Abstract
1. This study was performed to investigate the possible presence and role of the creatine kinase (CK) system in the contraction and relaxation of skinned guinea-pig uterus as well as the changes of the CK system during gestation. Experiments were performed on isolated longitudinal fibres of gravid and non-gravid myometrium. 2. Total CK activity increased from 74 +/- 11 to 196 +/- 39 IU (g wet wt)-1 during gestation. 3. The four isoenzymes of CK: muscle (MM), muscle-brain (MB), brain (BB) and mitochondrial (mt-CK) were found in myometrium. MM, MB and BB isoenzymes represented respectively 20.3 +/- 2.6, 10.3 +/- 4.4 and 72.7 +/- 2.2% of total activity. The distribution of isoenzymes did not significantly change with gestation, the contribution of mt-CK increasing from trace to 5% of total activity. 4. BB-CK was specifically bound to Triton X-100-skinned fibres with the non-gravid uterus containing 6.7 +/- 1.9 IU (g wet wt)-1 and the gravid uterus containing 44 +/- 13 IU (g wet wt)-1. 5. Active tension of Triton X-100-treated fibres increased from 6.06 +/- 0.68 to 19.3 +/- 1.9 mM mm-2 during gestation. 6. Submaximal tension (43.3 +/- 4.4% of maximal tension) can be developed in the absence of ATP and in the presence of 12 mM phosphocreatine (PCr) and 250 microM MgADP from endogenous CK in non-gravid uterine fibres while the gravid uterus was able to generate 65.4 +/- 3.9% of maximal tension via the CK system. 7. The endogenous CK system was able to relax the skinned fibres from high-tension rigor conditions by 47.3 +/- 4.2% of total relaxation in non-gravid fibres and 60.6 +/- 3.2% of total relaxation in gravid fibres. 8. Non-gravid and gravid uteri both contained mt-CK of 17.5 +/- 8.4 and 140 +/- 22 micrograms (g wet wt)-1 respectively as determined with antibodies against mt-CK. 9. Oxygen consumption was studied in fibres where the plasmalemma was solubilized with 50 micrograms ml-1 saponin. Maximal respiration was increased from 0.91 +/- 0.05 to 2.61 +/- 0.16 mumol oxygen min-1 (g dry wt)-1 in the gravid uterine fibres. However, creatine did not stimulate respiration in the uterine fibres treated with saponin. 10. It is concluded that the CK system undergoes qualitative as well as quantitative changes during gestation. BB-CK is specifically localized in the myofilaments and mt-CK is present in the uterine mitochondria.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





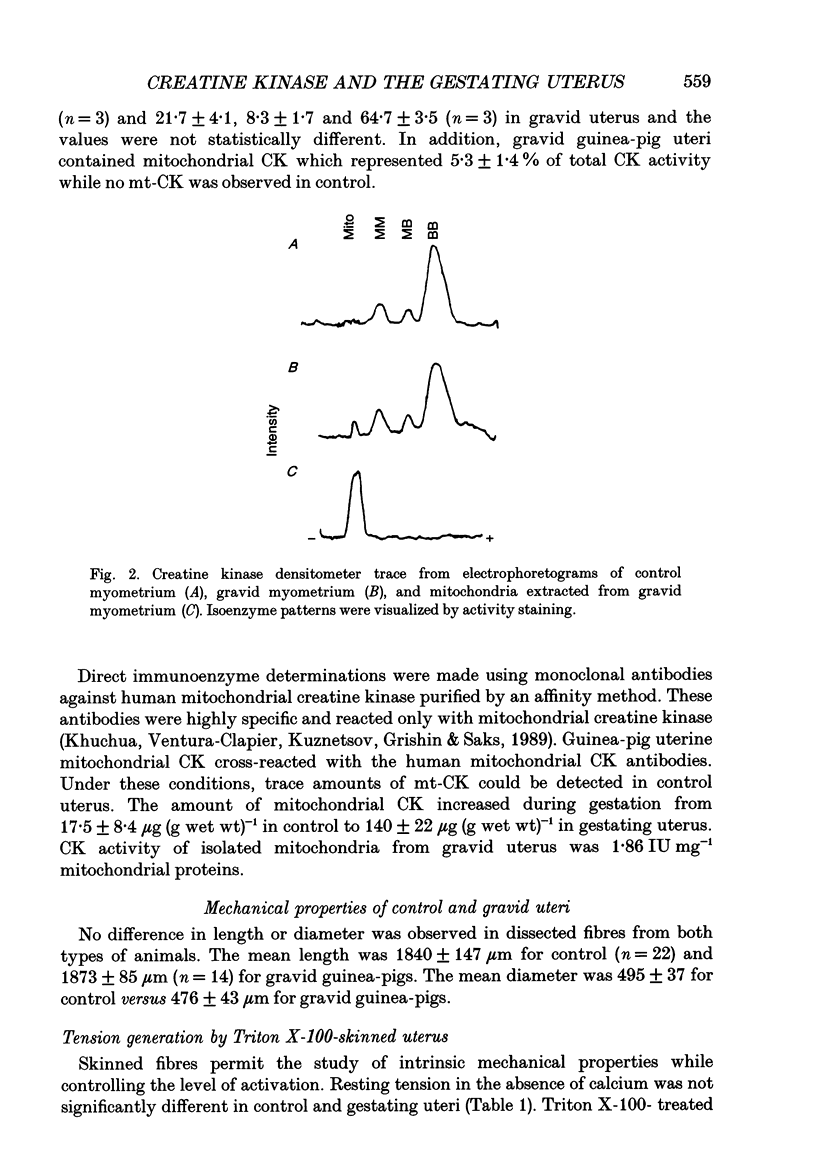

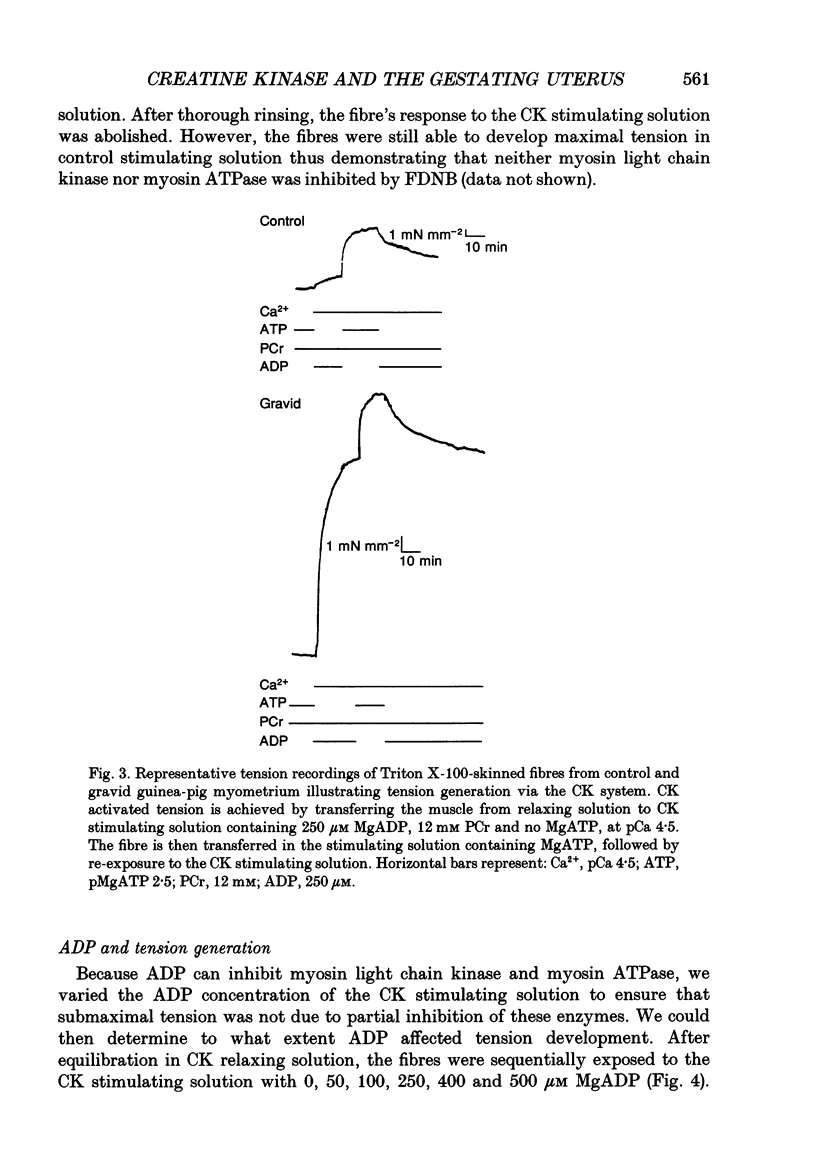

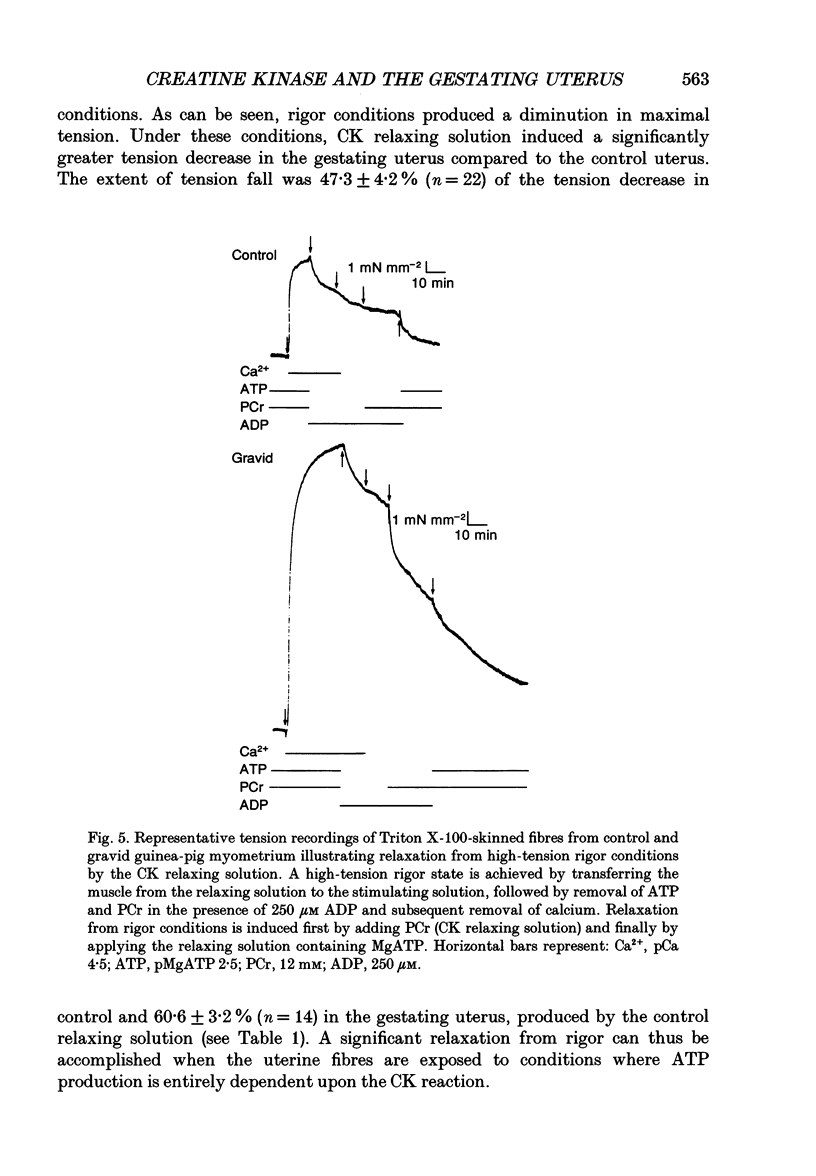
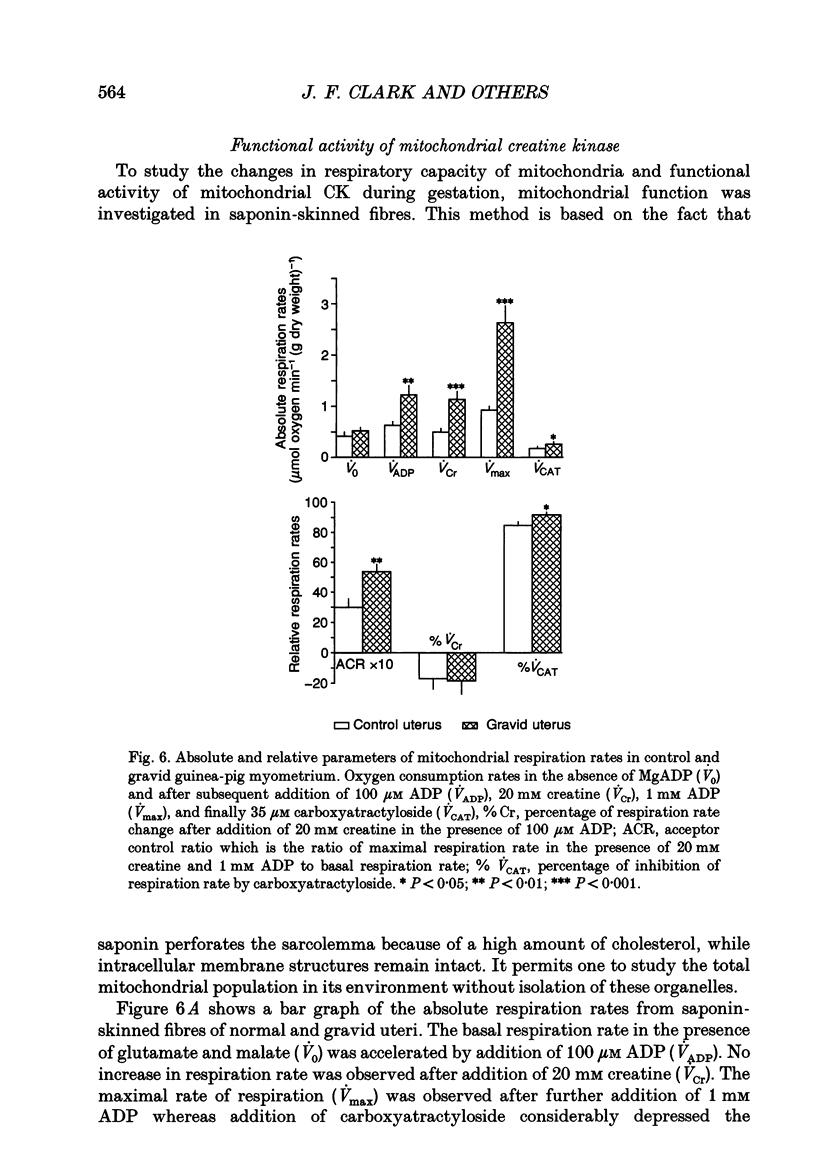








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessman S. P., Carpenter C. L. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- Breuiller-Fouche M., Doualla-Bell Kotto Maka F., Geny B., Ferre F. Alpha-1 adrenergic receptor: binding and phosphoinositide breakdown in human myometrium. J Pharmacol Exp Ther. 1991 Jul 1;258(1):82–87. [PubMed] [Google Scholar]
- Butler T. M., Pacifico D. S., Siegman M. J. ADP release from myosin in permeabilized smooth muscle. Am J Physiol. 1989 Jan;256(1 Pt 1):C59–C66. doi: 10.1152/ajpcell.1989.256.1.C59. [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Murphy R. A. Calcium-dependent stress maintenance without myosin phosphorylation in skinned smooth muscle. Science. 1983 Jul 29;221(4609):464–466. doi: 10.1126/science.6867722. [DOI] [PubMed] [Google Scholar]
- Chida K., Tsunenaga M., Kasahara K., Kohno Y., Kuroki T. Regulation of creatine phosphokinase B activity by protein kinase C. Biochem Biophys Res Commun. 1990 Nov 30;173(1):346–350. doi: 10.1016/s0006-291x(05)81063-0. [DOI] [PubMed] [Google Scholar]
- Clark J. F., Khuchua Z., Ventura-Clapier R. Creatine kinase binding and possible role in chemically skinned guinea-pig taenia coli. Biochim Biophys Acta. 1992 May 20;1100(2):137–145. doi: 10.1016/0005-2728(92)90074-c. [DOI] [PubMed] [Google Scholar]
- Degani H., Victor T. A., Kaye A. M. Effects of 17 beta-estradiol on high energy phosphate concentrations and the flux catalyzed by creatine kinase in immature rat uteri: 31P nuclear magnetic resonance studies. Endocrinology. 1988 Apr;122(4):1631–1638. doi: 10.1210/endo-122-4-1631. [DOI] [PubMed] [Google Scholar]
- Endo M., Yagi S., Iino M. Tension-pCa relation and sarcoplasmic reticulum responses in chemically skinned smooth muscle fibers. Fed Proc. 1982 May;41(7):2245–2250. [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Haas R. C., Strauss A. W. Separate nuclear genes encode sarcomere-specific and ubiquitous human mitochondrial creatine kinase isoenzymes. J Biol Chem. 1990 Apr 25;265(12):6921–6927. [PubMed] [Google Scholar]
- Hoerter J. A., Kuznetsov A., Ventura-Clapier R. Functional development of the creatine kinase system in perinatal rabbit heart. Circ Res. 1991 Sep;69(3):665–676. doi: 10.1161/01.res.69.3.665. [DOI] [PubMed] [Google Scholar]
- Hossle J. P., Schlegel J., Wegmann G., Wyss M., Böhlen P., Eppenberger H. M., Wallimann T., Perriard J. C. Distinct tissue specific mitochondrial creatine kinases from chicken brain and striated muscle with a conserved CK framework. Biochem Biophys Res Commun. 1988 Feb 29;151(1):408–416. doi: 10.1016/0006-291x(88)90608-0. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Paul R. J. Effects of hypoxia on high-energy phosphagen content, energy metabolism and isometric force in guinea-pig taenia caeci. J Physiol. 1990 May;424:41–56. doi: 10.1113/jphysiol.1990.sp018054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Paul R. J. Evidence for compartmentation of high energy phosphagens in smooth muscle. Prog Clin Biol Res. 1989;315:417–428. [PubMed] [Google Scholar]
- Ishida Y., Wyss M., Hemmer W., Wallimann T. Identification of creatine kinase isoenzymes in the guinea-pig. Presence of mitochondrial creatine kinase in smooth muscle. FEBS Lett. 1991 May 20;283(1):37–43. doi: 10.1016/0014-5793(91)80548-h. [DOI] [PubMed] [Google Scholar]
- Iyengar M. R., Fluellen C. E., Iyengar C. W. Increased creatine kinase in the hormone-stimulated smooth muscle of the bovine uterus. Biochem Biophys Res Commun. 1980 Jun 16;94(3):948–954. doi: 10.1016/0006-291x(80)91326-1. [DOI] [PubMed] [Google Scholar]
- Iyengar M. R., Fluellen C. E., Iyengar C. Creatine kinase from the bovine myometrium: purification and characterization. J Muscle Res Cell Motil. 1982 Jun;3(2):231–246. doi: 10.1007/BF00711944. [DOI] [PubMed] [Google Scholar]
- Izumi H., Ichihara J., Uchiumi Y., Shirakawa K. Gestational changes in mechanical properties of skinned muscle tissues of human myometrium. Am J Obstet Gynecol. 1990 Aug;163(2):638–647. doi: 10.1016/0002-9378(90)91216-y. [DOI] [PubMed] [Google Scholar]
- Jacobs H., Heldt H. W., Klingenberg M. High activity of creatine kinase in mitochondria from muscle and brain and evidence for a separate mitochondrial isoenzyme of creatine kinase. Biochem Biophys Res Commun. 1964 Aug 11;16(6):516–521. doi: 10.1016/0006-291x(64)90185-8. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Lehninger A. L. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J Biol Chem. 1973 Jul 10;248(13):4803–4810. [PubMed] [Google Scholar]
- Jacobus W. E., Saks V. A. Creatine kinase of heart mitochondria: changes in its kinetic properties induced by coupling to oxidative phosphorylation. Arch Biochem Biophys. 1982 Nov;219(1):167–178. doi: 10.1016/0003-9861(82)90146-1. [DOI] [PubMed] [Google Scholar]
- Khuchua Z. A., Ventura-Clapier R., Kuznetsov A. V., Grishin M. N., Saks V. A. Alterations in the creatine kinase system in the myocardium of cardiomyopathic hamsters. Biochem Biophys Res Commun. 1989 Dec 15;165(2):748–757. doi: 10.1016/s0006-291x(89)80030-0. [DOI] [PubMed] [Google Scholar]
- Kossmann T., Fürst D., Small J. V. Structural and biochemical analysis of skinned smooth muscle preparations. J Muscle Res Cell Motil. 1987 Apr;8(2):135–144. doi: 10.1007/BF01753989. [DOI] [PubMed] [Google Scholar]
- Kuznetsov A. V., Khuchua Z. A., Vassil'eva E. V., Medved'eva N. V., Saks V. A. Heart mitochondrial creatine kinase revisited: the outer mitochondrial membrane is not important for coupling of phosphocreatine production to oxidative phosphorylation. Arch Biochem Biophys. 1989 Jan;268(1):176–190. doi: 10.1016/0003-9861(89)90578-x. [DOI] [PubMed] [Google Scholar]
- Marc S., Leiber D., Harbon S. Carbachol and oxytocin stimulate the generation of inositol phosphates in the guinea pig myometrium. FEBS Lett. 1986 May 26;201(1):9–14. doi: 10.1016/0014-5793(86)80561-0. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Ochiai K., Tokutome S., Hachiya S., Umazume Y. Effects of Ca2+ and calmodulin on contraction of chemically skinned muscle fibers from pregnant rat uteri. Jpn J Physiol. 1986;36(6):1179–1191. doi: 10.2170/jjphysiol.36.1179. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Sweeney H. L., Kushmerick M. J. A simple analysis of the "phosphocreatine shuttle". Am J Physiol. 1984 May;246(5 Pt 1):C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- Michael C. A., Schofield B. M. The influence of the ovarian hormones on the actomyosin content and the development of tension in uterine muscle. J Endocrinol. 1969 Aug;44(4):501–511. doi: 10.1677/joe.0.0440501. [DOI] [PubMed] [Google Scholar]
- Nayler R. A., Sparrow M. P. Inhibition of cycling and noncycling cross bridges in skinned smooth muscle by vanadate. Am J Physiol. 1986 Feb;250(2 Pt 1):C325–C332. doi: 10.1152/ajpcell.1986.250.2.C325. [DOI] [PubMed] [Google Scholar]
- Paul R. J., Hewett T. E., Martin A. F. Myosin heavy chain isoforms and smooth muscle function. Adv Exp Med Biol. 1991;304:139–145. doi: 10.1007/978-1-4684-6003-2_13. [DOI] [PubMed] [Google Scholar]
- Paul R. J., Peterson J. W. Relation between length, isometric force, and O2 consumption rate in vascular smooth muscle. Am J Physiol. 1975 Mar;228(3):915–922. doi: 10.1152/ajplegacy.1975.228.3.915. [DOI] [PubMed] [Google Scholar]
- Quest A. F., Soldati T., Hemmer W., Perriard J. C., Eppenberger H. M., Wallimann T. Phosphorylation of chicken brain-type creatine kinase affects a physiologically important kinetic parameter and gives rise to protein microheterogeneity in vivo. FEBS Lett. 1990 Sep 3;269(2):457–464. doi: 10.1016/0014-5793(90)81215-a. [DOI] [PubMed] [Google Scholar]
- Reiss N. A., Kaye A. M. Identification of the major component of the estrogen-induced protein of rat uterus as the BB isozyme of creatine kinase. J Biol Chem. 1981 Jun 10;256(11):5741–5749. [PubMed] [Google Scholar]
- Saks V. A., Belikova Y. O., Kuznetsov A. V. In vivo regulation of mitochondrial respiration in cardiomyocytes: specific restrictions for intracellular diffusion of ADP. Biochim Biophys Acta. 1991 Jul 8;1074(2):302–311. doi: 10.1016/0304-4165(91)90168-g. [DOI] [PubMed] [Google Scholar]
- Saks V. A., Kuznetsov A. V., Kupriyanov V. V., Miceli M. V., Jacobus W. E. Creatine kinase of rat heart mitochondria. The demonstration of functional coupling to oxidative phosphorylation in an inner membrane-matrix preparation. J Biol Chem. 1985 Jun 25;260(12):7757–7764. [PubMed] [Google Scholar]
- Saks V. A., Rosenshtraukh L. V., Smirnov V. N., Chazov E. I. Role of creatine phosphokinase in cellular function and metabolism. Can J Physiol Pharmacol. 1978 Oct;56(5):691–706. doi: 10.1139/y78-113. [DOI] [PubMed] [Google Scholar]
- Schlegel J., Zurbriggen B., Wegmann G., Wyss M., Eppenberger H. M., Wallimann T. Native mitochondrial creatine kinase forms octameric structures. I. Isolation of two interconvertible mitochondrial creatine kinase forms, dimeric and octameric mitochondrial creatine kinase: characterization, localization, and structure-function relationships. J Biol Chem. 1988 Nov 15;263(32):16942–16953. [PubMed] [Google Scholar]
- Sparrow M. P., Mohammad M. A., Arner A., Hellstrand P., Rüegg J. C. Myosin composition and functional properties of smooth muscle from the uterus of pregnant and non-pregnant rats. Pflugers Arch. 1988 Oct;412(6):624–633. doi: 10.1007/BF00583764. [DOI] [PubMed] [Google Scholar]
- Veksler V. I., Kuznetsov A. V., Sharov V. G., Kapelko V. I., Saks V. A. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta. 1987 Jun 29;892(2):191–196. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]
- Veksler V. I., Murat I., Ventura-Clapier R. Creatine kinase and mechanical and mitochondrial functions in hereditary and diabetic cardiomyopathies. Can J Physiol Pharmacol. 1991 Jun;69(6):852–858. doi: 10.1139/y91-129. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R., Saks V. A., Vassort G., Lauer C., Elizarova G. V. Reversible MM-creatine kinase binding to cardiac myofibrils. Am J Physiol. 1987 Sep;253(3 Pt 1):C444–C455. doi: 10.1152/ajpcell.1987.253.3.C444. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Kaye A. M. mRNA for the rat uterine estrogen-induced protein. Translation in vitro and regulation by estrogen. J Biol Chem. 1981 Jan 10;256(1):23–26. [PubMed] [Google Scholar]
- Wallimann T., Eppenberger H. M. Localization and function of M-line-bound creatine kinase. M-band model and creatine phosphate shuttle. Cell Muscle Motil. 1985;6:239–285. doi: 10.1007/978-1-4757-4723-2_8. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Wyss M., Brdiczka D., Nicolay K., Eppenberger H. M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992 Jan 1;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]


