Abstract
The objective of this study was to examine the association between serum tacrolimus trough levels and the detection of BK viruria in kidney transplant recipients. We conducted a retrospective study and included kidney transplant recipients who underwent BK viruria screening during 2018–2021. Serum tacrolimus trough levels, urine BK viral load, and potential risk factors were collected. Statistical analysis was performed to identify the association between the serum tacrolimus trough levels and the detection of BK virus in urine (defined as positive BK viruria), as well as to determine the cumulative incidence and risk factors for BK viruria. Out of 243 recipients, 76 had positive BK viruria. The average serum tacrolimus trough level was significantly higher among the positive BK viruria group compared to the BK viruria-negative group. High HLA mismatch was identified as a risk factor for BK viruria. Approximately half of the recipients with average serum tacrolimus trough levels exceeding 10 ng/mL would develop BK viruria early. This study found an association between the average serum tacrolimus trough level and the detection of BK viruria. High HLA mismatch and an average serum tacrolimus trough level exceeding 10 ng/mL should be regarded as a risk for BK viruria.
Keywords: BK viruria, BK virus nephropathy, Risk factors, Therapeutic drug monitoring, Tacrolimus
Subject terms: Nephrology, Virology
Introduction
Kidney transplantation represents the foremost therapeutic approach for patients afflicted with end-stage kidney disease1. However, kidney transplant recipients face significant susceptibility to post-transplant complications, notably infections. The BK virus infection is particularly prominent among these complications, especially against the background of more potent immunosuppressive regimens2. BK virus infection can lead to BK virus-associated nephropathy (BKVAN), a threatening complication of kidney transplantation that stands as one of the most important causes of graft loss in kidney transplant recipients3.
BK virus infection primarily occurs during early childhood, causing mild respiratory illness, and remains latent in renal tubular epithelial cells4. When an individual’s immune system becomes compromised, the virus can reactivate and replicate within the kidney allograft4. After viral shedding, the BK virus becomes detectable in urine, denoted as BK viruria, and subsequently in the blood, referred to as BK viremia5. Regarding BK virus screening, quantitative real-time polymerase chain reactions (PCR) of urine or blood are widely used6. Although the 2019 Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice (AST-IDCOP) recommend BK viremia monitoring for its superior positive predictive value over BK viruria7,8, BK viruria is initially preferred for 2-stage BK virus screening in resource-limited settings due to its cost-effectiveness9,10. Monitoring BK viruria serves as an early marker for the development of BK viremia and BKVAN11,12. According to the 2024 International Consensus Guidelines on the Management of BK Polyomavirus in Kidney Transplantation, BK viuria screening can be used in resource-limited settings where plasma BK viral load screening is unavailable or inapplicable13. Detection of high levels of BK viruria (> 104 to 107 copies/mL) should prompt further testing for BK viremia to guide subsequent management. Furthermore, the presence of BK viruria will serve as an indicator to clinicians regarding the possibility of excessive immunosuppression; thus, it might be critical to modify the immunosuppression regimen to avert the development of BK viremia and BKVAN.
Tacrolimus has been a cornerstone immunosuppressant in kidney transplantation for over 20 years14,15. A meta-analysis has shown that graft loss is significantly reduced in recipients treated with tacrolimus16. However, with the increased use of tacrolimus over cyclosporine, a higher risk of BK virus infection has been observed in tacrolimus-treated transplant recipients has been observed17. In addition, evidence indicates that tacrolimus use may increase the risk of developing BKVAN18. Its use is further complicated by a narrow therapeutic window and high pharmacokinetic variability19. Excessive immunosuppression with tacrolimus may reactivate latent BK virus replication in renal tubular epithelial cells, possibly through mechanisms involving FKBP-12 or reinforcing infection within the allograft20. Conversely, inadequate exposure to tacrolimus can lead to acute or chronic graft rejection3.
Given the pivotal role of tacrolimus in kidney transplant recipients, it is imperative to determine the optimal tacrolimus concentrations to minimize the risk of BK virus reactivation in these patients. Therefore, the primary objective of this study was to examine the association between serum tacrolimus trough levels and the detection of BK virus in urine. The secondary objectives were to assess the cumulative incidence of BK viruria within 12 months post-transplantation and to identify other risk factors associated with BK viruria detection in kidney transplant recipients.
Materials and methods
Study design, setting, and population
A retrospective study was conducted at Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. The cohort entry date was February 8, 2018. The data were retrieved from kidney transplant recipients who underwent BK viruria screening between February 2018 and December 2021, regardless of the transplant date. Kidney transplant recipients who had at least a month of follow-up, at least one urine BK virus screening, and received tacrolimus within 6 months prior to the screening were included in this study. Individuals were excluded from the study if they were pregnant or nursing, received multiple organs or a previous organ transplant other than a kidney, experienced surgical complications (defined as acute postoperative complications occurring within 72 h, including hematoma formation, wound infection, injury to adjacent organs such as ureteral injury, or venous thrombosis), relocated immediately after transplantation, or died during the early post-transplantation period. Various variables were documented, including demographic information for recipients and donors, BK viral loads in urine and plasma, kidney biopsy results, and potential risk factors for BK viruria. These factors encompass donor, recipient, and transplant-related variables, including D+/R- CMV serostatus, deceased donor, male recipient, older recipient age, obesity (BMI > 30 kg/m2), a greater degree of HLA mismatch, prior sensitization, longer cold ischemic time, ABO incompatibility, delayed graft function defined as the requirement of dialysis during the first week after transplantation, acute graft rejection episodes, and immunosuppressive drugs21,22. The research protocol received approval from the Research Ethics Committee of the Faculty of Medicine, Chiang Mai University (No.146/2021).
BK virus screening protocol
The BK viruria screening was performed using quantitative real-time PCR, employing the QIAamp DNA Blood mini kit for DNA extraction (Qiagen, Hilden, Germany), the Argene PCR molecular kit (BioMerieux, France), and the Bio-Rad CFX96 machine and software for analysis (Bio-Rad, USA). Kidney transplant recipients were scheduled for follow-up assessments, including kidney function, tacrolimus though levels, and BK viruria screening at approximately 1, 3, 6, and 12 months after transplantation, followed by annual evaluations until 5 years post-transplantation. In this study, positive BK viruria was defined as the detection of the BK virus in urine (≥ 315 copies/mL). Time to BK viruria positivity was defined as the duration from the date of transplantation to the first positive BK viruria test result. Recipients without a positive test were censored at the date of their last follow-up.
Throughout the follow-up period, adjustments to immunosuppressive doses and regimens were made based on clinical symptoms, tacrolimus though levels, BK viruria levels, and allograft function. If recipients were identified as having a high clinical suspicion for BKVAN–either BK viruria > 104 copies/mL or unexplained allograft dysfunction–subsequent management followed the standard protocol. This included actions such as obtaining plasma BK viral load for stronger evidence of BK virus infection, with BK viremia defined as ≥ 218 copies/mL in blood, reducing the dose of immunosuppressive drugs, or switching to alternative immunosuppressive drugs such as everolimus or cyclosporine in case of persistent high-grade BK viruria and/or viremia. Ultimately, a graft biopsy was performed to confirm the definite diagnosis of BKVAN.
Average serum tacrolimus trough levels
In our institution, the ARCHITECT tacrolimus assay, a chemiluminescent microparticle immunoassay (CMIA) on the ARCHITECT iSystem, was used to determine tacrolimus levels in whole blood. To ensure that tacrolimus levels represented true troughs, patients were scheduled for morning clinic visits and instructed to take their last dose the evening prior. Blood samples were collected in EDTA tubes just before the next scheduled dose, typically 30 min to 1 h before administration, to ensure accurate trough readings.
Due to the dynamic nature of serum tacrolimus trough levels, we defined a variable for comparing tacrolimus exposure, referred to as the average serum tacrolimus trough level (Tacx). The calculation of Tacx was performed as follows: First, the endpoint was defined based on BK viruria status, specifically as either the first occurrence of positive BK viruria or the last occurrence of negative BK viruria. Second, data were collected on the day of the endpoint and traced back up to 180 days. In cases where the duration from the endpoint to the traced-back date was not exactly 180 days, we estimated the value as follows: (a) If the duration was more than 180 days, the nearest two points of serum tacrolimus trough levels around 180 days were used, and linear interpolation was applied to estimate the serum tacrolimus trough level at day 180; (b) If the duration was less than 180 days, the actual duration was used as the divisor to calculate the average serum tacrolimus trough level. Finally, we calculate the area under the curve (AUC) based on each serum tacrolimus trough level and the duration of use for each level using the following formula: AUC = ∑(Tacrolimus level × Δt), where Δt is the duration (in days) that each tacrolimus level was maintained.
Statistical analysis
The Statistical Package for the R program (Version 4.2.3) was used to analyze patient characteristics and outcomes. Continuous variables were summarized using measures of central tendency and dispersion (i.e., mean, median, standard deviation, and interquartile range). The Kolmogorov-Smirnov normality test was employed to evaluate the distribution of continuous variables. When distributions were normal, the Student’s t-test was used; otherwise, the Mann-Whitney U-test was applied. Categorical variables were analyzed using percentage-frequency distribution and compared across groups with Pearson’s chi-square test or Fisher’s exact test, as appropriate.
Predictive factors for BK viruria development were identified using multivariable logistic regression analysis. Variables with p-values < 0.2 in univariate analysis were included in the multivariable model to avoid excluding potentially relevant predictors. Adjusted odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated to quantify the strength of the association. Interaction terms were evaluated to explore potential effect modifications and sensitivity analyses were performed to assess the robustness of the model.
The Kaplan-Meier method was used to estimate and illustrate the cumulative probability of BK viuria positivity over time. Differences between groups stratified by average serum tacrolimus trough level ranges (i.e., Tacx <8 ng/ml, Tacx 8–10 ng/ml, and Tacx >10 ng/ml) were assessed using the log-rank test. A Cox proportional hazard model was employed to calculate hazard ratios (HRs) and corresponding 95% CIs. The proportional hazards assumption was tested using Schoenfeld residuals, ensuring model validity. The analysis was limited to patients who underwent kidney transplantation after the initiation of BK viruria screening at Maharaj Nakorn Chiang Mai Hospital, beginning in February 2018, so that the evaluation of time after transplantation would be more accurate.
A p-value of < 0.05 was considered statistically significant for all tests. For variables included in the multivariable models, CIs were prioritized to assess the precision of the effect estimate.
Results
Out of the initial 246 kidney transplant recipients screened for BK virus infection, 243 were included in the study. Three recipients were excluded due to the absence of tacrolimus-based therapy. Among the included recipients, 76 tested positive for BK viruria. The demographic characteristics of the included recipients are shown in Table 1. Most kidney transplant recipients (92.5%) received induction therapy, namely thymoglobulin (68.9%), basiliximab (29.7%), and others (e.g., alemtuzumab or rituximab) (1.4%). No significant difference in the rate of induction therapy administration between patients with high HLA mismatch and those with low HLA mismatch was observed.
Table 1.
Demographic characteristics of kidney transplant recipients.
| Variables | Total (n = 243) |
BK viruria-positive (n = 76) |
BK viruria-negative (n = 167) |
p-value |
|---|---|---|---|---|
| Recipients | ||||
| Age at the time of transplantation (years, mean ± SD) | 43.1 ± 11.2 | 44.0 ± 10.1 | 42.7 ± 11.7 | 0.412 |
| Male sex | 151/243 (62.1%) | 55/76 (72.4%) | 96/167 (57.5%) | 0.027 |
| BMI at the time of transplantation (kg/m2, mean ± SD) | 21.9 ± 3.6 | 22.4 ± 4.1 | 21.7 ± 3.4 | 0.119 |
| Re-transplantation | 21/243 (8.6%) | 11/76 (14.5%) | 10/167 (6.00%) | 0.029 |
| Living donor | 94/237 (39.7%) | 31/73 (42.5%) | 63/164 (38.4%) | 0.556 |
| Comorbidities | ||||
| Hypertension | 180/237 (76.0%) | 52/73 (71.2%) | 128/164 (78.1%) | 0.257 |
| Diabetes mellitus | 29/237 (12.2%) | 8/73 (11.0%) | 21/164 (12.8%) | 0.689 |
| Hyperlipidemia | 27/237 (11.4%) | 9/73 (12.3%) | 18/164 (11.0%) | 0.762 |
| Chronic viral hepatitis B or C | 22/237 (9.3%) | 10/73 (13.7%) | 12/164 (7.3%) | 0.118 |
| CMV serostatus (n = 139) | ||||
| R+ | 133 | 38 | 95 | 0.317 |
| D-/R- | 1 | 0 | 1 | 0.499 |
| D+/R- | 5 | 2 | 3 | 0.671 |
| EBV serostatus (n = 70) | ||||
| R+ | 70 | 26 | 44 | 0.210 |
| D-/R- | 0 | 0 | 0 | 1.000 |
| D+/R- | 0 | 0 | 0 | 1.000 |
| Cold ischemic time (hours, mean ± SD) | 13.4 ± 9.3 | 13.6 ± 9.8 | 13.4 ± 9.1 | 0.873 |
| History of acute allograft rejection | 31/243 (12.5%) | 15/76 (19.7%) | 16/167 (9.6%) | 0.030 |
| Presence of delayed graft function | 82/237 (34.6%) | 19/73 (26.0%) | 63/164 (37.7%) | 0.064 |
| Mismatch | ||||
| ABO incompatible transplantation | 3/235 (1.3%) | 1/73 (1.4%) | 2/162 (1.2%) | 1.000 |
| Human leukocyte antigen (HLA) mismatch | ||||
| Low HLA mismatch (0–3 mismatches) | 208/239 (87.0%) | 56/74 (75.7%) | 152/165 (92.1%) | < 0.001 |
| High HLA mismatch (4–6 mismatches) | 31/239 (13.0%) | 18/74 (24.3%) | 13/165 (7.9%) | < 0.001 |
| Panel-reactive antibody ≥ 20% | ||||
| Class I | 31/210 (14.8%) | 12/68 (17.7%) | 19/142 (13.4%) | 0.347 |
| Class II | 21/210 (10.5%) | 9/68 (13.2%) | 13/142 (9.2%) | 0.366 |
| Donors | ||||
| Age at the time of transplantation (years, mean ± SD) | 38.11 ± 12.09 | 38.42 ± 12.56 | 37.98 ± 11.90 | 0.793 |
| Male sex | 149/235 (63.4%) | 47/73 (64.4%) | 102/162 (63.0%) | 0.834 |
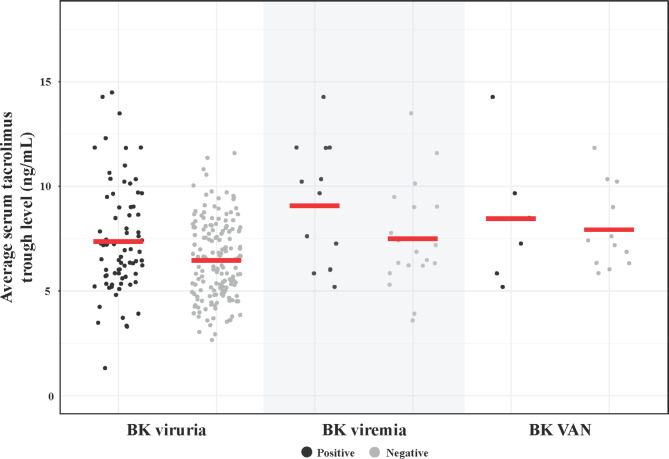
The average serum tacrolimus trough level was significantly higher in the BK viruria-positive group compared to the BK viruria-negative group (7.37 ± 2.61 vs. 6.47 ± 1.85, p = 0.002) (Fig. 1). At discharge, kidney function outcomes showed similar creatinine levels and estimated glomerular filtration rate (eGFR) values between the BK viruria-positive and BK viruria-negative groups. By the last follow-up, creatinine and eGFR values remained comparable between the two groups, with no significant differences observed.
Fig. 1.
Comparison of average serum tacrolimus trough levels among kidney transplant recipients based on BK virus infection outcomes: BK viruria, BK viremia, and BKVAN, subsequently categorized into positive and negative groups for each outcome.
Of the 76 recipients identified as BK viruria-positive, 32 underwent additional testing for BK viremia, and of these, 13 (40.6%) tested positive for BK viremia. The average serum tacrolimus trough level in the BK viremia positive group was higher than in the BK viremia negative group, but the difference did not reach statistical significance (9.08 ± 2.93 vs. 7.49 ± 2.49, p = 0.108). Notably, the average serum tacrolimus trough level was significantly higher in the BK viremia-positive group compared to the BK viruria-negative group (9.08 ± 2.93 vs. 6.47 ± 1.85, p < 0.001).
Eighteen recipients, all of whom had previously tested positive for BK viruria, underwent a kidney biopsy, and 6 of them (33.3%) were diagnosed as having BKVAN. The average serum tacrolimus trough level in recipients with BKVAN was higher than in those without BKVAN, but the difference was not statistically significant (8.45 ± 3.29 vs. 7.92 ± 1.97, p = 0.671). Importantly, the average serum tacrolimus trough level was significantly higher in the BKVAN group compared to the BK viruria-negative group (8.45 ± 3.29 vs. 6.47 ± 1.85, p = 0.013).
Table 2 presents the results of univariate and multivariable logistic regression analyses conducted to identify factors associated with BK viruria. The analysis evaluated 11 potential risk factors assessed for their association with BK virus infection. In the univariate analysis, average serum tacrolimus trough levels, use of mycophenolic acid, presence of delayed graft function, history of acute graft rejection, high HLA mismatch, and body mass index (BMI) were identified as potentially associated with BK viruria (p < 0.2). In the multivariable logistic regression analysis, only two factors demonstrated a significant association with the occurrence of BK viruria: average serum tacrolimus trough levels (OR = 1.244, 95% CI = 1.081 to 1.436, p = 0.002) and high HLA mismatch (OR = 3.388, 95% CI = 1.479 to 7.942, p = 0.004).
Table 2.
Univariate and multivariable logistic regression analysis for potential factors associated with BK viruria.
| Factors | Univariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Average serum tacrolimus trough level (ng/mL) | 1.214 | 1.069–1.386 | 0.003 | 1.244 | 1.081–1.440 | 0.002 |
| Use of mycophenolic acid | 4.777 | 0.891–88.476 | 0.139 | 3.302 | 0.581–62.461 | 0.268 |
| Use of corticosteroid | 1.409 | 0.563–4.026 | 0.487 | |||
| Use of everolimus | 0.424 | 0.064–1.661 | 0.276 | |||
| Presence of delayed graft function | 0.564 | 0.301–1.025 | 0.066 | 0.551 | 0.281–1.046 | 0.074 |
| Recipient age (years) | 1.010 | 0.986–1.035 | 0.410 | |||
| History of acute allograft rejection | 1.632 | 0.944–2.830 | 0.077 | 1.732 | 0.950–3.147 | 0.069 |
| High HLA mismatch | 3.758 | 1.741–8.325 | < 0.001 | 3.388 | 1.479–7.942 | 0.004 |
| Deceased donor | 0.845 | 0.483–1.486 | 0.556 | |||
| CMV D+/R- serostatus | 1.477 | 0.192–9.095 | 0.673 | |||
| Body mass index (kg/m2) | 1.061 | 0.985–1.144 | 0.120 | 1.049 | 0.966–1.138 | 0.250 |
Figure 2 depicts the cumulative incidence of positive BK viruria among three groups of kidney transplant recipients, categorized by different ranges of average serum tacrolimus trough level (Tacx). This analysis included 153 recipients who underwent kidney transplantation after the initiation of urine BK virus screening at Maharaj Nakorn Chiang Mai Hospital. Among these recipients, 47 individuals (30.7%) tested positive for BK viruria, while 106 individuals (69.3%) tested negative. The average follow-up duration was 913 ± 665 days (range: 132–3269 days) for the BK viruria-positive group and 679 ± 946 days (range: 34–6217 days) for the BK viruria-negative group, with no significant difference in follow-up duration between the groups (p = 0.702).
Fig. 2.
Kaplan–Meier curve depicting the cumulative incidence of BK viruria within 12 months post-transplantation, stratified by three ranges of average serum tacrolimus trough levels (Tacx).
The overall cumulative rate of BK viruria, calculated at 2-month intervals between kidney transplantation and 12 months post-transplantation using the Kaplan-Meier method, increased steadily over time. Recipients in the higher Tacx group were more likely to have BK virus detected in their urine, both in terms of frequency and earlier onset. The risk of BK viruria detection in the Tacx >10 ng/mL group was significantly higher compared to the Tacx <8 ng/mL group (HR = 14.83, 95% CI = 6.93 to 31.70, p < 0.001). In contrast, the difference in the risk of BK viruria detection between the Tacx 8–10 ng/mL group and Tacx <8 ng/mL group was not statistically significant (HR = 2.02, 95% CI = 0.91 to 4.50, p = 0.09). Recipients in the Tacx >10 ng/mL group had a BK viruria detection rate as high as 51.0% (95% CI = 32.0–80.0%) at 2 months and 75.0% (95% CI = 56.0–100.0%) at 4 months. In contrast, the cumulative incidence of BK viruria in the Tacx 8–10 ng/mL group and the Tacx <8 ng/mL group reached only 33.0% (95% CI = 17.0–62.0%) and 12.0% (95% CI = 6.8–21.0%) at 12 months, respectively.
Discussion
The present study identified a significant association between average serum tacrolimus trough levels and BK virus detection in the urine of kidney transplant recipients. Notably, approximately half of the recipients with average serum tacrolimus trough levels exceeding 10 ng/mL developed BK viruria early, around two months after kidney transplantation. This observation may be attributed to the potent immunosuppressive effects of tacrolimus, which could directly stimulate polyomavirus reactivation within renal tubular epithelial cells. Our findings are consistent with previously reported results, underscoring the association between tacrolimus use and an increased risk of BK virus infection in kidney transplant recipients6,23,24. For instance, Borni-Duval et al. demonstrated a link between heightened immunosuppression and BK virus infection in patients with elevated tacrolimus trough levels25. Similarly, a retrospective study conducted in China reported a linear relationship between changes in urine BK virus load and tacrolimus trough levels26.
Although evidence indicates a strong association between tacrolimus overexposure and BK virus infection, limited data are available to establish a specific cut-off value for serum tacrolimus trough levels that would serve as a warning for clinicians25,27. In our study cohort, average serum tacrolimus trough levels exceeding 10 ng/mL were significantly associated with a higher incidence of BK virus infection, as evidenced by BK viruria. Supporting previous research, recipients treated with a combination of tacrolimus and mycophenolate mofetil had the highest incidence of viremia and BKVAN, particularly when tacrolimus trough levels at three months post-transplant exceeded 10 ng/mL24. This threshold may serve as a potential cut-off point, indicating the need to consider modifying the tacrolimus-based immunosuppressive regimen during post-transplant care. However, reducing immunosuppressive therapy should be approached with caution to carefully balance the risk of BK virus infection with the risk of acute graft rejection28.
Our study also underscores the importance of tacrolimus drug-level monitoring to identify the risk of BK virus infection at an earlier stage. From a cost-effectiveness perspective, implementing more frequent monitoring of tacrolimus levels, particularly for those exceeding the 10 ng/mL threshold, could reduce the need for expensive interventions associated with BK virus progression, such as antiviral treatments, further diagnostic testing, or kidney biopsies. However, the increased frequency of monitoring may impose additional healthcare costs, especially in resource-limited settings. Further studies that assess the economic impact of this approach, including cost-benefit analyses, would be valuable to determine whether the clinical benefits outweigh the costs associated with routine monitoring and adjustments of tacrolimus levels.
In our study, we identified high HLA mismatch as another risk factor associated with BK viruria. The association between high HLA mismatch and BK viruria may arise from the need for more intensive immunosuppression in kidney transplant recipients with high HLA mismatch to prevent acute graft rejection, thereby increasing the risk of BK virus infection25,29. Several previous studies support the association between high HLA mismatch and an increased risk of BK virus infection, as indicated by BK viremia29–31and/or BKVAN32,33. However, some studies report conflicting results34–37. According to the Transplantation Society International BK Polyomavirus Consensus Group, certain HLA class I (absence of A2, B7, B8, B51, B44, B13, CW7) and HLA class II types (DR15) are risk factors for BK viremia. Additionally, HLA-E01:03 (compared to the protective HLA-E01:01) has been identified as a risk factor for BKVAN13. Nevertheless, the evidence level for HLA mismatch as a risk factor remains graded as low.
We acknowledge the following limitations of the present study. First, the definition of BK viruria-positive in our study may differ from those used in other studies37–40. This discrepancy should be considered when interpreting and comparing our results with those of previous research. In this study, BK viruria was defined as the detection of BK virus in urine with a viral load of ≥ 315 copies/mL, a threshold much lower than that recommended in international guidelines for predicting BK viremia or biopsy-confirmed BKVAN38,39. While this lower threshold may facilitate earlier identification of BK virus reactivation and enable timely immunosuppressive adjustments, it also increases the risk of overtreatment for subclinical cases, underscoring the need for careful clinical judgment to avoid unnecessary interventions that could lead to acute graft rejection. However, because the primary objective of this study was to examine the association between tacrolimus concentrations and the presence of BK virus in urine—rather than the relationship between BK viruria and BK viremia or BKVAN development—we chose to use the assay’s lowest detectable value. Second, serum concentrations of other immunosuppressive drugs, including mycophenolate mofetil and prednisolone, were not routinely monitored. As a result, our model could not account for their potential effects. In addition, adherence assessments—such as compliance with immunosuppressive medications and clinic visits—were not documented, which could influence outcomes. Future studies should address these factors. Third, while an average serum tacrolimus trough level exceeding 10 ng/mL over 6 months may suggest a potential risk for BK virus activation, particularly BK viruria, it is important to note that this association does not imply causation. Fourth, the relatively small number of transplant recipients who underwent kidney biopsy resulted in a limited number of documented BKVAN cases. This limitation reduced the statistical power to establish a definitive association between serum tacrolimus levels and biopsy-confirmed BKVAN.
In conclusion, this study found an association between average serum tacrolimus trough levels and the detection of BK viruria in kidney transplant recipients. By proposing a specific tacrolimus threshold (> 10 ng/mL) strongly associated with early BK viruria development, this research provides a clinically actionable metric for monitoring and potentially adjusting immunosuppressive therapy. Furthermore, the study highlights high HLA mismatch as an additional risk factor, enhancing the understanding of the multifactorial nature of BK virus infection. The findings also demonstrate the temporal dynamics of BK virus reactivation, emphasizing the need for personalized immunosuppressive regimens to optimize recipient management and improve kidney transplant outcomes. In cases where the average serum tacrolimus trough level exceeds 10 ng/ml, modifications to immunosuppressive regimens should be carefully considered, with caution to avoid excessive reductions that could lead to acute rejection.
Acknowledgements
The authors would like to express their gratitude to the staff at the Transplant and Dialysis Unit, Medical Nursing Division, Maharaj Nakorn Chiang Mai Hospital, and at the Department of Pharmacology, Faculty of Medicine, Chiang Mai University, for their contributions to data collection and administrative support. This research was partially supported by Chiang Mai University.
Author contributions
Napatsorn Kraivisitkul: Methodology, Investigation, Data Curation, Writing – Original Draft. Kajohnsak Noppakun: Conceptualization, Methodology, Writing – Review & Editing, Supervision. Chotiwit Sakuludomkan: Methodology, Validation, Formal analysis, Investigation, Data Curation, Visualization. Supavit Jirawattanapong: Investigation, Data Curation. Siriaran Kwangsukstith: Investigation, Writing – Original Draft. Nahathai Dukaew: Methodology, Validation, Investigation. Naruemon Suyayai: Investigation, Data Curation. Mingkwan Na Takuathung: Conceptualization, Methodology, Investigation. Nut Koonrungsesomboon: Conceptualization, Methodology, Writing – Original Draft, Supervision, Project Administration.
Funding
The authors did not receive support from any organization for the submitted work.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Research Ethics Committee of the Faculty of Medicine, Chiang Mai University (No.146/2021).
Interests to declare
The authors have no relevant financial or non-financial interests to disclose.
Informed consent
Patient consent was waived due to the retrospective nature of this study. The Research Ethics Committee of the Faculty of Medicine, Chiang Mai University, granted a waiver of informed consent.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abecassis, M. et al. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol.3(2), 471–480 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamboti, J. S. BK virus nephropathy in renal transplant recipients. Nephrol. (Carlton). 21 (8), 647–654 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Shen, C. L., Wu, B. S., Lien, T. J., Yang, A. H. & Yang, C. Y. BK polyomavirus nephropathy in kidney transplantation: balancing rejection and infection. Viruses13(3), 487 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambalathingal, G. R., Francis, R. S., Smyth, M. J., Smith, C. & Khanna, R. BK Polyomavirus: clinical aspects, Immune Regulation, and emerging therapies. Clin. Microbiol. Rev.30 (2), 503–528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung, A. Y., Chan, M., Tang, S. C., Liang, R. & Kwong, Y. L. Real-time quantitative analysis of polyoma BK viremia and viruria in renal allograft recipients. J. Virol. Methods. 103 (1), 51–56 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Brennan, D. C. et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am. J. Transpl.5 (3), 582–594 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Hirsch, H. H., Randhawa, P. S., Practice & ASTIDCo BK Polyomavirus in solid organ transplantation-guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transpl.33 (9), e13528 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Bucay, A., Ramirez-Andrade, S. E., Gordon, C. E., Francis, J. M. & Chitalia, V. C. Advances in BK Virus complications in Organ Transplantation and Beyond. Kidney Med.2 (6), 771–786 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma, M. K. et al. Screening algorithm for BK Virus-Associated Nephropathy using sequential testing of urinary cytology: a probabilistic model analysis. Am. J. Nephrol.42 (6), 410–417 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Boan, P. et al. Optimal use of plasma and urine BK viral loads for screening and predicting BK nephropathy. BMC Infect. Dis.16, 342 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babel, N. et al. Sustained BK Viruria as an early marker for the development of BKV-associated nephropathy: analysis of 4128 urine and serum samples. Transplantation88 (1), 89–95 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Komorniczak, M., Krol, E., Lizakowski, S. & Debska-Slizien, A. Screening for Polyomavirus Viruria like Early detection of human polyomavirus infection and replication: the results of a single-Center Observation. Transpl. Proc.54 (4), 989–994 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Kotton, C. N. et al. The Second International Consensus guidelines on the management of BK Polyomavirus in kidney transplantation. Transplantation108 (9), 1834–1866 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier-Kriesche, H. U. et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am. J. Transpl.6 (5 Pt 2), 1111–1131 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Gatault, P. et al. Reduction of extended-release Tacrolimus Dose in Low-Immunological-Risk kidney transplant recipients increases risk of rejection and appearance of Donor-Specific antibodies: a randomized study. Am. J. Transpl.17 (5), 1370–1379 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Webster, A. C., Woodroffe, R. C., Taylor, R. S., Chapman, J. R. & Craig, J. C. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ331 (7520), 810 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch, H. H. et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am. J. Transpl.13 (1), 136–145 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benavides, C. A. et al. BK virus-associated nephropathy in sirolimus-treated renal transplant patients: incidence, course, and clinical outcomes. Transplantation84 (1), 83–88 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Oberbauer, R. et al. Optimization of tacrolimus in kidney transplantation: New pharmacokinetic perspectives. Transpl. Rev. (Orlando). 34 (2), 100531 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Hirsch, H. H., Yakhontova, K., Lu, M. & Manzetti, J. BK Polyomavirus replication in renal tubular epithelial cells is inhibited by Sirolimus, but activated by Tacrolimus through a pathway Involving FKBP-12. Am. J. Transpl.16 (3), 821–832 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawinski, D. & Goral, S. BK virus infection: an update on diagnosis and treatment. Nephrol. Dial Transpl.30 (2), 209–217 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Demey, B. et al. Risk factors for BK virus viremia and nephropathy after kidney transplantation: a systematic review. J. Clin. Virol.109, 6–12 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Huang, G. et al. Prospective study of polyomavirus BK replication and nephropathy in renal transplant recipients in China: a single-center analysis of incidence, reduction in immunosuppression and clinical course. Clin. Transpl.24 (5), 599–609 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Hirsch, H. H., Randhawa, P., Practice & ASTIDCo BK Polyomavirus in solid organ transplantation. Am. J. Transpl.13 (Suppl 4), 179–188 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Borni-Duval, C. et al. Risk factors for BK virus infection in the era of therapeutic drug monitoring. Transplantation95 (12), 1498–1505 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Zhou, Y. et al. [Characteristics of BK polymavirus infection in kidney transplant recipients]. Nan Fang Yi Ke Da Xue Xue Bao. 39 (1), 120–124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mengel, M. et al. Incidence of polyomavirus-nephropathy in renal allografts: influence of modern immunosuppressive drugs. Nephrol. Dial Transpl.18 (6), 1190–1196 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Funahashi, Y. BK virus-associated nephropathy after renal transplantation. Pathogens10(2), 150 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awadalla, Y., Randhawa, P., Ruppert, K., Zeevi, A. & Duquesnoy, R. J. HLA mismatching increases the risk of BK virus nephropathy in renal transplant recipients. Am. J. Transpl.4 (10), 1691–1696 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Hassig, A. et al. Association of BK viremia with human leukocyte antigen mismatches and acute rejection, but not with type of calcineurin inhibitor. Transpl. Infect. Dis.16 (1), 44–54 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Gately, R. et al. Incidence, risk factors, and outcomes of kidney transplant recipients with BK Polyomavirus-Associated Nephropathy. Kidney Int. Rep.8 (3), 531–543 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burek Kamenaric, M., Ivkovic, V., Kovacevic Vojtusek, I. & Zunec, R. The role of HLA and KIR immunogenetics in BK virus infection after kidney transplantation. Viruses12(12), 1417 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsch, H. H. et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl. J. Med.347 (7), 488–496 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Bohl, D. L. et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am. J. Transpl.5 (9), 2213–2221 (2005). [DOI] [PubMed] [Google Scholar]
- 35.El-Husseini, A. et al. Impact of human leukocyte antigen and calculated panel reactive antibody on BK viremia in kidney transplant recipients: a single-center experience and literature review. Transpl. Infect. Dis.21 (4), e13071 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Kavuzlu, M., Basturk, B., Atac, F. B., Aliskan, H. E. & Kantaroglu, B. Investigation of the Relationship between BK Virus and Human leukocyte antigens in kidney transplant recipients. Exp. Clin. Transpl.18 (Suppl 1), 51–54 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Skulratanasak, P. et al. BK Virus infection in Thai kidney transplant recipients: a single-center experience. Transpl. Proc.50 (4), 1077–1079 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Pang, X. L., Doucette, K., LeBlanc, B., Cockfield, S. M. & Preiksaitis, J. K. Monitoring of polyomavirus BK virus viruria and viremia in renal allograft recipients by use of a quantitative real-time PCR assay: one-year prospective study. J. Clin. Microbiol.45 (11), 3568–3573 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chon, W. J. et al. High-level viruria as a screening tool for BK virus nephropathy in renal transplant recipients. Kidney Res. Clin. Pract.35 (3), 176–181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viscount, H. B. et al. Polyomavirus polymerase chain reaction as a surrogate marker of polyomavirus-associated nephropathy. Transplantation84 (3), 340–345 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.