchers used a sterilized syringe to draw 10 ml of fasting blood samples from the subjects through the vein, as well as 10 ml of blood samples within 120 min after oral administration of 75 g of glucose. The electrochemical method was used to detect the blood samples of the subjects to obtain the concentrations of fasting blood glucose (FPG) and postprandial blood glucose (PBG), the colorimetric method was used to detect the levels of total cholesterol (TC) and triglycerides (TG) in the blood, and the chemical analysis method was used to detect the levels of high-density lipoprotein (HDL) and low-density lipoprotein (LDL) in the blood.
we used enzyme-linked immunosorbent assay (ELISA)41 to detect the levels of various sex hormones in fasting blood samples of these subjects to measure blood sex hormones (follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), testosterone (T)) in perimenopausal and postmenopausal women. The collection time of sex hormone blood samples of perimenopausal women must be 6 months after amenorrhea or more than 3 cycles.
Statistical analysis
SPSS 21.0 (Statistical Package for Social Sciences, Inc., Chicago, IL, USA) was used for data analysis and significance testing42, and Graphpad prism5 (Statistical and Graphing Software, China) was used for graphics43. Normally distributed data are expressed as mean ± standard deviation (SD), while discrete data are expressed as median (interquartile range). Chi-square tests assessed whether there were significant differences in the proportions of relevant diseases between groups44. Bivariate correlation analysis evaluated the effects of hormones and age on four indicators: TC, LDL, T1, and T2. All statistical tests in this study were performed at a significance level of 0.05, with Bonferroni correction for multiple comparisons45.
Results
Subjects
A total of 2148 postmenopausal and perimenopausal female patients were recruited from West China Second University Hospital from 2020 to 2024, and 637 were excluded from the study (Table 1). Finally, 1511 women met the screening criteria for follow-up study, including 1103 perimenopausal women and 408 postmenopausal women. Among the 1108 perimenopausal women, 98 women had cardiovascular disease, and 268 women had osteopenia and osteoporosis. Among the 1108 postmenopausal women, 68 women had cardiovascular disease, and 86 women had osteopenia and osteoporosis. This result indicates that perimenopausal and postmenopausal females have an increased probability of suffering from cardiovascular and skeletal system diseases, and the risk of suffering from skeletal system diseases is much greater than that of cardiovascular diseases.
Table 1.
Prevalence rate of cardiovascular and skeletal disorders in perimenopausal and postmenopausal women at different age groups.
| Age | Number | Cardiovascular diseases | Skeletal system | |||
|---|---|---|---|---|---|---|
| Peri-M | Post-M | Peri-M | Post-M | Peri-M | Post-M | |
| 40–44 | 541 | 66 | 26 (4.81%) | 0 | 108 (19.96%) | 13 (19.69%) |
| 45–49 | 461 | 142 | 53 (11.47%) | 21 (14.79%) | 129 (27.98%) | 59 (41.55%) |
| 50–54 | 101 | 200 | 19 (18.81%) | 47 (23.50%) | 31 (30.69%) | 99 (49.50%) |
| Total | 1103 | 408 | 98 (8.88%) | 68 (16.66%) | 268 (24.30%) | 171 (41.91%) |
Note: Peri-M means perimenopausal and Post-M means postmenopausal.
To explore the relationship between age and cardiovascular and skeletal system diseases inperimenopausal and postmenopausal women, we analyzed the incidence of cardiovascular and skeletal system diseases at different age stages. The number of perimenopausal women suffering from cardiovascular diseases and skeletal diseases was 98 and 268, respectively. The number of postmenopausal women suffering from cardiovascular diseases and skeletal diseases was 68 and 171, respectively. The study found that the risk ofperimenopausal and postmenopausal women suffering from cardiovascular and skeletal system diseases increased with increasing age, and the incidence of postmenopausal women was significantly higher than that of perimenopausal women. This result indicates that both age and menstrual status have an impact on women’s risk of disease.
Metabolic related diseases in perimenopausal and postmenopausal women
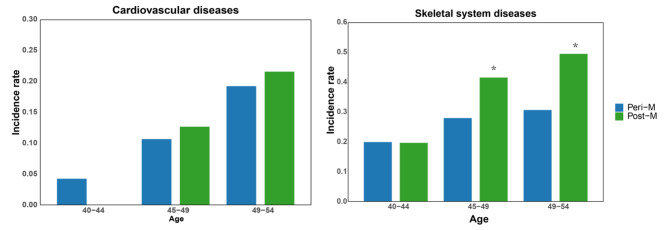
In order to further analyze the impact of age on the prevalence of cardiovascular and skeletal system diseases in perimenopausal and postmenopausal women, we further analyzed the prevalence rates at different age stages (Fig. 1). In cardiovascular diseases, the incidence rates of perimenopausal women in the 40–44 group, 45–49 group and 50–54 group were 26 (4.81%), 53 (11.47%) and 19 (18.81%), while the incidence rates of postmenopausal women were 21 (14.79%), 47 (23.50%) and 68 (16.66%). skeletal diseases, the incidence rates of menopausal women in the 40–44 group, 45–49 group and 50–54 group were 108 (19.96%), 129 (27.98%) and 31 (30.69%), while the incidence rates of perimenopausal women were 13 (19.69%), 59 (41.55%) and 99 (49.50%). The results of the study showed that the risk of cardiovascular disease and bone disease in perimenopausal and postmenopausal women increased with age, and the probability of postmenopausal women suffering from the disease was higher than that of perimenopausal women.
Fig. 1.
The prevalence rate of cardiovascular and skeletal diseases in perimenopausal and postmenopausal women at different age groups (A) Cardiovascular disease. (B) Skeletal diseases. Note: * means p < 0.05, Peri-M means perimenopausal, Post-M means postmenopausal.
Correlation analysis of the association between cardiovascular disease and perimenopausal and postmenopausal women showed that women’s risk of cardiovascular disease was not significantly related to their menstrual cycle stage. Correlation analysis of skeletal system diseases shows that the incidence rate of women aged 45 to 49 and 50 to 54 years old is related to the menstrual stage, and the incidence rate of postmenopausal women is significantly higher than that of perimenopausal women. This shows that women’s physiological stage will have an impact on women’s skeletal system diseases.
Physiological and biochemical indicators of perimenopausal and postmenopausal women
To explore the effects of various physiological and biochemical indicators on sexual function in perimenopausal and postmenopausal women, we analyzed the physiological and biochemical indicators of these two groups of women (Table 2). The results showed that there was no significant difference in FPG, PBG, BMI, WC, HC, BFR, TG and HDL between perimenopausal and postmenopausal women (p > 0.05).
Table 2.
Physiological and biochemical indicators of perimenopausal and postmenopausal women.
| Peri-M Mean ± SD |
Post-M Mean ± SD |
P value | |
|---|---|---|---|
| E2(pg/ml) | 65.76(31.83–93.85) | 38.08(11.9–60.85) | 0.000*** |
| T(ng/ml) | 0.41(0.23–0.45) | 0.39(0.24–0.46) | 0.258 |
| FSH(IU/L) | 44.84(10.20–73.10) | 74.07(50.53–95.43) | 0.000*** |
| LH(IU/L) | 25.93(18.13–42.12) | 37.62(27.15–46.92) | 0.000*** |
| TC(mmol/L) | 4.79 ± 0.84 | 5.00 ± 0.81 | 0.000*** |
| TG(mmol/L) | 1.29(0.82–1.56) | 1.39(0.88–1.66) | 0.193 |
| HDL(mmol/L) | 1.66 ± 0.39 | 1.64 ± 0.37 | 0.458 |
| LDL(mmol/L) | 2.68 ± 0.81 | 2.84 ± 0.75 | 0.000*** |
| FPG(mmol/L) | 5.32(5.0–5.6) | 5.33(5.00–5.65) | 0.305 |
| PBG(mmol/L) | 6.65(5.62–7.18) | 6.67(5.93–7.82) | 0.921 |
| BMI(kg/m2) | 22.61 ± 2.65 | 22.51 ± 2.87 | 0.085 |
| WC(cm) | 75 (70–80) | 76(69–80) | 0.768 |
| HC(cm) | 91(87–94) | 91(86–93) | 0.567 |
| WHR | 0.83 ± 0.05 | 0.83 ± 0.05 | 0.897 |
| BFR | 32.97(29.81–35.42) | 32.33(27.13–34.75) | 0.203 |
| L2-L4(BMD) | 1.15 ± 0.16 | 1.07 ± 0.16 | 0.000*** |
| T1 | −0.03 ± 1.19 | −0.37 ± 1.24 | 0.000*** |
| Neck of femur(BMD) | 0.93 ± 0.13 | 0.87 ± 0.12 | 0.000*** |
| T2 | −0.33 ± 1.01 | −0.59 ± 0.98 | 0.000*** |
Note: Normally distributed data were expressed as means ± SD, skewed distribution were reported as median (interquartile range), *** indicated that p is less than 0.001.
However, the difference in sex hormone levels between perimenopausal and postmenopausal women is very significant. The levels of E2, FSH and LH in perimenopausal women were 65.76 pg/ml, 44.8436 IU/L and 25.93IU/L respectively, while the levels in postmenopausal women were 38.08 pg/ml, 74.07 IU/L and 37.62 IU/L respectively. This result shows that E2 in postmenopausal women is significantly lower than that in perimenopausal women (p < 0.001), while FSH and LH are significantly higher than in perimenopausal women (p < 0.001). Among the blood lipid indicators, only the content of TC was significantly different between the two groups (p < 0.001), which were 4.79 mmol/L and 5.00 mmol/L respectively. In addition, there were significant differences in four bone mineral density indicators between perimenopausal and postmenopausal women (p < 0.001). The L2-L4 BMD, T1 value, femoral neck BMD, and T2 value in perimenopausal women were 1.15, −0.03, 0.93, and − 0.33, while the corresponding values in postmenopausal women were 1.07, −0.37, 0.87, and − 0.59. These results indicated that there are differences in certain physiological indicators between perimenopausal and postmenopausal women, which may be related to the occurrence of various metabolic diseases.
Physiological and biochemical indicators of perimenopausal and postmenopausal women at different ages
The differences in various physiological and biochemical indicators of perimenopausal and postmenopausal women of different age groups were further analyzed to explore the effect of age on metabolic-related diseases in women (Table 3). The three sex hormone indicators (E2, FSH and LH) showed significant differences in perimenopausal and postmenopausal women of different age groups. The values of E2 in perimenopausal and postmenopausal women aged 40–44, 45–49 and 50–54 were 72.81 pg/ml, 45.23 pg/ml and 23.06 pg/ml. FSH was 41.31 IU/L, 51.9 IU/L and 77.38 IU/L in these three age groups, while LH was 23.54 IU/L, 29.26 IU/L and 39.91 IU/L. This result shows that E2 of perimenopausal and postmenopausal women decreases with age, while FSH and LH increase with age. The three blood lipid indexes TC (p < 0.001), TG (p < 0.05), and LDL (p < 0.001) in perimenopausal and postmenopausal women of different age groups all showed an increasing trend with age, and there were significant differences with age. Among them, the content of TC in different age stages was 4.76 mmol/L, 4.84 mmol/L, and 5.04mmol/L. The values of L2-L4, T1, T2 and femoral neck in perimenopausal and postmenopausal women of different age groups decreased with age, and there were significant differences with age in L2-L4 (p < 0.001), T1 (p < 0.001), femoral neck (p < 0.05), and T2 (p < 0.05). There were no significant differences in T, TG, HDL, FPG, BMI, HC, WC, WHR, and BFR between perimenopausal and postmenopausal women of different age groups (p > 0.05).
Table 3.
Physiological and biochemical indicators of perimenopausal and postmenopausal women at different ages.
| 40–44 Mean ± SD |
45–49 Mean ± SD |
50–54 Mean ± SD |
P value | |
|---|---|---|---|---|
| E2(pg/ml) | 72.81(35.21–91.35) | 45.23(14.10–86.90) | 23.06(11.80–35.55) | 0.000*** |
| T(ng/ml) | 0.38(0.23–0.44) | 0.36(0.21–0.42) | 0.32(0.19–0.44) | 0.976 |
| FSH(IU/L) | 41.31(8.28–78.54) | 21.90(13.10–73.25) | 77.38(35.28–93.24) | 0.000*** |
| LH(IU/L) | 23.54(8.52–45.36) | 29.26(12.34–49.3) | 39.91(26.32–52.41) | 0.000*** |
| TC(mmol/L) | 4.76 ± 0.82 | 4.84 ± 0.80 | 5.04 ± 0.76 | 0.000*** |
| TG(mmol/L) | 1.01(0.68–1.47) | 1.27 (0.96–1.82) | 1.38(1.23–1.86) | 0.045* |
| HDL(mmol/L) | 1.65 ± 0.36 | 1.68 ± 0.39 | 1.65 ± 0.36 | 0.563 |
| LDL(mmol/L) | 2.67 ± 0.78 | 2.71 ± 0.75 | 2.89 ± 0.68 | 0.000*** |
| FPG(mmol/L) | 5.34(4.91–5.46) | 5.34(4.96–5.61) | 5.34(4.92–5.58) | 0.321 |
| PBG(mmol/L) | 6.62 (5.59–6.57) | 6.66(6.19–6.71) | 6.87(6.67–6.97) | 0.000*** |
| BMI(kg/m2) | 22.51 ± 2.69 | 22.72 ± 2.68 | 22.45 ± 2.65 | 0.996 |
| WC(cm) | 75(70–79) | 75(70–80) | 76(71–81) | 0.253 |
| HC(cm) | 90.15 ± 5.80 | 90.82 ± 6.03 | 90.75 ± 6.04 | 0.358 |
| WHR | 0.83 ± 0.05 | 0.83 ± 0.05 | 0.83 ± 0.05 | 0.758 |
| BFR | 32.78(29.52–35.36) | 33.39(29.85–35.60) | 33.01(30.10–35.43) | 0.291 |
| L2-L4(BMD) | 1.15 ± 0.12 | 1.14 ± 0.15 | 1.05 ± 0.13 | 0.000*** |
| T1 | −0.05 ± 1.12 | −0.23 ± 1.20 | −0.81 ± 1.32 | 0.000*** |
| Neckof femur(BMD) | 0.92 ± 0.12 | 0.89 ± 0.12 | 0.87 ± 0.12 | 0.025* |
| T2 | −0.30 ± 1.03 | −0.39 ± 1.01 | −0.60 ± 1.01 | 0.031* |
Note: Normally distributed data were expressed as means ± SD, skewed distribution were reported as median (interquartile range), *** indicated that p is less than 0.001.
Effects of age and E2 on perimenopausal and postmenopausal women related indicators
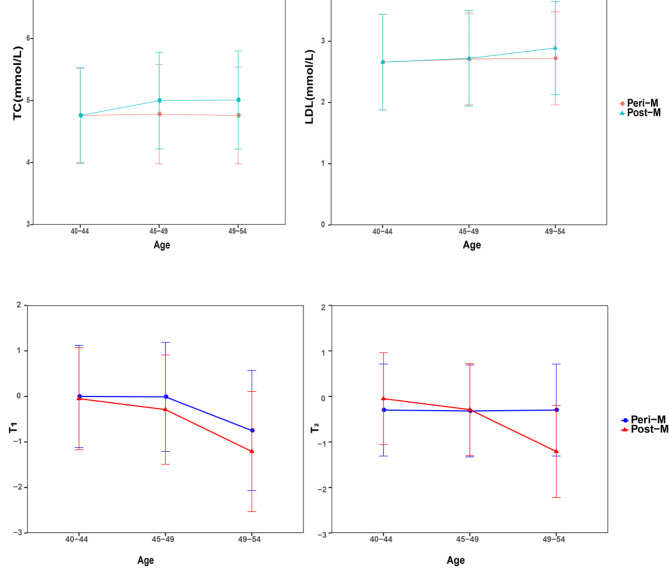
In order to further explore the impact of different ages on the physiological indicators of perimenopausal and menopausal women and the development of cardiovascular disease and osteoporosis, we combined the significantly related physiological indicators of women of different ages and selected 4 indicators for further analysis. TC and LDL are related indicators of cardiovascular disease. Excessive levels can lead to atherosclerosis and increase the risk of heart disease and stroke. T1 and T2 are important indicators for assessing bone density and bone health. The T1 value usually refers to the bone density of the lumbar spine, while the T2 value is related to the bone density of the femur or other parts. Low T1 and T2 values generally indicate an increased risk of osteoporosis. The four indicators of perimenopausal women and postmenopausal women aged 40 to 44 years are basically the same (Fig. 2), the TC and LDL of postmenopausal women are higher than those of perimenopausal women with age increases, while the values of T1 and T2 lower than perimenopausal women.
Fig. 2.
Distribution of four indicators among perimenopausal and postmenopausal women at different ages. (A) TC. (B) LDL. (C) T1. (D) T2.
This study further analyzed the correlation between E2 and age with the four indicators. The results showed that among perimenopausal and menopausal women, E2 and age were significantly related to the four indicators (p < 0.001) (Table S2). It was found that the correlation coefficient between age and the four indicators in perimenopausal and menopausal women were higher than the correlation coefficients between E2 and the four indicators (Table 4). This result suggests that age plays a key role in the development of metabolic diseases in perimenopausal and postmenopausal women.
Table 4.
The differences of correlation in the E2 and age with four indicators.
| r | TC | LDL | T1 | T2 | ||||
|---|---|---|---|---|---|---|---|---|
| Peri-M | Post-M | Peri-M | Post-M | Peri-M | Post-M | Peri-M | Post-M | |
| E2 | 0.0453 | −0.0523 | −0.0021 | −0.0613 | 0.0461 | 0.1155 | 0.0364 | 0.1246 |
| Age | 0.5321 | 0.2103 | 0.0904 | 0.1876 | −0.2045 | −0.1798 | −0.0312 | −0.0405 |
Discussion
This study analyzed the incidence of metabolic diseases (cardiovascular diseases and skeletal system diseases) in 1,151 perimenopausal and postmenopausal women at different ages. The study found that the risk of postmenopausal women was significantly higher than that of perimenopausal women. In addition, the estrogen and bone density of perimenopausal and postmenopausal women showed a downward trend with age, while TC and LDL levels increased.
Cardiovascular disease
The incidence of cardiovascular disease is closely related to age46. The elasticity of human blood vessels decreases and the degree of arteriosclerosis increases with age growing, which leads to an increased risk of cardiovascular disease47. And some studies have found that the incidence of heart disease, high blood pressure and stroke is significantly higher in the elderly than in young people48.
A woman’s menstrual cycle also has an important impact on cardiovascular disease risk49. Estrogen plays a key role in female physiology and has a protective effect on the cardiovascular system49. Changes in estrogen levels during different phases of the menstrual cycle may affect lipid profiles, vascular function, and inflammatory responses. Estrogen levels drop significantly in perimenopausal and postmenopausal women, which results in a significantly increased risk of cardiovascular disease. The main risk factor for cardiovascular disease is dyslipidemia, which is mainly manifested by the increase in TC, TG and LDL levels, and the decrease in HDL levels. The results of this study indicate that TC and LDL levels increase significantly in postmenopausal women, and these levels may further increase with age. Studies have found that the decline in estrogen levels in women during perimenopause and postmenopause can lead to an increased risk of cardiovascular disease50. Especially in postmenopausal women, the incidence of myocardial infarction and coronary heart disease has increased significantly51.
Estrogen is involved in many aspects of cardiovascular regulation, such as blood lipid regulation, vasodilation, antioxidant and anti-inflammatory effects52. Estrogen can reduce the risk of atherosclerosis by lowering total cholesterol and LDL levels and increasing HDL levels53. Moreover, estrogen can promote the function of vascular endothelial cells, enhance the elasticity and dilation capacity of blood vessels, and thus improve hemodynamics54. Estrogen levels drop significantly in perimenopausal and postmenopausal women. Women of childbearing age have higher estrogen levels during their menstrual cycle, which provides them with relatively good cardiovascular protection. Perimenopausal and postmenopausal women face a higher risk of cardiovascular disease, especially myocardial infarction and coronary heart disease55. This phenomenon indicates that estrogen deficiency is an important factor leading to the increased risk of cardiovascular disease in postmenopausal women56. Changes in estrogen levels can also affect endothelial function and inflammatory response. Studies have found that endothelial cell function in postmenopausal women decreases, which leads to increased levels of inflammation. A decrease in estrogen can lead to lipid metabolism disorders in the human body and aggravate dyslipidemia57.
Skeletal system disease
Osteoporosis is a disease characterized by reduced bone density and microarchitectural destruction of bone tissue which leads to bone fragility and an increased risk of fractures58. Changes in bone density are closely related to age, and the disease is more common in older people, especially women59. The arrival of menopause has a significant impact on bone density. Estrogen plays an important role in maintaining bone density by inhibiting bone resorption and promoting bone formation to maintain bone health60,61. Women’s bone density usually remains at a relatively high level before menopause. However, significant decreases in estrogen levels lead to accelerated bone resorption that can cause a rapid decrease in bone density in perimenopausal and postmenopausal women62. In this study, we found that the BMD of the lumbar spine and femoral neck in perimenopausal and postmenopausal women decreases with age, and the incidence of osteopenia and osteoporosis in postmenopausal women are higher than that in perimenopausal women. Studies have found that postmenopausal women’s bone density decline by 10–20% in the first five years63. During this period, perimenopausal and postmenopausal women have a significantly increased risk of osteoporosis, especially fractures of the spine, hips, and wrists.
Decreased bone density is a common problem faced by postmenopausal women, especially in the lumbar spine and femoral neck64. The bone density of perimenopausal and postmenopausal women is significantly lower than that of younger women65. The main reason for this phenomenon is the significant decrease in estrogen levels after menopause which causes the skeletal system to lose its important protective role. Additionally, women in early menopause face a higher risk of osteoporosis and fractures than women of childbearing age66. Because women of childbearing age usually have relatively stable estrogen levels, their bone density remains at a high level. Studies have found that women of childbearing age generally have higher bone density and stronger bone repair capabilities67. Women of childbearing age have a significantly lower risk of fractures than postmenopausal women when facing the same external stress.
Conclusion
Perimenopausal and postmenopausal women have a significantly increased risk of metabolic diseases, especially cardiovascular disease and osteoporosis. And as age increases, the risk of disease continues to increase for these groups of women. This study found that the incidence of cardiovascular disease and osteoporosis in menopausal women is higher than that in perimenopausal women, and the incidence of osteoporosis is closely related to women’s physiological stage. In addition, estrogen levels and bone mineral density were significantly lower in perimenopausal and postmenopausal women than in normal women, while TC and LDL levels were elevated. The study also found that there is a significant correlation between estrogen and TC, LDL and bone density. This study not only provides a scientific basis for understanding the health risks of perimenopausal and postmenopausal women, but also provides important guidance for clinical research and the formulation of public health policies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Xiaoyan Luo and Liangzhi Xu conceived and designed the research. Hong Mou analyzed the data and wrote the original draft. Jun Zhang and Yichuan Guo revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Scientific research project on cadre health care in Sichuan Province (ZH2021-1701).
Data availability
The raw data during the current study are available in supplementary material table S1.
Declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
Not applicable.
Informed consent
This study was approved by West China Second Medical College of Sichuan University.
Ethical statement
The study protocol was approved by the medical ethics committee of West China Second University Hospital, Sichuan University in accordance with ethical guidelines.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liangzhi Xu, Email: liangzxu@126.com.
Xiaoyan Luo, Email: luoxiaoyan0917@126.com.
References
- 1.Marnocha, S. K., Bergstrom, M. & Dempsey, L. F. The lived experience of perimenopause and menopause. Contemp. Nurse. 37, 229–240 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Shuster, L. T., Rhodes, D. J., Gostout, B. S., Grossardt, B. R. & Rocca, W. A. Premature menopause or early menopause: long-term health consequences. Maturitas65, 161–166 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Troìa, L., Martone, S., Morgante, G. & Luisi, S. Management of perimenopause disorders: hormonal treatment. Gynecol. Endocrinol.37, 195–200 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Tandon, V. R., Sharma, S., Mahajan, A., Mahajan, A. & Tandon, A. Menopause and Sleep disorders. J. mid-life Health. 13, 26–33. 10.4103/jmh.jmh_18_22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lialy, H. E., Mohamed, M. A., AbdAllatif, L. A., Khalid, M. & Elhelbawy, A. Effects of different physiotherapy modalities on insomnia and depression in perimenopausal, menopausal, and post-menopausal women: a systematic review. BMC Womens Health. 23, 363. 10.1186/s12905-023-02515-9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nash, D. et al. Blood lead, blood pressure, and hypertension in perimenopausal and postmenopausal women. Jama289, 1523–1532 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Gast, G. C. M. et al. Vasomotor menopausal symptoms are associated with increased risk of coronary heart disease. Menopause18, 146–151 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Pérez, J. M., Palacios, S., Chavida, F. & Pérez, M. Severity of menopausal symptoms and cardiovascular and osteoporosis risk factors. Climacteric16, 226–234 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Ko, S. H. & Jung, Y. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients13, 4556 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko, S. H. & Kim, H. S. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients12, 202 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryczkowska, K., Adach, W., Janikowski, K., Banach, M. & Bielecka-Dabrowa, A. Menopause and women’s cardiovascular health: is it really an obvious relationship? Archives Med. Science: AMS. 19, 458 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie, G. et al. The effects of menopause hormone therapy on lipid profile in postmenopausal women: a systematic review and meta-analysis. Front. Pharmacol.13, 850815 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han, Y. et al. Menopausal impact on the association between thyroid dysfunction and lipid profiles: a cross-sectional study. Front. Endocrinol.13, 853889 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentile, M. et al. Association between very low-density lipoprotein cholesterol (VLDL-C) and carotid intima-media thickness in postmenopausal women without overt cardiovascular disease and on LDL-C target levels. J. Clin. Med.9, 1422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anagnostis, P. et al. Menopause symptom management in women with dyslipidemias: an EMAS clinical guide. Maturitas135, 82–88 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Zhang, L. et al. Estrogen protects vasomotor functions in rats during catecholamine stress. Front. Cardiovasc. Med.8, 679240 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun, H. J., Wu, Z. Y., Nie, X. W. & Bian, J. S. Role of endothelial dysfunction in Cardiovascular diseases: the Link between inflammation and hydrogen sulfide. Front. Pharmacol.10, 1568. 10.3389/fphar.2019.01568 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Black, D. M. & Rosen, C. J. Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med.374, 254–262. 10.1056/NEJMcp1513724 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Di Medio, L. & Brandi, M. L. in Advances in Clinical Chemistry Vol. 105 101–140Elsevier, (2021). [DOI] [PubMed]
- 20.Luo, X., Zhang, J., Guo, Y. & Xu, L. The association study of body composition and hormone levels with glucose, lipid and bone density in perimenopausal and postmenopausal women. (2024).
- 21.Barnsley, J. et al. Pathophysiology and treatment of osteoporosis: challenges for clinical practice in older people. Aging Clin. Exp. Res.33, 759–773 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pala, R., Anju, V., Dyavaiah, M., Busi, S. & Nauli, S. M. Nanoparticle-mediated drug delivery for the treatment of cardiovascular diseases. Int. J. Nanomed., 3741–3769 (2020). [DOI] [PMC free article] [PubMed]
- 23.Shufelt, C. L. & Manson, J. E. Menopausal hormone therapy and cardiovascular disease: the role of formulation, dose, and route of delivery. J. Clin. Endocrinol. Metabolism. 106, 1245–1254 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grodstein, F. et al. Hormone replacement therapy & cardiovascular disease. ACOG Clin. Rev.2, 6 (1997). [Google Scholar]
- 25.Vigneswaran, K. & Hamoda, H. Hormone replacement therapy - current recommendations. Best Pract. Res. Clin. Obstet. Gynecol.81, 8–21. 10.1016/j.bpobgyn.2021.12.001 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Gambacciani, M. & Levancini, M. Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Menopause Review/Przegląd Menopauzalny. 13, 213–220 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cranney, A. & Wells, G. A. Hormone replacement therapy for postmenopausal osteoporosis. Clin. Geriatr. Med.19, 361–370 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Khalil, R. A. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem. Pharmacol.86, 1627–1642 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genazzani, A. R., Monteleone, P., Giannini, A. & Simoncini, T. Hormone therapy in the postmenopausal years: considering benefits and risks in clinical practice. Hum. Reprod. Update. 27, 1115–1150 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Tarlatzis, B. C. & Zepiridis, L. Perimenopausal conception. Ann. N. Y. Acad. Sci.997, 93–104 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Belchetz, P. E. Hormonal treatment of postmenopausal women. N. Engl. J. Med.330, 1062–1071 (1994). [DOI] [PubMed] [Google Scholar]
- 32.Atsma, F., Bartelink, M. L. E., Grobbee, D. E. & van der Schouw, Y. T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause13, 265–279 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Stevenson, J. C. A woman’s journey through the reproductive, transitional and postmenopausal periods of life: impact on cardiovascular and musculo-skeletal risk and the role of estrogen replacement. Maturitas70, 197–205 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Dosi, R., Bhatt, N., Shah, P. & Patell, R. Cardiovascular disease and menopause. J. Clin. Diagn. Research: JCDR. 8, 62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Böhm, M. et al. Achieved diastolic blood pressure and pulse pressure at target systolic blood pressure (120–140 mmHg) and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Eur. Heart J.39, 3105–3114 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Lu, L., Liu, M., Sun, R., Zheng, Y. & Zhang, P. Myocardial infarction: symptoms and treatments. Cell Biochem. Biophys.72, 865–867 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Jain, R. K. & Vokes, T. Dual-energy X-ray absorptiometry. J. Clin. Densitometry. 20, 291–303 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Di Carli, M. F. & Hachamovitch, R. New technology for noninvasive evaluation of coronary artery disease. Circulation115, 1464–1480 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Brinton, R. D., Yao, J., Yin, F., Mack, W. J. & Cadenas, E. Perimenopause as a neurological transition state. Nat. Reviews Endocrinol.11, 393–405 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kyle, U. G. et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin. Nutr.23, 1226–1243 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Butler, J. E. Enzyme-linked immunosorbent assay. J. Immunoass.21, 165–209 (2000). [DOI] [PubMed] [Google Scholar]
- 42.George, D. & Mallery, P. IBM SPSS Statistics 26 step by step: A Simple Guide and Reference (Routledge, 2019). [Google Scholar]
- 43.Motulsky, H. Prism 5 statistics guide, GraphPad Software 31, 39–42 (2007). (2007).
- 44.McHugh, M. L. The chi-square test of independence. Biochemia Med.23, 143–149 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisstein, E. W. Bonferroni correction. (2004). https://mathworld.wolfram.com/
- 46.Newman, A. B. et al. Successful aging: effect of subclinical cardiovascular disease. Arch. Intern. Med.163, 2315–2322 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Vatner, S. F. et al. Vascular stiffness in aging and disease. Front. Physiol.12, 762437 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nwabuo, C. C. et al. Long-term cumulative blood pressure in young adults and incident heart failure, coronary heart disease, stroke, and cardiovascular disease: the CARDIA study. Eur. J. Prev. Cardiol.28, 1445–1451 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Kelly, A. C. et al. Pregnancy and reproductive risk factors for cardiovascular disease in women. Circul. Res.130, 652–672 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertoia, M. L. et al. Risk factors for sudden cardiac death in post-menopausal women. J. Am. Coll. Cardiol.60, 2674–2682 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Hulley, S. et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Jama280, 605–613 (1998). [DOI] [PubMed] [Google Scholar]
- 52.Lavoie, J. M. Dynamics of hepatic and intestinal cholesterol and bile acid pathways: the impact of the animal model of estrogen deficiency and exercise training. World J. Hepatol.8, 961 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, H. H., Liu, M., Clegg, D. J., Portincasa, P. & Wang, D. Q.-H. New insights into the molecular mechanisms underlying effects of estrogen on cholesterol gallstone formation. Biochim. et Biophys. Acta (BBA)-Molecular Cell. Biology Lipids. 1791, 1037–1047 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phelps, T., Snyder, E., Rodriguez, E., Child, H. & Harvey, P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biology sex. Differences. 10, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmisano, B. T., Zhu, L. & Stafford, J. M. Role of estrogens in the regulation of liver lipid metabolism. Sex. Gend. Factors Affecting Metabolic Homeost. Diabetes Obes., 227–256 (2017). [DOI] [PMC free article] [PubMed]
- 56.Després, J. P. Body fat distribution and risk of cardiovascular disease: an update. Circulation126, 1301–1313 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Duntas, L. H. & Brenta, G. A renewed focus on the association between thyroid hormones and lipid metabolism. Front. Endocrinol.9, 386799 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lakshmanan, K., Dhanalakshmi, M., Ganesan, A. & Myneni, S. Bone health after menopause: effect of surgical menopause on bone mineral density and osteoporosis. Int. J. Reprod. Contracept. Obstet. Gynecol.10, 1820–1824 (2021). [Google Scholar]
- 59.Kim, S. & Won, C. W. Sex-different changes of body composition in aging: a systemic review. Arch. Gerontol. Geriatr.102, 104711 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Cheng, C. H., Chen, L. R. & Chen, K. H. Osteoporosis due to hormone imbalance: an overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int. J. Mol. Sci.23, 1376 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uehara, I. A., Soldi, L. R. & Silva, M. J. B. Current perspectives of osteoclastogenesis through estrogen modulated immune cell cytokines. Life Sci.256, 117921 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Turner, R. T., Riggs, B. L. & Spelsberg, T. C. Skeletal effects of estrogen. Endocr. Rev.15, 275–300 (1994). [DOI] [PubMed] [Google Scholar]
- 63.Shieh, A. et al. Associations of age at menopause with postmenopausal bone mineral density and fracture risk in women. J. Clin. Endocrinol. Metabolism. 107, e561–e569 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hemmati, E. et al. Prevalence of primary osteoporosis and low bone mass in postmenopausal women and related risk factors. J. Educ. Health Promotion10 (2021). [DOI] [PMC free article] [PubMed]
- 65.Gil-Montoya, J. A. et al. Association between low bone mineral density and periodontitis in generally healthy perimenopausal women. J. Periodontol.92, 95–103 (2021). [DOI] [PubMed] [Google Scholar]
- 66.Moberg, L., Hamrefors, V., Fedorowski, A. & Rogmark, C. Early menopause and weight loss are significant factors associated with risk of future fracture in middle-aged women. BMC Musculoskelet. Disord.23, 779 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nichols, D. L., Sanborn, C. F. & Essery, E. V. Bone density and young athletic women: an update. Sports Med.37, 1001–1014 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data during the current study are available in supplementary material table S1.