Abstract
Background and Objectives
Central sensitization (CS) is believed to play a role in many chronic pain conditions. Direct non‐invasive recording from single nociceptive neurons is not feasible in humans, complicating CS establishment. This review discusses how secondary hyperalgesia (SHA), considered a manifestation of CS, affects physiological measures in healthy individuals and if these measures could indicate CS. It addresses controversies about heat sensitivity changes, the role of tactile afferents in mechanical hypersensitivity and detecting SHA through electrical stimuli. Additionally, it reviews the potential of neurophysiological measures to indicate CS presence.
Databases and Data Treatment
Four databases, PubMed, ScienceDirect, Scopus and Cochrane Library, were searched using terms linked to ‘hyperalgesia’. The search was limited to research articles in English conducted in humans until 2023.
Results
Evidence for heat hyperalgesia in the SHA area is sparse and seems to depend on the experimental method used. Minimal or no involvement of tactile afferents in SHA was found. At the spinal level, the threshold of the nociceptive withdrawal reflex (RIII) is consistently reduced during experimentally induced SHA. The RIII area and the spinal somatosensory potential (N13‐SEP) amplitude are modulated only with long‐lasting nociceptive input. At the brain level, pinprick‐evoked potentials within the SHA area are increased.
Conclusions
Mechanical pinprick hyperalgesia is the most reliable behavioural readout for SHA, while the RIII threshold is the most sensitive neurophysiological readout. Due to scarce data on reliability, sensitivity and specificity, none of the revised neurophysiological methods is currently suitable for CS identification at the individual level.
Significance
Gathering evidence for CS in humans is a crucial research focus, especially with the increasing interest in concepts such as ‘central sensitization‐like pain’ or ‘nociplastic pain’. This review clarifies which readouts, among the different behavioural and neurophysiological proxies tested in experimental settings, can be used to infer the presence of CS in humans.
1. INTRODUCTION
Central sensitization (CS) is defined by the International Association for the Study of Pain (IASP) as the ‘increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold input’. CS is believed to contribute to many chronic pain conditions, suggesting a need for objective criteria for establishing CS (Arendt‐Nielsen et al., 2018; Nijs et al., 2021; Woolf, 2011). Following the IASP definition, CS can be measured by recording the responsiveness of nociceptive neurons in the central nervous system (CNS). The IASP defines a nociceptive neuron as ‘a central or peripheral neuron of the somatosensory nervous system that is capable of encoding noxious stimuli’. In humans, it is not possible, non‐invasively, to record from single nociceptive neurons in the CNS. The neurophysiological techniques that are available to record CNS activity in humans, including evoked potentials and reflexes, only record the activity of (large) pools of neurons and are probably unlikely to capture the activity of nociceptive specific neurons only.
Secondary hyperalgesia (SHA), the increase in evoked pain in the skin surrounding a cutaneous injury (Sandkühler, 2009), is the best‐documented manifestation of CS and can be induced experimentally. There are a dozen experimental methods to induce SHA, the description of which goes beyond the scope of this review and has been detailed elsewhere (Quesada et al., 2021).
The aim of this review was to critically discuss how the presence of CS evaluated by the manifestation of SHA affects physiological measures in healthy volunteers and ultimately if those measures could be used to infer the presence of CS.
We discussed the changes in perception elicited by stimuli belonging to different modalities, after the application of experimental methods used to induce SHA, with the aim to address three controversial issues: (1) Is there a change in heat sensitivity in the area of SHA? (2) Is there a contribution of tactile afferents to SHA? (3) Can we detect SHA using electrical stimuli?
The literature suggests mixed results regarding secondary heat hyperalgesia. An animal study on inflammatory arthritis tested whether SHA depends on the class of peripheral nociceptor (C‐ or A‐nociceptor) rather than the type of stimulation (mechanical vs. heat) (Hsieh et al., 2015). They found SHA was evoked by A‐nociceptor thermal stimulation, suggesting it is A‐nociceptor dependent, not stimulus modality dependent. We systematically searched the literature to investigate the consistency of secondary heat hyperalgesia.
Since mechanical pinprick stimuli activate low‐threshold mechanoreceptors (Cohen & Vierck, 1993; Johansson & Vallbo, 1979; Vallbo & Johansson, 1984), we explored whether these receptors contribute to changes in mechanical pinprick sensitivity.
Finally, previous studies suggest that secondary mechanical hyperalgesia is mediated by A‐fibre nociceptors (Ziegler et al., 1999). We explored whether electrical stimuli, supposed to selectively activate A‐fibre nociceptors, could identify secondary mechanical hyperalgesia.
Lastly, we reviewed the changes in different electrophysiological measures originating from the central and peripheral nervous systems, assessed in the context of experimentally induced SHA, to determine if those measures could be used to infer the presence of CS.
2. METHODS
After an initial exploratory search according to the three different questions we aimed to address, and according to the changes in neurophysiological measures occurring in the context of SHA, we performed systematic research of the literature. We adopted a systematic approach to building this narrative review (see the flow diagram of systematic searches in Figure 1). Four databases including PubMed, ScienceDirect, Scopus and Cochrane Library were searched using the terms: ‘hyperalgesia’ or ‘secondary hyperalgesia’ or ‘pinprick hyperalgesia’ or ‘mechanical hyperalgesia’ and ‘human’. Search was limited to research articles conducted on healthy volunteers and published in English until 2023. Additionally, to specifically look for research articles focusing on our research questions and reporting on the neurophysiological outcomes we aimed to discuss, successive searches were then performed including the same research terms in addition to ‘thermal’ or ‘heat’ or ‘reflex’ or ‘tactile’ or ‘touch’ or ‘electrical’ or ‘potentials’ or ‘sympathetic’ or ‘pupil’ or ‘skin conductance’. After merging all records, we discarded duplicates. Reports were excluded if they were not original research articles (reason 1 in the flow diagram) and if the outcomes were not obtained by stimuli delivered in the area of secondary hyperalgesia delineated using pinprick stimuli (reason 2). Relevant reports cited in the screened research articles have been reviewed and considered eligible if matching the research criteria mentioned above. Each article was reviewed by at least two authors. If there was no agreement on the extracted results, a review by the third author was necessary. After this process, we finally included 68 studies in total.
FIGURE 1.
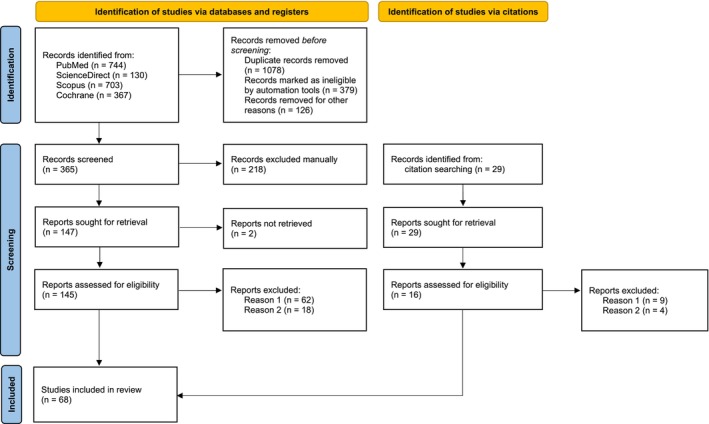
Flow diagram of the systematic searches of databases according to PRISMA guidelines (Page et al., 2021). Reason 1 refers to the type of article included (original research papers conducted in healthy volunteers) and reason 2 refers to the fact that the outcomes were obtained from stimuli delivered in the area of secondary hyperalgesia, which was identified using pinprick stimuli.
3. BEHAVIOURAL CHARACTERISTICS OF SECONDARY HYPERALGESIA
SHA seems most prominent for sharp mechanical stimuli, such as pinprick stimuli (Meyer & Campbell, 1981). Given the fact that mechanical pinprick stimuli are often not perceived as painful in normal conditions, that is, when delivered to non‐sensitized skin, strictly speaking, the increase in perceived pinprick sensitivity after sensitization cannot be labelled as secondary hyperalgesia or allodynia (pain evoked by non‐nociceptive stimuli), and hyperesthesia would be preferable (IASP). Indeed, to label the increased perception of sharp mechanical punctate stimuli as hyperalgesia, those pinprick stimuli should be perceived as painful at baseline, that is, before sensitization is induced. To label the change in perception elicited by mechanical pinprick stimuli as allodynia those sharp indenting pinprick stimuli should not activate peripheral nociceptors, which is clearly the case (Greenspan & McGillis, 1991; Magerl et al., 1998; Ziegler et al., 1999) (see also dynamic allodynia related to the activation of low threshold mechanoreceptors Section 2.2). In this review, we will follow a mechanistic approach, and will consider any increase in punctate mechanical pinprick sensitivity that develops at the skin surrounding the site at which intense and/or repeated nociceptive stimulation was applied as the same phenomenon as SHA.
3.1. Heat sensitivity in the area of SHA
Whether SHA is also present for heat stimuli is controversial. Table 1 summarizes studies that have assessed changes in heat pain threshold and perceived heat sensitivity to suprathreshold heat stimuli in the area of SHA. Secondary heat hyperalgesia has been mostly studied after conditioning with transcutaneous electrical stimulation, heat injury (burn and UVB) and capsaicin application (intradermal and topical) (Table 1). Regarding transcutaneous electrical stimulation, five studies used high‐frequency electrical stimulation (HFS) and one study used low‐frequency stimulation (LFS). The two studies that applied HFS or LFS with an intensity corresponding to 10 times the individual detection threshold (to a single electric pulse) did not find an increase in perceived heat sensitivity in the area of SHA. Three out of four studies that applied HFS at an intensity corresponding to 20 times the individual detection threshold found an increase in perceived heat sensitivity after HFS. Also, unpublished data reveals a change in heat sensitivity after HFS when HFS was delivered with an intensity of 20 times the detection threshold (Figure 2a). Two out of these three studies, and the unpublished data, found a higher perceived heat sensitivity at the conditioned arm compared to the control arm, but no increase compared to baseline measurement at the conditioned arm. In these studies, a decrease in heat sensitivity after HFS at the control arm was observed and attributed to habituation. Therefore, the higher heat sensitivity observed in the conditioned arm probably reflected a lack of habituation. When heat sensitivity was assessed within the same body part by comparing ratings before versus after the delivery of HFS only one study out of five showed an increase. One study (van den Broeke, Lenoir, & Mouraux, 2016), using long‐lasting heat stimuli (30 s), did not find any significant change in heat sensitivity in the area of SHA. Nevertheless, in this study using HFS, the perceived heat intensity within the first 5 s after stimulus onset was increased in the conditioned arm as compared to the control arm. None of the studies that applied transcutaneous HFS or LFS assessed heat pain thresholds in the area of SHA. Taken together, the most consistent result seems a lack of habituation in heat perception to brief radiant heat stimuli at the experimental arm after high‐intensity HFS when comparisons in heat sensitivity were performed between the sensitized arm and a control site. Notably, all these positive studies were conducted by the same group.
TABLE 1.
Summary of studies investigating heat sensitivity in the area of secondary mechanical hyperalgesia.
| Reference | Conditioning model | Conditioning intensity | Site | Sample size | Apparatus and stimulating surface | Pain threshold decrease | Ramping rate, baseline temperature | Perceived intensity increase | Intensity and duration of the test stimulus | Comparisons factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Lenoir et al. (2018) | HFS | 20× DT | Forearm | 18 | CO2 laser, 0.07 mm2 | n.a. | n.a. | YES | 10.3 ± 3.2 mJ/mm2 | Between arms, decrease at control arm 20 and 45 min after vs. before, no change in HFS arm |
| van den Broeke, Lenoir, and Mouraux (2016) | HFS | 20× DT | Forearm | 20 | CO2 laser, 0.28 cm2 | n.a. | n.a. | NO | 47.6°C (45–49°C), 30 s | Control vs. HFS arms, before vs. 20 min after |
| van den Broeke and Mouraux (2014a) | HFS | 20× DT | Forearm | 17 | CO2 laser, 0.11 cm2 | n.a. | n.a. | YES | 11.4 ± 2.8 mJ/mm2 for 50 ms | 20 min (T1) and 45 min (T2) after vs. before (T0), same site |
| van den Broeke and Mouraux (2014b) | HFS | 20× DT | Forearm | 15 | CO2 laser, 0.28 cm2 | n.a. | n.a. | YES | 43 ± 1°C | Between arms, increase at HFS arm and decrease at control arm at 20 and 45 min after vs. before |
| Vo and Drummond (2016) | HFS | 10× DT | Forearm | 22 | Metal probe, 1.77 cm2 | n.a. | n.a. | NO | 44 ± 0.2°C for 7 s | Between arms; after vs. before at HFS arm |
| Vo and Drummond (2014) | LFS | 10× DT | Forearm | 30 | Metal probe, 1.77 cm2 | n.a. | n.a. | NO | 44 ± 0.2°C for 7 s | After vs. before, same site |
| Geber et al. (2007) | Intradermal LFS | 30 mA | Forearm | 10 | Thermode, 7.84 cm2 | NO | 1°C/s, 32°C | n.a. | n.a. | After vs. before, same site |
| Scheuren et al. (2023) | Repetitive heat pain | 60 stimuli at 48°C, 6 s | Forearm | 20 |
Thermode, 9 cm2 Thermode, 1.2 cm2 |
NO |
10°C/s, 32°C 200°C/s, 35°C |
NO | 60°C, 150 to 600 ms | After vs. before, experimental vs. control condition |
| Jürgens et al. (2014) | Repetitive heat pain | 60 stimuli at 48°C, 6 s | Forearm | 18 | Thermode, 2.56 cm2 | NO | 1°C/s, 32°C | n.a. | 48°C, 2 s | Ipsilateral vs. contralateral sites, after vs. before experimental condition |
| Lotsch and Ultsch (2018) | UVB | 2× MED | Forearm | 28 | Thermode, 9 cm2 | YES | 1°C/s, 32°C | n.a. | n.a. | After vs. before, same site |
| Gustorff et al. (2013) | UVB | 3× MED | Upper leg | 22 | Thermode, 2.56 cm2 | NO | 1°C/s, 32°C | n.a. | n.a. |
UVB vs. control site (contralateral) |
| Gustorff et al. (2004) | UVB | 3× MED | Upper leg | 8 | Thermode, 3.24 cm2 | NO | 0.8°C/s, 32°C | n.a. | max 54°C | UVB vs. control site (contralateral) |
| Slimani et al. (2018) | Burn | 47°C, 7 min | Anterior‐medial calf | 20 | CO2 laser, 0.3 cm2 | n.a. | 35°C pre‐heated | NO | 100 ms, 38–53°C | Burn vs. control group, 1 h and 24 h after burn |
| Pedersen and Kehlet (1998) | Burn | 47°C, 7 min | Anterior lower leg | 15 | Thermode, 3.75 cm2 | n.a. | n.a. | YES | Ramping from 40 to 45°C (5 s) followed by 45°C (5 s) | Burn vs. control site, 1 and 2 h after vs. baseline |
| Møiniche et al. (1993) | Burn | 49°C, 5 min | Calf | 8 | Thermode, 3.75 cm2 | YES | 1°C/s, 32°C | n.a. | n.a. | After (3, 6, 24 h) vs. baseline, same site |
| Raja et al. (1984) | Burn | 53°C, 30 s | Hand | 8 | CO2 laser, 0.56 cm2 | n.a. | n.a. | NO | 41–49°C for 3 s | After vs. before, same site |
| Thalhammer and LaMotte (1982) | Burn | 56°C, 7 s | Forearm | 4 | Copper cylinder 0.8 cm2 | YES | n.a. | YES | 51°C, 5 s, 0.03 cm2, ISI 30 s | Before vs. after, same site, 4 individuals |
| Yucel et al. (2004) | Burn | 47°C, 7 min | Forearm | 12 | Xenon lamp, 0.47 cm2 | YES | n.a | n.a. | 0.8, 1, 1.2× PainTh, 500 ms | Burn vs. contralateral control site |
| Kilo et al. (1994) | Freeze lesion | −28°C, 20–40 s | Upper leg, forearm, hand dorsum | 24 | Thermode, 4 cm2 | YES | 0.5°C/s, 30°C | n.a. | n.a. | After vs. before, same sites |
| Geber et al. (2007) | Capsaicin (intradermal) | 50 μg | Forearm | 10 | Thermode, 7.84 cm2 | NO | 1°C/s, 32°C | n.a. | n.a. | After vs. before, same site |
| Sumikura et al. (2005) | Capsaicin (intradermal) | 100 μg | Forearm | 6 | Xenon lamp, 0.25 cm2 | n.a. | n.a. | YES | 0.8, 1, 1.2× PainTh, 350, 500, 750 ms | After vs. before, same site |
| Sumikura et al. (2003) | Capsaicin (intradermal) | 250 μg | Forearm | 8 | Thermode, 3.14 cm2 | n.a. | n.a. | YES | 1.2× PainTh, 500 ms | After vs. before, same site |
| Serra et al. (1998) | Capsaicin (intradermal) | 100 μg, 1% | Forearm | 27 | Thermode, 1 cm2 | n.a. | n.a. | YES | 47°C for 5 s | After vs. before, same site |
| Ali et al. (1996) | Capsaicin (intradermal) | 50 μg, 1% | Forearm | 16 | CO2 laser, 0.4 cm2 | NO | 1°C/s, 38°C | NO | 48°C for 2 s | After vs. before, same site |
| LaMotte et al. (1991) | Capsaicin (intradermal) | 100 μg, 1% | Forearm | 40 | Thermode, 0.78 cm2 | n.a. | n.a. | YES | 38°C for 5 s | After vs. before, same site |
| Simone and Ochoa (1991) | Capsaicin (intradermal) | 100 μg, 1% | Forearm | 10 | Thermode, 0.36 mm2 | NO | 9°C/s, 28°C | n.a. | Capsaicin vs. contralateral control site | |
| Yucel et al. (2004) | Capsaicin (intradermal) | 50 μg, 1% | Forearm | 12 | Xenon lamp, 0.47 cm2 | YES | n.a | n.a. | 0.8, 1, 1.2× PainTh, 500 ms | Capsaicin vs. contralateral control site |
| De Schoenmacker et al. (2021) | Capsaicin (topical) | 1 mL, 0.075% | Forearm | 21 |
YAP laser, 0.2 cm2 thermode, 5.7 cm2 |
n.a. n.a. |
n.a. 70°C/s, 35°C |
NO NO |
2.85 J, 5 mm, 5 ms 52°C |
Before vs. after, sham vs. capsaicin condition, same site |
| Hughes et al. (2020) | Capsaicin (topical) | 1% | Lateral calf | 14 | Thermode 2.56 or 9 cm2 | NO | 32°C, 1°C/s | NO | After vs. before, same site | |
| Lotsch and Ultsch (2018) | Capsaicin (topical) | Cream 150 mg, 0.2% | Forearm | 78 | Thermode, 9 cm2 | YES | 1°C/s, 32°C | n.a. | n.a. | After vs. before, same site |
| Hullemann et al. (2015) | Capsaicin (topical) | 1 mL, 0.6% | Hand dorsum | 15 |
Thermode, 9 cm2 YAP laser, 0.2 cm2 |
YES n.a. |
1°C/s, 32°C n.a. |
n.a. YES |
n.a. 191.9 ± 30.2 mJ/mm2, 5 ms |
After vs. before, same site |
| Drummond and Blockey (2009) | Capsaicin (topical) | 0.02 M 0.6% | Hand | 27 | Radiant heat lamp | NO | 0.5°C/s, 30°C | n.a. | 30 to 48°C, 0.5°C/s | After vs. before, same site |
| Valeriani et al. (2005) | Capsaicin (topical) | 1 mL, 3% a | Upper lip | 10 | CO2 laser, 0.25 cm2 | n.a. | n.a. | NO | 4.5 W, 30 ms | After vs. before, same site |
| Valeriani et al. (2003) | Capsaicin (topical) | 1 mL, 3% a | Hand dorsum | 10 | CO2 laser, 0.03 cm2 | n.a. | n.a. | NO | 11.1 ± 2.1 (right), 10.8 ± 1.9 (left) W, 10 ms | After vs. before, same site |
| Yucel et al. (2002) | Capsaicin (topical) | 1.5 g, 1% | Forearm | 13 | Thermode, 4 cm2 | n.a. | n.a. | YES |
45, 47, 49°C for 1.5 s (for 1, 5, 8°C/s ramps) |
After vs. before, same site |
| Arendt‐Nielsen et al. (1996) | Capsaicin (topical) | 1% b | Lower leg | 10 |
Thermode, 3 cm2 argon laser, 0.07 cm2 |
NO YES |
1°C/s, 30°C single pulses, 200 msec |
n.a. YES |
n.a. 0.8, 1, 1.2, 1.4× PainTh, 200 ms |
After vs. before, same site |
| Andersen et al. (1996) | Capsaicin (topical) | 1%, 6.25 cm2 | Foot dorsum | 17 | Thermode, 3.84 cm2 | NO | 1°C/s, 35°C | n.a. | n.a. | After vs. before, same site |
Note: Table summarizing the results of studies investigating heat sensitivity in the area of secondary hyperalgesia. Secondary hyperalgesia was induced by means of different conditioning models: HFS (high‐frequency stimulation of the skin), LFS (low‐frequency stimulation of the skin), UVB (ultraviolet‐B irradiation), burn, intradermal injection or topical application of capsaicin. To assess if heat sensitivity was enhanced in the area of secondary hyperalgesia, we report here if the heat threshold was decreased and/or if the perceived intensity for suprathreshold heat stimuli was enhanced by writing YES or NO in the corresponding columns. In the last column on the right part of the table, we detailed what were the compared conditions used to respond to this question, e.g., sensitized versus control body sites or before versus after sensitization on the same body site. Results are reported when significant effects or differences have been reported in the corresponding studies.
Abbreviations: DT, detection threshold; MED, minimal erythema dose of ultraviolet‐B; PainTh, pain threshold.
The percentage refers to the concentration of the capsicum oleoresin which corresponds to 0.3% capsaicin concentration.
The report does not mention the nature of the capsaicin solution or cream applied for 1 h.
FIGURE 2.
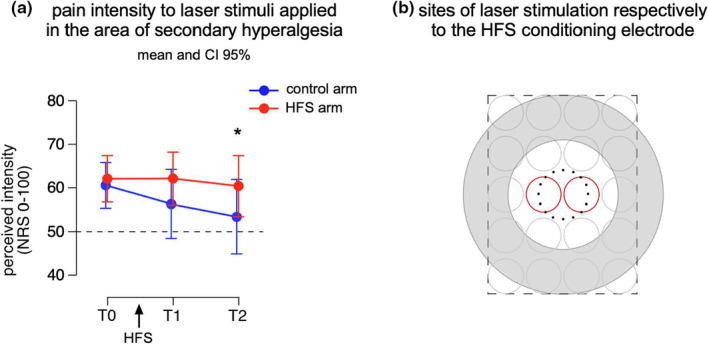
(a) Pain intensity to laser (Nd‐YAP) pulses delivered in the area of secondary hyperalgesia before (T0), 20 and 45 min after high‐frequency stimulation (HFS, 20× detection threshold) of the skin was delivered. Participants used a numerical rating scale (NRS) ranging from 0 (no perception) to 100 (the maximal imaginable pain) with 50 representing the border between non‐painful and painful sensations. In red: on the volar forearm onto which HFS was applied and in blue: on the contralateral arm. Sixteen participants (10 women; aged 20–32 years; 24.5 ± 3.8 years [mean ± SD]) were asked to rate 20 stimuli whose intensity (4.0 ± 0.5 J, [mean ± SD]) was individually adjusted to be qualified as clearly painful (NRS > 50/100). (b) Predefined sites for laser stimulation according to the location where the HFS electrode was positioned. The same template was drawn on both volar forearms. Laser pulses were in a random order on the 20 possible circles with a random inter‐stimulus‐interval (3–5 s). The laser pulses delivered at the location indicated by the two red circles were withdrawn from analysis. Mean and 95% confidence intervals are displayed. The asterisk indicates a significant difference in the perceived intensity between the control and HFS arm at T2. (Unpublished data Lenoir et al., 2019).
Regarding the studies that used capsaicin, eight used intradermal capsaicin injection and 10 applied capsaicin topically. Among the studies that applied capsaicin intradermally four studies investigated heat pain thresholds: three did not observe any significant change and one observed a decrease. Five studies assessed if there was a change in heat perception and four of those found an increase. Of the 10 studies that applied capsaicin topically six assessed changes in pain thresholds including one in which both contact and radiant heat thresholds were measured. Three experiments found a decrease in pain threshold and four experiments did not find any change. Change in heat sensitivity was assessed in eight different experimental settings among seven out of 10 studies using topical capsaicin. Among them only three studies found an increase. Taken together, changes in perceived heat sensitivity seem most prominent after intradermal capsaicin injection and suprathreshold contact heat stimuli.
The results regarding repetitive heat pain, burn injury or UVB conditioning methods are more inconsistent. Five out of eight studies that used heat pain or burn assessed changes in pain thresholds. Three of these found a decrease in threshold and two found no change. Five of the studies that used heat pain or burn assessed changes in perceived heat sensitivity. Two found an increase and three did not. Three studies that used UVB assessed pain thresholds. Two studies did not observe any change while one did. None of the UVB studies assessed changes in perceived heat sensitivity.
In summary, the perceived intensity elicited by heat stimuli is not consistently changed in the area of SHA. When present, it involves mainly an increase in perceived intensity to suprathreshold stimuli rather than a change in heat pain threshold. Changes in heat sensitivity are mostly reported following intradermal capsaicin injection or transcutaneous HFS. Changes in heat sensitivity are noted particularly for brief radiant heat stimuli (delivered by infra‐red laser stimulators) after HFS and for brief contact heat stimuli (delivered by the thermode) after intradermal capsaicin injection. This pattern is likely biased by the frequent use of laser stimuli in HFS studies and contact heat in capsaicin studies. Overall, the changes in heat sensitivity in the area of SHA involve a lack of habituation at the sensitized site as compared to the control site rather than an increase compared to the baseline.
3.2. Tactile mechanical sensitivity in the area of secondary hyperalgesia
SHA clearly manifests itself by the change in perception of sharp punctate mechanical stimuli, such as pinprick stimuli. However, mechanical indenting stimuli (with diameter in the 0.5 mm range and of intensity dozens of mN) which activate high‐threshold mechano‐nociceptors (HTMs) unavoidably activate also low‐threshold mechanoreceptors (LTMs) and notably slowly and rapidly adapting type I mechanoreceptors (Cohen & Vierck, 1993; Johansson & Vallbo, 1979; Vallbo & Johansson, 1984). This raises the question if there is a contribution of LTMs to the increase in mechanical sensitivity elicited by static indenting stimuli. Table 2 summarizes studies that have assessed the involvement of tactile mechanical sensitivity in the area of SHA. Figure 3 shows the results of an unpublished study in which tactile stimuli and pinprick stimuli of the same intensity were applied to the area of SHA induced by HFS. In that study, no increase in the perceived intensity elicited by the tactile stimuli were found. In contrast, the perception of mechanical pinprick stimuli was clearly increased. These results, thus, suggest that LTMs, activated by tactile stimuli of different intensities do not contribute significantly to SHA. In the same line, Vollert et al. (2018) found no evidence for changes in both mechanical detection thresholds using rounded tip punctate stimuli or vibrations in the area of SHA. One study showing a change in the perception of tactile stimuli specifically using modified von Frey filaments with round tips reported those changes for stimuli delivered at intensities higher than 32 mN which might increase the probability of recruiting HTMs concomitantly to LTMs. Indeed, an increase in sensations qualified as pricking and painful can be observed for tactile stimuli delivered at high intensities after HFS (Figure 4a,b). Another study reported a decrease in the threshold for vibration using topical capsaicin in two different concentrations (0.005% and 0.01%) (Andrews et al., 1999). This study included only six participants and explored vibration using a vibration stimulator applied with a concomitant pressure (650 g) in addition to the vibrating stimulus, therefore likely affecting deep tissues and not comparable with other studies.
TABLE 2.
Summary of studies investigating tactile sensitivity in the area of secondary mechanical hyperalgesia.
| Reference first author, year | Conditioning model | Conditioning intensity | Site | Sample size | Apparatus and stimulating surface | Threshold decrease | Intensity, surface of stimulus | Perceived intensity increase | Intensity, duration, surface of stimulus | Comparisons factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Vollert et al. (2018) | Capsaicin (intradermal) | 100 μg, 10 μL | Forearm | 36 | Modified von Frey filaments (round tips) tuning fork |
NO NO b |
0.25 to 512 mN, 0.5 mm d 64 Hz, 8/8 scale d |
n.a. n.a. |
n.a. n.a. |
Capsaicin vs. control site (contralateral) |
| van den Broeke et al. (2014) | HFS | 20× DT | Forearm | 17 | Mechanical transducer | n.a. | n.a. | NO | 300 Hz,50 ms, 4 cm2 | 20 min (T1) and 45 min (T2) after vs. before (T0), between HFS and control arms |
| Gustorff et al. (2013) | UVB | 3× MED | Upper leg | 22 |
Modified von Frey filaments (round tips) tuning fork |
NO NO |
0.25 to 512 mN, 0.5 mm d 64 Hz, 8/8 scale d |
n.a. n.a. |
n.a. n.a. |
UVB vs. control site (contralateral) |
| De Col and Maihöfner (2008) | Trancutaneous electrical stimulation a | Pain NRS 5/10 | Forearm | 50 |
Modified von Frey filaments (round tips) |
NO b | 0.25 to 512 mN, 0.5 mm d | n.a. | n.a. | After vs. before |
| Stammler et al. (2008) | Trancutaneous electrical stimulation a | Pain NRS 5/10 | Forearm | 12 | Modified von Frey filaments (round tips) | NO b | 0.25 to 512 mN, 0.5 mm d | n.a. | n.a. | After vs. before |
| Magerl et al. (1998) | Capsaicin (intradermal) | 40 μg, 12.5 μL | Forearm | 12 | Modified von Frey filaments (round tips) | YES c | 0.5 to 4100 mN, 1.1 mm | YES | 256 mN, 1.1 mm | After vs. before, capsaicin vs. control site |
| Andrews et al. (1999) | Capsaicin (topical) | 0.05 mg/mL 0.005% | Forearm | 6 | Vibration | YES | 1 cm 100 Hz | n.a. | n.a. | Capsaicine vs. control |
| Andrews et al. (1999) | Capsaicin (topical) | 0.1 mg/mL 0.01% | Forearm | 6 | Vibration | YES | 1 cm 100 Hz | n.a. | n.a. | Capsaicine vs. control |
Note: Table summarizing the results of studies investigating static tactile sensitivity in the area of secondary hyperalgesia. Secondary hyperalgesia was induced by means of different conditioning models: HFS (High‐frequency stimulation of the skin), UVB (ultraviolet‐B irradiation), transcutaneous electrical stimulation (35 min at different frequencies and stimulation patterns), intradermal injection or topical application of capsaicin. Similar conventions as in table heat (to assess if static tactile sensitivity was enhanced in the area of secondary hyperalgesia, we report here if the threshold was decreased and/or if perceived intensity for suprathreshold heat stimuli was enhanced by writing YES or NO in the corresponding columns. In the last column on the right part of the table, we detailed what were the compared conditions used to respond to this question, for example, between sensitized and control body sites or before versus after sensitization on the same body site). Results are reported when significant effects or differences have been reported in the corresponding studies.
Abbreviations: DT, detection threshold; MED, minimal erythema dose of ultraviolet‐B.
See references for conditioning stimulation parameters.
A significant increase in the tactile detection threshold was observed.
No change in perceived intensity up to 32 mN, and no change in pain incidence up to 8 mN.
DFNS standardized protocol (see Rolke et al., 2006).
FIGURE 3.
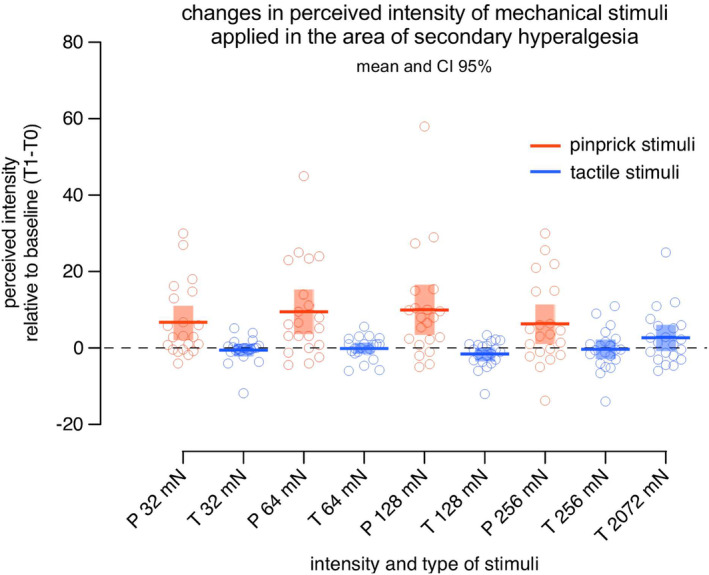
Increased perceived intensity for different pinprick mechanical stimuli and not for tactile stimuli delivered in the area of secondary hyperalgesia induced by HFS. The change in perceived intensity was computed by subtracting the ratings obtained before HFS (baseline T0) from the ratings obtained 20 min after HFS (T1). Participants used a numerical rating scale (NRS) ranging from 0 (no perception) to 100 (the maximal imaginable sensation) and additionally provided descriptors for the quality of the sensation and if the sensation was painful or not. Pinprick stimuli with a blunt tip (P; in red; diameter 0.35 mm) and tactile stimuli with a round tip (T; in blue; diameter 2 mm) of four intensities (32, 64, 128 and 256 mN) were delivered in a counterbalanced manner onto the skin surrounding the site where the HFS electrode was positioned. In addition, a tactile stimulator with a round tip of 2072 mN which matched the pressure elicited by the pinprick 64 mN was also used. Mean and 95% confidence intervals are displayed. Across all intensities, the perception of pinprick stimuli (in red) was enhanced after HFS which was not the case with the tactile stimuli (in blue) of the same intensities or stimuli exerting the same pressure when applied onto the skin. (21 participants; 11 women; aged 18–29 years; 21.1 ± 2.7 years [mean ± SD]). (Unpublished data Lenoir et al., 2021).
FIGURE 4.
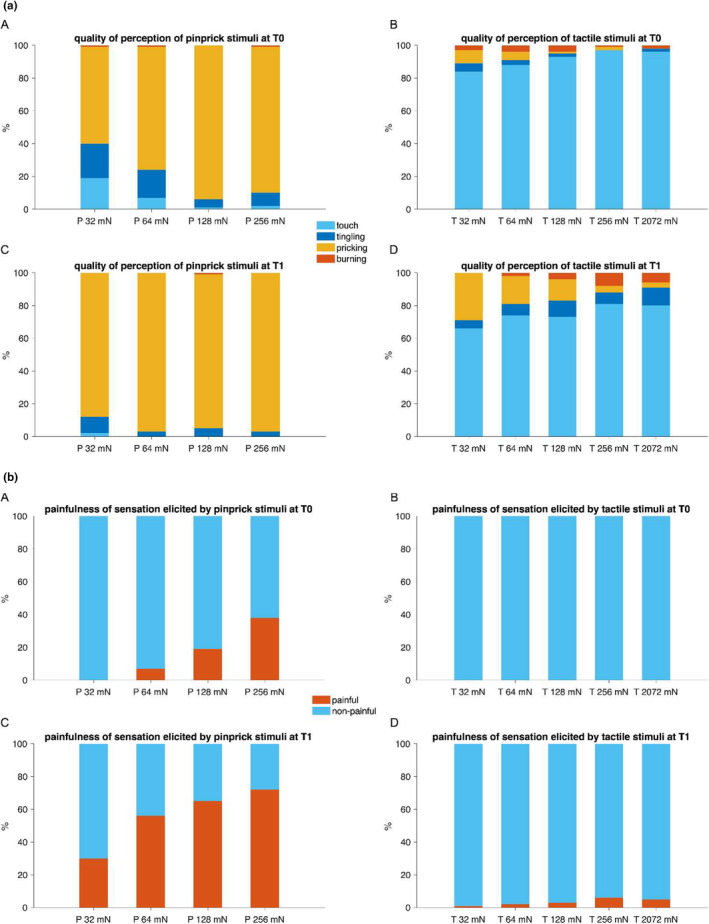
(a) Proportion of each descriptor used to describe the quality of perception of the pinprick and tactile stimuli delivered in the area of secondary hyperalgesia before (T0) and after (T1) HFS. (a, c) Pinprick stimuli (P; stimulator with blunt tip; diameter 0.35 mm) and (b, d) tactile stimuli (T; stimulator with round tip; diameter 2 mm) of four intensities (32, 64, 128 and 256 mN) were delivered in a counter‐balanced manner onto the skin surrounding the site where the HFS electrode was positioned. In addition, a tactile stimulator of 2072 mN which matched the pressure elicited by the pinprick 64 mN was also used. Descriptors considered to qualify are displayed in light blue for ‘touch’, dark blue for ‘tingling’, in orange for ‘pricking’ and red for ‘burning’. When applied to sensitized skin the quality of pinprick stimuli and to a lesser extent of tactile stimuli was changed with pinprick stimuli more often perceived as ‘pricking’ and tactile ones more often perceived as ‘pricking’ and ‘burning’. (21 participants; 11 women; aged 18–29 years; 21.1 ± 2.7 years [mean ± SD]). (Unpublished data Lenoir, van den Broeke et al., 2021). (b) Proportion of stimuli perceived as ‘painful’ and ‘non‐painful’ before (T0) and after (T1) HFS. (a, c) Pinprick stimuli (P; stimulator with blunt tip; diameter 0.35 mm) and (b, d) tactile stimuli (T; stimulator with round tip; diameter 2 mm) of four intensities (32, 64, 128 and 256 mN) were delivered in a counter‐balanced manner onto the skin surrounding the site where the HFS electrode was positioned. In addition, a tactile stimulator of 2072 mN which matched the pressure elicited by the pinprick 64 mN was also used. Proportions of stimuli perceived as ‘painful’ and ‘non‐painful’ are displayed respectively in blue and red. When applied to sensitized skin pinprick stimuli of different intensities are in 30%–70% of the cases perceived as ‘painful’ which is the case for only 6% maximum of the cases for tactile stimuli. The increase of pinprick stimuli perceived as painful after HFS is clearly visible which is not the case for tactile stimuli (21 participants; 11 women; aged 18–29 years; 21.1 ± 2.7 years [mean ± SD]). (Unpublished data Lenoir et al., 2021).
3.3. Can we detect secondary hyperalgesia using electrical stimuli?
Unlike thermal and mechanical, electrical stimuli bypass peripheral receptor transduction and activate directly voltage‐gated ion channels. It should be stressed that the characteristics of the electrical test stimuli, such as the electrode type, the stimulation intensity and the pattern of stimulation are crucial to establish the relative selectivity of the electrical stimulus. In other words, those parameters will determine how preferentially the stimulus will recruit nociceptive versus non‐nociceptive nerve fibre (La Cesa et al., 2018; Legrain & Mouraux, 2013; Mouraux et al., 2010, 2014; Poulsen et al., 2022).
Transcutaneous electrical stimulation through surface electrodes elicits an electric current that propagates deep in the dermis. Depending on the intensity, this stimulation can activate preferentially large diameter non‐nociceptive fibre in association with thinly myelinated nociceptive fibres. Such stimulation has been used in three studies. In the first study (van den Broeke et al., 2010) the authors did not find any significant increase in the perceived intensity elicited by transcutaneous electrical stimuli in the area of SHA induced by HFS as compared to baseline, for electrical stimulation delivered at an intensity below the pain threshold (50% of pain threshold intensity). However, the authors reported a significant difference in the perceived intensity after HFS between the HFS arm and the control arm. The authors concluded that sensations mediated by A‐delta fibres might be affected (less habituation) in sensitized skin. Yucel et al. (2004) used transcutaneous electrical stimulation delivered with surface electrodes to assess changes in pain thresholds after SHA was induced using both intradermal capsaicin and a heat injury model. They found a decreased electrical pain threshold in both experimental methods using a pulse train of five pulses of 1 ms delivered at 200 Hz which was perceived as a single stimulus and was intended to mainly recruit small, myelinated fibres. More recently, Hughes et al. (2020) used transcutaneous electrical stimuli delivered with surface electrodes to assess changes in pain thresholds after SHA was induced using topical capsaicin application. They found lowered pain thresholds for electrical stimuli delivered in the area of SHA. Interestingly, the authors observed that the magnitude of hyperalgesia to electrical stimuli was far from being uniformly distributed within the area of SHA. In this study, it is likely that the electrical stimulation delivered at a high intensity (approximately 18 mA) and by means of surface electrodes recruited small and large diameter fibres, it is therefore difficult to conclude about the type of afference responsible for the observed perceptual increase to electrical stimuli delivered in the area of SHA.
A multipin electrode designed to preferentially activate cutaneous free nerve endings and used for conditioning stimulation in HFS protocols (Klein et al., 2004) has been used recently to investigate pain perception elicited by painful single electrical stimuli (intensity approximately 1.7 mA) in the area of SHA induced by HFS (van den Broeke et al., 2021). The authors reported an overall significant decrease in pain perception to electrical stimuli after HFS at both the control and HFS sites. Although pain ratings were globally significantly decreased after HFS, the participants reported significantly higher pain ratings for stimuli delivered to the area of SHA as compared to the control site. This study showed again that perception of electrical stimuli was affected in sensitized versus control sites, similarly as in van den Broeke et al. (2010).
Two studies used low‐intensity intra‐epidermal electrical stimulation (IES, Inui et al., 2002) to investigate the contribution of A‐delta fibre nociceptors to SHA (Biurrun Manresa et al., 2018; Liang et al., 2016). Liang et al. (2016) showed that the intensity of perception elicited by IES (intensity at twice the detection threshold ~0.5 mA (personal communication)) was significantly increased in participants who developed ‘robust SHA’ (referred to as ‘responders’) after intradermal capsaicin injection. However, using HFS to induce SHA, Biurrun Manresa et al. (2018) did not find evidence for an increase in the perceived intensity of these stimuli (intensity at twice the detection threshold ~0.14 mA) in the area of SHA. These apparently contradictory results might be due to the different methods used to induce SHA, that is, intradermal capsaicin injection versus HFS, and/or to the difference in IES intensity, which was considerably higher in the study of Liang et al. (2016). Regarding this latter, Legrain and Mouraux (2013) have shown that with higher stimulation intensities, intra‐epidermal electrical stimulation loses its selectivity for activating nociceptive afferents.
4. ELECTROPHYSIOLOGICAL MEASURES
4.1. Spinal cord activity assessed by the nociceptive flexion reflex
The nociceptive flexion reflex (NFR) is a polysynaptic and multi‐segmental spinal reflex that induces a complex flexion synergy of the stimulated limb, whose anatomical substrate is entirely located at the spinal level (Sandrini et al., 2005). It can be elicited by transcutaneous electrical stimulation of either the upper or lower limb, however, it has been mainly investigated in the lower limb. The withdrawal reflex consists of two separate components, namely the RII component generated by the activation of large‐myelinated fibres (Hugon, 1973) and the RIII component elicited at higher intensities due to the additional recruitment of thinly myelinated fibres (Ertekin et al., 1975). Due to the close correspondence existing between the RIII reflex threshold and the subjective pain threshold (Willer et al., 1984), this component has been considered by some researchers as an ‘objective’ measure of experimental pain in humans (Sandrini et al., 2005). The recording of the RIII is performed using an electromyogram (EMG) recording device. Several protocols have been proposed to induce the reflex, the more common setup being the stimulation of the peripheral nerve with a train of five 1‐ms electrical square pulses delivered at a frequency of 200 Hz. The reflex response is obtained from the recording of EMG activity of the flexor muscle using surface electrodes. The reflex assessment foresees first the estimation of the threshold defined as the intensity evoking a stable response at a rate of 50–60% after 20–30 stimuli, using a staircase method (Andersen et al., 2005; Sandrini et al., 1993). The reflex recording is then performed using a stimulation intensity multiple of that of the threshold. Several parameters of the RIII can be assessed, including threshold, amplitude, latency, receptive field, temporal summation, response rate and habituation (Andersen et al., 1995; Arendt‐Nielsen et al., 1994; Biurrun Manresa et al., 2014; Dimitrijević et al., 1972; Willer, 1977). Several studies explored the capability of the RIII to detect spinal excitability changes induced by different experimental methods to induce SHA, which are summarized in Table 3. Nearly all the studies explored the lower limb RIII reflex after stimulation of either the tibial or the peroneal nerve, except the study by Linde et al. (2021), where the authors explored the upper limb after stimulation of the index finger.
TABLE 3.
Summary of studies investigating NFR modulation by secondary hyperalgesia models.
| Reference | Conditioning stimulus | Ongoing pain | SHA testing | Site | Sample size | Intensity and duration of test stimulus | Recording site | Pain rating | Reflex thr↓ | Reflex AUC ↑ | Comparison factors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grönroos and Pertovaara (1993) | Capsaicine 1% (topical) | YES | YES a | Foot dorsum | 7 | Sural: train of 4 pulses (1 ms duration) delivered over 40 ms | Ipsilateral biceps femoris | n.a | YES | n.a |
Before vs. 45 min after H reflex |
| Andersen et al. (1995) | Capsaicine 0.1% (topical) | NO | NO | Foot dorsum | 8 | Sural: train of 5 pulses (1 ms duration), 200 Hz | Ipsilateral biceps femoris/rectus femoris | NO | n.a | NO | Before vs. 60 min after H reflex |
| Capsaicine 0.1% (topical) + Aβ activity in SHA area (40 Hz electrical stimulation/rotating V.Freys) | NO | NO | Foot dorsum | 8 | Sural: train of 5 pulses (1 ms duration), 200 Hz | Ipsilateral biceps femoris/rectus femoris | NO | n.a | NO | Before vs. 60 min after H reflex | |
| Capsaicine 0.1% (topical) + continuous heat (38°C) in PHA | YES | NO | Foo t dorsum | 8 | Sural: train of 5 pulses (1 ms duration), 200 Hz |
Ipsilateral biceps femoris /rectus femoris |
YES | n.a | YES | Before vs. 60 min after H reflex | |
| Capsaicine 0.1% (topical) + heat (38°C, 30 s) +40 Hz electrical stimulation (in SHA area) | YES | NO | Foot dorsum | 8 | Sural: train of 5 pulses (1 ms duration), 200 Hz | Ipsilateral biceps femoris/rectus femoris | YES | n.a | YES | Before vs. 60 min after H reflex | |
| Andersen et al. (1996) | Capsaicine 1% (topical) | n.a. | YES | Foot dorsum | 17 | Sural: train of 5 pulses (1 ms duration), 200 Hz | Ipsilateral biceps femoris/rectus femoris | NO | n.a | NO |
Before vs. 60 min after Placebo vs. ketamine |
| Capsaicine 1% (topical) + heat (32–40°C) | YES | YES | Foot dorsum | 17 | Sural: train of 5 pulses (1 ms duration), 200 Hz | Ipsilateral biceps femoris/rectus femoris | YES | n.a | YES |
Before vs. 60 min after placebo vs. ketamine |
|
| Capsaicine 1% (topical) + heat (32–40°C) + 40 Hz electrical stimulation (in SHA area) | YES | YES | Foot dorsum | 17 | Sural: train of 5 pulses (1 ms duration), 200 Hz | Ipsilateral biceps femoris/rectus femoris | NO | n.a | YES | Before vs. 60 min after Placebo vs. ketamine | |
| Biurrun Manresa et al. (2014) | Capsaicine 0.1% (intradermal) | YES | NO | Flexor digitorum brevis muscle | 14 | Foot sole: train of 5 pulses (1 ms duration), 200 Hz | Tibialis anterior | n.a | YES | n.a | Before vs. 60 min after |
| Leone et al. (2021) | Capsaicine 0.1% (topical) | NO | YES | Foot dorsum | 12 | Sural: train of 5 pulses (1 ms duration), 200 Hz | Ipsilateral biceps femoris | YES | YES | NO |
Before vs. after Active vs. control |
| Linde et al. (2021) | Capsaicine 0.075% (topical) | YES | YES a | Lateral forearm | 20 | Palmar index finger: train of 5 pulses (1 ms duration), 200 Hz | Flexor carpi ulnaris | n.a | YES | n.a |
Before vs. after Active vs. control |
| Ellrich and Treede (1998) | Tonic Heat (from 32 to 46°C 90 s× 8 temperatures) | YES | NO | Lateral lower leg | 11 | Medial Plantar: train of 5 pulses (0.1 ms duration), 200 Hz | Ipsilateral tibialis anterior | n.a | n.a | YES | Before vs. after |
| Guekos et al. (2023) | Tonic Heat (from 32 to 46°C 90 s× 8 temperatures) | YES | NO | Lateral lower leg | 16 | Sural: train of 5 pulses (1 ms duration), 200 Hz | Ipsilateral biceps femoris | NO | n.a | NO | Before vs. after |
| Leone et al. (2021) | HFS (5 trains, 100 Hz, 20× dT) | NO | YES | Foot dorsum | 14 | Sural: train of 5 pulses (1 ms duration), 200 Hz | Ipsilateral biceps femoris | YES | YES | NO |
Before vs. after Active vs. control |
| Biurrun Manresa et al. (2010) | HFS (5 trains, 100 Hz, 10× dT) | NO | YES | Foot dorsum | 13 |
Medial dorsal cutaneous branch of the superficial peroneal nerve: train of 5 pulses (1 ms duration), 200 Hz |
Ipsilateral biceps femoris | NO | n.a | NO |
Before vs. after (each 10 min, 7 times) Maximum response before vs. after 10 min |
| Biurrun Manresa et al. (2010) | LFS (1 train, 1000 pulses, 10× dT) | NO | YES | Foot dorsum | 13 | Medial dorsal cutaneous branch of the superficial peroneal nerve: train of 5 pulses (1 ms duration), 200 Hz | Ipsilateral biceps femoris | NO | n.a | YES |
Before vs. after (Each 10 min, 7 times) Maximum response before vs. after 10 min |
Note: Results are reported when significant effects or differences have been reported in the corresponding studies.
The authors only tested the presence of allodynia to light tactile stimulus.
4.1.1. Reflex threshold and pain threshold modulation
When tested, the reflex threshold at the sensitized side exhibited a decrease in each of the five experimental settings reported in four studies, regardless of the experimental method used to induce SHA (capsaicin or HFS) and the considered comparisons, that is, before versus after the conditioning paradigm (Biurrun Manresa et al., 2014; Grönroos & Pertovaara, 1993; Leone et al., 2021; Linde et al., 2021) or sensitized versus control side (Leone et al., 2021; Linde et al., 2021). Based on the consistency of the results, the reflex threshold can be considered a reliable proxy of spinal excitability changes that occur in a sensitized state induced by different methods of secondary hyperalgesia. Only one study Leone et al. (2021) reported data on pain thresholds, that is, the lowest stimulation intensities causing a painful sensation, and reflex thresholds together, and as expected (Willer, 1977) found a correlation between the two measures (r = 0.738; p < 0.0001, unpublished results).
4.1.2. Modulation of the reflex area under the curve (AUC)
The EMG activity resulting in the flexor muscle after the nociceptive stimulation can be quantified using the reflex AUC after the signal of a selected interval of interest being rectified. The assessment of reflex AUC changes in humans, using different experimental methods to induce SHA, provided conflicting results. Some authors were able to detect an increase in the AUC only when an ongoing continuous afferent input activating nociceptive primary afferents was provided to the spinal cord concomitantly to the recording of the reflex. Andersen et al. (1995) showed that topical application of capsaicin (0.1%) alone did not positively modulate the reflex AUC. However, when a concomitant nociceptive input was delivered in the area of primary hyperalgesia evoking an ongoing pain sensation, a facilitation of the reflex was observed (Table 3). The authors replicated their results in a subsequent study (Andersen et al., 1996). Ellrich and Treede (1998) described an increased AUC after the induction of SHA by tonic heat stimulation, eliciting an ongoing painful sensation. However, this observation was not confirmed in a recent replication study using tonic heat to induce sensitization (Guekos et al., 2023). It should be stressed that in this latter study the induction of SHA was not tested by the presence of mechanical hyperalgesia in the area surrounding the conditioning site. The authors could not detect any change in pain ratings and concluded that the conditioning method was likely unsuccessful in affecting spinal excitability. Biurrun Manresa et al. (2010) provided evidence of facilitation of the reflex in terms of increased AUC after the induction of SHA by LFS (Biurrun Manresa et al., 2010). Of note, the authors detected an increased AUC after the cessation of the conditioning stimulus and therefore in the absence of any ongoing painful sensation. All the other studies, exploring a modulation of the reflex AUC after the induction of SHA with different methods of sensitization, either topical capsaicin alone or HFS, did not find any modulation of the reflex AUC after inducing sensitization (Table 3). The heterogeneity of the experimental settings and methods used to calculate the area (root mean square vs. integral of the rectified EMG, non‐standardized windows of interest), may account for the discrepancies between studies and prevent drawing conclusions on the ability of this parameter to detect the spinal excitability changes induced by different methods of sensitization. In all but one study (Biurrun Manresa et al., 2010), the reflex AUC is modulated when the reflex is tested during an ongoing pain sensation produced by the sensitization procedure. The authors also observed a modulation of the AUC after the sensitization procedure (LFS) without any ongoing pain sensation. A possible explanation could be that LFS provided a prolonged peripheral nociceptive input likely mimicking peripheral activity present during pain. Two studies reported information on a possible relationship between the increase in perceived intensity elicited by pinprick stimuli in the area of SHA and the changes in the reflex threshold or AUC. Leone et al. (2021) found no correlation between the hyperalgesia score and the decrease in the reflex threshold neither for capsaicin nor for HFS (unpublished data). Biurrun Manresa et al. (2010) tested the time course of both pinprick perception and reflex AUC every 10 min, from 20 min before LFS to 60 min after. Though a correlation analysis has not been performed, the authors showed that the two variables behaved differently.
Several studies provided data on test–retest reliability for the RIII threshold (Micalos et al., 2009; Lewis et al., 2012; Rhudy & France, 2011) describing a between session ICC of 0.72 and 0.82, and a test–retest correlation coefficient of 0.83, respectively. One study reported data on the reliability of the RIII pain rating, describing a between session ICC of 0.88 (Lewis et al., 2012). No data are available so far on the test–retest reliability of the RIII variables modulation during pain methods induction.
4.2. Spinal cord activity assessed by spinal somatosensory evoked potentials
The N13 is a spinal component of the somatosensory evoked potentials mediated by non‐nociceptive Aβ fibres, elicited by electrical stimulation of mixed nerves above the motor threshold (evoking a muscular twitch) and, best recorded at the level of the C6 spinous process referenced to the glottis (Desmedt & Cheron, 1981, 1982).
The origin of this component has long been debated (Cracco, 1973; Jones, 1977; Matthews et al., 1974), due to distortions introduced by the use of a midfrontal (Fz) scalp reference. The subsequent introduction of a non‐cephalic reference, which allowed the recording of the cervical potential without the contamination by scalp potentials (Desmedt, 1984), indicated a stable transversely oriented generator in the dorsal horn of the cervical cord near the spinal entry, compatible with the synaptic activity of collateral fibres of the ascending dorsal column axons as described in cats (Scheibel & Scheibel, 1968). These conclusions came from the evidence that the N13 reversed its polarity when recorded at the anterior aspect of the neck (Desmedt & Cheron, 1981) and remained unaffected in a patient with a spinal lesion above C4 with complete destruction of the dorsal funiculus (Mauguière & Courjon, 1981). In patients with syringomyelia, which pathology is an expanding cavity in the central spinal canal compressing the spinothalamic tract neurons that decussate in the anterior white commissure and preserving the distally located posterior columns, a reduced or absent N13 has been shown, even if it is not mediated by peripheral nociceptive fibres activation. These results suggest that the N13 might reflect the postsynaptic activity of cells receiving, at least in part, spinothalamic tract afferent input (Restuccia & Mauguière, 1991). The properties of the N13 in humans are very similar to those of the ‘intermediary cord potentials’ in animals. Gasser and Graham (1933) first described a series of slow potentials that could be recorded from the surface of the spinal cord in response to stimulation of dorsal roots which are thought to reflect the activation of interneurons in lamina IV and V of the dorsal horn in response to large‐myelinated fibres input (Beall et al., 1977). Thanks to the evidence described above and the similarities with animals' spinal potentials, the N13 has been recently reconsidered and proposed as a possible readout of dorsal horn excitability changes occurring during central sensitization. Di Lionardo et al. (2021) demonstrated that spinal excitability changes triggered by topical capsaicin (0.1%) application and testified by the presence of an area of secondary hyperalgesia, increased the amplitude of the N13 component in 10 healthy participants. Furthermore, with a double‐blind placebo‐controlled crossover design, the authors showed that pregabalin, a drug with proven efficacy at the dorsal horn level (Tuchman et al., 2010), was able to prevent the increase in the N13 amplitude. A subsequent study by the same group (Di Pietro et al., 2021) demonstrated a negative modulation of the N13 component amplitude by a heterotopic noxious conditioning stimulation paradigm. The amplitude of the N13 was tested before, during and after a cold pressor test. The ANOVA on the amplitude of the N13 showed a significant effect of time across the three time points disclosing an N13 amplitude reduction by 25% during the cold pressor test as compared with before. Since the heterotopic noxious conditioning stimulation simultaneously modulated pain processing remotely, as tested using pressure pain threshold, plausibly affecting wide dynamic range (WDR) neuron excitability (Leone & Truini, 2019), the authors concluded that the N13 negative modulation could be explained by a modulation of WDR neurons' activity and, therefore, suggested WDR as a possible generator of the N13.
Recent research tested a modulation of the N13 component by different experimental pain methods inducing SHA, namely low‐ and high‐frequency electrical stimulation (LFS and HFS) (Leone et al., 2023). The authors found an increased amplitude of the N13 after LFS, on both active and control sides but no effect of HFS. The authors interpreted their results as LFS and HFS being able to trigger central sensitization through different mechanisms: thanks to its peculiar stimulation parameters LFS may induce dorsal horn excitability changes through two distinct mechanisms, namely wind‐up and heterotopic facilitation, while HFS may be able to induce heterotopic facilitation only.
Taken together, these albeit limited evidence, suggest WDR neurons as possible generators of the N13 component of somatosensory evoked potential, which in turn could be a useful readout of dorsal horn excitability changes occurring during central sensitization. However, the ability to detect a modulation of the N13 seems to depend on the experimental pain method used to induce CS, possibly requiring a long‐lasting low‐frequency ongoing afferent input to the spinal level.
Test–retest reliability has been assessed in a few studies, after stimulation of the median and tibial nerve, and showed overall good reliability for amplitudes (Spearman correlation coefficient = 0.69, [84]; ICC = 0.94) (Romani et al., 1996). No data are available on the test–retest reliability of the N13 amplitude increase. No correlation has been found between the N13 amplitude increase and the magnitude of change of mechanical pinprick sensitivity after capsaicin application (p = 0.6; Pearson r = −0.14) (Di Lionardo et al., 2021).
4.3. Cortical activity assessed by pinprick‐evoked brain potentials
Calibrated mechanical pinprick stimuli delivered onto the skin elicit pinprick‐evoked brain potentials (PEPs) in the electroencephalogram (EEG). Evoked potentials are voltage polarity fluctuations time‐locked to the onset of a stimulus. It is yet unclear what is the functional significance of PEPs.
PEPs consist of a biphasic wave involving a negative peak that reaches a maximum of around 100–150 ms after stimulus onset and is followed by a positive peak reaching its maximum around 250–300 ms (Iannetti et al., 2013). Both PEPs peaks are maximal at central EEG electrodes.
The latencies of PEPs are compatible with the peripheral conduction velocities of A‐fibres. Mechanical pinprick stimuli inevitably activate both A‐fibre high‐threshold mechanoreceptors (HTMs) and A‐fibre low‐threshold mechanoreceptors (LTMs, Nagi et al., 2019) which makes therefore the interpretation of PEPs ambiguous regarding mediating primary afferent population. When the same mechanical pinprick stimuli are delivered to skin that has developed increased pinprick sensitivity in response to either topical capsaicin application, HFS or heat, they elicit, depending on the study, an increase in the amplitude of either the negative (Iannetti et al., 2013; Scheuren et al., 2020) or positive peak (van den Broeke et al., 2015, 2017; van den Broeke, Lambert, et al., 2016) (Table 4).
TABLE 4.
Summary of studies investigating amplitudes and/or latencies of pinprick evoked potentials (PEPs).
| Reference | Stimulation method | Conditioning model | Body location | Sample size | Pinprick intensity (mN) | N2 amplitude increase | N2 latency (ms) | P2 amplitude increase | P2 latency (ms) | Comparison factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Iannetti et al. (2013) | Manual | Capsaicine intradermal | Hand dorsum | 10 | 128 | YES | 111 ± 8 a | NO | 245 ± 17 | Ipsilateral vs. contralateral sites, after vs. before |
| van den Broeke et al. (2015) | Manual | Capsaicine intradermal |
Ventral forearm |
16 | AVG | NO | YES | Ipsilateral vs. contralateral sites, after vs. before | ||
| van den Broeke, Lambert, et al. (2016) | Manual | HFS 20× DT | Forearm | 16 |
64 90 |
NO NO |
YES NO |
Control vs. HFS arms, before vs. 20, 45 min after | ||
| van den Broeke et al. (2017) | Manual | HFS 20× DT | Forearm | 16 |
64 96 |
NO NO |
YES NO |
Ipsilateral vs. contralateral sites, after vs. before | ||
| van den Broeke et al. (2019) | Robotic | HFS 20× DT | Forearm | 14 |
64 96 |
NO NO |
136 ± 43 b 137 ± 48 |
NO NO |
After vs. before | |
| van den Broeke et al. (2020) | Robotic | HFS 20× DT | Forearm | 16 | 64 | YES | 136 ± 25 | YES | 259 ± 40 | After vs. before |
| Scheuren et al. (2020) | Manual |
Burn 48°, 6 s |
Forearm | 20 | 256 | YES | 66.4 ± 20.8 c | YES | Ipsilateral vs. contralateral sites, after vs. before | |
| Gousset et al. (2023) | Robotic | HFS 20× DT | Forearm | 20 | 512 | YES | 150 ± 30 | YES | 310 ± 60 | After vs. before |
Post‐hoc corrected for trigger delay.
Baseline control arm.
Value obtained from the first 15 stimuli applied at baseline in the control condition.
To make mechanical pinprick stimulation operator‐independent and to reduce across trial variability of the applied force, van den Broeke et al. (2020) developed a robot‐controlled mechanical pinprick stimulator. Indeed, as can be seen in Figure 5, the applied normal and tangential forces when the pinprick stimuli are applied manually show much more variability across trials compared to pinprick stimuli delivered with the robot‐controlled pinprick stimulator. When robot‐controlled mechanical pinprick stimuli were used to elicit PEPs, van den Broeke et al. (2020) found that the PEP negative peak reached its maximum at around 136 ms and the positive amplitude at around 250 . When applying the pinprick stimuli within the area of increase pinprick sensitivity they found an increase in the amplitude of both peaks (Gousset et al., 2023; van den Broeke et al., 2020). However, one study (van den Broeke et al., 2019) did not observe an increase in the amplitude of PEP peaks after HFS, despite using robot‐controlled mechanical pinprick stimulation. In that study, participants provided a single average rating of all pinprick stimuli at the end of each block. In the studies where an increase in the amplitude of PEPs was observed, the task was different: participants had to rate each single pinprick stimulus individually. It could be that providing a rating only at the end of the block may have rendered the pinprick stimuli less task relevant. One possibility, although speculative, might be that the reduced task relevance prevented the increase in PEPs. However, this should be tested in future studies.
FIGURE 5.
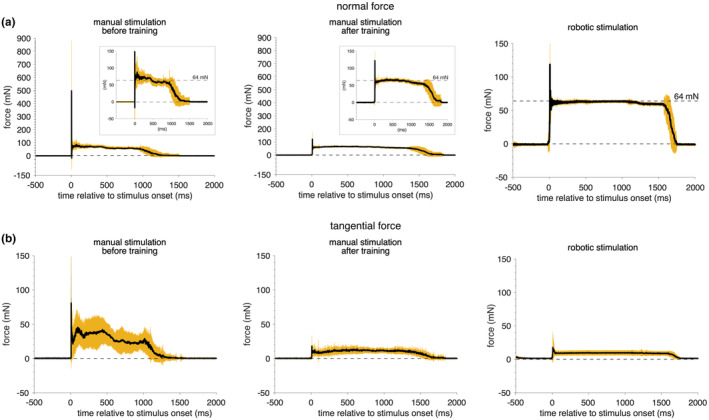
Time course of the force recorded from a force sensor during manually or robotically delivered 64 mN pinprick stimuli on a force sensor ([mean ± SD]). (a) The normal force (along the perpendicular axis of the stimulation) and (b) the tangential force (perpendicular to the axis of stimulation) were recorded during manual or robotic delivery of 30 pinprick stimuli (64 mN) on a 6‐axis strain‐gauge force‐torque transducer. In the manual condition, the experimenter delivered the pinprick stimuli before and after a training period. The training aimed at improving the perpendicular orientation of the pinprick stimulator with respect to the skin with minimal lateral movements (tangential force), and at as constant as possible speed, with a contact held for approximately 1 s. Note after training the reduced overshoot of normal force at the onset of the stimulation. By comparing the inserts in (a), note after training the more constant normal force of 64 mN applied during stimulation and its reduced variability. In (b), note after training that the tangential force is reduced by a factor of 10. After training both lateral and tangential force values induced by manual stimulation are closer to the forces induced by robotic stimulation. Robot‐controlled pinprick stimulation leads to even less variable normal and tangential force and offers the advantage of constant stimulation over time in contrast to the performance of a human experimenter that could suffer from its fatigue throughout an experiment. (Unpublished data Lambert et al., 2017)
It has been shown that vibrotactile stimuli—predominantly activating LTMs—delivered to the area of SHA elicited an increase in the magnitude of the negative amplitude of the vibrotactile evoked potential reaching its maximum around 130 ms (van den Broeke & Mouraux, 2014a). The positive amplitude elicited by vibrotactile stimuli was not increased and no changes were observed in the perceived intensity of these stimuli. These results suggest that part of the increase in the negative component of PEPs could be modality unspecific whereas the increase in the positive component could be modality specific.
No correlations have been observed between the increase in perceived intensity elicited by pinprick stimuli in the area of SHA and the changes in the amplitude of the negative and positive peaks of PEPs (van den Broeke et al., 2020). Nevertheless, the increase in pinprick perception elicited from the area of SHA tends to follow the same time course as the increase in PEP positivity (van den Broeke, Lambert, et al., 2016). No study has assessed the test–retest reliability, sensitivity and specificity of the increase in PEPs in the context of SHA.
Triccas et al. assessed the test–retest reliability of the negative–positive peak recorded at Cz and elicited by 256 mN pinprick stimulation delivered to the hand dorsum. They found an intra‐class correlation coefficient (ICC and 95% CI) of 0.573 (0.151, 0.897) for the right hand and 0.624 (0.262, 0.832) for the left hand (Triccas et al., 2022). Also, Rosner et al. (2020) assessed the test–retest reliability of the negative–positive peak at Cz after 256 mN pinprick stimulation delivered to the hand dorsum and found an ICC of 0.27.
4.3.1. Pinprick‐evoked EEG responses analysed in the time‐frequency domain
When analysing mechanical pinprick‐evoked EEG responses in the time‐frequency domain, van den Broeke et al. (2017, 2019) and Triccas et al. (2022) found that pinprick stimuli elicited a phase‐locked low‐frequency (<5 Hz) response between 150 and 400 ms after stimulus onset, followed by a reduction of alpha‐band activity (7–10 Hz) between 100 and 600 ms. Triccas et al. (2022) assessed test–retest reliability and found that the low‐frequency response had an almost perfect relative reliability (ICC = 0.98), which was not the case for PEP amplitudes.
van den Broeke et al. (2017) showed that mechanical pinprick stimuli, when applied to the area of increased pinprick sensitivity, elicited an increase in the low‐frequency response but did not change alpha‐band activity. They further showed that the part of the low‐frequency response was phase‐locked to the stimulus and, thus, mostly reflected PEPs. In a later study, van den Broeke et al. (2019) did not find a significant increase in the low‐frequency response when the pinprick stimuli were applied in the area of increased pinprick sensitivity, which is in line with the lack of increase in PEPs as discussed in Section 3.1.3. Future studies should assess the reliability of the increase in low‐frequency response.
Gousset et al. recently investigated if scalp‐recorded gamma‐band activity (30–100 Hz) elicited by strong pinprick stimulation (512 mN) is associated with SHA (Gousset et al., 2023). However, they only observed pinprick‐evoked gamma‐band activity in a subgroup of participants and this activity was not significantly increased when pinprick stimuli were applied to the area of SHA. This conclusion is supported by a re‐analysis of the data using different parameters (Jaltare et al., 2023).
Also in a previous study, van den Broeke et al. (2017) assessed if manually applied 64 mN pinprick stimulation to the area of SHA increased scalp‐recorded gamma‐band activity. However, the authors did not observe any increase in cortical‐related gamma‐band activity after HFS.
4.4. Brainstem autonomic activity assessed by pupil dilation response
Pupil size has been shown to reflect in primates the activity of neurons of the locus coeruleus (Joshi et al., 2016) which is thought to play an important role in the regulation of arousal (Berridge, 2008) and which activity is increased in response to a nociceptive stimulus (Samuels & Szabadi, 2008).
van den Broeke et al. (2019) were the first to record the pupil dilation response (PDR) elicited by robot‐controlled mechanical pinprick stimulation before and after the experimental induction of SHA using HFS. They found that pinprick stimulation (64 and 96 mN) applied to non‐sensitized skin of the ventral forearm elicited a dilation of the pupil that reached its maximum between 1 and 2 s after stimulus onset. They further showed that when the same pinprick stimuli were delivered to the area of SHA the pupil dilated even more. No increase in pupil dilation response was observed at the control site (contralateral arm) after the induction of SHA. However, the PDR elicited by the 64 mN delivered to the area of SHA was on average larger than the one elicited by the 96 mN, while the increase in perceived intensity was on average similar between both pinprick stimulation intensities, suggesting that there was no correlation between the two variables. This finding should be interpreted with caution as the sample of participants was small.
Of note, the increase in PDR associated with SHA was present without any accompanying increase in PEPs, suggesting that the PDR is more sensitive (or less dependent on task relevance as discussed for PEPs in Section 3.1.3) to detect changes in central nervous system processing of mechanical nociceptive input than PEPs. No studies have assessed the test–retest reliability of the increase in PDR associated with SHA and no data on the specificity and sensitivity are available.
4.5. Brainstem autonomic activity assessed by sympathetic skin response
The sympathetic skin response (SSR) is measured as a change in electric potential, in relation to the modification of skin conductance due to sweat gland's activity, in response to a stimulus (Vetrugno et al., 2003). SSR has been used as a means to indirectly assess the changes in the autonomic nervous system activity, and in particular of the sympathetic tone, in the context of sensitization. Scheuren et al. (2020) recorded the SSR elicited by pinprick stimulation (256 mN) before and after the induction of SHA using repetitive heat stimulation. Together with an increase in pinprick ratings, they found increased amplitudes of the SSR in response to pinprick stimulation applied in the area of SHA compared to both baseline and control conditions.
In a follow‐up study, the same group did not replicate this increase in SSR elicited by pinprick stimulation in the area of SHA but found a lack of habituation (compared to the control condition) only (Scheuren et al., 2023). However, in the second study the pain intensity of all pinprick and heat stimuli was individually adapted to NRS‐4, therefore the two studies are not directly comparable. Interestingly, also heat stimuli applied to the area of SHA elicited a lack of habituation of the SSR amplitude.
No data is available about the test–retest reliability of the increase in SSR nor about sensitivity and specificity.
5. DISCUSSION
5.1. Experimentally induced central sensitization involves predominantly a facilitation of mechanical nociceptive pathways
5.1.1. Heat sensitivity
The presence of heat hyperalgesia seems to depend on the method used to induce SHA (intradermal capsaicin and HFS) and the stimulus to assess it (Table 1). There is no obvious explanation for this observation. The fact that heat hyperalgesia manifests with the use of experimental models that induce greater pain (Quesada et al., 2021) leads us to think that the intensity of the conditioning stimulus (peripheral nociceptive input) and the characteristics of the type of afferent might play a role.
5.1.2. Do tactile afferents play a role in SHA?
The quality and perceived intensity elicited by static indenting non‐nociceptive tactile stimuli are not changed in the area of SHA. This suggests that A‐fibre low threshold mechanoreceptors may have a negligible contribution to SHA. This conclusion does not preclude the involvement of LTMs in dynamic mechanical allodynia (Baron, 2000; Cervero et al., 1994; Pfau et al., 2011; Simone & Ochoa, 1991).
5.1.3. Can we detect SHA using electrical stimuli?
Our findings suggest that changes in perceived intensity to electrical stimuli in the SHA area can vary with stimulus characteristics. In the case of suprathreshold electrical stimuli (above pain threshold), the change involves a difference compared to the control site rather than an increase compared to baseline. According to the dual process theory proposed by Groves and Thompson (1970), habituation and sensitization processes interact, leading to the net outcome of behaviour. A difference between arms but not between before and after, might suggest either reduced habituation or weak sensitization.
Low‐intensity intraepidermal stimulation, which has been shown to selectively activate A‐fibre nociceptors, does not detect secondary mechanical hyperalgesia.
High‐intensity electrical stimuli (which are non‐selective for activating types of primary afferents) can increase perception, possibly due to the synchronous activation of many fibres. This synchronous recruitment might be perceived differently than natural mechanoreceptor activation, leading to distinct perceptual experiences (Graczyk et al., 2016).
Currently, it is impossible to conclude which nerve fibres mediate SHA from transcutaneous electrical stimulation results.
5.2. Physiological measures, what do they reflect?
5.2.1. RIII reflex and the N13 SSEP
During RIII recording, distal electrical stimulation activates small‐myelinated fibres, transmitting afferent input to the spinal cord dorsal horn. Interneurons, with a primary role of WDR, are involved, as shown in animal studies (Li & Chen, 2004). The output connects to motor neurons (Lundberg, 1979), and the RIII is subject to dynamic supraspinal modulation (Sandrini et al., 2005). Given the complexity of the reflex generation, it is difficult to establish how and which of these neurons are facilitated during the induction of SHA. While the threshold of the reflex is systematically decreased after different SHA induction methods, the reflex AUC appears to be modulated only if a continuous afferent input, whether this is due to the presence of continuous pain or by mimicking the primary afferents firing rate through a long‐lasting LFS, is provided to the spinal cord (Table 3), suggesting that the two outcomes are not equally affected in sensitized contexts.
The N13 does not reflect the activity of spinal nociceptive specific neurons (Restuccia & Mauguière, 1991). However, evidence suggest that this response might reflect the activity of WDR neurons. It was shown to be increased in amplitude by experimental methods inducing SHA through a prolonged peripheral nociceptive input, making it a suitable readout of spinal excitability changes (Di Lionardo et al., 2021; Leone et al., 2023). However, the low signal‐to‐noise ratio, the small amplitude of the response, the dependence of its modulation on specific experimental methods and the limited data available on its test–retest reliability imply that this neurophysiological tool is still in its infancy and requires further studies. Nevertheless, the N13 recorded at the group level might be useful to assess drug effects on dorsal horn excitability in pharmacological trials. Future studies should replicate the modulation of this response by selected pain methods used to induce CS.
Based on this information, we can conclude that the threshold of the RIII reflex is a reliable readout of spinal changes occurring in the context of SHA induction. However, modulation of the reflex AUC only occurs when there is ongoing nociceptive afferent input to the spinal cord.
5.2.2. Pinprick‐evoked potentials
Studies have shown that mechanical pinprick stimuli delivered to the area of SHA elicit an increase in the amplitude of both the negative and positive peaks of PEPs. The available data suggest that the increase in the positive peak is stimulus specific and site specific, though it seems to be task dependent. Data suggest that pinprick‐evoked gamma‐band activity is not useful for assessing altered processing of mechanical nociceptive input in the context of SHA. One study has found that the pinprick‐evoked low‐frequency EEG response (<5 Hz) has a high reliability and therefore future studies should investigate the reliability of the increase of this low‐frequency response in the context of SHA.
As sensitivity and specificity are unknown, the usefulness of these EEG features for detecting CS remains to be clarified.
5.2.3. Pupil dilation response and sympathetic skin response
The PDR elicited by mechano‐nociceptive stimuli may reflect changes in locus coeruleus, a brainstem structure thought to be also involved in arousal (Joshi et al., 2016). Mechanical stimuli delivered to the area of SHA elicit a larger PDR as compared to pinprick stimuli delivered before sensitization or compared to the control site. Interestingly, whereas the increase in PEPs seems task‐dependent, the PDR is less (van den Broeke et al., 2019).
The SSR is increased when elicited both by mechanical pinprick and heat stimulation delivered to the area of SHA (Scheuren et al., 2020, 2023), indicating that SSR modulation is not modality specific and more related to a general increase in the state of arousal.
5.3. Lack of association between changes in perception and physiological measures
Correlations between changes in perception and neurophysiological responses are not systematically observed, indicating that there is no one‐to‐one relationship between them. The fact that various experimental pain models seem to elicit different effects with variable time courses supports the idea of multiple pathways involved (Di Lionardo et al., 2021; Gousset et al., 2020). In addition to the long‐lasting sensitization of mechanical nociceptive pathways, pain models also appear to induce short‐term changes that are not specific to a particular sensory modality. These short‐term changes could result from higher‐level cognitive processes (van den Broeke et al., 2014) or reflect changes in spinal excitability unrelated to changes in mechanical pinprick processing (Leone et al., 2023).
5.4. Different methods to induce CS might differently affect spinal circuitries
All revised experimental pain methods trigger an increased pinprick sensitivity in the surrounding skin, but they impact behavioural and spinal responses differently. This indicates that the different experimental pain stimuli may affect spinal circuits differently. Change in heat sensitivity, for example, is more common with intense pain during the experimental model (Quesada et al., 2021). The amplitudes of spinal‐evoked responses change only with prolonged spinal afferent input. Since hyperexcitability in spinal neurons has been shown mainly with specific methods such as intradermal capsaicin and HFS (Patel et al., 2024; Randić et al., 1993; Simone & Ochoa, 1991), it questions whether other models can cause similar dorsal horn changes. The different experimental pain methods might explain some inconsistent findings. Future research should use methods with better‐defined spinal mechanisms to induce SHA, such as capsaicin and electrical stimulation.
5.5. Methodological considerations and recommendations
The use of numerical rating scales (NRS) is known to generate non‐ratio scales as opposed to visual analogue scales (VAS) (Myles & Urquhart, 2005; Price et al., 1983, 1994). Therefore, to obtain continuous data with similar mathematical properties that could be analysed with standard statistical methods and be compared across studies, the use of VAS should be preferred. To standardize the rating scales and their use, importance should be given to the definition of the anchors. The use of an intensity VAS in addition to a quality assessment using descriptors of the sensation should be preferred. During assessment at multiple time points, keeping visible the previous VAS used by the participant could allow him/her to have a reference for subsequent ratings without relying on the memory of the perception elicited by previous stimuli. A precise description of the instruction should systematically be reported to standardize experimental setups and allow fair comparisons between studies. Some other factors might play an important role such as the testing order between different modalities, and the body sites explored (Leone et al., 2021).
Studies in this review required SHA to be evidenced by pinprick stimuli. SHA assessment involves increased perceived pinprick intensity and an area. Research shows that while pinprick sensitivity generally increases across methods (except UVB models) the area of hyperalgesia can vary by stimulation quality, intensity and application site (Quesada et al., 2021). These findings highlight how increased pinprick sensitivity around the pain application site is an essential indicator of pain model success. Accurately mapping the hyperalgesia area before collecting pinprick ratings is crucial to minimize biases.
6. CONCLUSION
The most reliable behavioural readout for SHA is pinprick hyperalgesia. This should ideally be assessed using visual rating scales, after outlining the hyperalgesic area. Currently, the best neurophysiological readout for evaluating spinal hyperexcitability induced by sensitizing methods is the RIII reflex threshold. Animal studies with direct recordings from the dorsal horn are necessary to clarify the excitability mechanisms induced by different experimental models. Until such data is available, it is preferable to use methods whose mechanisms are at least partially understood and reproducible, that is, methods such as topical application or injection of capsaicin and electrical stimulation of the skin.
Test–retest reliability, sensitivity and specificity data of all the revised neurophysiological outcomes are scarce, therefore none of these methods is currently indicated for CS identification at the individual level. The clinical use is further limited by the fact that a comparison between the healthy side and the affected side in patients is not possible given the fact that central sensitization thought to be linked to persistent pain might be widespread (Arendt‐Nielsen et al., 2018). It is limited, at present, to the comparison between populations. It is worth mentioning that Quantitative Sensory Testing according to DFNS standards including clustering (Vollert et al., 2017) has the characteristics to provide reliable information at the individual level. This battery of tests was beyond the scope of this review as it does not constitute an objective neurophysiological tool.
AUTHOR CONTRIBUTIONS
The authors contributed equally to the review by revising papers, drafting the manuscript and discussing the results.
Leone, C. M. , Lenoir, C. , & van den Broeke, E. N. (2025). Assessing signs of central sensitization: A critical review of physiological measures in experimentally induced secondary hyperalgesia. European Journal of Pain, 29, e4733. 10.1002/ejp.4733
Caterina M. Leone, Cedric Lenoir, and Emanuel N. van den Broeke contributed equally.
REFERENCES
- Ali, Z. , Meyer, R. A. , & Campbell, J. N. (1996). Secondary hyperalgesia to mechanical but not heat stimuli following a capsaicin injection in hairy skin. Pain, 68, 401–411. [DOI] [PubMed] [Google Scholar]
- Andersen, O. K. , Felsby, S. , Nicolaisen, L. , Bjerring, P. , Jensen, T. S. , & Arendt‐Nielsen, L. (1996). The effect of ketamine on stimulation of primary and secondary hyperalgesic areas induced by capsaicin—a double‐blind, placebo‐controlled, human experimental study. Pain, 66, 51–62. [DOI] [PubMed] [Google Scholar]
- Andersen, O. K. , Gracely, R. H. , & Arednt‐Nielsen, L. (1995). Facilitation of the human nociceptive reflex by stimulation of Aβ‐fibres in a secondary hyperalgesic area sustained by nociceptive input from the primary hyperalgesic area. Acta Physiologica Scandinavica, 155, 87–97. [DOI] [PubMed] [Google Scholar]
- Andersen, O. K. , Spaich, E. G. , Madeleine, P. , & Arendt‐Nielsen, L. (2005). Gradual enlargement of human withdrawal reflex receptive fields following repetitive painful stimulation. Brain Research, 1042, 194–204. [DOI] [PubMed] [Google Scholar]
- Andrews, K. , Baranowski, A. , & Kinnman, E. (1999). Sensory threshold changes without initial pain or alterations in cutaneous blood flow, in the area of secondary hyperalgesia caused by topical application of capsaicin in humans. Neuroscience Letters, 266, 45–48. [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen, L. , Andersen, O. K. , & Jensen, T. S. (1996). Brief, prolonged and repeated stimuli applied to hyperalgesic skin areas: A psychophysical study. Brain Research, 712, 165–167. [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen, L. , Brennum, J. , Sindrup, S. , & Bak, P. (1994). Electrophysiological and psychophysical quantification of temporal summation in the human nociceptive system. European Journal of Applied Physiology and Occupational Physiology, 68, 266–273. [DOI] [PubMed] [Google Scholar]
- Arendt‐Nielsen, L. , Morlion, B. , Perrot, S. , Dahan, A. , Dickenson, A. , Kress, H. G. , Wells, C. , Bouhassira, D. , & Mohr Drewes, A. (2018). Assessment and manifestation of central sensitisation across different chronic pain conditions. European Journal of Pain, 22, 216–241. [DOI] [PubMed] [Google Scholar]
- Baron, R. (2000). Peripheral neuropathic pain: From mechanisms to symptoms. The Clinical Journal of Pain, 16, S12–S20. [DOI] [PubMed] [Google Scholar]
- Beall, J. E. , Applebaum, A. E. , Foreman, R. D. , & Willis, W. D. (1977). Spinal cord potentials evoked by cutaneous afferents in the monkey. Journal of Neurophysiology, 40, 199–211. [DOI] [PubMed] [Google Scholar]
- Berridge, C. W. (2008). Noradrenergic modulation of arousal. Brain Research Reviews, 58, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biurrun Manresa, J. , Kæseler Andersen, O. , Mouraux, A. , & van den Broeke, E. N. (2018). High frequency electrical stimulation induces a long‐lasting enhancement of event‐related potentials but does not change the perception elicited by intra‐epidermal electrical stimuli delivered to the area of increased mechanical pinprick sensitivity. PLoS One, 13, e0203365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biurrun Manresa, J. A. , Finnerup, N. S. B. , Johannesen, I. L. , Biering‐Sørensen, F. , Jensen, T. S. , Arendt‐Nielsen, L. , & Andersen, O. K. (2014). Central sensitization in spinal cord injured humans assessed by reflex receptive fields. Clinical Neurophysiology, 125, 352–362. [DOI] [PubMed] [Google Scholar]
- Biurrun Manresa, J. A. , Mørch, C. D. , & Andersen, O. K. (2010). Long‐term facilitation of nociceptive withdrawal reflexes following low‐frequency conditioning electrical stimulation: A new model for central sensitization in humans. European Journal of Pain, 14, 822–831. [DOI] [PubMed] [Google Scholar]
- Cervero, F. , Meyer, R. A. , & Campbell, J. N. (1994). A psychophysical study of secondary hyperalgesia: Evidence for increased pain to input from nociceptors. Pain, 58, 21–28. [DOI] [PubMed] [Google Scholar]
- Cohen, R. H. , & Vierck, C. J. (1993). Population estimates for responses of cutaneous mechanoreceptors to a vertically indenting probe on the glabrous skin of monkeys. Experimental Brain Research, 94, 105–119. [DOI] [PubMed] [Google Scholar]
- Cracco, R. Q. (1973). Spinal evoked response: Peripheral nerve stimulation in man. Electroencephalography and Clinical Neurophysiology, 35, 379–386. [DOI] [PubMed] [Google Scholar]
- De Col, R. , & Maihöfner, C. (2008). Centrally mediated sensory decline induced by differential C‐fiber stimulation. Pain, 138, 556–564. [DOI] [PubMed] [Google Scholar]
- De Schoenmacker, I. , Berry, C. , Blouin, J.‐S. , Rosner, J. , Hubli, M. , Jutzeler, C. R. , & Kramer, J. L. K. (2021). An intensity matched comparison of laser‐ and contact heat evoked potentials. Scientific Reports, 11, 6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt, J. E. (1984). Non‐cephalic reference makes it possible to avoid errors in the interpretation of somatosensory evoked potentials in man. Revue d'Électroencéphalographie et de Neurophysiologie Clinique, 13, 349–366. [DOI] [PubMed] [Google Scholar]
- Desmedt, J. E. , & Cheron, G. (1981). Prevertebral (oesophageal) recording of subcortical somatosensory evoked potentials in man: The spinal P13 component and the dual nature of the spinal generators. Electroencephalography and Clinical Neurophysiology, 52, 257–275. [DOI] [PubMed] [Google Scholar]
- Desmedt, J. E. , & Cheron, G. (1982). Somatosensory evoked potentials in man: Subcortical and cortical components and their neural basis. Annals of the New York Academy of Sciences, 388, 388–411. [DOI] [PubMed] [Google Scholar]
- Di Lionardo, A. , Di Stefano, G. , Leone, C. , Di Pietro, G. , Sgro, E. , Malara, E. , Cosentino, C. , Mollica, C. , Blockeel, A. J. , Caspani, O. , Garcia‐Larrea, L. , Mouraux, A. , Treede, R. D. , Phillips, K. G. , Valeriani, M. , & Truini, A. (2021). Modulation of the N13 component of the somatosensory evoked potentials in an experimental model of central sensitization in humans. Scientific Reports, 11, 20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro, G. D. , Di Stefano, G. D. , Leone, C. , Lionardo, A. D. , Sgrò, E. , Blockeel, A. J. , Caspani, O. , Garcia‐Larrea, L. , Mouraux, A. , Phillips, K. G. , Treede, R. D. , Valeriani, M. , & Truini, A. (2021). The N13 spinal component of somatosensory evoked potentials is modulated by heterotopic noxious conditioning stimulation suggesting an involvement of spinal wide dynamic range neurons. Neurophysiologie Clinique=Clinical Neurophysiology, 51, 517–523. [DOI] [PubMed] [Google Scholar]
- Dimitrijević, M. R. , Faganel, J. , Gregorić, M. , Nathan, P. W. , & Trontelj, J. K. (1972). Habituation: Effects of regular and stochastic stimulation. Journal of Neurology, Neurosurgery, and Psychiatry, 35, 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond, P. D. , & Blockey, P. (2009). Topically applied capsaicin inhibits sensitivity to touch but not to warmth or heat‐pain in the region of secondary mechanical hyperalgesia. Somatosensory & Motor Research, 26, 75–81. [DOI] [PubMed] [Google Scholar]
- Ellrich, J. , & Treede, R. D. (1998). Convergence of nociceptive and non‐nociceptive inputs onto spinal reflex pathways to the tibialis anterior muscle in humans. Acta Physiologica Scandinavica, 163, 391–401. [DOI] [PubMed] [Google Scholar]
- Ertekin, C. , Ertekin, N. , & Karcioglu, M. (1975). Conduction velocity along human nociceptive reflex afferent nerve fibres. Journal of Neurology, Neurosurgery, and Psychiatry, 38, 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser, H. S. , & Graham, H. T. (1933). Potentials produced in the spinal cord by stimulation of dorsal roots. American Journal of Physiology, 103, 303–320. [Google Scholar]
- Geber, C. , Fondel, R. , Krämer, H. H. , Rolke, R. , Treede, R. D. , Sommer, C. , & Birklein, F. (2007). Psychophysics, flare, and neurosecretory function in human pain models: Capsaicin versus electrically evoked pain. The Journal of Pain, 8, 503–514. [DOI] [PubMed] [Google Scholar]
- Gousset, S. , Mouraux, A. , & van den Broeke, E. N. (2020). Burst‐like conditioning electrical stimulation is more efficacious than continuous stimulation for inducing secondary hyperalgesia in humans. Journal of Neurophysiology, 123, 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousset, S. , Torta, D. , Mouraux, A. , Lambert, J. , & van den Broeke, E. (2023). Pinprick‐induced gamma‐band oscillations are not a useful electrophysiological marker of pinprick hypersensitivity in humans. Clinical Neurophysiology, 153, 102–110. [DOI] [PubMed] [Google Scholar]
- Grönroos, M. , & Pertovaara, A. (1993). Capsaicin‐induced central facilitation of a nociceptive flexion reflex in humans. Neuroscience Letters, 159, 215–218. [DOI] [PubMed] [Google Scholar]
- Graczyk, E. L. , Schiefer, M. A. , Saal, H. P. , Delhaye, B. P. , Bensmaia, S. J. , & Tyler, D. J. (2016). The neural basis of perceived intensity in natural and artificial touch. Science Translational Medicine, 8, 362ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan, J. D. , & McGillis, S. L. (1991). Stimulus features relevant to the perception of sharpness and mechanically evoked cutaneous pain. Somatosensory & Motor Research, 8, 137–147. [DOI] [PubMed] [Google Scholar]
- Groves, P. M. , & Thompson, R. F. (1970). Habituation: A dual‐process theory. Psychological Review, 77, 419–450. [DOI] [PubMed] [Google Scholar]
- Guekos, A. , Grata, A. C. , Hubli, M. , Schubert, M. , & Schweinhardt, P. (2023). Are changes in nociceptive withdrawal reflex magnitude a viable central sensitization proxy? Implications of a replication attempt. Clinical Neurophysiology, 145, 139–150. [DOI] [PubMed] [Google Scholar]
- Gustorff, B. , Anzenhofer, S. , Sycha, T. , Lehr, S. , & Kress, H. G. (2004). The sunburn pain model: The stability of primary and secondary hyperalgesia over 10 hours in a crossover setting. Anesthesia and Analgesia, 98, 173–177. [DOI] [PubMed] [Google Scholar]
- Gustorff, B. , Sycha, T. , Lieba‐Samal, D. , Rolke, R. , Treede, R. D. , & Magerl, W. (2013). The pattern and time course of somatosensory changes in the human UVB sunburn model reveal the presence of peripheral and central sensitization. Pain, 154, 586–597. [DOI] [PubMed] [Google Scholar]
- Hsieh, M.‐T. , Donaldson, L. F. , & Lumb, B. M. (2015). Differential contributions of A‐ and C‐nociceptors to primary and secondary inflammatory hypersensitivity in the rat. Pain, 156, 1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, S. W. , Basra, M. , Chan, C. , Parr, C. , Wong, F. , Gomes, S. , & Strutton, P. H. (2020). Capsaicin‐induced changes in electrical pain perception threshold can be used to assess the magnitude of secondary hyperalgesia in humans. Pain Medicine (Malden, Mass), 21, 2830–2838. [DOI] [PubMed] [Google Scholar]
- Hugon, M. (1973). Methodology of the Hoffmann reflex in man. In Desmedt J. E. (Ed.), Human reflexes, pathophysiology of motor systems, methodology of human reflexes. S.Karger AG. [Google Scholar]
- Hullemann, P. , Watfeh, R. , Shao, Y. Q. , Nerdal, A. , Binder, A. , & Baron, R. (2015). Peripheral sensitization reduces laser‐evoked potential habituation. Neurophysiologie Clinique. 45, 457–467. [DOI] [PubMed] [Google Scholar]
- Iannetti, G. D. , Baumgartner, U. , Tracey, I. , Treede, R. D. , & Magerl, W. (2013). Pinprick‐evoked brain potentials: A novel tool to assess central sensitization of nociceptive pathways in humans. Journal of Neurophysiology, 110, 1107–1116. [DOI] [PubMed] [Google Scholar]
- Inui, K. , Tran, T. D. , Hoshiyama, M. , & Kakigi, R. (2002). Preferential stimulation of Adelta fibers by intra‐epidermal needle electrode in humans. Pain, 96, 247–252. [DOI] [PubMed] [Google Scholar]
- Jürgens, T. P. , Sawatzki, A. , Henrich, F. , Magerl, W. , & May, A. (2014). An improved model of heat‐induced hyperalgesia—Repetitive phasic heat pain causing primary hyperalgesia to heat and secondary hyperalgesia to pinprick and light touch. PLoS One, 9, e99507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaltare, K. P. , Torta, D. , & van den Broeke, E. (2023). Preprocessing choices do not affect the chances of observing increased scalp recorded gamma‐band oscillations in the context of mechanical hypersensitivity. 10.31219/osf.io/ujvcy [DOI]
- Johansson, R. S. , & Vallbo, A. B. (1979). Detection of tactile stimuli. Thresholds of afferent units related to psychophysical thresholds in the human hand. The Journal of Physiology, 297, 405–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S. J. (1977). Short latency potentials recorded from the neck and scalp following median nerve stimulation in man. Electroencephalography and Clinical Neurophysiology, 43, 853–863. [DOI] [PubMed] [Google Scholar]
- Joshi, S. , Li, Y. , Kalwani, R. M. , & Gold, J. I. (2016). Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron, 89, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilo, S. , Schmelz, M. , Koltzenburg, M. , & Handwerker, H. O. (1994). Different patterns of hyperalgesia induced by experimental inflammation in human skin. Brain, 117(Pt 2), 385–396. [DOI] [PubMed] [Google Scholar]
- Klein, T. , Magerl, W. , Hopf, H. C. , Sandkuhler, J. , & Treede, R. D. (2004). Perceptual correlates of nociceptive long‐term potentiation and long‐term depression in humans. The Journal of Neuroscience, 24, 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Cesa, S. , Di Stefano, G. , Leone, C. , Pepe, A. , Galosi, E. , Alu, F. , Fasolino, A. , Cruccu, G. , Valeriani, M. , & Truini, A. (2018). Skin denervation does not alter cortical potentials to surface concentric electrode stimulation: A comparison with laser evoked potentials and contact heat evoked potentials. European Journal of Pain, 22, 161–169. [DOI] [PubMed] [Google Scholar]
- Lambert, J. , Mouraux, A . & van den Broeke, E. N . (2017). Comparison of force parameters during manual and robot‐assisted pinprick stimulation. Internal report (UCLouvain). Unpublished [Google Scholar]
- LaMotte, R. H. , Shain, C. N. , Simone, D. A. , & Tsai, E. F. (1991). Neurogenic hyperalgesia: Psychophysical studies of underlying mechanisms. Journal of Neurophysiology, 66, 190–211. [DOI] [PubMed] [Google Scholar]
- Legrain, V. , & Mouraux, A. (2013). Activating selectively and reliably nociceptive afferents with concentric electrode stimulation: Yes we can! Provided that low stimulus intensities are used! Clinical Neurophysiology, 124, 424. [DOI] [PubMed] [Google Scholar]
- Lenoir, C. , Plaghki, L. , Mouraux, A. , & van den Broeke, E. N. (2018). Quickly‐responding C‐fibre nociceptors contribute to heat hypersensitivity in the area of secondary hyperalgesia. The Journal of Physiology, 596, 4443–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir, C. , Vizzari, V. , Huang, G. , Mouraux, A. & van den Broeke, E. N. , (2019). Transcutaneous high frequency electrical stimulation in humans enhances heat pain perception but exerts no effect on concomitantly recorded C fibre related brain potentials. Internal report (UCLouvain). Unpublished [Google Scholar]
- Lenoir, C. , Demol, J. , Mouraux, A . & van den Broeke, E. N. (2021). Is there a contribution of low‐threshold mechanoreceptors to mechanical sensitivity in the area of secondary hyperalgesia induced by high frequency electrical stimulation ? Internal report (UCLouvain). Unpublished [Google Scholar]
- Leone, C. , Di Lionardo, A. , Di Pietro, G. , Di Stefano, G. , Falco, P. , Blockeel, A. J. , Caspani, O. , Garcia‐Larrea, L. , Mouraux, A. , Phillips, K. G. , Treede, R. D. , & Truini, A. (2021). How different experimental models of secondary hyperalgesia change the nociceptive flexion reflex. Clinical Neurophysiology, 132, 2989–2995. [DOI] [PubMed] [Google Scholar]
- Leone, C. , Di Pietro, G. , Salman, Y. , Galosi, E. , Di Stefano, G. , Caspani, O. , Garcia‐Larrea, L. , Mouraux, A. , Treede, R.‐D. , & Truini, A. (2023). Modulation of the spinal N13 SEP component by high‐ and low‐frequency electrical stimulation. Experimental pain models matter. Clinical Neurophysiology, 156, 28–37. [DOI] [PubMed] [Google Scholar]
- Leone, C. , & Truini, A. (2019). The CPM effect: Functional assessment of the diffuse noxious inhibitory control in humans. Journal of Clinical Neurophysiology, 36, 430–436. [DOI] [PubMed] [Google Scholar]
- Lewis, G. N. , Rice, D. A. , Jourdain, K. , & McNair, P. J. (2012). Influence of stimulation location and posture on the reliability and comfort of the nociceptive flexion reflex. Pain Research & Management, 17, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K.‐C. , & Chen, J. (2004). Altered pain‐related behaviors and spinal neuronal responses produced by s.c. injection of melittin in rats. Neuroscience, 126, 753–762. [DOI] [PubMed] [Google Scholar]
- Liang, M. , Lee, M. C. , O'Neill, J. , Dickenson, A. H. , & Iannetti, G. D. (2016). Brain potentials evoked by intraepidermal electrical stimuli reflect the central sensitization of nociceptive pathways. Journal of Neurophysiology, 116, 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde, L. D. , Bent, L. R. , Dickey, J. P. , Kumbhare, D. A. , & Srbely, J. Z. (2021). Exploring the effect of capsaicin‐induced central sensitization on the upper limb nociceptive withdrawal reflex threshold. Experimental Brain Research, 239, 3405–3415. [DOI] [PubMed] [Google Scholar]
- Lotsch, J. , & Ultsch, A. (2018). Machine learning in pain research. Pain, 159, 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg, A. (1979). Multisensory control of spinal reflex pathways. Progress in Brain Research, 50, 11–28. [DOI] [PubMed] [Google Scholar]
- Magerl, W. , Wilk, S. H. , & Treede, R. D. (1998). Secondary hyperalgesia and perceptual wind‐up following intradermal injection of capsaicin in humans. Pain, 74, 257–268. [DOI] [PubMed] [Google Scholar]
- Matthews, W. B. , Beauchamp, M. , & Small, D. G. (1974). Cervical somato‐sensory evoked responses in man. Nature, 252, 230–232. [DOI] [PubMed] [Google Scholar]
- Mauguière, F. , & Courjon, J. (1981). The origins of short‐latency somatosensory evoked potentials in humans. Annals of Neurology, 9, 607–611. [DOI] [PubMed] [Google Scholar]
- Meyer, R. A. , & Campbell, J. N. (1981). Myelinated nociceptive afferents account for the hyperalgesia that follows a burn to the hand. Science (New York, N.Y.), 213, 1527–1529. [DOI] [PubMed] [Google Scholar]
- Micalos, P. S. , Drinkwater, E. J. , Cannon, J. , Arendt‐Nielsen, L. , & Marino, F. E. (2009). Reliability of the nociceptive flexor reflex (RIII) threshold and association with pain threshold. European Journal of Applied Physiology, 105, 55–62. [DOI] [PubMed] [Google Scholar]
- Møiniche, S. , Dahl, J. B. , & Kehlet, H. (1993). Time course of primary and secondary hyperalgesia after heat injury to the skin. British Journal of Anaesthesia, 71, 201–205. [DOI] [PubMed] [Google Scholar]
- Mouraux, A. , Iannetti, G. D. , & Plaghki, L. (2010). Low intensity intra‐epidermal electrical stimulation can activate Adelta‐nociceptors selectively. Pain, 150, 199–207. [DOI] [PubMed] [Google Scholar]
- Mouraux, A. , Marot, E. , & Legrain, V. (2014). Short trains of intra‐epidermal electrical stimulation to elicit reliable behavioral and electrophysiological responses to the selective activation of nociceptors in humans. Neuroscience Letters, 561, 69–73. [DOI] [PubMed] [Google Scholar]
- Myles, P. S. , & Urquhart, N. (2005). The linearity of the visual analogue scale in patients with severe acute pain. Anaesthesia and Intensive Care, 33, 54–58. [DOI] [PubMed] [Google Scholar]
- Nagi, S. S. , Marshall, A. G. , Makdani, A. , Jarocka, E. , Liljencrantz, J. , Ridderstrom, M. , Shaikh, S. , O'Neill, F. , Saade, D. , Donkervoort, S. , Foley, A. R. , Minde, J. , Trulsson, M. , Cole, J. , Bonnemann, C. G. , Chesler, A. T. , Bushnell, M. C. , McGlone, F. , & Olausson, H. (2019). An ultrafast system for signaling mechanical pain in human skin. Science Advances, 5, eaaw1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs, J. , George, S. Z. , Clauw, D. J. , Fernández‐de‐las‐Peñas, C. , Kosek, E. , Ickmans, K. , Fernández‐Carnero, J. , Polli, A. , Kapreli, E. , Huysmans, E. , Cuesta‐Vargas, A. I. , Mani, R. , Lundberg, M. , Leysen, L. , Rice, D. , Sterling, M. , & Curatolo, M. (2021). Central sensitisation in chronic pain conditions: Latest discoveries and their potential for precision medicine. The Lancet Rheumatology, 3, e383–e392. [DOI] [PubMed] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , Akl, E. A. , Brennan, S. E. , Chou, R. , Glanville, J. , Grimshaw, J. M. , Hróbjartsson, A. , Lalu, M. M. , Li, T. , Loder, E. W. , Mayo‐Wilson, E. , McDonald, S. , … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R. , Taylor, J. L. , Dickenson, A. H. , McMahon, S. B. , & Bannister, K. (2024). A back‐translational study of descending interactions with the induction of hyperalgesia by high‐frequency electrical stimulation in rat and human. Pain, 165, 1978–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, J. L. , & Kehlet, H. (1998). Secondary hyperalgesia to heat stimuli after burn injury in man. Pain, 76, 377–384. [DOI] [PubMed] [Google Scholar]
- Pfau, D. B. , Klein, T. , Putzer, D. , Pogatzki‐Zahn, E. M. , Treede, R. D. , & Magerl, W. (2011). Analysis of hyperalgesia time courses in humans after painful electrical high‐frequency stimulation identifies a possible transition from early to late LTP‐like pain plasticity. Pain, 152, 1532–1539. [DOI] [PubMed] [Google Scholar]
- Poulsen, A. H. , van den Berg, B. , Arguissain, F. , Tigerholm, J. , Buitenweg, J. R. , Andersen, O. K. , & Mørch, C. D. (2022). Novel surface electrode design for preferential activation of cutaneous nociceptors. Journal of Neural Engineering, 19, 016010. [DOI] [PubMed] [Google Scholar]
- Price, D. D. , Bush, F. M. , Long, S. , & Harkins, S. W. (1994). A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain, 56, 217–226. [DOI] [PubMed] [Google Scholar]
- Price, D. D. , McGrath, P. A. , Rafii, A. , & Buckingham, B. (1983). The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain, 17, 45–56. [DOI] [PubMed] [Google Scholar]
- Quesada, C. , Kostenko, A. , Ho, I. , Leone, C. , Nochi, Z. , Stouffs, A. , Wittayer, M. , Caspani, O. , Brix Finnerup, N. , Mouraux, A. , Pickering, G. , Tracey, I. , Truini, A. , Treede, R. D. , & Garcia‐Larrea, L. (2021). Human surrogate models of central sensitization: A critical review and practical guide. European Journal of Pain, 25, 1389–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja, S. N. , Campbell, J. N. , & Meyer, R. A. (1984). Evidence for different mechanisms of primary and secondary hyperalgesia following heat injury to the glabrous skin. Brain, 107(Pt 4), 1179–1188. [DOI] [PubMed] [Google Scholar]
- Randić, M. , Jiang, M. C. , & Cerne, R. (1993). Long‐term potentiation and long‐term depression of primary afferent neurotransmission in the rat spinal cord. The Journal of Neuroscience, 13, 5228–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restuccia, D. , & Mauguière, F. (1991). The contribution of median nerve SEPs in the functional assessment of the cervical spinal cord in syringomyelia. A study of 24 patients. Brain, 114(Pt 1B), 361–379. [DOI] [PubMed] [Google Scholar]
- Rhudy, J. L. , & France, C. R. (2011). Reliability and validity of a brief method to assess nociceptive flexion reflex (NFR) threshold. The Journal of Pain, 12, 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolke, R. , Magerl, W. , Campbell, K. A. , Schalber, C. , Caspari, S. , Birklein, F. , & Treede, R. D. (2006). Quantitative sensory testing: A comprehensive protocol for clinical trials. European Journal of Pain, 10, 77–88. [DOI] [PubMed] [Google Scholar]
- Romani, A. , Bergamaschi, R. , Versino, M. , Zilioli, A. , Sartori, I. , Callieco, R. , Montomoli, C. , & Cosi, V. (1996). Estimating reliability of evoked potential measures from residual scores: An example using tibial SSEPs. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section, 100, 204–209. [DOI] [PubMed] [Google Scholar]
- Rosner, J. , Scheuren, P. S. , Stalder, S. A. , Curt, A. , & Hubli, M. (2020). Pinprick evoked potentials‐reliable Acquisition in Healthy Human Volunteers. Pain Medicine (Malden, Mass), 21, 736–746. [DOI] [PubMed] [Google Scholar]
- Samuels, E. R. , & Szabadi, E. (2008). Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part I: Principles of functional organisation. Current Neuropharmacology, 6, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkühler, J. (2009). Models and mechanisms of hyperalgesia and allodynia. Physiological Reviews, 89, 707–758. [DOI] [PubMed] [Google Scholar]
- Sandrini, G. , Arrigo, A. , Bono, G. , & Nappi, G. (1993). The nociceptive flexion reflex as a tool for exploring pain control systems in headache and other pain syndromes. Cephalalgia, 13, 21–27. [DOI] [PubMed] [Google Scholar]
- Sandrini, G. , Serrao, M. , Rossi, P. , Romaniello, A. , Cruccu, G. , & Willer, J. C. (2005). The lower limb flexion reflex in humans. Progress in Neurobiology, 77, 353–395. [DOI] [PubMed] [Google Scholar]
- Scheibel, M. E. , & Scheibel, A. B. (1968). Terminal axonal patterns in cat spinal cord. II. The dorsal horn. Brain Research, 9, 32–58. [DOI] [PubMed] [Google Scholar]
- Scheuren, P. S. , Bösch, S. , Rosner, J. , Allmendinger, F. , Kramer, J. L. K. , Curt, A. , & Hubli, M. (2023). Priming of the autonomic nervous system after an experimental human pain model. Journal of Neurophysiology, 130, 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuren, P. S. , Rosner, J. , Curt, A. , & Hubli, M. (2020). Pain‐autonomic interaction: A surrogate marker of central sensitization. European Journal of Pain, 24, 2015–2026. [DOI] [PubMed] [Google Scholar]
- Serra, J. , Campero, M. , & Ochoa, J. (1998). Flare and hyperalgesia after intradermal capsaicin injection in human skin. Journal of Neurophysiology, 80, 2801–2810. [DOI] [PubMed] [Google Scholar]
- Simone, D. A. , & Ochoa, J. (1991). Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain, 47, 285–294. [DOI] [PubMed] [Google Scholar]
- Slimani, H. , Plaghki, L. , Valenti, P. , Werner, M. U. , Kehlet, H. , & Kupers, R. (2018). Aδ and not C fibers mediate thermal hyperalgesia to short laser stimuli after burn injury in man. Pain, 159, 2331–2338. [DOI] [PubMed] [Google Scholar]
- Stammler, T. , De Col, R. , Seifert, F. , & Maihöfner, C. (2008). Functional imaging of sensory decline and gain induced by differential noxious stimulation. NeuroImage, 42, 1151–1163. [DOI] [PubMed] [Google Scholar]
- Sumikura, H. , Andersen, O. K. , Drewes, A. M. , & Arendt‐Nielsen, L. (2003). Spatial and temporal profiles of flare and hyperalgesia after intradermal capsaicin. Pain, 105, 285–291. [DOI] [PubMed] [Google Scholar]
- Sumikura, H. , Miyazawa, A. , Yucel, A. , Andersen, O. K. , & Arendt‐Nielsen, L. (2005). Secondary heat hyperalgesia detected by radiant heat stimuli in humans: Evaluation of stimulus intensity and duration. Somatosensory & Motor Research, 22, 233–237. [DOI] [PubMed] [Google Scholar]
- Thalhammer, J. G. , & LaMotte, R. H. (1982). Spatial properties of nociceptor sensitization following heat injury of the skin. Brain Research, 231, 257–265. [DOI] [PubMed] [Google Scholar]
- Triccas, L. T. , Camilleri, K. P. , Tracey, C. , Mansoureh, F. H. , Benjamin, W. , Francesca, M. , Leonardo, B. , Dante, M. , & Geert, V. (2022). Reliability of upper limb pin‐prick stimulation with electroencephalography: Evoked potentials, spectra and source localization. Frontiers in Human Neuroscience, 16, 881291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchman, M. , Barrett, J. A. , Donevan, S. , Hedberg, T. G. , & Taylor, C. P. (2010). Central sensitization and Ca(V)α₂δ ligands in chronic pain syndromes: Pathologic processes and pharmacologic effect. The Journal of Pain, 11, 1241–1249. [DOI] [PubMed] [Google Scholar]
- Valeriani, M. , Arendt‐Nielsen, L. , Le Pera, D. , Restuccia, D. , Rosso, T. , De Armas, L. , Maiese, T. , Fiaschi, A. , Tonali, P. , & Tinazzi, M. (2003). Short‐term plastic changes of the human nociceptive system following acute pain induced by capsaicin. Clinical Neurophysiology, 114, 1879–1890. [DOI] [PubMed] [Google Scholar]
- Valeriani, M. , Tinazzi, M. , Le Pera, D. , Restuccia, D. , De Armas, L. , Maiese, T. , Tonali, P. , & Arendt‐Nielsen, L. (2005). Inhibitory effect of capsaicin evoked trigeminal pain on warmth sensation and warmth evoked potentials. Experimental Brain Research, 160, 29–37. [DOI] [PubMed] [Google Scholar]
- Vallbo, A. B. , & Johansson, R. S. (1984). Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Human Neurobiology, 3, 3–14. [PubMed] [Google Scholar]
- van den Broeke, E. N. , de Hemptinne, P. , Mercken, M. , Torta, D. M. , Lambert, J. , & Mouraux, A. (2020). Central sensitization of nociceptive pathways demonstrated by robot‐controlled pinprick‐evoked brain potentials. Clinical Neurophysiology, 131, 2491–2498. [DOI] [PubMed] [Google Scholar]
- van den Broeke, E. N. , de Vries, B. , Lambert, J. , Torta, D. M. , & Mouraux, A. (2017). Phase‐locked and non‐phase‐locked EEG responses to pinprick stimulation before and after experimentally‐induced secondary hyperalgesia. Clinical Neurophysiology, 128, 1445–1456. [DOI] [PubMed] [Google Scholar]
- van den Broeke, E. N. , Geene, N. , van Rijn, C. M. , Wilder‐Smith, O. H. , & Oosterman, J. (2014). Negative expectations facilitate mechanical hyperalgesia after high‐frequency electrical stimulation of human skin. European Journal of Pain, 18, 86–91. [DOI] [PubMed] [Google Scholar]
- van den Broeke, E. N. , Hartgerink, D. M. , Butler, J. , Lambert, J. , & Mouraux, A. (2019). Central sensitization increases the pupil dilation elicited by mechanical pinprick stimulation. Journal of Neurophysiology, 121, 1621–1632. [DOI] [PubMed] [Google Scholar]
- van den Broeke, E. N. , Lambert, J. , Huang, G. , & Mouraux, A. (2016). Central sensitization of mechanical nociceptive pathways is associated with a long‐lasting increase of pinprick‐evoked brain potentials. Frontiers in Human Neuroscience, 10, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broeke, E. N. , Lenoir, C. , & Mouraux, A. (2016). Secondary hyperalgesia is mediated by heat‐insensitive A‐fibre nociceptors. The Journal of Physiology, 594, 6767–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broeke, E. N. , & Mouraux, A. (2014a). High‐frequency electrical stimulation of the human skin induces heterotopical mechanical hyperalgesia, heat hyperalgesia, and enhanced responses to nonnociceptive vibrotactile input. Journal of Neurophysiology, 111, 1564–1573. [DOI] [PubMed] [Google Scholar]
- van den Broeke, E. N. , & Mouraux, A. (2014b). Enhanced brain responses to C‐fiber input in the area of secondary hyperalgesia induced by high‐frequency electrical stimulation of the skin. Journal of Neurophysiology, 112, 2059–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broeke, E. N. , Mouraux, A. , Groneberg, A. H. , Pfau, D. B. , Treede, R. D. , & Klein, T. (2015). Characterizing pinprick‐evoked brain potentials before and after experimentally induced secondary hyperalgesia. Journal of Neurophysiology, 114, 2672–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broeke, E. N. , Urdí, M. , Mouraux, A. , Biurrun Manresa, J. A. , & Torta, D. M. E. (2021). High‐frequency electrical stimulation of cutaneous nociceptors differentially affects pain perception elicited by homotopic and heterotopic electrical stimuli. Journal of Neurophysiology, 126, 1038–1044. [DOI] [PubMed] [Google Scholar]
- van den Broeke, E. N. , van Rijn, C. M. , Biurrun Manresa, J. A. , Andersen, O. K. , Arendt‐Nielsen, L. , & Wilder‐Smith, O. H. (2010). Neurophysiological correlates of nociceptive heterosynaptic long‐term potentiation in humans. Journal of Neurophysiology, 103, 2107–2113. [DOI] [PubMed] [Google Scholar]
- Vetrugno, R. , Liguori, R. , Cortelli, P. , & Montagna, P. (2003). Sympathetic skin response: Basic mechanisms and clinical applications. Clinical Autonomic Research, 13, 256–270. [DOI] [PubMed] [Google Scholar]
- Vo, L. , & Drummond, P. D. (2014). Analgesia to pressure‐pain develops in the ipsilateral forehead after high‐ and low‐frequency electrical stimulation of the forearm. Experimental Brain Research, 232, 685–693. [DOI] [PubMed] [Google Scholar]
- Vo, L. , & Drummond, P. D. (2016). Involvement of alpha2‐adrenoceptors in inhibitory and facilitatory pain modulation processes. European Journal of Pain, 20, 386–398. [DOI] [PubMed] [Google Scholar]
- Vollert, J. , Magerl, W. , Baron, R. , Binder, A. , Enax‐Krumova, E. K. , Geisslinger, G. , Gierthmühlen, J. , Henrich, F. , Hüllemann, P. , Klein, T. , Lötsch, J. , Maier, C. , Oertel, B. , Schuh‐Hofer, S. , Tölle, T. R. , & Treede, R. D. (2018). Pathophysiological mechanisms of neuropathic pain: Comparison of sensory phenotypes in patients and human surrogate pain models. Pain, 159, 1090–1102. [DOI] [PubMed] [Google Scholar]
- Vollert, J. , Maier, C. , Attal, N. , Bennett, D. L. H. , Bouhassira, D. , Enax‐Krumova, E. K. , Finnerup, N. B. , Freynhagen, R. , Gierthmühlen, J. , Haanpää, M. , Hansson, P. , Hüllemann, P. , Jensen, T. S. , Magerl, W. , Ramirez, J. D. , Rice, A. S. C. , Schuh‐Hofer, S. , Segerdahl, M. , Serra, J. , … Baron, R. (2017). Stratifying patients with peripheral neuropathic pain based on sensory profiles: Algorithm and sample size recommendations. Pain, 158, 1446–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer, J. C. (1977). Comparative study of perceived pain and nociceptive flexion reflex in man. Pain, 3, 69–80. [DOI] [PubMed] [Google Scholar]
- Willer, J. C. , Roby, A. , & Le Bars, D. (1984). Psychophysical and electrophysiological approaches to the pain‐relieving effects of heterotopic nociceptive stimuli. Brain, 107(Pt 4), 1095–1112. [DOI] [PubMed] [Google Scholar]
- Woolf, C. J. (2011). Central sensitization: Implications for the diagnosis and treatment of pain. Pain, 152, S2–s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel, A. , Andersen, O. K. , Nielsen, J. , & Arendt‐Nielsen, L. (2002). Heat hyperalgesia in humans: Assessed by different stimulus temperature profiles. European Journal of Pain, 6, 357–364. [DOI] [PubMed] [Google Scholar]
- Yucel, A. , Miyazawa, A. , Andersen, O. K. , & Arendt‐Nielsen, L. (2004). Comparison of hyperalgesia induced by capsaicin injection and controlled heat injury: Effect on temporal summation. Somatosensory & Motor Research, 21, 15–24. [DOI] [PubMed] [Google Scholar]
- Ziegler, E. A. , Magerl, W. , Meyer, R. A. , & Treede, R. D. (1999). Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A‐fibre nociceptor input. Brain, 122(Pt 12), 2245–2257. [DOI] [PubMed] [Google Scholar]


