Summary
Plasma membrane (PM) nanodomains have emerged as pivotal elements in the regulation of plant cellular functions and signal transduction. These nanoscale membrane regions, enriched in specific lipids and proteins, behave as regulatory/signaling hubs spatially and temporally coordinating critical cellular functions. In this review, we first examine the mechanisms underlying the formation and maintenance of PM nanodomains in plant cells, highlighting the roles of PM lipid composition, protein oligomerization and interactions with cytoskeletal and cell wall components. Then, we discuss how nanodomains act as organizing centers by mediating protein–protein interactions that orchestrate essential processes such as symbiosis, defense against pathogens, ion transport or hormonal and reactive oxygen species (ROS) signaling. Finally, we introduce the concept of nanoenvironments, where localized physicochemical variations are generated in the very close proximity of PM nanodomains, in response to stimuli. After decoding by a dedicated machinery likely localized in the vicinity of nanodomains, this enrichment of secondary messengers, such as ROS or Ca2+, would allow specific downstream cellular responses. This review provides insights into the dynamic nature of nanodomains and proposes future research to better understand their contribution to the intricate signaling networks that govern plant development and stress responses.
Keywords: lipids, nanodomain, nanoenvironment, plant, plasma membrane, protein–protein interactions, signaling
I. Introduction
In the crowded environment of the cell, where for instance protein concentration can reach several hundred mg ml−1 in the cytoplasm, highly complex biochemical reactions are constantly ongoing (Zimmerman & Trach, 1991; Liu et al., 2022). To be efficient, timely and spatially defined, biochemical reactions need to be compartmentalized. This phenomenon is best exemplified in the case of membranes that define cells and organelles. Indeed, their surfaces participate in biochemical reaction networks by confining proteins or lipids, aligning reaction partners and directly controlling enzymatic activities. At the cellular level, membrane‐localized reactions shape cellular membranes and could generate signaling gradients that originate from the plasma membrane (PM) and extend into the cytoplasm and the nucleus. Therefore, membranes appear as essential platforms from which many cellular processes are scaffolded, leading ultimately to developmental and physiological responses of organisms.
At the molecular scale, the thermic agitation should lead to an even concentration of the membrane constituents, meaning that at any place of the membrane, the quantity of biomolecules should be the same. However, after decades of studies, biological membranes appear instead to be highly heterogeneous both spatially and temporally. Membranes are now considered as a juxtaposition of domains of small size composed of different lipids and proteins. Throughout this review, we will refer to them by the term nanodomain as a local assembly of proteins and/or lipids that display a nanoscale size (< 1 μm in diameter) (Jaillais et al., 2024). Nanodomains are highly diverse in their composition and are present in virtually all the types of membranes including the PM, the endoplasmic reticulum and chloroplast thylakoid membranes (Johnson et al., 2014; Gao et al., 2019; Smokvarska et al., 2020). Nanodomains were shown to be involved in the regulation of many cellular processes by acting as regulatory/signaling platforms (Demir et al., 2013; Gronnier et al., 2017; Platre et al., 2019). Contrary to what was sometimes proposed in the past, nanodomains do not imply predefined physicochemical properties like being enriched in sphingolipids, sterols or phosphoinositides, even if these lipids can be components, among others, of some nanodomains. Similarly, nanodomains are not obligatory associated with specific lipid order nor detergent resistant membrane (DRM) fraction (Mongrand et al., 2010; Tanner et al., 2011).
In recent years, plant PM nanodomains have emerged as key elements to regulate a variety of biological functions by shaping molecular reactions and acting on cell signaling. In this review, we will first detail the genesis and maintenance of plant PM nanodomains. Second, we will illustrate their biological roles as regulatory/signaling hubs gathering functionally related proteins. Finally, we will emphasize the ability of PM nanodomains to create in their very close proximity localized variations of physicochemical parameters, hereafter called nanoenvironments that may act on signal transduction processes.
II. Membrane and nanodomain emerging properties
Biomolecules binding to membranes have a drastic effect on their kinetic of biochemical interactions (Küchler et al., 2016; Leonard et al., 2023). First, membrane binding is changing biomolecule local concentration. For instance, for an Arabidopsis differentiated root cell displaying a cytoplasmic volume of c. 4000 μm3 (Dünser et al., 2019), the corresponding PM inner leaflet only represents 75 μm3 (with 5 nm PM thickness). This is increasing the local reactant concentration to 50 times (Fig. 1a). The exact same reasoning can be followed for biomolecules clustering in nanodomains where the volume will be even smaller (Fig. 1a). Membrane binding also reduces the dimensionality of molecules. Indeed, when a protein is bound to the membrane, its rotation is restricted to the axis perpendicular to the membrane surface, which enhances biochemical reactions by aligning reaction partners (Kholodenko et al., 2000). Thus, confining proteins in membranes or within nanodomains will modify biomolecules apparent affinity, enabling protein interactions that would be impossible in solution (McCloskey & Poo, 1986). Second, membrane binding can act on diffusion, which is typically decreased by 100–1000 times between the cytoplasm and membranes (Fig. 1b; McCloskey & Poo, 1986). In addition, biomolecule clusterization in nanodomains could decrease their diffusion even more. In extreme cases, reactants could be virtually immobilized and their reaction rate will then tend to zero (Fig. 1b). Membrane association and nanodomain organization is also acting on rotational motion of biomolecules and could help to co‐align interactants (Kholodenko et al., 2000). In the case of reactants that are reversibly organized in nanodomains, interaction kinetics will depend on the time they spend in nanodomains. Because nanodomain organization may increase local concentration and decrease diffusion rate, fast nanodomain association rate will favor protein–protein interactions. Conversely, the rate of nanodomain association can become limiting for slow binding reactions. Therefore organization of biomolecules in nanodomains have a drastic effect on interaction kinetics and consequently on signaling pathways (Küchler et al., 2016; Mishra & Johnson, 2021).
Fig. 1.
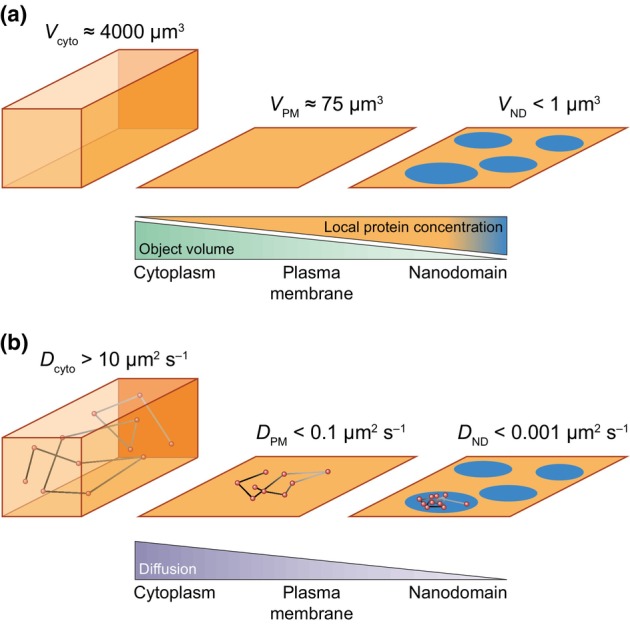
Confinement in nanodomains influences biochemical reactions by increasing the local concentration of biomolecules, such as proteins, as well as reducing their diffusion. (a) Confining proteins in a thin layer at the membrane surface or within membrane nanodomains increases their concentration. (b) Association of proteins with membranes and even more so within nanodomains drastically reduces their diffusion. V and D correspond to volume and diffusion, respectively. cyto, cytoplasm; ND, nanodomain; PM, plasma membrane.
III. Nanodomains: pre‐existing structures and/or auto‐assembly?
As proposed by Jaillais et al. (2024), nanodomain constituents can be divided in two classes: ‘driver’ molecules that are necessary to form or maintain nanodomains and ‘client’ molecules that clusterize in nanodomains but could be dispensable for their maintenance. This concept is illustrated by the following examples. In response to auxin, the small GTPase Rho of Plant6 (ROP6) organizes in nanodomains that are enriched in the anionic lipid phosphatidylserine (PS) that helps ROP6 stabilization in nanodomains through electrostatic interaction with its C‐terminal lysine and arginine amino acids. Importantly, before any auxin stimulation, nanodomains of PS pre‐exist in the membrane. Thus, PS and ROP6 act as ‘driver’ and ‘client’ molecules, respectively (Platre et al., 2019). Some proteins named Remorins (REM) organize in PM nanodomains (Jarsch et al., 2014) and were also demonstrated to act as ‘driver’ molecules. Indeed, in Medicago truncatula, Symbiotic Remorin 1 (SYMREM1) is necessary to induce the organization of the receptor Lysine Motif Kianse3 (LYK3) into nanodomains, following rhizobial inoculation (Liang et al., 2018). This mechanism will be detailed hereafter.
Alternatively, to pre‐existing nanodomains, some nanodomains are de novo constituted in membranes. In this case, nanodomains are formed/maintained by a series of feedback loops creating reaction–diffusion processes (Schweisguth & Corson, 2019; Landge et al., 2020). In tip growing cells, like pollen tubes and root hairs, polar domains are proposed to be formed by self‐assembly (Klahre & Kost, 2006; Hwang et al., 2008, 2010; Luo et al., 2017). Self‐assembly seems also to drive the formation of nanodomains. Phosphatidylinositol 4‐phosphate 5‐kinases (PIP5Ks) physically interact with the Rho‐GTPase Nicotiana tabacum Ras‐related C3 botulinum toxin substrate 5 (NtRAC5) and create local phosphatidylinositol‐4,5‐bisphosphate (PI(4,5)P2) rich nanodomains in the pollen tube. In turn, this local enrichment in PI(4,5)P2 may favor NtRAC5 recruitment and stabilize actin through the activation of RAC effector proteins like ROP‐interactive CRIB motif‐containing protein (RIC) (Wu et al., 2001; Fratini et al., 2021; Heilmann & Heilmann, 2022). Armadillo Repeat Only (ARO) proteins form nanodomains and participate in the stabilization of growth sites in root hair cells (Kulich et al., 2020). ARO interacts with anionic lipids, ROP1 and ROP1 Enhancer (RENGAP). Because RENGAP proteins are negative regulators of ROPs, they are thought to act as negative feedback loops on ROP signaling in nanodomains (Kulich et al., 2020). Nagashima et al. (2018) proposed that feedback interactions between ROP, their activators guanidine exchange factors (GEFs) and their inhibitors GTPase activating proteins (GAPs), was sufficient to create periodic organization in the PM (Nagashima et al., 2018). More recently, the role of reaction–diffusion processes in ROP nanodomain formation was nicely extended to six different Arabidopsis ROPs (Sternberg et al., 2021; Deinum & Jacobs, 2024). In conclusion, the formation and maintenance of membrane nanodomains could arise from both self‐assembly and pre‐existing structures in plant PM.
IV. Lipids are key regulators of PM organization into nanodomains
In this section, we will describe the different cellular structures and mechanisms that are involved in the formation/maintenance of PM nanodomains (Fig. 2a). Several hundred of lipid species co‐exist in plant PM (Bahammou et al., 2024). Understanding how they act on membrane organization and therefore participate in signaling processes is a major challenge. In the past, the characterization of PM lipid domains relied mostly on the use of DRM fractionation and localization of chemically stained lipids in liposomes. So far, in vivo analyses of lipid organization in nanodomains remain scarce, especially in the plant field. However, approaches using inhibitors, biosensors and mutants already suggested that some membrane heterogeneities are maintained by a complex lipid homeostasis as exemplified by PS and ROP6 nanodomains, as explained previously (Fig. 2b). In vitro studies and molecular modeling showed that certain lipid interactions, for instance between sterols and sphingolipids, can create a liquid‐ordered phase (Gronnier et al., 2018; Mamode Cassim et al., 2019; Jaillais & Ott, 2020). Di‐4‐ANEPPDHQ (ANEPP) and other solvatochromic probes emerged as an interesting way to characterize membrane order phase in vivo (Gronnier et al., 2017; Grosjean et al., 2018; Huang et al., 2019; Pan et al., 2020). ANEPP is a ratiometric fluorescent probe that displays different emission p in liquid‐ordered phase and liquid‐disordered phase (non‐nanodomain). The use of ANEPP revealed a decrease of lipid order in the PM after methyl‐β‐cyclodextrin treatment, a sterol‐depleting agent or in the sterol biosynthesis mutant fackel‐J79 (Pan et al., 2020). This suggests that lipid homeostasis is acting on membrane ordering and therefore potentially on membrane nanodomain formation/maintenance in plants. In the future, it will be interesting to extend those observations at the nanodomain scale as it was done in the animal field (Klymchenko, 2017; Pelletier et al., 2023). The role of sterols in the formation of PM nanodomains was also highlighted by other works showing for instance that the nanoclustering of Solanum tuberosum StREM1;3 is altered in the presence of the sterol synthesis inhibitor fenpropimorph (Gronnier et al., 2017). In a similar manner, the nanodomain organization of the auxin‐related Transmembrane Receptor Kinase 1 (TMK1) is dependent on sterols (Pan et al., 2020). Even if they label lipids in an indirect way, the emergence of specific lipid biosensors allows studying lipid localization in living cells. Among others, sensors are now available in plant cells for PS, PI(4,5)P2, phytosterol, and were all shown to form nanometric clusters in the PM (Furt et al., 2010; Platre et al., 2019; Fratini et al., 2021; Ukawa et al., 2022). Interestingly, certain lipids can probably be locally produced in plant PM by dedicated protein complexes anchored in nanodomains, for example PI4Kα or PI(4,5)P‐kinases (Fratini et al., 2021; Noack et al., 2022). In the future, lipid biosensors will provide an interesting way to study the importance of lipids in PM nanodomain organization. Performing ANEPP staining on giant unilamellar vesicles (GUVs) prepared with a mixture of the different classes of lipids found in the PM of BY‐2 cells, Grosjean et al. (2018) showed that the lipid composition influenced the GUV membrane order. In addition, the same GUVs containing only lipids displayed a different membrane order compared with giant vesicles of native PM, containing lipids and proteins, suggesting that proteins had an impact on membrane order. Those observations highlight the tight interplay between lipids and proteins in membranes and how it influences membrane properties.
Fig. 2.
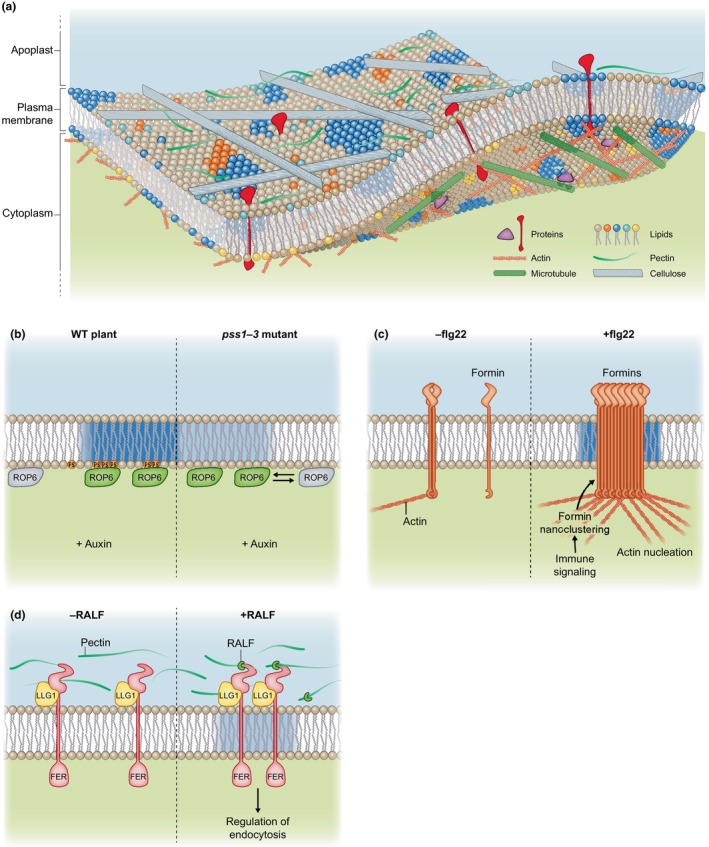
Mechanisms insuring the formation and the maintenance of plasma membrane (PM) nanodomains in plant cells. (a) Schematic representation of plant cell PM heterogeneity. The PM is highly heterogeneous and made up of a wide range of lipids and proteins. These elements can arrange into nanodomains of varying complexity. The cell wall, mostly composed of cellulose and pectin, has an important effect on protein organization in nanodomains. On the cytoplasmic side, the cytoskeleton, made up mainly of microtubules and actin microfilaments, also contributes to the stability of nanodomains. (b) Upon auxin stimulation of Arabidopsis root cells, Rho of Plant6 (ROP6) organizes in PM nanodomains. These domains contain phosphatidylserine (PS) that helps ROP6 stabilization through electrostatic interactions. In the absence of PS, as in the pss1‐3 mutant, ROP6 nanodomains are still present, but ROP6 stability in the domains is decreased (as represented by an inverted double arrow). (c) In response to flagellin, actin polymerization proteins named type‐I formin oligomerize and arrange in PM nanoclusters. This local condensation and stabilization activate formins that in turn induce actin nucleation. (d) The receptor‐like kinase FERONIA (FER) interacts with both pectins and rapid alkalinization factor (RALF) and acts as a cell growth regulator. Interestingly, upon abiotic stimulation, RALF and pectin phase separate and recruit FER together with the co‐receptor Lorelei‐Like GPI‐anchored protein 1 (LLG1) into PM nanodomains. This complex stimulates global endocytosis ensuring plant resilience under stress. WT, wild‐type.
V. Role of protein oligomerization in nanodomain formation
In addition to PM lipid composition, protein oligomerization recently appeared as an important feature for nanodomain organization. REMs and members of the Stomatin/Prohibitin/Flotillin/HflK/C (SPFH) domain‐containing protein family arrange in nanodomains and were proposed to act as scaffolding proteins (Browman et al., 2007; Lefebvre et al., 2010; Martinière & Zelazny, 2021). Indeed, these two families of proteins form large oligomers that likely participate in the formation/maintenance of certain nanodomains (Daněk et al., 2016; Legrand et al., 2023). StREM1.3 trimer formation through their coiled‐coiled domain is needed for PM association (Martinez et al., 2019). AtREM1.3 multimerization is also maintained by the intrinsically disordered N‐terminal domain (Marín et al., 2012). Genetic, structural biology and modeling approaches showed that the C‐terminal anchor (REM‐CA) of StREM1.3 binds to sterols and phosphatidylinositol phosphate (PIP) insuring StREM1.3 nanodomains formation (Perraki et al., 2012; Gronnier et al., 2017; Legrand et al., 2019). From those interesting observations, it was tempting to speculate that StREM1.3 nanodomain formation may change PIP and/or sterol nanoclustering in plant cells. In accordance with this idea, REM overexpression increases membrane ordering and rem1.2 × rem1.3 mutant displays an attenuated SA‐induced membrane ordering, as demonstrated using ANEPP (Huang et al., 2019). Another proof of the role of REM in nanodomain organization came from in vitro experiments showing that StREM1.3 drives lipid reorganization in reconstituted membranes, leading to the formation of REM‐enriched nanodomains, a process that is influenced by the phosphorylation of StREM1.3 intrinsically disordered domain by the Calcium‐dependent Protein Kinase 3 (CPK3) (Jolivet et al., 2023; Legrand et al., 2023). Although not directly related to nanodomain organization, REM oligomerization was recently shown to induce membrane blebs in protoplasts, suggesting a role of REM oligomerization in membrane topology (Su et al., 2023).
So far, the role of SPFH domain‐containing proteins in the organization of certain proteins or lipids in nanodomains was not formally demonstrated. However, as detailed hereafter, Flotillin 4 (FLOT4) was proposed to act as a central hub to organize symbiotic signaling in M. truncatula PM nanodomains (Liang et al., 2018). Flotillins are known to oligomerize via their C‐terminal domains (Yu et al., 2017) and Hypersensitive Induced Reaction (HIR) proteins, a plant specific SPFH protein family, are able to form large homo‐ and hetero‐oligomers in vivo, although the molecular function of such oligomerization process remains to be determined (Qi et al., 2011). Interestingly, in bacteria, the SPFH domain‐containing proteins HflK and HflC form a gigantic circular 24‐mer complex, featuring a laterally segregated membrane nanodomain (20 nm in diameter) bordered by transmembrane domains of HflK/C (C. Ma et al., 2022). This study provides new exciting insights in the contribution of SPFH domain‐containing proteins in the formation of membrane nanodomains.
VI. Cortical cytoskeleton meshwork and nanodomains
Plasma membrane organization not only emerges from its lipid and protein composition but also from its interaction with the surrounding structures. This idea was theorized by the group of Kusumi (Tomishige et al., 1998; Ritchie et al., 2003; Kusumi et al., 2005). In the picket and fence model, protein and lipid diffusion in the PM is strongly influenced by the cytoskeleton, which is in direct contact with the PM, thanks to a putative binding between cytoskeleton and membrane‐anchored proteins. According to this model, actin filaments act as a barrier to diffusion of lipids and proteins from the inner face of the PM. These fences limit diffusion and therefore could participate in the creation of domains. In addition, the putative transmembrane proteins that hold actin microfilaments at the PM act as pickets and because they extend over the two lipid layers would also limit the movement of lipids even in the outer membrane leaflet. Interestingly, in this model, protein and lipid diffusion is constrained not because of specific interaction with the membrane skeleton, but thanks to the steric hindrance generated by the pickets and fences.
Compared with animal models, the steric hindrance effect of the cytoskeleton in plant cells seems to be limited, as the diffusion of artificial PM protein is not affected by cytoskeleton depolymerization drugs (Lenne et al., 2006; Martinière et al., 2012). Moreover, certain proteins known to be organized in nanodomains, for example FLOT2, HIR1 and PIN‐formed 3 (PIN3), are not affected by a disruption of the cytoskeleton (McKenna et al., 2019; Daněk et al., 2020). However, in other cases, like with the auxin efflux transporter PIN2 that forms nanodomains in the apico‐basal root cell membrane, 3D confocal imaging and immuno‐gold labeling show that PIN2 nanoclustering is sensitive to pharmacological and genetic removal of microtubules (Kleine‐Vehn et al., 2011; Li et al., 2021). Destabilization of actin also induces a reduction in SYMREM1 nanodomain density in the PM (Liang et al., 2018). In addition, the actin and microtubule cytoskeleton also acts on the diffusion of nanodomains containing the pathogen receptor Flagellin‐Sensing 2 (FLS2) (McKenna et al., 2019). In conclusion, the effect of the cytoskeleton as a diffusion barrier in plant cells seems to depend on the proteins and we think that an in‐depth analysis is still required to precisely decipher these mechanisms.
Some examples in the literature show specific interactions between the cytoskeleton and given biomolecules, which consequently control nanodomain organization. Cellulose Synthase (CesA) complexes are responsible for cellulose microfibril secretion and form nanodomains in the PM (Desprez et al., 2007; Crowell et al., 2009). Their function is tightly associated with microtubules and actin. Cellulose Synthase Interacting 1/POM2 and Korrigan (KOR) form bridges between CesA complexes and microtubules, participating in CesA nanodomain maintenance and/or dynamics in the PM (Paredez et al., 2008; Bringmann et al., 2012; Vain et al., 2014). During immune signaling, type‐I formin, that contains single pass transmembrane domain and an intrinsic actin bundling activity, undergoes nanodomain formation connecting actin cytoskeleton regulation and membrane organization (Fig. 2c; Ma et al., 2021; Z. Ma et al., 2022). Similarly, PIP5K nanodomains in pollen tube regulate actin dynamics and organization by recruitment of RAC5 (Fratini et al., 2021). This shows that RAC5 or formin nanodomain organization can shape actin microfilaments. Networked 3C (NET3C), Kinesin Light Chain‐Related protein‐1 (KLCR1) and IQ‐Domain 2 (IQD2) together with Vesicle‐Associated Protein 27 (VAP27) arrange in nanodomains at ER/PM contact sites (Wang et al., 2014; Zang et al., 2021). This complex forms oligomers and are associated with actin microfilaments and microtubules. Interestingly, in this case, the cytoskeleton seems to be important for NET3C and VAP27 turn‐over within nanodomains, suggesting a role for the cytoskeleton to immobilized those proteins in nanodomains (Wang et al., 2014). We can hypothesize that the membrane nucleation machinery of the cytoskeleton may both polymerize actin or tubulin and simultaneously act as a priming event to initiate the formation of nanodomains.
VII. Cell wall/PM interactions act as an additive layer of regulation for nanodomain organization
Plant cells are surrounded by a cell wall composed of a tangle of polysaccharides such as cellulose, hemicellulose and pectin. This structure enables the cell to maintain its integrity under the high internal turgor pressure. As a result, the PM is in direct contact with the cell wall. This is exemplified by protein complexes that directly link cell wall components and the PM such as CesA (Wilson et al., 2021). This proximity influences the diffusion of artificial PM proteins, suggesting that the cell wall may act as a corral to limit protein lateral mobility and therefore influences their distribution in the PM (Martinière et al., 2012). A reverse genetic screen raised for the identification of PIN polarity regulators identified an allele of CesA3, suggesting that PIN polarity and CesA are functionally connected (Feraru et al., 2011). Indeed, further investigations showed that PIN2 diffusion and cluster formation were impaired in plasmolyzed cells and after treatment with isoxaben, an inhibitor of cellulose deposition in cells (Feraru et al., 2011; Li et al., 2021). Interestingly, the lateral diffusion and the nanodomain organization of FLOT2, HIR1, FLS2, PIN3 and Plasma membrane Intrinsic Protein 2;1 (PIP2;1) proteins are affected by cell wall perturbations (Feraru et al., 2011; Hosy et al., 2015; McKenna et al., 2019; Daněk et al., 2020). Cell wall can also act on the PM organization through signaling processes involving FERONIA (FER), a prototypical isoform of Catharanthus roseus receptor‐like kinase gene family. FER interacts with both pectins and rapid alkalinization factor (RALF) and is acting as a cell growth regulator (Fig. 2d; Haruta et al., 2014; Li et al., 2015; Feng et al., 2018; Dünser et al., 2019; Cheung, 2024). Interestingly, upon abiotic stimulation, RALF and pectin phase separate and recruit FER together with the co‐receptor Lorelei‐Like GPI‐anchored protein 1 (LLG1) into nanodomains (Fig. 2d). This complex regulates endocytosis of noncognate receptors and therefore their downstream signaling pathways (Liu et al., 2024). Together, those observations show that, in plant cells, the cell wall has a crucial effect on protein organization in nanodomains. This regulatory layer can be likened to an inverted ‘picket and fence’ model.
VIII. Functional link between PM nanodomain organization and endocytosis
The internalization of proteins from the PM is mainly achieved by clathrin‐mediated endocytosis (CME) in plant cells (Dhonukshe et al., 2007). Clathrin and other proteins from the CME machinery such as the Adaptor Protein‐2 (AP‐2) and the TPLATE complexes were shown to arrange in PM nanoclusters using Total Internal Reflection Fluorescence (TIRF) microscopy (Gadeyne et al., 2014; Johnson & Vert, 2017; Wang et al., 2020). Interestingly, the formation of CME nanoclusters occurs sequentially, and the TPLATE complex is recruited in PM foci, preceding the recruitment of AP‐2 complex, clathrin and dynamin‐related proteins (Gadeyne et al., 2014). Dual‐color variable‐angle TIRF microscopy analyses showed that clathrin (most of the time Clathrin Light Chain (CLC)) can co‐localize in nanodomains with many PM cargo proteins including the brassinosteroid receptor Brassinosteroid Insensitive 1 (BRI1), the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase named Respiratory Burst Oxidase Homologue D (RBOHD), the ammonium transporter AMT1;3 and PIP2;1 aquaporin (Wang et al., 2013, 2015; Hao et al., 2014). Co‐localization between CME machinery and cargo proteins in certain nanodomains probably reflects endocytic hot spots in the PM and highlights the functional link that can exist between nanodomain organization and CME in plant cells. Endocytosis can modify the dynamics of nanodomain‐organized proteins. In response to high ammonium concentration, AMT1;3 was found to be internalized from the PM (Wang et al., 2013). Before internalization, the size and fluorescence intensity of AMT1;3 nanodomains increased, and concomitantly, the overall number of nanoclusters and their residence time decreased. Interestingly, CME is implicated in the dynamics of AMT1.3 nanodomains since in the clathrin heavy chain 2 (chc2) mutant, the size and the fluorescence intensity of AMT1;3 nanoclusters increased (Wang et al., 2013). A similar enrichment in nanodomains before internalization was revealed for PIP2;1 by single‐particle tracking photoactivated localization microscopy (sptPALM) (Martinière et al., 2019). Indeed, a hyperosmotic treatment not only induced PIP2;1 molecule diffusion but also enhanced their local density, concomitantly to an increase in PIP2;1 internalization in root cells (Martinière et al., 2019).
The role of the nanodomain‐organized Flot1 protein in a putative endocytic pathway highlights the functional link between PM nanodomains and endocytosis in plants (Li et al., 2012). Indeed Flot1 co‐localized in PM nanodomains with numerous cargo proteins and was proposed to be involved in the internalization of BRI1, among others, since interfering with Flot1 functionality partially disturbed BRI1 endocytosis (Wang et al., 2015; Zhang et al., 2019). The Flot1‐mediated endocytic pathway probably coexists with CME and may be activated in response to environmental stimuli, as it was proposed for instance for PIP2.1 in response to salt stress (Li et al., 2012).
IX. Organization of proteins in nanodomains to coordinate/modulate their functions
One of the functions of nanodomains is to gather in confined environment proteins that cooperate, and possibly physically interact, to achieve a given cellular process. In this paragraph, we will present selected examples relative to symbiosis, defense against pathogens, ion transport, hormonal and reactive oxygen species (ROS) signaling that illustrate this phenomenon.
1. Biotic interactions
As mentioned previously, REMs participate in PM nanodomain organization. A scaffolding function of REMs in symbiotic processes was initially highlighted by Lefebvre et al. (2010) who showed that SYMREM1 interacts with symbiotic receptors involved in the perception of bacterial signaling molecules and regulate bacterial infection. FLOTs were also demonstrated to be required for the establishment of symbiosis and, interestingly, the co‐localization between FLOT4 and the symbiotic receptor LYK3 in PM nanodomains from root hairs was shown to be strongly stimulated after bacterial infection (Fig. 3a; Haney & Long, 2010; Haney et al., 2011). Later works highlighted that both FLOT4 and SYMREM1 proteins are required to ensure symbiosis by immobilizing LYK3 in PM nanodomains (Fig. 3a; Liang et al., 2018). First, infection by symbiotic bacteria induces the expression of SYMREM1 that is recruited into nanodomains via a process requiring FLOT4 that probably acts as a central hub during primary nanodomain assembly. Second, SYMREM1 interacts with ligand‐activated LYK3 to stabilize the receptor into nanodomains and prevent its endocytosis, thus ensuring root hair infection.
Fig. 3.
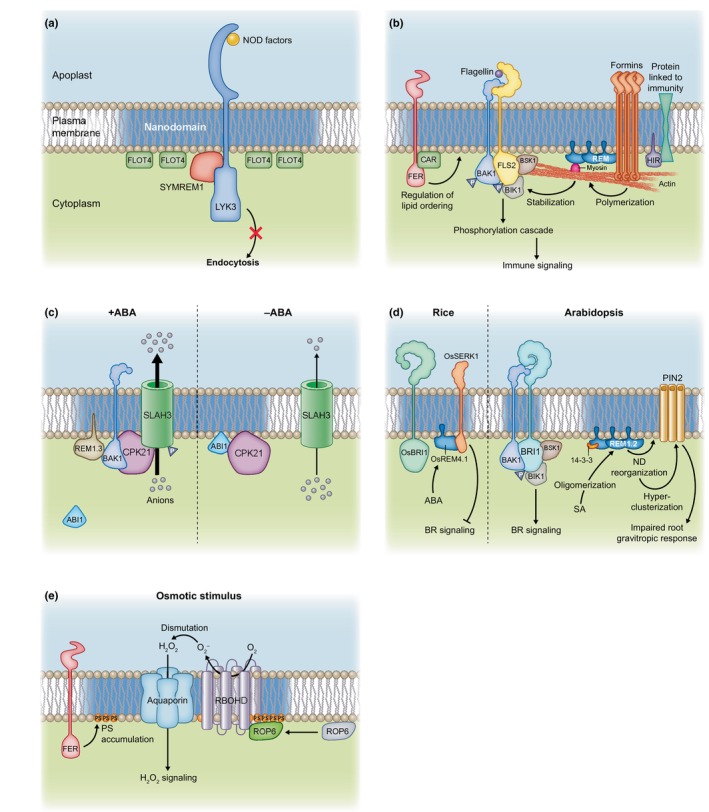
Nanodomain organization of proteins to generate regulatory/signaling hubs involved in different biological processes. (a) Symbiosis is regulated by nanodomain‐recruited protein machinery. Flotillin 4 (FLOT4)/SYMREM1 proteins act as a hub to stabilize the symbiotic receptor Lysine Motif Kianse3 (LYK3) that is involved in the perception of NOD factors. This mechanism prevents LYK3 endocytosis, thus ensuring root hair infection and the establishment of symbiosis. (b) Role of nanodomain‐organized proteins in plant immunity. In plasma membrane (PM) nanodomains, Brassinosteroid insensitive 1‐Associated receptor Kinase 1 (BAK1), Brassinosteroid Signaling Kinase 1 (BSK1) and Botrytis‐Induced Kinase 1 (BIK1) proteins interact with the flagellin receptor Flagellin‐Sensing 2 (FLS2) to initiate a phosphorylation cascade resulting in immune signaling. FLS2 nanodomains also contain Remorins (REM). FERONIA (FER) and CAR proteins physically interact and the rapid alkalinization factor 1 (RALF1)‐FER pathway induces an accumulation of CAR proteins that are recruited to the PM to stabilize the PM liquid‐ordered phase, which in turn allows the formation of the FLS2–BAK1 complex. Myosin, that allows the transport of cargoes along actin filaments, interacts with REM to recruit and stabilize BIK1 in FLS2‐containing nanodomains, which facilitates FLS2–BIK1 complex formation. Formins oligomerize and arrange in PM nanoclusters in response to flagellin, which induces their activation and further actin polymerization. This mechanism depends on the oligomerization of REM proteins that physically interact with formins. Hypersensitive Induced Reaction (HIR) are scaffolding nanodomain‐organized proteins that interact with proteins linked to immunity, although the meaning of these interactions remains to be determined. Triangles with the letter P represent phosphorylation. (c) The formation and dissociation of protein complexes in PM nanodomains regulate ion transport. The anion channel Slow Anion channel 1 Homologue 3 (SLAH3) can interact with its activating kinase CPK21 in PM nanodomains containing REM1.3, in an abscisic acid (ABA) dependent manner. In this condition, SLAH3 is fully active. In absence of ABA, the phosphatase Protein Phosphatase 2C/Abscisic acid Insensitive 1 (PP2C/ABI1) inhibits the interaction between CPK21 and SLAH3 and also probably induces a displacement of SLAH3 outside of nanodomains. This results in a reduction in SLAH3‐mediated transport. (d) Nanodomains in hormonal signaling. In rice, ABA upregulates the expression of OsREM4.1 that interacts with OsSERK1, an orthologue of Arabidopsis BAK1, to inhibit its interaction with the receptor OsBRI1. This represses OsSERK1‐catalyzed transphosphorylation of OsBRI1, thus inhibiting brassinosteroid (BR) signaling. This mechanism likely occurs in PM nanodomains. In Arabidopsis, BRI1 arranges in PM nanodomains where it interacts with BAK1, BSK1 and BIK1 to regulate BR signaling. Salicylic acid (SA) induces an accumulation of REM as well as their higher‐order oligomerization in the PM in a 14‐3‐3 protein‐dependent manner. This likely increases the liquid‐ordered phase of the PM. The SA‐mediated reorganization of nanodomains also induces a hyper‐clusterization of PIN2 at the PM resulting in an impaired root gravitropic response. (e) Reactive oxygen species (ROS) signaling in response to an osmotic signal is initiated through the cooperation of nanodomain‐organized proteins. In response to an osmotic constraint, Rho of Plant6 (ROP6) accumulates in PM nanodomains where it physically interacts with and activates Respiratory Burst Oxidase Homologue D (RBOHD) that produces superoxide ions (O2 −) in the apoplast. O2 − ions are dismutated to hydrogen peroxide (H2O2) that is then likely transported across the PM by aquaporins to ensure intracellular signaling. Osmotically induced ROS production is regulated by the receptor kinase FER that stimulates phosphatidylserine (PS) PM accumulation and nanoclustering, which in turn favors ROP6 nano‐partitioning. For clarity, not all the mechanisms presented in the corresponding section of this review were illustrated in this figure.
Plant perception of pathogens involves PM‐localized immune receptors and co‐receptors that physically interact in PM nanodomains. Following binding to the bacterial flagellin, FLS2 heteromerizes with the co‐receptor Brassinosteroid insensitive 1‐Associated receptor Kinase 1 (BAK1) to initiate phosphorylation cascades resulting in immune signaling (Chinchilla et al., 2007; Couto & Zipfel, 2016; Stegmann et al., 2017). In Arabidopsis cells, FLS2 protein arranges in stable PM nanodomains that partially co‐localize with REM1.2 and REM1.3 (Fig. 3b; Bücherl et al., 2017; Hurst et al., 2023; Cui et al., 2024). BAK1 nanoclustering in PM was also further highlighted (Gronnier et al., 2022). Interestingly, the receptor‐like cytoplasmic kinases Brassinosteroid Signaling Kinase 1 (BSK1) and Botrytis‐Induced Kinase 1 (BIK1), which are involved in the transduction of the perception of flagellin via phosphorylation events, were also shown to organize in nanodomains where they physically interact with FLS2, as demonstrated by bimolecular fluorescence complementation assays (Fig. 3b; Bücherl et al., 2017). FER interacts with both FLS2 and BAK1 and acts as a scaffold to regulate the formation of the FLS2–BAK1 immune complex (Stegmann et al., 2017). Recently, FER was shown to regulate the PM nanoscale organization of FLS2 and BAK1, albeit in an opposite manner since FLS2 and BAK1 proteins were more and less mobile in fer mutant, respectively (Fig. 3b; Gronnier et al., 2022). In addition, it was proposed that FER‐mediated regulation relies on the control of lipid ordering phase in the PM via a regulation of the lipid‐binding proteins named C2 domain ABA Related (CAR) (Fig. 3b; Chen et al., 2023). FER and CAR proteins physically interact and the RALF1–FER pathway induces an accumulation of CAR proteins that are recruited to the PM to stabilize the PM liquid‐ordered phase. This, in turn, affects FLS2–BAK1 complex formation (Fig. 3b). Similarly to FER, leucine‐rich repeat extensins (LRX) bind RALF and are also implicated in the formation of flagellin‐induced FLS2–BAK1 complex and regulate BAK1 organization in the PM and immune signaling (Gronnier et al., 2022). Recently, Myosin XI, which acts as a molecular motor allowing the transport of cargoes along actin filaments, was shown to interact with REM1.3 and to recruit and stabilize BIK1 in FLS2‐containing nanodomains (Fig. 3b; Wang et al., 2024). This phenomenon facilitates FLS2–BIK1 complex formation and results in the activation of BIK1‐dependent defense responses upon flagellin perception. Bacterial infections are known to induce a remodeling of actin in plant cells in order to coordinate cellular processes required for plant defense (Henty‐Ridilla et al., 2013). As mentioned previously, this mechanism involves type‐I formins that oligomerize and arrange in PM nanoclusters in response to flagellin (Fig. 3b; Ma et al., 2021). This local condensation and stabilization activate formins that in turn induce actin polymerization. Recently, formin nanoclustering and actin polymerization in response to pathogens were shown to depend on REMs (Fig. 3b; Z. Ma et al., 2022). This mechanism relies on the capacity of REM1.2 to oligomerize through its intrinsically disordered region and to physically interact with formins. Interestingly, this signaling module can be hijacked during infection by Xanthomonas campestris. Indeed, the effector XopR was shown to manipulate multiple steps of actin assembly, including formin‐mediated nucleation (Sun et al., 2021).
The scaffolding nanodomain‐organized proteins, HIR, are involved in the defense against various pathogens in different plant species (Qi et al., 2011; Li et al., 2019; Mei et al., 2020). HIR proteins were shown to physically interact with proteins linked to immunity, such as (1) the immune receptor Resistant to Pseudomonas synrigae 2 (RPS2) that is involved in effector‐triggered immunity in Arabidopsis (Qi et al., 2011), (2) the rice receptor kinase named Leucine‐Rich Repeat protein 1 (OsLRR1) that has a protective role against bacterial infection (Zhou et al., 2009) and (3) the Pleiotropic Drug Resistance 8 (PDR8)/Penetration3 (PEN3), a camalexin exporter that confers pathogen resistance (Fig. 3b; Lv et al., 2017; Aryal et al., 2023). So far, the meaning of these interactions remains to be determined but we can hypothesize that HIR proteins are probably important to create or maintain specialized nanodomain‐localized protein hubs involved in the regulation of plant immunity. Intriguingly, proteins from pathogens were shown to interact with HIR but also with other nanodomain‐organized proteins such as REMs. Those are well known to limit the cell‐to‐cell spread of virus in plants (Raffaele et al., 2009; Perraki et al., 2018), in a phosphorylation‐dependent manner involving the CPK3 kinase (Jolivet et al., 2023; Legrand et al., 2023). Thus, the filamentous hemagglutinin‐like protein (Fha1) from X. campestris interacts with pepper HIR proteins (Choi et al., 2013). Similarly, StREM1.3 and NbREM4 interacts with the potyviral movement protein cylindrical inclusion (CI) and the Pseudomonas type‐III effector protein HopZ1a, respectively (Albers et al., 2019; Rocher et al., 2022). Although the biological function of the interactions between HIR/REM and pathogen effectors remains largely unknown, it probably reflects a way for pathogens to disturb the plant immunity and favor infection as illustrated by the Turnip mosaic virus that mediates REM1.2 degradation through the interaction with the viral protein VPg (Cheng et al., 2020). In the same way, the rice stripe virus (RSV)‐encoded movement protein NSvc4 can interact with NbREM1 to interfere with its S‐acylation, which affects NbREM1 PM targeting and thus prevents REM‐mediated defense against RSV (Fu et al., 2018).
2. Ion transport
Apart from symbiosis and plant immune response, the formation and dissociation of protein complexes among PM nanodomains were shown to regulate ion transport. The anion channel Slow Anion channel 1 Homologue 3 (SLAH3) can interact with its activating kinase CPK21 in PM nanodomains containing AtREM1.3 (Fig. 3c; Demir et al., 2013). Interestingly, this interaction is stimulated by abscisic acid (ABA) as demonstrated by Förster resonance energy transfer (FRET). Upon co‐expression with CPK21, SLAH3 abundance in DRM (considered in this study as nanodomains) increases which was interpreted by the authors as a stimulated recruitment of SLAH3 in membrane nanodomains. At the opposite, co‐expression with the Protein Phosphatase 2C/Abscisic acid Insensitive 1 (PP2C/ABI1) leads to a shift of both CPK21 and SLAH3 from DRM to detergent‐sensitive membranes (DSM, considered in this study as non‐nanodomains) (Fig. 3c). On a functional point of view, measurements of currents in Xenopus oocytes demonstrated that CPK21 activates SLAH3, which results in anion efflux whereas the co‐expression with ABI1 inhibits the interaction between CPK21 and SLAH3, thus preventing SLAH3‐mediated anion currents (Fig. 3c; Demir et al., 2013).
3. Hormonal regulations
In plants, hormonal regulation is linked to the nanodomain organization of proteins as illustrated by the brassinosteroid receptor BRI1, which clustering in the PM of Arabidopsis cells is crucial for brassinosteroid signaling (Wang et al., 2015; Bücherl et al., 2017). Interestingly, although BRI1 and FLS2 receptors employ common downstream interacting signaling components such as BAK1, BSK1 and BIK1 proteins, they organize in distinct PM nanodomains (Fig. 3d; Bücherl et al., 2017). Bücherl et al. proposed that this spatial separation of BRI1 and FLS2 in distinct domains might explain their signaling specificity in response to brassinosteroid and flagellin, respectively. An original work showed that REM can act as molecular switches to regulate the antagonistic interactions between brassinosteroid and ABA during plant development (Gui et al., 2016). In rice, ABA upregulates the expression of OsREM4.1 that interacts with the kinase domain of Oryza sativa Somatic Embryogenesis Receptor 1 (OsSERK1), an orthologue of Arabidopsis BAK1, to inhibit its interaction with the receptor OsBRI1 and repress OsSERK1‐catalyzed transphosphorylation of OsBRI1 (Fig. 3d). Interestingly, this negative regulation of brassinosteroid output can be counterbalanced by OsBRI1 itself that phosphorylates OsREM4.1 to reduce its binding affinity to OsSERK1 (Gui et al., 2016). In Arabidopsis, the defense hormone salicylic acid (SA) induces an accumulation of REM proteins as well as their higher‐order oligomerization and their arrangement in large clusters in the PM that are dependent on 14‐3‐3 proteins that physically interact with REM1.2 (Fig. 3d; Huang et al., 2019). This mechanism was proposed to be responsible for the increase in the liquid‐ordered phase of the PM observed upon SA treatment. On a functional point of view, SA‐stimulated higher‐ordered lipids, which are enriched in plasmodesmata, may decrease plasmodesmata membrane plasticity to restrict their opening and reduce virus spreading, as proposed by the authors (Huang et al., 2019). The SA‐mediated reorganization of nanodomains also affects auxin signaling by impairing the lateral diffusion and the endocytosis of PIN2 auxin transporter, which results in the hyper‐clusterization of PIN2 at the PM (Fig. 3d; Ke et al., 2021). As a result, the auxin‐dependent root gravitropic response is impaired in Arabidopsis upon SA stimulation. The nanodomain organization of PIN proteins is dependent on the PM lipid composition (Kleine‐Vehn et al., 2011), but may also involve protein–protein interactions. Indeed PIN1 interacts with the ATP‐binding cassette (ABC) transporter ABCB19 (Blakeslee et al., 2007) and ABCB19 mutation induces a shift of PIN1 from DRM to DSM (Titapiwatanakun et al., 2009). However, these biochemical analyses still need to be validated by microscopy approaches. Interestingly, in a similar manner to SA, auxin was also demonstrated to modulate the PM nanodomain organization since: (1) auxin biosynthesis mutants exhibit reduced lipid ordering in the PM of Arabidopsis pavement cells and (2) auxin induces the nanoclustering of the auxin‐related receptor kinase TMK1 and increases the size of FLOT1‐containing nanodomains (Pan et al., 2020).
4. Reactive oxygen species
Reactive oxygen species signaling in plant cells is initiated through the cooperation of nanodomain‐organized proteins and is regulated by environmental factors. A key player is the PM‐localized NADPH oxidase named RBOHD that clusters in nanodomains. Upon activation, RBOHD generates superoxide in the apoplast, which turns to H2O2 by dismutation (Hao et al., 2014). Then, H2O2 is likely transported across the PM by channels such as PIP aquaporins (Bienert et al., 2007; Bienert & Chaumont, 2014; Rodrigues et al., 2017). In response to an osmotic signal, ROP6 is accumulating in PM nanodomains where it physically interacts with and activates RBOHD to produce ROS that will act as secondary messengers, inducing an adaptive response of the plant to the osmotic constraint (Fig. 3e; Smokvarska et al., 2020). Interestingly, although auxin also induces ROP6 nanoclustering (Platre et al., 2019), the corresponding nanodomains do not contain RBOHD and differ from osmotically induced ROP6 nanodomains. This suggests that ROP6 nano‐partitioning at the PM ensures signal specificity downstream of independent stimuli (Fig. 3e; Smokvarska et al., 2020). As recently demonstrated, osmotically induced ROS production is regulated by the receptor kinase FER. It controls ROP6 nanoclustering through the modulation of PS nanodomain density (Fig. 3e; Platre et al., 2019; Smokvarska et al., 2023). Thus, FER signaling regulates the strength of ROP6 signaling by controlling the density of ROP6 nanodomains (Smokvarska et al., 2023). The production of ROS in response to pathogens is essential for plant immunity (Kadota et al., 2014). Upon flagellin perception, BIK1 directly interacts with and phosphorylates RBOHD to stimulate its activity (Kadota et al., 2014; Li et al., 2014). However, although both proteins were independently shown to arrange in PM nanodomains (Hao et al., 2014; Bücherl et al., 2017; Smokvarska et al., 2020), the interplay between their physical association and their nanodomain organization remains to be determined.
X. From nanodomains to nanoenvironments
Molecular processes are compartmentalized inside the cell for proper functioning. The nanoscale organization of the PM brings the importance of this compartmentalization at further scale. Indeed, the co‐clustering of biomolecules involved in signaling, as described upon osmotic signal with the ROP6 GTPase and the ROS producing enzymes RBOHD/F (Smokvarska et al., 2020), raises questions about the role of the localized production of secondary messengers. An appealing hypothesis is that this localized ROS production leads to the formation of ROS nanoenvironments at the immediate vicinity of the PM that is mandatory for the establishment of molecular responses (Fig. 4a). Such nanometric RBOH‐dependent ROS production/accumulation involved in signaling was documented in tobacco leaves (Lherminier et al., 2009). Using CeCl3 staining under transmission electron microscopy, the authors described nanometric ROS accumulation at the immediate vicinity of the PM, on the cytosolic face, in response to the cryptogein, a fungal elicitor. Those cerium deposits of 100 nm in diameter reflecting ROS accumulation appear within minutes in elicited cells in a RBOH‐dependent manner (Lherminier et al., 2009). In the context of lateral root emergence, a similar pattern of CeCl3 precipitates was shown at the contact between endoderm and the lateral root primordia. In this case, RBOH activity was suggested to help lateral root emergence (Orman‐Ligeza et al., 2016). A local H2O2 accumulation was also demonstrated during the differentiation of endodermal cells and the Casparian strip formation (Lee et al., 2013; Fujita et al., 2020). Whereas a lateral sequestration of ROS in the plane of the PM is clear from localization of sub‐micrometric CeCl3 precipitates, it remains undetermined whether H2O2 nanoenvironments exist on both sides of the PM. This is likely the case since superoxide produced by RBOH and the resulting H2O2 accumulate in the apoplast, but then H2O2 is probably transported across the PM by PIP aquaporins, as mentioned previously, thus generating H2O2 hot spots on the cytoplasmic side. Recently, Kritsiligkou et al. (2023) showed interesting insights in yeast that illustrate the existence of H2O2 nanoenvironments (Kritsiligkou et al., 2023). They fused the H2O2 sensor, HyPer7 to the C‐terminus of all different open reading frames of the yeast genome. This approach led to the identification of differentially oxidized individual proteins and protein complexes and illustrated a compartmentalization of the ROS signaling that was more sophisticated than previously admitted.
Fig. 4.
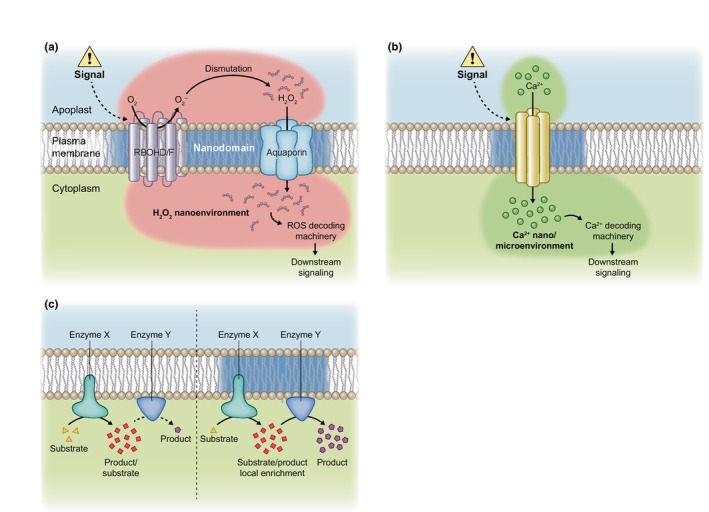
Nanoenvironments to generate signaling pathways. (a) Model for signal‐induced reactive oxygen species (ROS) nanoenvironment at the plasma membrane (PM). Upon stimulus, nicotinamide adenine dinucleotide phosphate oxidases RBHOD and F are activated leading to the production of superoxide ions (O2 −) in the apoplast that are then dismutated to H2O2, which in turn is likely transported inside the cell by aquaporins. We propose that this mechanism leads to a highly localized oxidation in both the apoplast and the cytoplasm at the immediate vicinity of the PM that we call H2O2 nanoenvironment. This H2O2 accumulation in the cytoplasm may cause the oxidation of molecular actors of the ROS decoding machinery that would be, for some of them, organized in clusters at the cytosolic face of the PM. This activates the downstream signaling. (b) Model for signal‐induced calcium nano/microenvironment at the PM. Following the same principle than for H2O2 nanoenvironment, upon stimulus, calcium channels putatively present in PM nanodomains are activated leading to a localized accumulation of calcium ion in the cytosol close to nanodomains. Molecular actors of the calcium decoding machinery that may be, for some of them, organized in PM clusters would then be activated. (c) Model for nanometric organization of enzymatic complexes to generate channeling processes. Efficiency of enzymatic reaction relies on biochemical environment and substrate availability. Here, we propose that clustered enzymatic complexes could be a way to locally enrich substrates, especially in the case of multi‐step reactions involving several enzymes, to optimize the production of certain molecules. We can imagine that the relocalization of certain enzymes among enzymatic clusters upon signals could be a way to activate the pathway.
A local enrichment of secondary messengers is not exclusive to ROS. Localized accumulation of calcium ions forming ‘calcium hot spots’ ranging from a few nanometer scale to several micrometers have been first documented in neuronal cells (Augustine et al., 1992). Generated aside calcium channels at the inner side of the PM, they are usually referred to as calcium microdomains (Fig. 4b). Interestingly, calcium release at the PM activates Syntaxin 1, a SNARE protein that regulates exocytosis. Calcium acts as a charge bridge that specifically and reversibly connects multiple Syntaxin 1 and PIP(4;5)P (Milovanovic et al., 2015, 2016). Consequently, calcium microdomains may drive vesicle fusions in specific zones of the PM. Studies of certain cation channels like Two‐Pore Channel 1 (TPC1) showed that their full activation would need high cytosolic Ca2+ concentration that would be detrimental for cell survival in case of global calcium release (Pottosin & Dobrovinskaya, 2018). However, a local accumulation of calcium among a nano/microdomain would circumvent this problem by allowing both the activation of the channel and cell survival. Most evidences concerning local accumulation of secondary messengers creating nanoenvironments are indirect in plants. Undoubtedly, the recent technical advances in super‐resolution microscopy combined with new genetically encoded sensors for secondary messengers like HyPer7 for H2O2 (Ugalde et al., 2021) and GCaMP‐based sensors for calcium (Waadt et al., 2017), will allow in the future to formally demonstrate the existence of these nanoenvironments.
Signaling pathways emerging from nanoenvironments obligatory require a localized decoding machinery (Fig. 4a,b). The ROS signaling decoding machinery would rely on sensing proteins, containing cysteines highly sensitive to H2O2, that transmit the primary oxidation to protein thiols and so on to downstream target proteins (Meyer et al., 2021; Mittler et al., 2022). This requires strong proximity of the involved molecular actors and protein–protein interactions. Interestingly, some glutathione peroxidase (GPX), thioredoxin (TRX) or peroxiredoxin (PRX) could be membrane associated and would typically participate in oxidation of target biomolecules (Delaunay et al., 2002; Attacha et al., 2017; Bi et al., 2022). Interestingly, the LRR receptor kinase H2O2‐induced CA2+ increases 1 (HPCA1) is activated by H2O2 application through the covalent modification of its extracellular cysteine residues leading to its autophosphorylation (Wu et al., 2020). This demonstrates that H2O2 decoding could happen at the PM and may rise from apoplastic H2O2 nanoenvironments. The calcineurin B‐like (CBL) proteins are important actors of the calcium decoding machinery in plants. Together with CBL‐interacting protein kinases (CIPKs), they form protein complexes that phosphorylate target proteins mediating molecular responses. CBL proteins are localized to the PM through lipid modifications (myristoylation and S‐acylation) and are major candidates for calcium decoding proteins among nano/microdomains (Batistič et al., 2008). CPK proteins are other molecular actors of the calcium decoding machinery in plants. Interestingly, CPK3 activation by virus induces a decrease of its diffusion in the PM and leads to its confinement in nanodomains. A similar behavior is observed using a constitutively active form of CPK3, linking the kinase activation to its PM organization (Jolivet et al., 2023). The study of such precise organization of signaling decoding complexes, for example CBL/CPK for Ca2+ signaling or GPX/TRX/PRX for ROS signaling is a promising field of research that gives new insights in the spatiotemporal molecular responses of plant cells. Given the numerous cross talks between ROS and calcium signaling, it is likely that ROS and calcium nanoenvironments could overlap and regulate each other depending on situations.
In a more general way, nanometric accumulation of molecules may participate in the metabolite channeling of enzymatic activities, also called metabolons. Metabolite channeling processes consist in the transfer of the product of a proximal activity directly to a distal activity as a substrate, without equilibration with the bulk solvent, thereby enhancing the efficiency of the kinetic process (Fig. 4c; Kuzmak et al., 2019; Pareek et al., 2021; Dahmani et al., 2023). At this stage, the exact role of membrane nanodomains in metabolite channeling remains poorly explored in plants.
XI. Conclusion and future prospective
Our view of biological membranes has constantly evolved since the lipid bilayer model proposed in the 1920s by Gorter & Grendel (1925) and the fluid mosaic model by Singer & Nicolson (1972). Nowadays, the membrane's lateral heterogeneity in lipids and proteins forming nanodomains is well accepted.
As fluorescent microscopy techniques advance toward higher resolution, the study of plant nanodomains has emerged as a key area in plant cell biology, helped by the development of genetically encoded biosensors. Because nanodomains induce high local concentration and/or low diffusion of lipids and proteins, they are predicted to shape chemical reactions and would bring new avenues to understand cellular processes.
In the plant biology field, numerous studies have demonstrated that plant membrane proteins can be organized in nanodomains (Ott, 2017; Gronnier et al., 2018; Jaillais & Ott, 2020; Martinière & Zelazny, 2021; Jaillais et al., 2024). Nevertheless, our understanding of how membrane nanodomain organization participates in cellular function has just started to emerge. Nanodomains have a scaffolding function that mediates protein–protein interactions necessary for diverse processes such as symbiosis, immune signaling or ion transport. This scaffolding role can participate in the regulation of protein post‐translational modification, for example phosphorylation (Demir et al., 2013; Chu et al., 2021). Thus, specific nanodomains may act in segregating kinases or phosphatases together with their target proteins. Nanodomain organization of membranes may participate in signal specificity. Even if they share common downstream signaling components, FLS2 and BRI1 form distinct nanodomains in the PM. This could explain why they maintain distinct signaling output in immunity and steroid‐mediated growth, respectively (Bücherl et al., 2017). Importantly, nanodomains should be seen as highly dynamic structures whose organization is modified by environmental stimuli as exemplified by ROP6 protein that organizes into nanodomains to ensure ROS signaling in response to osmotic or auxin stimulations (Platre et al., 2019; Pan et al., 2020; Smokvarska et al., 2020).
Nanodomain organization and maintenance are complex and require specific lipids, oligomerization of ‘driver’ proteins, the cytoskeleton and the cell wall. One of the main objectives in the future will be to understand how these different parameters are orchestrated and interconnected. Concerning the interplay between lipids and proteins, an interesting aspect to explore is the capacity of some ‘driver’ proteins, such as REMs, to directly interact with lipids to potentially shape nanodomains (Perraki et al., 2012; Legrand et al., 2023). Another very interesting aspect would be to determine to what extent microtubules and the cell wall, which are in constant dialog during cell growth, cooperate to organize nanodomains. Along this line, recent studies showed a functional interplay between stress sensing, CesA complex localization/dynamics and microtubule organization (McFarlane et al., 2021; Kesten et al., 2022).
Secondary messengers such as Ca2+ and ROS participate in a myriad of signaling processes. They are at the neck of hourglass signaling pathways, being induced by various stimuli and leading to specific downstream cellular responses. Even if it remains rather speculative at this stage, in this review we propose that plant nanodomains may participate in the formation of local chemical nanoenvironments, in the absence of any diffusion barriers like membranes. Such heterogeneity in Ca2+ and H2O2 concentrations or difference in the pH could be later decoded locally by cells. This hypothesis is supported by indirect evidence: the nanodomain organization of proteins involved in secondary messenger production, for example RBOHs (Hao et al., 2014; Smokvarska et al., 2020); a local enrichment of certain secondary messengers close to the PM (Lherminier et al., 2009; Orman‐Ligeza et al., 2016; Fujita et al., 2020); and PM‐localized protein involved in secondary messenger decoding, for example GPX/TRX, CBLs/CPKs (Batistič et al., 2008; Attacha et al., 2017; Jolivet et al., 2023). According to this model, and because of their proximity in the same type of nanodomains, the activation of a given secondary messenger producer may locally induce the appropriate decoding machinery, and would thus contribute to the generation of a specific signaling signature.
Competing interests
None declared.
Disclaimer
The New Phytologist Foundation remains neutral with regard to jurisdictional claims in maps and in any institutional affiliations.
Acknowledgements
AP and AM are funded by the French National Agency (ANR) CellOsmo (ANR‐19‐CE20‐0008‐01). CM and AM are funded by the I‐Site Optosens from Montpellier University. This work has also benefited from another ANR funding (ANR‐23‐CE20‐0022, HIRAQIM project, to EZ) and another I‐site funding from Montpellier University (HIROS project, to EZ).
Contributor Information
Alexandre Martinière, Email: alexandre.martiniere@cnrs.fr.
Enric Zelazny, Email: enric.zelazny@cnrs.fr.
References
- Albers P, Üstün S, Witzel K, Kraner M, Börnke F. 2019. A Remorin from Nicotiana benthamiana interacts with the Pseudomonas type‐III effector protein HopZ1a and is phosphorylated by the immune‐related kinase PBS1. Molecular Plant–Microbe Interactions 32: 1229–1242. [DOI] [PubMed] [Google Scholar]
- Aryal B, Xia J, Hu Z, Stumpe M, Tsering T, Liu J, Huynh J, Fukao Y, Glöckner N, Huang H‐Y et al. 2023. An LRR receptor kinase controls ABC transporter substrate preferences during plant growth‐defense decisions. Current Biology 33: 2008–2023. [DOI] [PubMed] [Google Scholar]
- Attacha S, Solbach D, Bela K, Moseler A, Wagner S, Schwarzländer M, Aller I, Müller SJ, Meyer AJ. 2017. Glutathione peroxidase‐like enzymes cover five distinct cell compartments and membrane surfaces in Arabidopsis thaliana . Plant, Cell & Environment 40: 1281–1295. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Adler EM, Charlton MP, Hans M, Swandulla D, Zipser K. 1992. Presynaptic calcium signals during neurotransmitter release: detection with fluorescent indicators and other calcium chelators. Journal of Physiology 86: 129–134. [DOI] [PubMed] [Google Scholar]
- Bahammou D, Recorbet G, Mamode Cassim A, Robert F, Balliau T, Van Delft P, Haddad Y, Mongrand S, Fouillen L, Simon‐Plas F. 2024. A combined lipidomic and proteomic profiling of Arabidopsis thaliana plasma membrane. The Plant Journal 119: 1570–1595. [DOI] [PubMed] [Google Scholar]
- Batistič O, Sorek N, Schültke S, Yalovsky S, Kudla J. 2008. Dual fatty acyl modification determines the localization and plasma membrane targeting of CBL/CIPK Ca2+ signaling complexes in Arabidopsis. Plant Cell 20: 1346–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G, Hu M, Fu L, Zhang X, Zuo J, Li J, Yang J, Zhou J‐M. 2022. The cytosolic thiol peroxidase PRXIIB is an intracellular sensor for H2O2 that regulates plant immunity through a redox relay. Nature Plants 8: 1160–1175. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Chaumont F. 2014. Aquaporin‐facilitated transmembrane diffusion of hydrogen peroxide. Biochimica et Biophysica Acta 1840: 1596–1604. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Møller ALB, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP. 2007. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. Journal of Biological Chemistry 282: 1183–1192. [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J et al. 2007. Interactions among PIN‐FORMED and P‐glycoprotein auxin transporters in Arabidopsis. Plant Cell 19: 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann M, Li E, Sampathkumar A, Kocabek T, Hauser M‐T, Persson S. 2012. POM‐POM2/cellulose synthase interacting1 is essential for the functional association of cellulose synthase and microtubules in Arabidopsis. Plant Cell 24: 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman DT, Hoegg MB, Robbins SM. 2007. The SPFH domain‐containing proteins: more than lipid raft markers. Trends in Cell Biology 17: 394–402. [DOI] [PubMed] [Google Scholar]
- Bücherl CA, Jarsch IK, Schudoma C, Segonzac C, Mbengue M, Robatzek S, MacLean D, Ott T, Zipfel C. 2017. Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. eLife 6: e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhou H, Xu F, Yu M, Coego A, Rodriguez L, Lu Y, Xie Q, Fu Q, Chen J et al. 2023. CAR modulates plasma membrane nano‐organization and immune signaling downstream of RALF1‐FERONIA signaling pathway. New Phytologist 237: 2148–2162. [DOI] [PubMed] [Google Scholar]
- Cheng G, Yang Z, Zhang H, Zhang J, Xu J. 2020. Remorin interacting with PCaP1 impairs Turnip mosaic virus intercellular movement but is antagonised by VPg. New Phytologist 225: 2122–2139. [DOI] [PubMed] [Google Scholar]
- Cheung AY. 2024. FERONIA: a receptor kinase at the core of a global signaling network. Annual Review of Plant Biology 75: 345–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JDG, Felix G, Boller T. 2007. A flagellin‐induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500. [DOI] [PubMed] [Google Scholar]
- Choi HW, Kim DS, Kim NH, Jung HW, Ham JH, Hwang BK. 2013. Xanthomonas filamentous hemagglutinin‐like protein Fha1 interacts with pepper hypersensitive‐induced reaction protein CaHIR1 and functions as a virulence factor in host plants. Molecular Plant–Microbe Interactions 26: 1441–1454. [DOI] [PubMed] [Google Scholar]
- Chu L‐C, Offenborn JN, Steinhorst L, Wu XN, Xi L, Li Z, Jacquot A, Lejay L, Kudla J, Schulze WX. 2021. Plasma membrane calcineurin B‐like calcium‐ion sensor proteins function in regulating primary root growth and nitrate uptake by affecting global phosphorylation patterns and microdomain protein distribution. New Phytologist 229: 2223–2237. [DOI] [PubMed] [Google Scholar]
- Couto D, Zipfel C. 2016. Regulation of pattern recognition receptor signalling in plants. Nature Reviews Immunology 16: 537–552. [DOI] [PubMed] [Google Scholar]
- Crowell EF, Bischoff V, Desprez T, Rolland A, Stierhof Y‐D, Schumacher K, Gonneau M, Höfte H, Vernhettes S. 2009. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 21: 1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Qian H, Yin J, Xu C, Luo P, Zhang X, Yu M, Su B, Li X, Lin J. 2024. Single‐molecule analysis reveals the phosphorylation of FLS2 governs its spatiotemporal dynamics and immunity. eLife 12: RP91072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmani I, Qin K, Zhang Y, Fernie AR. 2023. The formation and function of plant metabolons. The Plant Journal 114: 1080–1092. [DOI] [PubMed] [Google Scholar]
- Daněk M, Angelini J, Malínská K, Andrejch J, Amlerová Z, Kocourková D, Brouzdová J, Valentová O, Martinec J, Petrášek J. 2020. Cell wall contributes to the stability of plasma membrane nanodomain organization of Arabidopsis thaliana FLOTILLIN2 and HYPERSENSITIVE INDUCED REACTION1 proteins. The Plant Journal 101: 619–636. [DOI] [PubMed] [Google Scholar]
- Daněk M, Valentová O, Martinec J. 2016. Flotillins, Erlins, and HIRs: from animal base camp to plant new horizons. Critical Reviews in Plant Sciences 35: 191–214. [Google Scholar]
- Deinum EE, Jacobs B. 2024. Rho of plants patterning: linking mathematical models and molecular diversity. Journal of Experimental Botany 75: 1274–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB. 2002. A thiol peroxidase is an H2O2 receptor and redox‐transducer in gene activation. Cell 111: 471–481. [DOI] [PubMed] [Google Scholar]
- Demir F, Horntrich C, Blachutzik JO, Scherzer S, Reinders Y, Kierszniowska S, Schulze WX, Harms GS, Hedrich R, Geiger D et al. 2013. Arabidopsis nanodomain‐delimited ABA signaling pathway regulates the anion channel SLAH3. Proceedings of the National Academy of Sciences, USA 110: 8296–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez T, Juraniec M, Crowell EF, Jouy H, Pochylova Z, Parcy F, Höfte H, Gonneau M, Vernhettes S. 2007. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana . Proceedings of the National Academy of Sciences, USA 104: 15572–15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dünser K, Gupta S, Herger A, Feraru MI, Ringli C, Kleine‐Vehn J. 2019. Extracellular matrix sensing by FERONIA and Leucine‐Rich Repeat Extensins controls vacuolar expansion during cellular elongation in Arabidopsis thaliana . EMBO Journal 38: e100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof Y‐D, Friml J. 2007. Clathrin‐mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Current Biology 17: 520–527. [DOI] [PubMed] [Google Scholar]
- Feng W, Kita D, Peaucelle A, Cartwright HN, Doan V, Duan Q, Liu M‐C, Maman J, Steinhorst L, Schmitz‐Thom I et al. 2018. The FERONIA receptor kinase maintains cell‐wall integrity during salt stress through Ca2+ signaling. Current Biology 28: 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraru E, Feraru MI, Kleine‐Vehn J, Martinière A, Mouille G, Vanneste S, Vernhettes S, Runions J, Friml J. 2011. PIN polarity maintenance by the cell wall in Arabidopsis. Current Biology 21: 338–343. [DOI] [PubMed] [Google Scholar]
- Fratini M, Krishnamoorthy P, Stenzel I, Riechmann M, Matzner M, Bacia K, Heilmann M, Heilmann I. 2021. Plasma membrane nano‐organization specifies phosphoinositide effects on Rho‐GTPases and actin dynamics in tobacco pollen tubes. Plant Cell 33: 642–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Xu Y, Li C, Li Y, Wu J, Zhou X. 2018. Rice stripe virus interferes with S‐acylation of Remorin and induces its autophagic degradation to facilitate virus infection. Molecular Plant 11: 269–287. [DOI] [PubMed] [Google Scholar]
- Fujita S, De Bellis D, Edel KH, Köster P, Andersen TG, Schmid‐Siegert E, Dénervaud Tendon V, Pfister A, Marhavý P, Ursache R et al. 2020. SCHENGEN receptor module drives localized ROS production and lignification in plant roots. EMBO Journal 39: e103894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furt F, König S, Bessoule J‐J, Sargueil F, Zallot R, Stanislas T, Noirot E, Lherminier J, Simon‐Plas F, Heilmann I et al. 2010. Polyphosphoinositides are enriched in plant membrane rafts and form microdomains in the plasma membrane. Plant Physiology 152: 2173–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeyne A, Sánchez‐Rodríguez C, Vanneste S, Di Rubbo S, Zauber H, Vanneste K, Van Leene J, De Winne N, Eeckhout D, Persiau G et al. 2014. The TPLATE adaptor complex drives clathrin‐mediated endocytosis in plants. Cell 156: 691–704. [DOI] [PubMed] [Google Scholar]
- Gao G, Zhu C, Liu E, Nabi IR. 2019. Reticulon and CLIMP‐63 regulate nanodomain organization of peripheral ER tubules. PLoS Biology 17: e3000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter E, Grendel F. 1925. On bimolecular layers of lipoids on the chromocytes of the blood. Journal of Experimental Medicine 41: 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronnier J, Crowet J‐M, Habenstein B, Nasir MN, Bayle V, Hosy E, Platre MP, Gouguet P, Raffaele S, Martinez D et al. 2017. Structural basis for plant plasma membrane protein dynamics and organization into functional nanodomains. eLife 6: e26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronnier J, Franck CM, Stegmann M, DeFalco TA, Abarca A, von Arx M, Dünser K, Lin W, Yang Z, Kleine‐Vehn J et al. 2022. Regulation of immune receptor kinase plasma membrane nanoscale organization by a plant peptide hormone and its receptors. eLife 11: e74162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronnier J, Gerbeau‐Pissot P, Germain V, Mongrand S, Simon‐Plas F. 2018. Divide and rule: plant plasma membrane organization. Trends in Plant Science 23: 899–917. [DOI] [PubMed] [Google Scholar]
- Grosjean K, Der C, Robert F, Thomas D, Mongrand S, Simon‐Plas F, Gerbeau‐Pissot P. 2018. Interactions between lipids and proteins are critical for organization of plasma membrane‐ordered domains in tobacco BY‐2 cells. Journal of Experimental Botany 69: 3545–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui J, Zheng S, Liu C, Shen J, Li J, Li L. 2016. OsREM4.1 interacts with OsSERK1 to coordinate the interlinking between abscisic acid and brassinosteroid signaling in rice. Developmental Cell 38: 201–213. [DOI] [PubMed] [Google Scholar]
- Haney CH, Long SR. 2010. Plant flotillins are required for infection by nitrogen‐fixing bacteria. Proceedings of the National Academy of Sciences, USA 107: 478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney CH, Riely BK, Tricoli DM, Cook DR, Ehrhardt DW, Long SR. 2011. Symbiotic rhizobia bacteria trigger a change in localization and dynamics of the Medicago truncatula receptor kinase LYK3. Plant Cell 23: 2774–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H, Fan L, Chen T, Li R, Li X, He Q, Botella MA, Lin J. 2014. Clathrin and membrane microdomains cooperatively regulate RbohD dynamics and activity in Arabidopsis. Plant Cell 26: 1729–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR. 2014. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343: 408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann M, Heilmann I. 2022. Regulators regulated: different layers of control for plasma membrane phosphoinositides in plants. Current Opinion in Plant Biology 67: 102218. [DOI] [PubMed] [Google Scholar]
- Henty‐Ridilla JL, Shimono M, Li J, Chang JH, Day B, Staiger CJ. 2013. The plant actin cytoskeleton responds to signals from microbe‐associated molecular patterns. PLoS Pathogens 9: e1003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E, Martinière A, Choquet D, Maurel C, Luu D‐T. 2015. Super‐resolved and dynamic imaging of membrane proteins in plant cells reveal contrasting kinetic profiles and multiple confinement mechanisms. Molecular Plant 8: 339–342. [DOI] [PubMed] [Google Scholar]
- Huang D, Sun Y, Ma Z, Ke M, Cui Y, Chen Z, Chen C, Ji C, Tran TM, Yang L et al. 2019. Salicylic acid‐mediated plasmodesmal closure via Remorin‐dependent lipid organization. Proceedings of the National Academy of Sciences, USA 116: 21274–21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CH, Turnbull D, Xhelilaj K, Myles S, Pflughaupt RL, Kopischke M, Davies P, Jones S, Robatzek S, Zipfel C et al. 2023. S‐acylation stabilizes ligand‐induced receptor kinase complex formation during plant pattern‐triggered immune signaling. Current Biology 33: 1588–1596. [DOI] [PubMed] [Google Scholar]
- Hwang J‐U, Vernoud V, Szumlanski A, Nielsen E, Yang Z. 2008. A tip‐localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Current Biology 18: 1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J‐U, Wu G, Yan A, Lee Y‐J, Grierson CS, Yang Z. 2010. Pollen‐tube tip growth requires a balance of lateral propagation and global inhibition of Rho‐family GTPase activity. Journal of Cell Science 123: 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Bayer E, Bergmann DC, Botella MA, Boutté Y, Bozkurt TO, Caillaud M‐C, Germain V, Grossmann G, Heilmann I et al. 2024. Guidelines for naming and studying plasma membrane domains in plants. Nature Plants 10: 1172–1183. [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Ott T. 2020. The nanoscale organization of the plasma membrane and its importance in signaling: a proteolipid perspective. Plant Physiology 182: 1682–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsch IK, Konrad SSA, Stratil TF, Urbanus SL, Szymanski W, Braun P, Braun K‐H, Ott T. 2014. Plasma membranes are subcompartmentalized into a plethora of coexisting and diverse microdomains in Arabidopsis and Nicotiana benthamiana . Plant Cell 26: 1698–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Vert G. 2017. Single event resolution of plant plasma membrane protein endocytosis by TIRF microscopy. Frontiers in Plant Science 8: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Vasilev C, Olsen JD, Hunter CN. 2014. Nanodomains of cytochrome b6f and photosystem II complexes in spinach grana thylakoid membranes. Plant Cell 26: 3051–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet M‐D, Deroubaix A‐F, Boudsocq M, Abel NB, Rocher M, Robbe T, Wattelet‐Boyer V, Huard J, Lefebvre D, Lu Y‐J et al. 2023. Interdependence of plasma membrane nanoscale dynamics of a kinase and its cognate substrate underlies Arabidopsis response to viral infection. eLife 12: RP90309. [Google Scholar]
- Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JD, Shirasu K, Menke F, Jones A et al. 2014. Direct regulation of the NADPH oxidase RBOHD by the PRR‐associated kinase BIK1 during plant immunity. Molecular Cell 54: 43–55. [DOI] [PubMed] [Google Scholar]
- Ke M, Ma Z, Wang D, Sun Y, Wen C, Huang D, Chen Z, Yang L, Tan S, Li R et al. 2021. Salicylic acid regulates PIN2 auxin transporter hyperclustering and root gravitropic growth via Remorin‐dependent lipid nanodomain organisation in Arabidopsis thaliana . New Phytologist 229: 963–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesten C, García‐Moreno Á, Amorim‐Silva V, Menna A, Castillo AG, Percio F, Armengot L, Ruiz‐Lopez N, Jaillais Y, Sánchez‐Rodríguez C et al. 2022. Peripheral membrane proteins modulate stress tolerance by safeguarding cellulose synthases. Science Advances 8: eabq6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko BN, Hoek JB, Westerhoff HV. 2000. Why cytoplasmic signalling proteins should be recruited to cell membranes. Trends in Cell Biology 10: 173–178. [DOI] [PubMed] [Google Scholar]
- Klahre U, Kost B. 2006. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell 18: 3033–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine‐Vehn J, Wabnik K, Martinière A, Łangowski Ł, Willig K, Naramoto S, Leitner J, Tanaka H, Jakobs S, Robert S et al. 2011. Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Molecular Systems Biology 7: 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymchenko AS. 2017. Solvatochromic and fluorogenic dyes as environment‐sensitive probes: design and biological applications. Accounts of Chemical Research 50: 366–375. [DOI] [PubMed] [Google Scholar]
- Kritsiligkou P, Bosch K, Shen TK, Meurer M, Knop M, Dick TP. 2023. Proteome‐wide tagging with an H2O2 biosensor reveals highly localized and dynamic redox microenvironments. Proceedings of the National Academy of Sciences, USA 120: e2314043120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küchler A, Yoshimoto M, Luginbühl S, Mavelli F, Walde P. 2016. Enzymatic reactions in confined environments. Nature Nanotechnology 11: 409–420. [DOI] [PubMed] [Google Scholar]
- Kulich I, Vogler F, Bleckmann A, Cyprys P, Lindemeier M, Fuchs I, Krassini L, Schubert T, Steinbrenner J, Beynon J et al. 2020. ARMADILLO REPEAT ONLY proteins confine Rho GTPase signalling to polar growth sites. Nature Plants 6: 1275–1288. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. 2005. Paradigm shift of the plasma membrane concept from the two‐dimensional continuum fluid to the partitioned fluid: high‐speed single‐molecule tracking of membrane molecules. Annual Review of Biophysics and Biomolecular Structure 34: 351–378. [DOI] [PubMed] [Google Scholar]
- Kuzmak A, Carmali S, von Lieres E, Russell AJ, Kondrat S. 2019. Can enzyme proximity accelerate cascade reactions? Scientific Reports 9: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landge AN, Jordan BM, Diego X, Müller P. 2020. Pattern formation mechanisms of self‐organizing reaction‐diffusion systems. Developmental Biology 460: 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Rubio MC, Alassimone J, Geldner N. 2013. A mechanism for localized lignin deposition in the endodermis. Cell 153: 402–412. [DOI] [PubMed] [Google Scholar]
- Lefebvre B, Timmers T, Mbengue M, Moreau S, Hervé C, Tóth K, Bittencourt‐Silvestre J, Klaus D, Deslandes L, Godiard L et al. 2010. A Remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proceedings of the National Academy of Sciences, USA 107: 2343–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand A, G‐Cava D, Jolivet M‐D, Decossas M, Lambert O, Bayle V, Jaillais Y, Loquet A, Germain V, Boudsocq M et al. 2023. Structural determinants of REMORIN nanodomain formation in anionic membranes. Biophysical Journal 122: 2192–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand A, Martinez D, Grélard A, Berbon M, Morvan E, Tawani A, Loquet A, Mongrand S, Habenstein B. 2019. Nanodomain clustering of the plant protein Remorin by solid‐state NMR. Frontiers in Molecular Biosciences 6: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenne P‐F, Wawrezinieck L, Conchonaud F, Wurtz O, Boned A, Guo X‐J, Rigneault H, He H‐T, Marguet D. 2006. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO Journal 25: 3245–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard TA, Loose M, Martens S. 2023. The membrane surface as a platform that organizes cellular and biochemical processes. Developmental Cell 58: 1315–1332. [DOI] [PubMed] [Google Scholar]
- Lherminier J, Elmayan T, Fromentin J, Elaraqui KT, Vesa S, Morel J, Verrier J‐L, Cailleteau B, Blein J‐P, Simon‐Plas F. 2009. NADPH oxidase‐mediated reactive oxygen species production: subcellular localization and reassessment of its role in plant defense. Molecular Plant–Microbe Interactions 22: 868–881. [DOI] [PubMed] [Google Scholar]
- Li C, Yeh F‐L, Cheung AY, Duan Q, Kita D, Liu M‐C, Maman J, Luu EJ, Wu BW, Gates L et al. 2015. Glycosylphosphatidylinositol‐anchored proteins as chaperones and co‐receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife 4: e06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, von Wangenheim D, Zhang X, Tan S, Darwish‐Miranda N, Naramoto S, Wabnik K, Rycke RD, Kaufmann WA, Gütl D et al. 2021. Cellular requirements for PIN polar cargo clustering in Arabidopsis thaliana . New Phytologist 229: 351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y et al. 2014. The FLS2‐associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host & Microbe 15: 329–338. [DOI] [PubMed] [Google Scholar]
- Li R, Liu P, Wan Y, Chen T, Wang Q, Mettbach U, Baluska F, Samaj J, Fang X, Lucas WJ et al. 2012. A membrane microdomain‐associated protein, Arabidopsis Flot1, is involved in a clathrin‐independent endocytic pathway and is required for seedling development. Plant Cell 24: 2105–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhao J, Zhai Y, Yuan Q, Zhang H, Wu X, Lu Y, Peng J, Sun Z, Lin L et al. 2019. The hypersensitive induced reaction 3 (HIR3) gene contributes to plant basal resistance via an EDS1 and salicylic acid‐dependent pathway. The Plant Journal 98: 783–797. [DOI] [PubMed] [Google Scholar]
- Liang P, Stratil TF, Popp C, Marín M, Folgmann J, Mysore KS, Wen J, Ott T. 2018. Symbiotic root infections in Medicago truncatula require Remorin‐mediated receptor stabilization in membrane nanodomains. Proceedings of the National Academy of Sciences, USA 115: 5289–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M‐CJ, Yeh F‐LJ, Yvon R, Simpson K, Jordan S, Chambers J, Wu H‐M, Cheung AY. 2024. Extracellular pectin‐RALF phase separation mediates FERONIA global signaling function. Cell 187: 312–330. [DOI] [PubMed] [Google Scholar]
- Liu X, Oh S, Kirschner MW. 2022. The uniformity and stability of cellular mass density in mammalian cell culture. Frontiers in Cell and Developmental Biology 10: 1017499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Yan A, Liu G, Guo J, Rong D, Kanaoka MM, Xiao Z, Xu G, Higashiyama T, Cui X et al. 2017. Exocytosis‐coordinated mechanisms for tip growth underlie pollen tube growth guidance. Nature Communications 8: 1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X, Jing Y, Xiao J, Zhang Y, Zhu Y, Julian R, Lin J. 2017. Membrane microdomains and the cytoskeleton constrain AtHIR1 dynamics and facilitate the formation of an AtHIR1‐associated immune complex. The Plant Journal 90: 3–16. [DOI] [PubMed] [Google Scholar]
- Ma C, Wang C, Luo D, Yan L, Yang W, Li N, Gao N. 2022. Structural insights into the membrane microdomain organization by SPFH family proteins. Cell Research 32: 176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Liu X, Nath S, Sun H, Tran TM, Yang L, Mayor S, Miao Y. 2021. Formin nanoclustering‐mediated actin assembly during plant flagellin and DSF signaling. Cell Reports 34: 108884. [DOI] [PubMed] [Google Scholar]
- Ma Z, Sun Y, Zhu X, Yang L, Chen X, Miao Y. 2022. Membrane nanodomains modulate formin condensation for actin remodeling in Arabidopsis innate immune responses. Plant Cell 34: 374–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamode Cassim A, Gouguet P, Gronnier J, Laurent N, Germain V, Grison M, Boutté Y, Gerbeau‐Pissot P, Simon‐Plas F, Mongrand S. 2019. Plant lipids: key players of plasma membrane organization and function. Progress in Lipid Research 73: 1–27. [DOI] [PubMed] [Google Scholar]
- Marín M, Thallmair V, Ott T. 2012. The intrinsically disordered N‐terminal region of AtREM1.3 Remorin protein mediates protein‐protein interactions. Journal of Biological Chemistry 287: 39982–39991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Legrand A, Gronnier J, Decossas M, Gouguet P, Lambert O, Berbon M, Verron L, Grélard A, Germain V et al. 2019. Coiled‐coil oligomerization controls localization of the plasma membrane REMORINs. Journal of Structural Biology 206: 12–19. [DOI] [PubMed] [Google Scholar]
- Martinière A, Lavagi I, Nageswaran G, Rolfe DJ, Maneta‐Peyret L, Luu D‐T, Botchway SW, Webb SED, Mongrand S, Maurel C et al. 2012. Cell wall constrains lateral diffusion of plant plasma‐membrane proteins. Proceedings of the National Academy of Sciences, USA 109: 12805–12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A, Fiche JB, Smokvarska M, Mari S, Alcon C, Dumont X, Hematy K, Jaillais Y, Nollmann M, Maurel C. 2019. Osmotic stress activates two reactive oxygen species pathways with distinct effects on protein nanodomains and diffusion. Plant Physiology 179: 1581–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A, Zelazny E. 2021. Membrane nanodomains and transport functions in plant. Plant Physiology 187: 1839–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey MA, Poo MM. 1986. Rates of membrane‐associated reactions: reduction of dimensionality revisited. Journal of Cell Biology 102: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HE, Mutwil‐Anderwald D, Verbančič J, Picard KL, Gookin TE, Froehlich A, Chakravorty D, Trindade LM, Alonso JM, Assmann SM et al. 2021. A G protein‐coupled receptor‐like module regulates cellulose synthase secretion from the endomembrane system in Arabidopsis. Developmental Cell 56: 1484–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JF, Rolfe DJ, Webb SED, Tolmie AF, Botchway SW, Martin‐Fernandez ML, Hawes C, Runions J. 2019. The cell wall regulates dynamics and size of plasma‐membrane nanodomains in Arabidopsis. Proceedings of the National Academy of Sciences, USA 116: 12857–12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y, Ma Z, Wang Y, Zhou X. 2020. Geminivirus C4 antagonizes the HIR1‐mediated hypersensitive response by inhibiting the HIR1 self‐interaction and promoting degradation of the protein. New Phytologist 225: 1311–1326. [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Dreyer A, Ugalde JM, Feitosa‐Araujo E, Dietz K‐J, Schwarzländer M. 2021. Shifting paradigms and novel players in Cys‐based redox regulation and ROS signaling in plants – and where to go next. Biological Chemistry 402: 399–423. [DOI] [PubMed] [Google Scholar]
- Milovanovic D, Honigmann A, Koike S, Göttfert F, Pähler G, Junius M, Müllar S, Diederichsen U, Janshoff A, Grubmüller H et al. 2015. Hydrophobic mismatch sorts SNARE proteins into distinct membrane domains. Nature Communications 6: 5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic D, Platen M, Junius M, Diederichsen U, Schaap IAT, Honigmann A, Jahn R, van den Bogaart G. 2016. Calcium promotes the formation of syntaxin 1 mesoscale domains through phosphatidylinositol 4,5‐bisphosphate. Journal of Biological Chemistry 291: 7868–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B, Johnson ME. 2021. Speed limits of protein assembly with reversible membrane localization. Journal of Chemical Physics 154: 194101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Zandalinas SI, Fichman Y, Van Breusegem F. 2022. Reactive oxygen species signalling in plant stress responses. Nature Reviews Molecular Cell Biology 23: 663–679. [DOI] [PubMed] [Google Scholar]
- Mongrand S, Stanislas T, Bayer EMF, Lherminier J, Simon‐Plas F. 2010. Membrane rafts in plant cells. Trends in Plant Science 15: 656–663. [DOI] [PubMed] [Google Scholar]
- Nagashima Y, Tsugawa S, Mochizuki A, Sasaki T, Fukuda H, Oda Y. 2018. A Rho‐based reaction‐diffusion system governs cell wall patterning in metaxylem vessels. Scientific Reports 8: 11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack LC, Bayle V, Armengot L, Rozier F, Mamode‐Cassim A, Stevens FD, Caillaud M‐C, Munnik T, Mongrand S, Pleskot R et al. 2022. A nanodomain‐anchored scaffolding complex is required for the function and localization of phosphatidylinositol 4‐kinase alpha in plants. Plant Cell 34: 302–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orman‐Ligeza B, Parizot B, de Rycke R, Fernandez A, Himschoot E, Van Breusegem F, Bennett MJ, Périlleux C, Beeckman T, Draye X. 2016. RBOH‐mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 143: 3328–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott T. 2017. Membrane nanodomains and microdomains in plant–microbe interactions. Current Opinion in Plant Biology 40: 82–88. [DOI] [PubMed] [Google Scholar]
- Pan X, Fang L, Liu J, Senay‐Aras B, Lin W, Zheng S, Zhang T, Guo J, Manor U, Van Norman J et al. 2020. Auxin‐induced signaling protein nanoclustering contributes to cell polarity formation. Nature Communications 11: 3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez AR, Persson S, Ehrhardt DW, Somerville CR. 2008. Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiology 147: 1723–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek V, Sha Z, He J, Wingreen NS, Benkovic SJ. 2021. Metabolic channeling: predictions, deductions, and evidence. Molecular Cell 81: 3775–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier R, Danylchuk DI, Benaissa H, Broch F, Vauchelles R, Gautier A, Klymchenko AS. 2023. Genetic targeting of solvatochromic dyes for probing nanoscale environments of proteins in organelles. Analytical Chemistry 95: 8512–8521. [DOI] [PubMed] [Google Scholar]
- Perraki A, Cacas J‐L, Crowet J‐M, Lins L, Castroviejo M, German‐Retana S, Mongrand S, Raffaele S. 2012. Plasma membrane localization of Solanum tuberosum Remorin from group 1, homolog 3 is mediated by conformational changes in a novel C‐terminal anchor and required for the restriction of potato virus X movement. Plant Physiology 160: 624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraki A, Gronnier J, Gouguet P, Boudsocq M, Deroubaix A‐F, Simon V, German‐Retana S, Legrand A, Habenstein B, Zipfel C et al. 2018. REM1.3's phospho‐status defines its plasma membrane nanodomain organization and activity in restricting PVX cell‐to‐cell movement. PLoS Pathogens 14: e1007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platre MP, Bayle V, Armengot L, Bareille J, Marquès‐Bueno MDM, Creff A, Maneta‐Peyret L, Fiche J‐B, Nollmann M, Miège C et al. 2019. Developmental control of plant Rho GTPase nano‐organization by the lipid phosphatidylserine. Science 364: 57–62. [DOI] [PubMed] [Google Scholar]
- Pottosin I, Dobrovinskaya O. 2018. Two‐pore cation (TPC) channel: not a shorthanded one. Functional Plant Biology 45: 83–92. [DOI] [PubMed] [Google Scholar]
- Qi Y, Tsuda K, Nguyen LV, Wang X, Lin J, Murphy AS, Glazebrook J, Thordal‐Christensen H, Katagiri F. 2011. Physical association of Arabidopsis hypersensitive induced reaction proteins (HIRs) with the immune receptor RPS2. Journal of Biological Chemistry 286: 31297–31307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele S, Bayer E, Lafarge D, Cluzet S, German Retana S, Boubekeur T, Leborgne‐Castel N, Carde J‐P, Lherminier J, Noirot E et al. 2009. Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs potato virus X movement. Plant Cell 21: 1541–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K, Iino R, Fujiwara T, Murase K, Kusumi A. 2003. The fence and picket structure of the plasma membrane of live cells as revealed by single molecule techniques. Molecular Membrane Biology 20: 13–18. [DOI] [PubMed] [Google Scholar]
- Rocher M, Simon V, Jolivet M‐D, Sofer L, Deroubaix A‐F, Germain V, Mongrand S, German‐Retana S. 2022. StREM1.3 REMORIN protein plays an agonistic role in Potyvirus cell‐to‐cell movement in N. benthamiana . Viruses 14: 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues O, Reshetnyak G, Grondin A, Saijo Y, Leonhardt N, Maurel C, Verdoucq L. 2017. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA‐ and pathogen‐triggered stomatal closure. Proceedings of the National Academy of Sciences, USA 114: 9200–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweisguth F, Corson F. 2019. Self‐organization in pattern formation. Developmental Cell 49: 659–677. [DOI] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL. 1972. The fluid mosaic model of the structure of cell membranes. Science 175: 720–731. [DOI] [PubMed] [Google Scholar]
- Smokvarska M, Bayle V, Maneta‐Peyret L, Fouillen L, Poitout A, Dongois A, Fiche J‐B, Gronnier J, Garcia J, Höfte H et al. 2023. The receptor kinase FERONIA regulates phosphatidylserine localization at the cell surface to modulate ROP signaling. Science Advances 9: eadd4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smokvarska M, Francis C, Platre MP, Fiche J‐B, Alcon C, Dumont X, Nacry P, Bayle V, Nollmann M, Maurel C et al. 2020. A plasma membrane nanodomain ensures signal specificity during osmotic signaling in plants. Current Biology 30: 4654–4664. [DOI] [PubMed] [Google Scholar]
- Stegmann M, Monaghan J, Smakowska‐Luzan E, Rovenich H, Lehner A, Holton N, Belkhadir Y, Zipfel C. 2017. The receptor kinase FER is a RALF‐regulated scaffold controlling plant immune signaling. Science 355: 287–289. [DOI] [PubMed] [Google Scholar]
- Sternberg H, Buriakovsky E, Bloch D, Gutman O, Henis YI, Yalovsky S. 2021. Formation of self‐organizing functionally distinct Rho of plants domains involves a reduced mobile population. Plant Physiology 187: 2485–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Rodriguez‐Franco M, Lace B, Nebel N, Hernandez‐Reyes C, Liang P, Schulze E, Mymrikov EV, Gross NM, Knerr J et al. 2023. Stabilization of membrane topologies by proteinaceous Remorin scaffolds. Nature Communications 14: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Zhu X, Li C, Ma Z, Han X, Luo Y, Yang L, Yu J, Miao Y. 2021. Xanthomonas effector XopR hijacks host actin cytoskeleton via complex coacervation. Nature Communications 12: 4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner W, Malinsky J, Opekarová M. 2011. In plant and animal cells, detergent‐resistant membranes do not define functional membrane rafts. Plant Cell 23: 1191–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titapiwatanakun B, Blakeslee JJ, Bandyopadhyay A, Yang H, Mravec J, Sauer M, Cheng Y, Adamec J, Nagashima A, Geisler M et al. 2009. ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. The Plant Journal 57: 27–44. [DOI] [PubMed] [Google Scholar]
- Tomishige M, Sako Y, Kusumi A. 1998. Regulation mechanism of the lateral diffusion of band 3 in erythrocyte membranes by the membrane skeleton. Journal of Cell Biology 142: 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde JM, Schlößer M, Dongois A, Martinière A, Meyer AJ. 2021. The latest HyPe(r) in plant H2O2 biosensing. Plant Physiology 187: 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukawa T, Banno F, Ishikawa T, Kasahara K, Nishina Y, Inoue R, Tsujii K, Yamaguchi M, Takahashi T, Fukao Y et al. 2022. Sphingolipids with 2‐hydroxy fatty acids aid in plasma membrane nanodomain organization and oxidative burst. Plant Physiology 189: 839–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vain T, Crowell EF, Timpano H, Biot E, Desprez T, Mansoori N, Trindade LM, Pagant S, Robert S, Höfte H et al. 2014. The cellulase KORRIGAN is part of the cellulose synthase complex. Plant Physiology 165: 1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Krebs M, Kudla J, Schumacher K. 2017. Multiparameter imaging of calcium and abscisic acid and high‐resolution quantitative calcium measurements using R‐GECO1‐mTurquoise in Arabidopsis. New Phytologist 216: 303–320. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhou Z, Zhou J‐M, Li J. 2024. Myosin XI‐mediated BIK1 recruitment to nanodomains facilitates FLS2‐BIK1 complex formation during innate immunity in Arabidopsis. Proceedings of the National Academy of Sciences, USA 121: e2312415121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mylle E, Johnson A, Besbrugge N, De Jaeger G, Friml J, Pleskot R, Van Damme D. 2020. High temporal resolution reveals simultaneous plasma membrane recruitment of TPLATE complex subunits. Plant Physiology 183: 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li H, Lv X, Chen T, Li R, Xue Y, Jiang J, Jin B, Baluška F, Šamaj J et al. 2015. Spatiotemporal dynamics of the BRI1 receptor and its regulation by membrane microdomains in living Arabidopsis cells. Molecular Plant 8: 1334–1349. [DOI] [PubMed] [Google Scholar]
- Wang P, Hawkins TJ, Richardson C, Cummins I, Deeks MJ, Sparkes I, Hawes C, Hussey PJ. 2014. The plant cytoskeleton, NET3C, and VAP27 mediate the link between the plasma membrane and endoplasmic reticulum. Current Biology 24: 1397–1405. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao Y, Luo W, Li R, He Q, Fang X, Michele RD, Ast C, von Wirén N, Lin J. 2013. Single‐particle analysis reveals shutoff control of the Arabidopsis ammonium transporter AMT1;3 by clustering and internalization. Proceedings of the National Academy of Sciences, USA 110: 13204–13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TH, Kumar M, Turner SR. 2021. The molecular basis of plant cellulose synthase complex organisation and assembly. Biochemical Society Transactions 49: 379–391. [DOI] [PubMed] [Google Scholar]
- Wu F, Chi Y, Jiang Z, Xu Y, Xie L, Huang F, Wan D, Ni J, Yuan F, Wu X et al. 2020. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 578: 577–581. [DOI] [PubMed] [Google Scholar]
- Wu G, Gu Y, Li S, Yang Z. 2001. A genome‐wide analysis of Arabidopsis Rop‐interactive CRIB motif‐containing proteins that act as Rop GTPase targets. Plant Cell 13: 2841–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Liu H, Dong Z, Xiao J, Su B, Fan L, Komis G, Šamaj J, Lin J, Li R. 2017. The dynamics and endocytosis of Flot1 protein in response to flg22 in Arabidopsis. Journal of Plant Physiology 215: 73–84. [DOI] [PubMed] [Google Scholar]
- Zang J, Klemm S, Pain C, Duckney P, Bao Z, Stamm G, Kriechbaumer V, Bürstenbinder K, Hussey PJ, Wang P. 2021. A novel plant actin‐microtubule bridging complex regulates cytoskeletal and ER structure at ER‐PM contact sites. Current Biology 31: 1251–1260. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cui Y, Yu M, Su B, Gong W, Baluška F, Komis G, Šamaj J, Shan X, Lin J. 2019. Phosphorylation‐mediated dynamics of nitrate transceptor NRT1.1 regulate auxin flux and nitrate signaling in lateral root growth. Plant Physiology 181: 480–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Cheung M‐Y, Zhang Q, Lei C‐L, Zhang S‐H, Sun SS‐M, Lam H‐M. 2009. A novel simple extracellular leucine‐rich repeat (eLRR) domain protein from rice (OsLRR1) enters the endosomal pathway and interacts with the hypersensitive‐induced reaction protein 1 (OsHIR1). Plant, Cell & Environment 32: 1804–1820. [DOI] [PubMed] [Google Scholar]
- Zimmerman SB, Trach SO. 1991. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli . Journal of Molecular Biology 222: 599–620. [DOI] [PubMed] [Google Scholar]


