ABSTRACT
The Dark Triad (DT), encompassing narcissism, Machiavellianism and psychopathy traits, poses significant societal challenges. Understanding the neural underpinnings of these traits is crucial for developing effective interventions and preventive strategies. Our study aimed to unveil the neural substrates of the DT by examining brain scans from 201 individuals (mean age: 32.43, 105 females) using the unsupervised learning algorithm transposed independent vector analysis (tIVA). tIVA, known for identifying complex patterns in neuroimaging data, detected 15 joint grey matter (GM) and white matter (WM) networks. Of these networks, four were associated with the DT. The first component comprises areas within the reward network, including the thalamus, caudate, anterior cingulate and prefrontal regions. The second component encompasses regions within the executive network, predominantly involving prefrontal and posterior areas. The third component includes regions within the default mode network (DMN), such as the angular gyrus, the precuneus and the posterior cingulate cortex. Lastly, the fourth component overlaps with areas of the visual network, primarily located in the occipital and temporal lobes. Within these networks, the reward‐related component correlated with narcissism, suggesting an association with the need for constant interpersonal rewards to enhance self‐esteem and grandiosity in narcissistic individuals. Conversely, the DM‐related component correlated with Machiavellianism, potentially reflecting the heightened strategic thinking employed by Machiavellian individuals for manipulation purposes. In line with established trends, sex differences emerged, with males displaying notably higher DT scores. Our findings offer insights into the intricate neurobiological bases of the DT personality and hold implications for future research and interventions.
Keywords: Dark Triad, personality traits, structural brain networks, transposed independent vector analysis, unsupervised machine learning

Abbreviations
- ACC
anterior cingulate cortex
- BSS
blind source separation
- CAT12
Computational Anatomy Toolbox
- DD
Dirty Dozen
- dlPFC
dorsolateral prefrontal cortex
- DMN
default mode network
- DT
Dark Triad
- DTI
diffusion tensor imaging
- DWI
diffusion‐weighted imaging
- fMRI
functional magnetic resonance imaging
- GM
grey matter
- ICA
independent component analysis
- MACH‐IV
Machiavellianism IV Scale
- MNI
Montreal Neurological Institute
- MP2RAGE
Magnetization Prepared 2 Rapid Acquisition Gradient Echoes
- mPFC
medial prefrontal cortex
- MRI
magnetic resonance imaging
- NIBS
non‐invasive brain stimulation
- NPI
Narcissistic Personality Inventory
- OFC
orbitofrontal cortex
- PCC
posterior cingulate cortex
- PFC
prefrontal cortex
- SD
standard deviation
- SD3
Short Dark Triad
- sMRI
structural magnetic resonance imaging
- SPM12
statistical parametric mapping
- SRP‐III
Self‐Report Psychopathy Scale
- tIVA
transposed independent vector analysis
- WM
white matter
1. Introduction
The term Dark Triad coined by Paulhus and Williams (2002) refers to three distinct personality characteristics linked to norm‐breaking, transgressive behaviour and manipulative tendencies (McDonald, Donnellan, and Navarrete 2012): narcissism, Machiavellianism and psychopathy. The extreme manifestations of these three underlying structures of the Dark Triad take a high toll in our society both when they are considered separately and together, accounting for burdensome financial, socioecological and psychological costs. For example, the engagement of psychopathic individuals in criminal activity causes societal monetary losses for almost $500 billion (Kiehl and Hoffman 2011). Furthermore, psychopathy has been found to be associated with a preference for volatile sexual encounters (Jonason et al. 2011; Jonason, Li, and Czarna 2013), which undermine the establishment of a romantic connection and may lead to unhappy relationships (Jonason, Li, and Buss 2010). Narcissism is associated with personal gains in the short term and with social detriments in the long term (Campbell et al. 2005). Finally, a study on a large Chinese sample (Zhu, Wang, and Geng 2021) showed how some of the components of Machiavellianism, such as distrust, were responsible for the unhealthy lifestyle conducted by individuals high on this trait.
Although they share some common characteristics, each trait of the Dark Triad has its own origins and definitions. Narcissism is characterized by a grandiose sense of entitlement, supremacy and superiority on the one hand and by a vulnerable sense of sensitivity, insecurity and inadequacy on the other hand, threatening interpersonal relationships (Cain, Pincus, and Ansell 2008; Jornkokgoud et al. 2023; Pincus, Cain, and Wright 2014). Machiavellians can be ruthless, cynic, deceptive and manipulative, exploiting others for short‐term personal advantage and lacking socio‐emotional comprehension and empathy (Ali, Sousa Amorin, and Chamorro‐Premuzic 2009; Barlow, Qualter, and Stylianou 2010; Brewer and Abell 2017; Rauthmann and Kolar 2013). Psychopathy is a severe personality disorder characterized by a constellation of detrimental traits (Glenn and Raine 2014). Traditionally, two overarching factors have been associated with psychopathy (Hare 2003): Factor 1, referring to the Interpersonal‐Affective facets and including callous, manipulative and lack‐of‐guilt tendencies, and Factor 2, referring to the Lifestyle‐Antisocial facets and including lack of self‐control and risk‐taking and antisocial behaviour (Glenn and Raine 2014; Hare 1996; Hare et al. 2000; Kiehl 2006). Recent perspectives on psychopathy view it as a spectrum of traits, ranging from subclinical to clinical levels. The Triarchic Model of Psychopathy (Patrick, Fowles, and Krueger 2009) identifies three core dimensions: boldness, meanness and disinhibition. Boldness involves traits like confidence, emotional stability and fearlessness. Meanness includes emotional detachment, callousness and a tendency to exploit others. Disinhibition is marked by impulsivity, recklessness and a propensity for externalizing behaviours. These dimensions are present to varying extents in existing psychopathy models and measures, such as the traditional two‐factor model (Patrick 2022). As clinical science increasingly adopts a dimensional approach to understanding and assessing personality disorders (APA 2013), a thorough understanding of subclinical traits can enhance the literature on personality pathology and improve the conceptualization of psychopathic traits.
These three Dark Triad traits have been measured separately, using the Machiavellianism‐IV Scale (MACH‐IV; Christie and Geis 1970), the Narcissistic Personality Inventory (NPI; Raskin and Hall 1979; Raskin and Terry 1988) and the Self‐Report Psychopathy Scale (SRP‐III; Paulhus, Neumann, and Hare 2009) to assess Machiavellianism, narcissism and psychopathy, respectively. Combining these three scales, two measures have been established to assess the three traits of the Dark Triad: the Dirty Dozen scale (DD; Jonason and Webster 2010), which includes just 12 items, and the Short Dark Triad (SD3; Jones and Paulhus 2014), which includes 27 items. These brief measures have the advantage of investigating each trait simultaneously while also reducing the amount of time needed for completion. Despite the fact that both measures have been demonstrated to have some significance in terms of statistical correlations with the individual scales (i.e., NPI, MACH‐IV and SRP‐III), the SD3 is more preferable and reliable in this respect (Maples, Lamkin, and Miller 2014).
Considering the neurobiological correlates of the Dark Triad, no existing study, to the best of our knowledge, has tried to investigate them as a single component. Only a few studies have looked at some of its sub‐components separately, leading to a still inchoate discovery of their underlying anatomical and functional alterations in the brain. For what concerns Machiavellianism, Verbeke et al. (2011) found that individuals high on this trait displayed significant variations in the basal ganglia, prefrontal cortex (PFC), insula, right hippocampus and left parahippocampal gyrus as opposed to individuals low on this trait. The basal ganglia, including the caudate and putamen, are crucial for learning, memory, social cognition and motivated behaviour (Davidson and Irwin 1999; Lieberman 2000; Verbeke et al. 2011). The PFC, particularly the orbital frontal cortex (OFC), is vital for planned social behaviour, decision‐making under uncertainty and emotional regulation (Elliott, Dolan, and Frith 2000; Lieberman 2007; Ohira et al. 2006; Tremblay and Schultz 1999). The insula has been demonstrated to processes emotional suppression, social risk decisions and risk perception (Ochsner et al. 2002; Preuschoff, Quartz, and Bossaerts 2008). The hippocampus is essential for memory formation and retrieval, with distinct involvement also noted in psychopathic behaviour (Laakso et al. 2001). All these are characteristics linked to Machiavellian traits (Verbeke et al. 2011). By the same token, in an MRI study exploring social cognition, Nestor et al. (2013) found that higher scores on the MACH‐IV were linked to more grey matter (GM) volume in the left lateral orbital gyrus.
Regarding narcissism, functional and structural alterations of insular regions seem to play an important role in the symptomatology exhibited by narcissistic individuals. As a matter of fact, the anterior insula has been established as a critical component of the neural network associated with empathy (Decety and Lamm 2006; Singer and Lamm 2009), which has been proposed to be diminished and dysfunctional in complex ways (Baskin‐Sommers, Krusemark, and Ronningstam 2014; Hart, Hepper, and Sedikides 2018; Urbonaviciute and Hepper 2020), if not lacking at all (Kernberg 1970; Kohut 1966), in narcissistic individuals. In this respect, Fan et al. (2011) found that reduced deactivation in the right anterior insula was correlated with increased levels of narcissism in an empathy condition during an fMRI study. Another study (Schulze et al. 2013), investigating 17 narcissistic personality disordered patients by using voxel‐based morphometry, found decreased GM volume in frontal‐paralimbic brain regions compared to healthy controls. In a recent investigation conducted by Jornkokgoud et al. (2023), the researchers employed machine learning techniques to delve into the structural configuration of the brain in individuals exhibiting narcissistic personality traits. The study revealed that a neural circuit encompassing the lateral and middle frontal gyri, the angular gyrus, Rolandic operculum and Heschl's gyrus effectively predicted the presence of narcissistic personality characteristics. The frontal gyrus and all the related areas have been shown to be linked to empathy and cognitive emotional processes (Li et al. 2020; Schulze et al. 2013). The Rolandic operculum has been associated with different disorders such as borderline personality disorder and addiction‐related disorders and plays a role in emotion processing and bodily self‐consciousness, potentially influencing emotional instability in narcissistic traits (Blefari et al. 2017; Schulze et al. 2013; Zhang et al. 2021). The angular gyrus is crucial for higher level social cognition and empathy and is implicated in abnormal self‐image and self‐other distinctions in narcissism (Decety and Lamm 2007; Ruby and Decety 2004). Lastly, the Heschl's gyrus, including the primary auditory cortex, is associated with auditory perception, language processing and possible abnormal internal dialogues in borderline personalities, with structural abnormalities linked to cognitive impairments and addiction‐related disorders (Karaali et al. 2016; Kasai et al. 2003; Zhang et al. 2021).
In psychopathic individuals, brain alterations have been observed as volume reductions and GM thinning in frontal, temporal and subcortical regions (Yang, Raine, Colletti, et al. 2009; Yang, Raine, Narr, et al. 2009; Yang et al. 2005). Specifically, the dorsolateral prefrontal cortex (dlPFC) and the orbitofrontal cortex (OFC) show significant structural and functional reductions compared to controls (De Oliveira‐Souza et al. 2008; Müller et al. 2008; Rilling et al. 2007). These prefrontal areas are crucial for decision‐making (Elliott, Dolan, and Frith 2000; Glenn and Raine 2014), understanding others' emotional states (Shamay‐Tsoory et al. 2005), regulating emotions (Ochsner et al. 2002), processing reward and punishment (Rolls 2000) and inhibiting responses (Völlm et al. 2006). Additionally, Muller et al. (2008) found GM reductions in the right superior temporal gyrus and the angular gyrus in psychopaths. The superior temporal gyrus is important for social cognition, perspective‐taking, mental state attribution and emotional attention (Müller et al. 2008), whereas the angular gyrus is involved in experiencing guilt and embarrassment, emotions that deter future antisocial behaviour (Glenn and Raine 2014; Takahashi et al. 2004). Subcortical regions, including the amygdala, parahippocampal gyrus, hippocampus and posterior cingulate, also show alterations in psychopathic individuals, affecting emotion regulation and empathy‐related functions (Ermer et al. 2013; Yang, Raine, Colletti, et al. 2009). Psychopathy is associated with deficits in responding to aversive stimuli, aversive conditioning, startle reflex augmentation, recognizing fearful expressions and experiencing moral emotions, all functions thought to rely on the functioning of the amygdala (Glenn and Raine 2014). Choy, Raine, and Schug (2022) recently found increased striatal volume in adult psychopaths, linked to traits such as reward seeking and impulsivity (Glenn and Raine 2014). Moreover, psychopathic individuals exhibit abnormal activation in the default mode network (DMN; Freeman et al. 2015; Juarez, Kiehl, and Calhoun 2012; Pujol et al. 2012), which is typically active during rest and self‐referential processing (Buckner and Carroll 2007; Northoff et al. 2006) but deactivates during externally‐focused tasks to engage ‘task‐positive’ networks with minimal interference (Raichle et al. 2001).
However, despite the efforts to map Machiavellianism, psychopathy and narcissism onto the brain, mixed and puzzling results hinder our understanding of how the brain is organized in individuals displaying all these traits. For example, Cope et al. (2012) found that the anterior cingulate cortex (ACC) was affected in people with psychopathic characteristics, whereas Glenn et al. (2010) had not found this result in a previous study. Similarly, the extent to which the activity of regions associated with empathy and emotion regulation (e.g., insula) is altered in narcissists is still unclear given their complex relationship (Baskin‐Sommers, Krusemark, and Ronningstam 2014). By the same token, whereas Bereczkei et al. (2013; 2015) found a heightened activation in the ACC of individuals with high Machiavellian traits during a trust game and interpreted it as the sign of an augmented emotional conflict between social norms and personal interest, Verbeke et al. (2011) did not find any structural, GM abnormality in the ACC in their study.
The mixed results in previous research may stem from methodological limitations. First, previous studies have predominantly relied on the use of mass‐univariate statistical techniques, which take into account alterations within single voxels rather than among multiple voxels. However, the contemporary understanding of brain functioning considers psychiatric and neurological symptomatology as associated with distributed patterns of voxel alterations rather than with isolated voxel anomalies (Biswal et al. 2010; Fox et al. 2005; Kennedy and Courchesne 2008; Sheffield and Barch 2016). Additionally, previous studies have relied on group‐level statistics, not allowing for drawing inferences at the individual level (Grecucci et al. 2022; Lapomarda, Grecucci, et al. 2021; Lapomarda, Pappaianni, et al. 2021; Scarpazza et al. 2020). Finally, the contribution of the white matter (WM) to the Dark Triad has yet to be considered.
Affective neuroscientists have recently shifted their attention towards the adoption of machine learning techniques. These techniques have the advantage to explore the intricate relationships among multiple brain voxels compared to traditional statistical methods, detecting subtle and distributed alterations in brain structure and function (Grecucci et al. 2022; Hebart and Baker 2018; Vieira, Pinaya, and Mechelli 2020). In other words, machine learning goes beyond assessing each individual voxel and comprehensively examines the relationship of multiple voxels simultaneously (Grecucci et al. 2022; Hebart and Baker 2018).
One type of machine learning methods is unsupervised learning, which allows researchers to unveil concealed patterns in complex datasets for a deeper understanding of large‐scale brain networks (Vieira, Pinaya, and Mechelli 2020). Unsupervised learning can also exploit data fusion strategies (Baggio et al. 2023), which, combining mutual information from different modalities such as GM and WM concentration, offer a holistic view of brain structure and function. Multivariate machine learning approaches hold promise for advancing our understanding of the complexities of the brain and its relationship with affective and personality processes.
From a methodological standpoint, the main purpose of this paper was to provide new evidence on the neural bases of DT traits by using unsupervised machine learning in the form of dimensionality reduction applied to GM and WM features in a data fusion perspective in combination with backward stepwise regression statistics. The idea was to use unsupervised learning to decompose the whole brain into independent circuits of covarying GM and WM and then to predict which brain circuit explained the DT. This way, reducing the number of features (e.g., voxels) had the advantage to limit demanding computations, ignore redundancy and only focus on meaningful information and reduce the risk of overfitting (Guyon and Elisseeff 2003).
First, unsupervised machine learning in the form of transposed independent vector analysis (tIVA) (Adali, Levin‐Schwartz, and Calhoun 2015a, 2015b; Lee et al. 2008) was used to decompose the brain into independent neural circuits. This brain decomposition method allows for a reduction of the dimensionality of the state space (≃105 brain voxels) into a few naturally grouping brain networks (Ghomroudi, Scaltritti, and Grecucci 2023; Grecucci et al. 2022). These networks are more biologically plausible than atlas‐based brain parcellations based on anatomical and histological features not based on structural or temporal covariation (regions that work together form a specialized network with a common spatial and temporal profile). This approach is in accordance with the fact that personality traits are not ascribed to one specific region in the brain, but they are distributed across different networks of brain areas (Biswal et al. 2010; Fox et al. 2005; Kennedy and Courchesne 2008; Sheffield and Barch 2016). In addition, the decision to include both GM and WM components in our analyses is motivated by the fact that they both can be informative and influenced by similar genetic factors (Baggio et al. 2023; Spalletta, Piras, and Gili 2018). As a matter of fact, pathological processes affect WM as well and are not only limited to GM (Spalletta, Piras, and Gili 2018). WM studies often use diffusion tensor imaging (DTI), which is a technique that, measuring the microstructural displacement of water molecules, assesses the integrity of WM fibres (Assaf and Pasternak 2007; Alba‐Ferrara and de Erausquin 2013). However, DTI is extremely sensitive to noise, and this might result in poor reproducible regions of interest (Radwan et al. 2022). In this regard, the relevance of adopting a combined GM–WM approach resides in the fact that considering pure WM concentrations has the advantage to evaluate distributed WM alterations without the restrictions imposed by specific tracts (Baggio et al. 2023).
We predicted that three brain circuits specifically could explain Dark Triad scores on the SD3: a circuit with areas at least partly overlapping with the reward network, primarily including the ACC, the OFC, the basal ganglia, the amygdala, the hippocampus and the thalamus (Haber and Knutson 2010); a circuit with areas at least partly overlapping with the executive network, primarily including the dlPFC and the lateral posterior parietal cortex; and a circuit with areas at least partly overlapping with the DMN, primarily including the medial PFC (mPFC), the posterior cingulate cortex (pCC), the precuneus and the angular gyrus (Raichle et al. 2001). We hypothesized these brain circuits based on their potential relevance to the DT traits and their associated functions in reward processing, executive control and thinking processes, which are aspects that we find deficient in individuals displaying these personality traits.
Furthermore, we did an exploratory analysis to examine whether the three networks predicted could be linked to specific subscales of the SD3. We expected to find a correspondence between narcissistic traits and specific brain regions coinciding with those within the reward network. Likewise, we expected to observe a correlation between Machiavellian traits and brain areas overlapping with the DMN. Additionally, we expected to identify a relationship between psychopathic traits and brain regions that intersect with both the reward and executive networks. This delineation of the expected associations aims to enhance the characterization of how distinct DT traits may be represented at a neurological level. As a matter of fact, we aim to advance our understanding of the neural correlates of the DT, both as a single and separate component, by considering the unique contribution of both GM and WM.
Additionally, we investigated how age and gender would affect these three networks in the brain. Previous studies (Cale and Lilienfeld 2002; Grijalva et al. 2015; Krampen et al. 1990; Nicholls et al. 2005) have consistently found that all the sub‐traits associated with the DT are more prevalent in males as opposed to females. Although these studies have examined sex differences in each specific DT sub‐trait, there remains a gap in understanding whether there are overall sex disparities in the DT as a collective construct. We expected to find a discernible sex‐based disparity in the collective manifestation of the DT traits, indicating an elevated presence of these traits in males relative to females.
2. Materials and Methods
2.1. Participants
Participants recruited for this study were 214 German native speakers, selected from the ‘MPI‐Leipzig Mind‐Brain‐Body’ database (OpenNeuro Dataset, https://openneuro.org, accession number ds000221; Babayan et al. 2020; Harvard Dataverse 2020), which consists of structural and functional MRI and behavioural data from 321 German‐speaking people. Participants of the ‘MPI‐Leipzig Mind‐Brain‐Body’ database were selected according to specific selection criteria (see below), including completion of the SD3, medical eligibility for magnetic resonance sessions and absence of past or present psychiatric and neurological disorders. Seventeen participants were excluded for the present study because they were 70 or older years of age to avoid possible ageing alteration effects, leading to a final sample of 201 participants (105 females) between the ages of 20 and 69 (M = 32.43, SD = 13.92).
2.1.1. Exclusion Criteria
History of psychiatric diseases that required inpatient treatment for longer than 2 weeks within the last 10 years (e.g., psychosis, attempted suicide and post‐traumatic stress disorder);
History of neurological disorders (incl. multiple sclerosis, stroke, epilepsy, brain tumour, meningoencephalitis, and severe concussion);
History of malignant diseases;
- Intake of one of the following medications:
-
○Any centrally active drugs (including Hypericum perforatum )
-
○Beta‐ and alpha‐blocker
-
○Cortisol
-
○Any chemotherapeutic or psychopharmacological medication;
-
○
Positive drug anamnesis (extensive alcohol, MDMA, amphetamines, cocaine, opiates, benzodiazepine and cannabis);
Extensive testing experience at the MPI‐CBS or other academic institution;
Past or present student of psychology;
- MRI exclusion criteria
-
○Any metallic implants, braces and non‐removable piercings
-
○Tattoos
-
○Pregnancy
-
○Claustrophobia
-
○Tinnitus
-
○Surgical operation in the last 3 months
-
○
Note: Adapted from Mendes et al. (2019).
Written informed consents were obtained by all participants, who received financial compensations for their participation. The study protocol was approved by the ethics committee of the University of Leipzig (097/15‐ff) (154/13‐ff) (Babayan et al. 2019; Mendes et al. 2019).
2.2. SD3 Questionnaire
The SD3 is a shorter and combined version of the MACH‐IV, the NPI and the SPR‐III and was created to assess the DT by focusing on the definitions and aspects of the three distinct traits (Jones and Paulhus 2014; Paulhus and Jones 2015). It is composed of 27 items—nine items for each of the three traits that form the DT—that can be rated on a 5‐point Likert scale (1 being strongly disagree to 5 being strongly agree) (Jones and Paulhus 2014). Examples of the items are ‘Make sure your plans benefit you, not others’ for Machiavellianism, ‘I know I am special because everyone tells me so’ for narcissism and ‘People who mess with me always regret it’ for psychopathy. For our purpose, the English version of the SD3 was translated into German. Reliability analyses for the SD3 were satisfactory, having obtained Cronbach's alpha of 0.68 for Machiavellianism (English original: α = 0.78), Cronbach's alpha of 0.65 for narcissism (English original: α = 0.77) and Cronbach's alpha of 0.59 for psychopathy (English original: α = 0.80). SD3 total scores were calculated by summing the scores of the individual DT traits.
The descriptive statistics for each dimension of the SD3 are presented in Table S1. The means, standard deviations, standard errors, minimums, maximums, ranges and 95% confidence intervals for each dimension are reported.
Paulhus (https://www.psytoolkit.org/survey‐library/short‐dark‐triad.html) proposed the normal ranges for each of the three traits (Table S2). The descriptive statistics revealed mean scores of 20.74 for Machiavellianism, 24.50 for narcissism and 18.66 for psychopathy. When comparing these scores to the normal ranges in Table S2, adjusted to account for the summing of 9 items per dimension, we see that our sample's scores fall within the normal range. Specifically, the scaled normal ranges for Machiavellianism, narcissism and psychopathy are mean scores of 27.9, 25.2 and 21.6 respectively, with corresponding thresholds for being outside the normal range at scores of 34.74 for Machiavellianism, 33.12 for narcissism and 30.6 for psychopathy. Given these comparisons, our participants' mean suggests that they exhibit normal levels of these traits.
2.3. MRI Data Acquisition
T1‐weighted images were acquired using a 3‐T Siemens MAGNETOM Verio scanner (Siemens Healthcare GmbH, Erlangen, Germany), which was built with a 32‐channel head coil. The data collection took place at the Day Clinic for Cognitive Neurology of the University Clinic Leipzig and the Max Planck Institute for Human and Cognitive and Brain Sciences (MPI CBS) in Leipzig, Germany. The original MPI‐Leipzig Mind‐Brain‐Body dataset comprised structural magnetic resonance imaging (sMRI), functional magnetic resonance imaging (fMRI) and diffusion‐weighted imaging (DWI) scans (Babayan et al. 2019), but, for the purposes of our study, we just considered the T1‐weighted images. The high‐resolution structural image was acquired by using a 3D MP2RAGE sequence (Marques et al. 2010) consisting of the following parameters: sagittal acquisition orientation, one 3D volume with 176 slices, TR = 5000 ms, TE = 2.92 ms, TI1 = 700 ms, TI2 = 2500 ms, FA1 = 4°, FA2 = 5°, pre‐scan normalization, echo spacing = 6.9 ms, bandwidth = 240 Hz/pixel, FOV = 256 mm, voxel size = 1 mm isotropic, GRAPPA acceleration factor 3, slice order = interleaved, duration = 8 min 22 s.
2.4. Preprocessing
After conducting a quality check on the images in order to exclude artefacts and before running any analyses, all data were preprocessed with the same pipeline using the segmentation operations offered by the Computational Anatomy Toolbox (CAT12, http://www.neuro.uni‐jena.de/cat/), a toolbox available for the Statistical Parametric Mapping software (SPM12, https://www.fil.ion.ucl.ac.uk/spm/software) in the MATLAB environment. This way, after reorienting each image and placing the anterior commissure as the origin, segmentation of GM, WM and cerebrospinal fluid was computed. The registration was computed utilizing Diffeomorphic Anatomical Registration through Exponential Lie algebra tools (DARTEL) (Ashburner 2007), which is a whole‐brain‐approach alternative to SPM12. Finally, DARTEL images were normalized to the MNI space using a spatial Gaussian smoothing of 12 (full width at half maximum of Gaussian smoothing kernel [12, 12, 12]) (Pappaianni et al. 2017).
2.5. tIVA
tIVA is a blind source separation (BSS) method, which provides a fully multivariate approach and enables fusion of data from multiple modalities, such as GM and WM and then decompose the brain into joint GM‐WM profiles (Adali, Levin‐Schwartz, and Calhoun 2015a, 2015b). The tIVA algorithm allows for the extraction of brain circuits that are maximally dependent across modalities while being independent among themselves within a modality, and this makes tIVA a more suitable algorithm when the correlation among components across modalities are significantly different as opposed to other solutions (e.g., joint independent component analysis; Adali, Levin‐Schwartz, and Calhoun 2015a, 2015b). We applied tIVA to structural data by using the Fusion ICA Toolbox (FIT, http://mialab.mrn.org/software/fit) (Calhoun et al. 2006) in the MATLAB 2018a environment (https://it.mathworks.com/products/matlab.html) (MATLAB (R2018a)) in order to compute the network decomposition, which consisted of separating the brain into naturally grouping circuits. Then, an estimation of the number of components for both modalities was conducted following default parameters. To investigate the reliability of each modality, the ICASSO (Himberg and Hyvärinen 2003; Himberg, Hyvärinen, and Esposito 2004) and the Infomax algorithm were used. The resulting output consisted of a matrix with the number of subjects (rows) and the loading coefficients for each component (columns). Loading coefficients represent how each component is expressed for every subject; for example, considering GM concentration, loading coefficients refer to how much GM concentration each subject has in each component compared to an average across the brain. Subsequently, the groupICA software (https://trendscenter.org/software/) was used to convert the independent components into Talairach coordinates in order to specify the brain areas associated with them. Finally, we took into consideration brain areas with both higher (positive values) and lower (negative values) GM and WM concentration and plotted them in Surf Ice (https://www.nitrc.org/projects/surfice/) (Rorden) (using a different template for GM and WM).
2.6. Stepwise Regression Analysis
After having decomposed the brains of our participants in spatially independent circuits, we entered the loading coefficients of each network for each participant in a regression model to predict DT scores from the identified brain networks. A backward stepwise regression model was used in JASP (Jasp Team (Version 0.16.2), 2022). All predictors were initially entered simultaneously and, then, were removed sequentially based on predefined criteria. The criteria for predictor entry were determined by a significance level of p < 0.05, whereas the removal criterion was set at a threshold of 0.1. Figure 1 shows a simplified representation of the pipeline (tIVA + regression) used to decompose the brains of our participants and predict the personality traits.
FIGURE 1.

Unsupervised machine learning tIVA was used to combine the two modalities (GM and WM) and to decompose the brain into the covarying GM‐WM independent circuits. Then backward stepwise regression was applied to predict DT scores.
3. Results
3.1. Unsupervised Machine Learning to Decompose the Brain Into Covarying GM–WM Circuits
The tIVA algorithm detected 15 distinct GM and WM brain networks that were independent yet correlated. These networks were derived in a data‐driven manner, allowing for the decomposition of participants' brains into individual components using the information theoretic criteria (Wax and Kailath 1985). Each estimated GM component had a corresponding estimated WM component that exhibited a similar pattern of concentration. Positive values in these networks indicated increased concentration of GM/WM, whereas negative values suggested decreased concentration. Fundamentally, the covariation between GM and WM components implied that when GM concentration was high in a specific circuit (e.g., tIVA‐GM12), the corresponding WM concentration in that circuit (tIVA‐WM12) was also high.
3.2. Backward Stepwise Regression
From the backward stepwise regression analysis, we extracted one significant winning model for the prediction of DT scores total, which included the least number of components (R = 0.252, R 2 = 0.064, adjusted R 2 = 0.044, RMSE = 8.956, F = 3.323, p = 0.012). This model included four tIVA components with a significant p value (tIVA4 = 0.032; tIVA5 = 0.020; tIVA12 = 0.047; tIVA13 = 0.040). See Figures 2, 3, 4, 5 for a visual representation of the network areas (see Tables S1–S4 for their anatomical denominations). It is worth noting to mention that, when age is included as a covariate, the results remain the same.
FIGURE 2.

tIVA‐4 threshold was set at z‐score > 2. From left to right are displayed brain plots of tIVA‐GM 4 of the left hemisphere in medial view, of the left hemisphere in lateral view and of both hemispheres in inferior view (top) and brain plots of tIVA‐WM 4 of the right hemisphere in lateral view, of the right hemisphere in medial view and of both hemispheres in superior view. Regions with increased GM and WM are represented with warm colours, whereas regions with decreased GM and WM are represented with cold colours.
FIGURE 3.
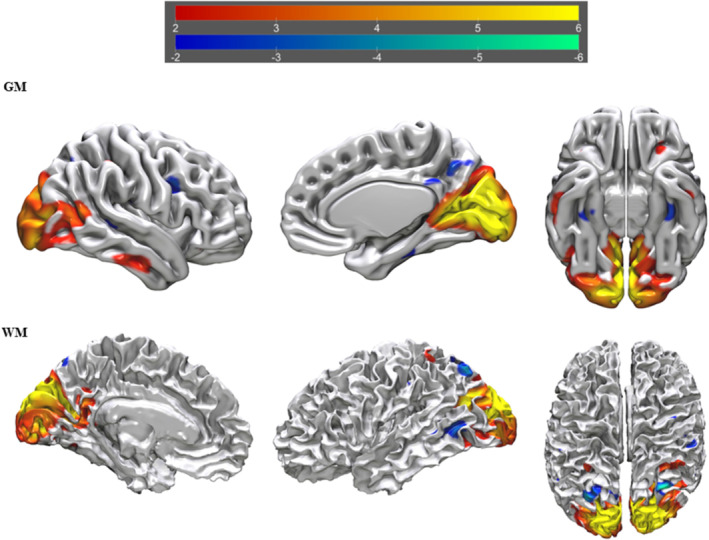
tIVA‐5 threshold was set at z‐score > 2. From left to right are displayed brain plots of tIVA‐GM 5 of the right hemisphere in lateral view, of the right hemisphere in medial view and of both hemispheres in inferior view (top) and brain plots of tIVA‐WM 5 of the left hemisphere in medial view, of the left hemisphere in lateral view and of both hemispheres in superior view. Regions with increased GM and WM are represented with warm colours, whereas regions with decreased GM and WM are represented with cold colours.
FIGURE 4.

tIVA‐12 threshold was set at z‐score > 2. From left to right are displayed brain plots of tIVA‐GM 12 of the right hemisphere in lateral view, of the right hemisphere in medial view and of both hemispheres in inferior view (top) and brain plots of tIVA‐WM 12 of the right hemisphere in lateral view, of the right hemisphere in medial view and of both hemispheres in superior view. Regions with increased GM and WM are represented with warm colours, whereas regions with decreased GM and WM are represented with cold colours.
FIGURE 5.

tIVA‐13 threshold was set at z‐score > 2. From left to right are displayed brain plots of tIVA‐GM 13 of the left hemisphere in medial view, of the left hemisphere in lateral view and of both hemispheres in superior view (top) and brain plots of tIVA‐WM 13 of the left hemisphere in medial view, of the left hemisphere in lateral view and of both hemispheres in superior view. Regions with increased GM and WM are represented with warm colours, whereas regions with decreased GM and WM are represented with cold colours.
Upon subsequent analyses, we conducted correlation analyses (see Figures S6 and S7 for the distributions of Dark Triad traits and for the choice of the statistical test based on these distributions) to examine the relationship between the 4 tIVA estimated components and the specific Dark Triad subscales (psychopathy, narcissism and Machiavellianism). Interestingly, we found that the tIVA‐4 component was negatively correlated with narcissistic traits (Spearman's ρ = −0.148, p < 0.037), whereas the tIVA‐13 component was positively correlated with Machiavellian traits (Spearman's ρ = 0.160, p < 0.024; Figure 6).
FIGURE 6.
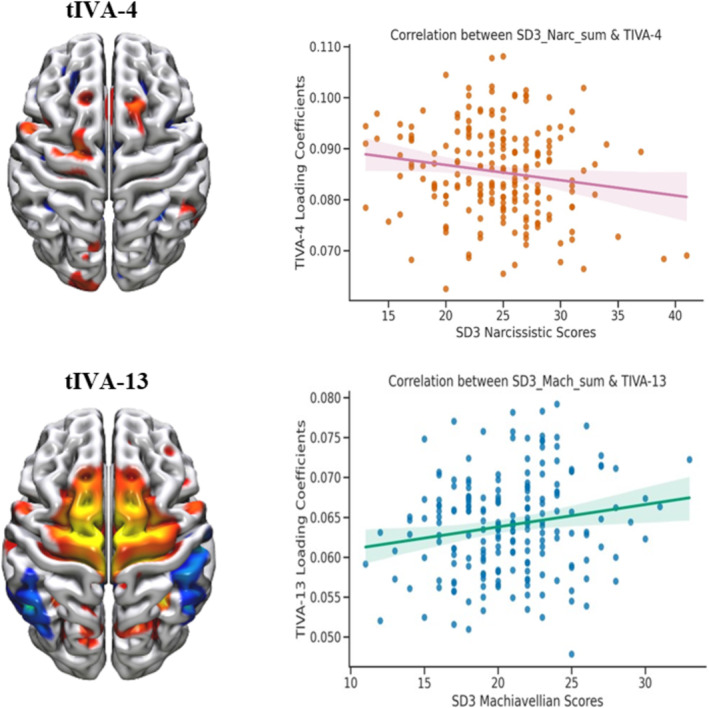
Brain circuits correlating with narcissism and Machiavellianism. Top: Brain plot depicting the tIVA‐4 component (left) and its correlation plot showing a negative association with narcissism scores (right). Bottom: Brain plot for the tIVA‐13 component (left) and its correlation plot showing a positive association with Machiavellianism scores (right).
3.3. Relationship Between Sex, Age and Dark Triad Traits
Participants were equally distributed by sex (96 females and 105 males), and the range of age in years went from 20 to 70. Participants had an average overall DT total score of 63.90 (SD = 9.16) out of a maximum of 135 on the SD3 questionnaire, with an average score of 20.74 (SD = 3.80), 24.50 (SD = 4.72) and 18.66 (SD = 4.27) on the Machiavellianism, narcissism and psychopathy subscales, respectively. Analysing the results separately for males and females, we found that males had an average DT total score of 65.73 and females had an average DT total score of 61.89. The SD3 scores of male participants (M = 65.73, SD = 9.20) were statistically significantly higher than the SD3 scores obtained by female participants (M = 61.89, SD = 8.73). For what concerns age effect on the DT, Pearson's correlation revealed that there was no significant association between age and Machiavellianism (r = 0.104; p = 0.142), between age and narcissism (r = −0.062; p = 0.384), between age and psychopathy (r = −0.120; p = 0.090) and between age and DT total (r = −0.045; p = 0.530).
4. Discussion
In our study, we investigated the neural correlates of the DT traits (narcissism, Machiavellianism and psychopathy) by using the unsupervised machine learning technique tIVA (Adali, Levin‐Schwartz, and Calhoun 2015a, 2015b) to determine the joint contribution of GM and WM concentration. To our knowledge, this is the first time that the tIVA algorithm has been applied to unravel the neurobiological underpinnings of the DT.
Through the utilization of tIVA, we were able to decompose, in a data‐driven perspective, the brain into independent neural circuits, which provided a more biologically plausible representation of the structural organization of the brain compared to traditional anatomical parcellations (Ghomroudi, Scaltritti, and Grecucci 2023; Grecucci et al. 2022). Our findings revealed significant associations between specific neural circuits and the DT. Of the15 naturally independent neural networks detected by the tIVA algorithm, four were predictive of DT traits. Specifically, the tIVA‐4 component comprises areas within the reward network, including the thalamus, caudate, anterior cingulate and prefrontal regions (see Table S1). The tIVA‐12 component encompasses regions within the executive network, predominantly involving prefrontal and posterior areas (see Table S3). The tIVA‐13 component consists of regions within the DMN, such as the angular gyrus, the precuneus and the posterior cingulate cortex (see Table S4). However, unexpected findings were observed with the tIVA‐5 component, which overlaps with areas of the visual network, primarily located in the occipital and temporal lobes (see Table S2). Below, we discuss our findings and their implications.
4.1. The Role of Reward, Default and Executive Networks in the Dark Triad
Interestingly, in examining the individual subscales of the DT and their relationships with the predicted neural networks, we noticed specific patterns. The tIVA‐4 circuit correlated with narcissistic traits, and the tIVA‐13 circuit was specifically linked to Machiavellian traits (see Figure 6). Furthermore, the tIVA‐4 component showed a reduced GM and WM concentration, whereas the tIVA‐13 component exhibited an increase in GM and WM concentration (see Figure 6). This distinction is noteworthy.
Narcissistic traits, characterized by the absence of certain qualities such as self‐control and empathy (Jornkokgoud et al. 2023; Vazire and Funder 2006) as well as by the relentless pursuit of rewards and attention to maintain self‐esteem and grandiosity (Buss and Chiodo 1991; Chester et al. 2016; Lakey et al. 2008), are well documented. The observed reduction in brain concentration in areas related to reward and emotion regulation might reflect these deficits (Decety and Lamm 2006; Singer and Lamm 2009). In contrast, the heightened thinking strategies linked to the manipulative nature of Machiavellian individuals (Jones and Paulhus 2011) could be explained by the elevated brain concentration in specific regions involved in decision making, evaluating pros and cons and formulating strategic plans for achieving long‐term goals (Ali, Sousa Amorin, and Chamorro‐Premuzic 2009; Barlow, Qualter, and Stylianou 2010; Wilson, Near, and Miller 1996).
Specifically, in terms of narcissism, our findings suggest that the anterior insula plays a crucial role in the constellation of traits displayed by narcissistic individuals. The structural alterations observed in the insular regions align with previous research that emphasizes the involvement of the insula in empathy and emotion regulation (Decety and Lamm 2006; Singer and Lamm 2009). Specifically, we observed reduced GM concentration in the right anterior insula, which was associated with higher levels of narcissism. This finding implies diminished empathic abilities in narcissistic individuals (Fan et al. 2011). Moreover, the decreased volume of GM and WM concentration in frontal‐paralimbic brain regions provides additional support for the notion that structural alterations contribute to narcissistic traits (Schulze et al. 2013). As a matter of fact, Chester et al. (2016) found that grandiose narcissistic individuals had a frontostriatal weakened connectivity, suggesting that it might reflect their pursual of external affirmation as a way to make up for the gap between their actual and expected self‐rewards.
In relation to Machiavellianism, our findings indicate significant variations in several brain regions, including the basal ganglia, PFC, insula, right hippocampus, left parahippocampal gyrus and areas of the DMN such as the angular gyrus, the precuneus and the PCC. These results are consistent with prior research (Collins and Persinger 2014; Nestor et al. 2013; Verbeke et al. 2011) that suggests that individuals high in Machiavellianism display altered neural functioning in regions associated with social cognition and emotional processing. Furthermore, our study demonstrates an altered involvement of the cingulate gyrus, supporting the findings of Bereczkei et al. (2013), who discovered increased activation in the ACC among individuals high in Machiavellianism during a trust game. This suggests that the cingulate gyrus plays a role in the emotional conflict experienced by individuals with high Machiavellian tendencies during social interactions. The increased concentration of GM and WM in these areas might suggest a relationship with the heightened thinking strategies of Machiavellians to manipulate others and successfully achieve their objectives. These findings open avenues for future research to further investigate this hypothesis.
Regarding the investigation of psychopathy, our study did not reveal a specific neural circuit strongly associated with this trait. Two plausible explanations may account for this outcome. Firstly, it is conceivable that the range of psychopathic scores within the study sample may not have exhibited as much variability as observed for the other two DT sub‐traits, potentially contributing to the absence of statistically significant findings. Secondly, it is possible that psychopathy does not solely rely on one circuit, but rather on a combination of the circuits under investigation. This latter hypothesis finds support in an unexpected observation: the involvement of the tIVA‐5 component, which overlapped with brain regions associated with the visual network. Existing literature (De Oliveira‐Souza et al. 2008; Tiihonen et al. 2008) suggests an imbalance between posterior and anterior brain regions in favour of the former in individuals with high psychopathic traits, potentially leading to reduced activity in the latter. Future studies may consider further investigation into this hypothesis.
4.2. Sex Differences in the Dark Triad
Furthermore, our analyses revealed that male participants had significantly higher scores on the DT traits as opposed to female participants. The significant sex differences observed align with previous research (Grijalva et al. 2015; Cale and Lilienfeld 2002; Klimstra et al. 2020; Krampen et al. 1990; Muris et al. 2017; Nicholls et al. 2005; Vize et al. 2018). This suggests that males tend to exhibit higher levels of DT traits compared to females. Recognizing and understanding sex differences within the DT can have implications for various domains, particularly in the context of psychology and interpersonal relationships (Jones and Paulhus 2014).
4.3. Implications of Understanding the Neural Correlates of the Dark Triad
In recent years, a consensus has emerged in research advocating biological interventions to reduce antisocial and aggressive behaviour (Beauchaine et al. 2008; Hübner and White 2016; Raine et al. 2015). A better comprehension of the neural correlates of the DT is pivotal in informing such interventions for individuals exhibiting pathological narcissistic, Machiavellian and psychopathic traits, which are often associated with antisocial and aggressive behaviour (McDonald, Donnellan, and Navarrete 2012; Paulhus and Williams 2002). For example, studies on non‐invasive brain stimulation (NIBS), a technique involving the application of electrical or magnetic fields to specific brain regions to modulate their activity, demonstrate its potential in reducing antisocial and aggressive tendencies and enhancing empathy and moral judgement by targeting areas of the PFC (Choy 2021; Choy, Raine, and Hamilton 2018; Sergiou et al. 2020). Therefore, one of the implications of understanding the brain networks underlying the DT could be the targeted application of NIBS to address the detrimental behaviours associated with the DT. However, research is needed to explore this application thoroughly and its ethical implications.
5. Conclusions and Limitations
The present study offers new insights into the neurobiology of the DT traits by utilizing a data fusion approach that combines unsupervised machine learning and traditional backward stepwise regression. This methodology allowed us to dissect the brain of our participants into independent neural circuits, revealing the distributed nature of DT traits across various brain regions. The inclusion of both GM and WM components enhanced the comprehensiveness of our findings and shed light on the structural alterations associated with these personality characteristics.
However, it is important to acknowledge some limitations of our study and to point to future directions. Firstly, we solely focused on structural brain features, and future research could explore the fusion of structural and functional MRI data, such as resting state or task‐related scans. Additionally, our study relied on self‐report measures, such as the SD3 questionnaire, which introduces the potential for response biases and social desirability effects. The accuracy of our measurements may have been influenced by participants underreporting or overreporting their DT traits. To enhance the validity of the findings, future studies should consider integrating self‐report measures with other assessment methods, such as behavioural observations or clinician ratings. Moreover, despite the focus of this study was on subclinical traits and, therefore, our sample comprised individuals who did not exhibit exceptionally high levels of DT traits, in future research, it may be worthwhile to compare individuals with high DT scores to those with low DT scores, allowing for a more comprehensive examination. Furthermore, although our sample size was larger than previous studies in this domain, larger samples would be beneficial to confirm and validate our results. Lastly, combining unsupervised and supervised machine learning techniques could facilitate the development of predictive models for individual differences in DT traits using GM and WM features, allowing for a generalization to the whole population because these models could be tested on new unobserved cases (Grecucci et al. 2022).
Ultimately, our study represents an application of data fusion and machine learning in examining the DT traits. Overall, these findings deepen our understanding of the DT by highlighting sex differences, trait stability across age and the neural correlates of specific traits. The insights gained might have important implications for clinical intervention strategies and future research directions, ultimately contributing to a more comprehensive understanding of the DT and its impact on individuals and society.
Author Contributions
Richard Bakiaj: conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, validation, visualization, writing – original draft. Clara Isabel Pantoja Muñoz: software, visualization. Andrea Bizzego: formal analysis, methodology, visualization. Alessandro Grecucci: conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, supervision, validation, visualization.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/ejn.16674.
Supporting information
Table S1 Descriptive statistics of the SD3.
Table S2. Normal ranges for the Dark Triad traits.
Table S3. Brain areas for tIVA‐4.
Table S4. Brain areas for tIVA‐5.
Table S5. Brain areas for tIVA‐12.
Table S6. Brain areas for tIVA‐13.
Figure S6. Histograms with kernel density estimate (KDE) plots to visualize the distribution of the data.
Figure S7. QQ plots for assessing normality of SD3 dimensions.
Table S7. Normality test for Dark Triad traits.
Associate Editor: Agustin Ibanez
Data Availability Statement
The dataset analysed during the current study is available in the MPI‐Leipzig Mind‐Brain‐Body repository (https://openneuro.org/datasets/ds000221/versions/00002). The complete LEMON and N&C Data can be downloaded through this link (https://openneuro.org/datasets/ds000221/versions/00002/download). All data were downloaded using S3: aws s3 sync ‐‐no‐sign‐request s3://openneuro.org/ds000221 ds000221‐download/ (accessed 1 November 2022).
References
- Adali, T. , Levin‐Schwartz Y., and Calhoun V. D.. 2015a. “Multimodal Data Fusion Using Source Separation: Two Effective Models Based on ICA and IVA and Their Properties.” Proceedings of the IEEE 103, no. 9: 1478–1493, 7206517. 10.1109/JPROC.2015.2461624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adali, T. , Levin‐Schwartz Y., and Calhoun V. D.. 2015b. “Multimodal Data Fusion Using Source Separation: Application to Medical Imaging.” Proceedings of the IEEE 103, no. 9: 1494–1506. 10.1109/jproc.2015.2461601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba‐Ferrara, L. , and de Erausquin G. A.. 2013. “What Does Anisotropy Measure? Insights From Increased and Decreased Anisotropy in Selective Fiber Tracts in Schizophrenia.” Frontiers in Integrative Neuroscience 7: 9. 10.3389/fnint.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, F. , Sousa Amorin I., and Chamorro‐Premuzic T.. 2009. “Empathy Deficits and Trait Emotional Intelligence in Psychopathy and Machiavellianism.” Personality and Individual Differences 47: 758–762. 10.1016/j.paid.2009.06.016. [DOI] [Google Scholar]
- APA (American Psychiatric Association) . 2013. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Ashburner, J. 2007. “A Fast Diffeomorphic Image Registration Algorithm.” NeuroImage 38, no. 1: 95–113. 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Assaf, Y. , and Pasternak O.. 2007. “Diffusion Tensor Imaging (DTI)‐Based White Matter Mapping in Brain Research: A Review.” Journal of Molecular Neuroscience 34, no. 1: 51–61. 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Babayan, A. , Baczkowski B., Cozatl R., et al. 2020. “MPI‐Leipzig‐Mind‐Brain‐Body (1.0.0) [Dataset].” Franklin Feingold. 10.18112/openneuro.ds000221.v1.0.0. [DOI]
- Babayan, A. , Erbey M., Kumral D., et al. 2019. “A Mind‐Brain‐Body Dataset of MRI, EEG, Cognition, Emotion, and Peripheral Physiology in Young and Old Adults.” Scientific Data 6, no. 1: 180308. 10.1038/sdata.2018.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio, T. , Grecucci A., Meconi F., and Messina I.. 2023. “Anxious Brains: A Combined Data Fusion Machine Learning Approach to Predict Trait Anxiety From Morphometric Features.” Sensors 23, no. 2: 610. 10.3390/s23020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow, A. , Qualter P., and Stylianou M.. 2010. “Relationships Between Machiavellianism, Emotional Intelligence, and Theory of Mind in Children.” Personality and Individual Differences 48, no. 1: 78–82. 10.1016/j.paid.2009.08.021. [DOI] [Google Scholar]
- Baskin‐Sommers, A. R. , Krusemark E. A., and Ronningstam E.. 2014. “Empathy in Narcissistic Personality Disorder: From Clinical and Empirical Perspectives.” Personality Disorders, Theory, Research, and Treatment 5, no. 3: 323–333. 10.1037/per0000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine, T. P. , Neuhaus E., Brenner S. L., and Gatzke‐Kopp L.. 2008. “Ten Good Reasons to Consider Biological Processes in Prevention and Intervention Research.” Development and Psychopathology 20, no. 3: 745–774. 10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereczkei, T. , Deak A., Papp P., Perlaki G., and Orsi G.. 2013. “Neural Correlates of Machiavellian Strategies in a Social Dilemma Task.” Brain and Cognition 82, no. 1: 108–116. 10.1016/j.bandc.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Biswal, B. B. , Mennes M., Zuo X., et al. 2010. “Toward Discovery Science of Human Brain Function.” Proceedings of the National Academy of Sciences of the United States of America 107, no. 10: 4734–4739. 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blefari, M. L. , Martuzzi R., Salomon R., et al. 2017. “Bilateral Rolandic Operculum Processing Underlying Heartbeat Awareness Reflects Changes in Bodily Self‐Consciousness.” European Journal of Neuroscience 45, no. 10: 1300–1312. 10.1111/ejn.13567. [DOI] [PubMed] [Google Scholar]
- Brewer, G. , and Abell L.. 2017. “Machiavellianism and Romantic Relationship Dissolution.” Personality and Individual Differences 106: 226–230. [Google Scholar]
- Buckner, R. L. , and Carroll D. C.. 2007. “Self‐Projection and the Brain.” Trends in Cognitive Sciences 11, no. 2: 49–57. 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buss, D. M. , and Chiodo L. M.. 1991. “Narcissistic Acts in Everyday Life.” Journal of Personality 59: 179–215. [DOI] [PubMed] [Google Scholar]
- Cain, N. M. , Pincus A. L., and Ansell E. B.. 2008. “Narcissism at the Crossroads: Phenotypic Description of Pathological Narcissism Across Clinical Theory, Social/Personality Psychology, and Psychiatric Diagnosis.” Clinical Psychology Review 28, no. 4: 638–656. 10.1016/j.cpr.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Cale, E. M. , and Lilienfeld S. O.. 2002. “Sex Differences in Psychopathy and Antisocial Personality Disorder. A Review and Integration.” Clinical Psychology Review 22, no. 8: 1179–1207. 10.1016/S0272-7358(01)00125-8. [DOI] [PubMed] [Google Scholar]
- Calhoun, V. D. , Adali T., Pearlson G. D., and Kiehl K. A.. 2006. “Neuronal Chronometry of Target Detection: Fusion of Hemodynamic and Event‐Related Potential Data.” NeuroImage 30, no. 2: 544–553. Elsevier BV. Retrieved from. 10.1016/2Fj.neuroimage.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Campbell, W. K. , Bush C. M., Brunell A. B., and Shelton J. B.. 2005. “Understanding the Social Costs of Narcissism: The Case of the Tragedy of the Commons.” Personality and Social Psychology Bulletin 31, no. 10: 1358–1368. 10.1177/0146167205274855. [DOI] [PubMed] [Google Scholar]
- Chester, D. S. , Lynam D. R., Powell D. K., and DeWall C. N.. 2016. “Narcissism Is Associated With Weakened Frontostriatal Connectivity: A DTI Study.” Social Cognitive and Affective Neuroscience 11, no. 7: 1036–1040. 10.1093/scan/nsv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy, O. 2021. “The Effect of Repeated Prefrontal Cortical Stimulation on Aggression: A Randomized Controlled Trial.” Brain Stimulation 14, no. 6: 1720. 10.1016/j.brs.2021.10.435. [DOI] [Google Scholar]
- Choy, O. , Raine A., and Hamilton R. H.. 2018. “Stimulation of the Prefrontal Cortex Reduces Intentions to Commit Aggression: A Randomized, Double‐Blind, Placebo‐Controlled, Stratified, Parallel‐Group Trial.” Journal of Neuroscience: The Official Journal of the Society for Neuroscience 38, no. 29: 6505–6512. 10.1523/JNEUROSCI.3317-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy, O. , Raine A., and Schug R. A.. 2022. “Larger Striatal Volume Is Associated With Increased Adult Psychopathy.” Journal of Psychiatric Research 149: 185–193. 10.1016/j.jpsychires.2022.03.006. [DOI] [PubMed] [Google Scholar]
- Christie, R. , and Geis F. L.. 1970. Studies in Machiavellianism. New York, NY: Academic Press. [Google Scholar]
- Collins, M. W. , and Persinger M. A.. 2014. “Enhanced Power Within the Default Mode Network in Normal Subjects With Elevated Scores on an Egocentric Scale.” Open Neuroimaging Journal 8: 5–10. 10.2174/1874440001408010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope, L. M. , Shane M. S., Segall J. M., et al. 2012. “Examining the Effect of Psychopathic Traits on Gray Matter Volume in a Community Substance Abuse Sample.” Psychiatry Research 204, no. 2–3: 91–100. 10.1016/j.pscychresns.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, R. J. , and Irwin W.. 1999. “The Functional Neuroanatomy of Emotion and Affective Style.” Trends in Cognitive Science 3: 11–21. 10.1016/S1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- De Oliveira‐Souza, R. , Hare R. D., Bramati I. E., et al. 2008. “Psychopathy as a Disorder of the Moral Brain: Fronto‐Temporo‐Limbic Grey Matter Reductions Demonstrated by Voxel‐Based Morphometry.” NeuroImage 40, no. 3: 1202–1213. 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Decety, J. , and Lamm C.. 2006. “Human Empathy Through the Lens of Social Neuroscience.” TheScientificWorldJOURNAL 6: 1146–1163. 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety, J. , and Lamm C.. 2007. “The Role of the Right Temporoparietal Junction in Social Interaction: How Low‐Level Computational Processes Contribute to Meta‐Cognition.” Neuroscientist 13, no. 6: 580–593. 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Elliott, R. , Dolan R. J., and Frith C. D.. 2000. “Dissociable Functions in the Medial and Lateral Orbitofrontal Cortex: Evidence From Human Neuroimaging Studies.” Cerebral Cortex 10: 308–317. 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Ermer, E. , Cope L. M., Nyalakanti P. K., Calhoun V. D., and Kiehl K. A.. 2013. “Aberrant Paralimbic Gray Matter in Incarcerated Male Adolescents With Psychopathic Traits.” Journal of the American Academy of Child and Adolescent Psychiatry 52, no. 1: 94–103.e3. 10.1016/j.jaac.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Y. , Wonneberger C., Enzi B., et al. 2011. “The Narcissistic Self and its Psychological and Neural Correlates: An Exploratory fMRI Study.” Psychological Medicine 41, no. 8: 1641–1650. 10.1017/S003329171000228X. [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder A. Z., Vincent J. L., Corbetta M., Van Essen D. C., and Raichle M. E.. 2005. “The Human Brain Is Intrinsically Organized Into Dynamic, Anticorrelated Functional Networks.” Proceedings of the National Academy of Sciences 102, no. 27: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, S. , Clewett D., Bennett C. L., Kiehl K. A., Gazzaniga M. S., and Miller M. I.. 2015. “The Posteromedial Region of the Default Mode Network Shows Attenuated Task‐Induced Deactivation in Psychopathic Prisoners.” Neuropsychology 29, no. 3: 493–500. 10.1037/neu0000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghomroudi, P. A. , Scaltritti M., and Grecucci A.. 2023. “Decoding Reappraisal and Suppression From Neural Circuits: A Combined Supervised and Unsupervised Machine Learning Approach.” Cognitive, Affective, & Behavioral Neuroscience 23: 1095–1112. 10.3758/s13415-023-01076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn, A. L. , and Raine A.. 2014. Psychopathy: An Introduction to Biological Findings and Their Implications. New York University Press. [Google Scholar]
- Glenn, A. L. , Raine A., Yaralian P. S., and Yang Y.. 2010. “Increased Volume of the Striatum in Psychopathic Individuals.” Biological Psychiatry 67, no. 1: 52–58. 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakey, C. E. , Rose P., Campbell W. K., and Goodie A. S.. 2008. “Probing the Link Between Narcissism and Gambling: The Mediating Role of Judgment and Decision‐Making Biases.” Journal of Behavioral Decision Making 21, no. 2: 113–137. 10.1002/bdm.582. [DOI] [Google Scholar]
- Grecucci, A. , Lapomarda G., Messina I., Monachesi B., Sorella S., and Siugzdaite R.. 2022. “Structural Features Related to Affective Instability Correctly Classify Patients With Borderline Personality Disorder. A Supervised Machine Learning Approach.” Frontiers in Psychiatry 13: 804440. 10.3389/fpsyt.2022.804440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Lee T. W., Jolesz F. A., and Yoo S. S.. 2008. “Independent Vector Analysis (IVA): Multivariate Approach for fMRI Group Study.” NeuroImage 40, no. 1: 86–109. 10.1016/j.neuroimage.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Grijalva, E. , Newman D. A., Tay L., et al. 2015. “Gender Differences in Narcissism: A Meta‐Analytic Review.” Psychological Bulletin 141, no. 2: 261–310. 10.1037/a0038231. [DOI] [PubMed] [Google Scholar]
- Guyon, I. , and Elisseeff A.. 2003. “An Introduction to Variable and Feature Selection.” Journal of Machine Learning Research 3: 1157–1182. [Google Scholar]
- Haber, S. N. , and Knutson B.. 2010. “The Reward Circuit: Linking Primate Anatomy and Human Imaging.” Neuropsychopharmacology 35, no. 1: 4–26. 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare, R. D. 1996. “Psychopathy: A Clinical Construct Whose Time Has Come.” Criminal Justice and Behavior 23, no. 1: 25–54. 10.1177/0093854896023001004. [DOI] [Google Scholar]
- Hare, R. D. 2003. The Hare Psychopathy Checklist‐Revised. 2nd ed. Toronto: MHS. [Google Scholar]
- Hare, R. D. , Clark D., Grann M., and Thornton D. J.. 2000. “Psychopathy and the Predictive Validity of the PCL‐R: An International Perspective.” Behavioral Sciences & the Law 18, no. 5: 623–645. 10.1002/1099-0798(200010)18:5. [DOI] [PubMed] [Google Scholar]
- Hart, C. A. , Hepper E. G., and Sedikides C.. 2018. “Understanding and Mitigating Narcissists' Low Empathy.” In Handbook of Trait Narcissism: Key Advances, Research Methods, and Controversies, 335–343. Springer. 10.1007/978-3-319-92171-6_36. [DOI] [Google Scholar]
- Harvard Dataverse . 2020. “Behavioral Data for “a Functional Connectome Phenotyping Dataset Including Cognitive State and Personality Measures” [Dataset].” https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/VMJ6NV. [DOI] [PMC free article] [PubMed]
- Hebart, M. N. , and Baker C. I.. 2018. “Deconstructing Multivariate Decoding for the Study of Brain Function.” NeuroImage 180: 4–18. 10.1016/j.neuroimage.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg, J. , and Hyvärinen A.. 2003. “Icasso: Software for Investigating the Reliability of ICA Estimates by Clustering and Visualization.” 10.1109/nnsp.2003.1318025. [DOI]
- Himberg, J. , Hyvärinen A., and Esposito F.. 2004. “Validating the Independent Components of Neuroimaging Time Series via Clustering and Visualization.” NeuroImage 22, no. 3: 1214–1222. 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hübner, D. , and White L.. 2016. “Neurosurgery for Psychopaths? An Ethical Analysis.” AJOB Neuroscience 7: 140–149. [Google Scholar]
- Jonason, P. K. , Li N. P., and Buss D. M.. 2010. “The Costs and Benefits of the Dark Triad: Implications for Mate Poaching and Mate Retention Tactics.” Personality and Individual Differences 48: 373–378. [Google Scholar]
- Jonason, P. K. , Li N. P., and Czarna A. Z.. 2013. “Quick and Dirty: Some Psychosocial Costs Associated With the Dark Triad in Three Countries.” Evolutionary Psychology 11, no. 1: 147470491301100. 10.1177/147470491301100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonason, P. K. , Valentine K. A., Li N. P., and Harbeson C. L.. 2011. “Mate‐Selection and the Dark Triad: Facilitating a Short‐Term Mating Strategy and Creating a Volatile Environment.” Personality and Individual Differences 51: 759–763. [Google Scholar]
- Jonason, P. K. , and Webster G. D.. 2010. “The Dirty Dozen: A Concise Measure of the Dark Triad.” Psychological Assessment 22, no. 2: 420–432. [DOI] [PubMed] [Google Scholar]
- Jones, D. N. , and Paulhus D. L.. 2011. “Differentiating the Dark Triad Within the Interpersonal Circumplex.” In Handbook of Interpersonal Psychology: Theory, Research, Assessment, and Therapeutic Interventions, edited by Horowitz L. M. and Strack S., 249–267. John Wiley & Sons, Inc. [Google Scholar]
- Jones, D. N. , and Paulhus D. L.. 2014. “Introducing the Short Dark Triad (SD3): A Brief Measure of Dark Personality Traits.” Assessment 21, no. 1: 28–41. 10.1177/1073191113514105. [DOI] [PubMed] [Google Scholar]
- Jornkokgoud, K. , Baggio T., Faysal M., et al. 2023. “Predicting Narcissistic Personality Traits From Brain and Psychological Features: A Supervised Machine Learning Approach.” Social Neuroscience 18: 257–270. [DOI] [PubMed] [Google Scholar]
- Juarez, M. , Kiehl K. A., and Calhoun V. D.. 2012. “Intrinsic Limbic and Paralimbic Networks Are Associated With Criminal Psychopathy.” Human Brain Mapping 34, no. 8: 1921–1930. 10.1002/hbm.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaali, K. , Aytaç G., Şenol U., and Sindel M.. 2016. “Heschl's Transverse Gyri: Anatomy and Morphological Variations.” Akdeniz Tıp Dergisi 2, no. 3: 149–152. [Google Scholar]
- Kasai, K. , Shenton M. E., Salisbury D. F., et al. 2003. “Progressive Decrease of Left Heschl Gyrus and Planum Temporale Gray Matter Volume in First‐Episode Schizophrenia: A Longitudinal Magnetic Resonance Imaging Study.” Archives of General Psychiatry 60, no. 8: 766–775. 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, D. P. , and Courchesne E.. 2008. “The Intrinsic Functional Organization of the Brain Is Altered in Autism.” NeuroImage 39, no. 4: 1877–1885. 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kernberg, O. F. 1970. “Factors in the Psychoanalytic Treatment of Narcissistic Personalities.” Journal of the American Psychoanalytic Association 18, no. 1: 51–85. 10.1177/000306517001800103. [DOI] [PubMed] [Google Scholar]
- Kiehl, K. A. 2006. “A Cognitive Neuroscience Perspective on Psychopathy: Evidence for Paralimbic System Dysfunction.” Psychiatry Research 142, no. 2–3: 107–128. 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl, K. A. , and Hoffman M. B.. 2011. “The Criminal Psychopath: History, Neuroscience, Treatment and Economics.” Jurimetrics 51: 355–397. [PMC free article] [PubMed] [Google Scholar]
- Klimstra, T. A. , Jeronimus B. F., Sijtsema J. J., and Denissen J. J. A.. 2020. “The Unfolding Dark Side: Age Trends in Dark Personality Features.” Journal of Research in Personality 85: 103915. 10.1016/j.jrp.2020.103915. [DOI] [Google Scholar]
- Kohut, H. 1966. “Forms and Transformations of Narcissism.” Journal of the American Psychoanalytic Association 14, no. 2: 243–272. 10.1177/000306516601400201. [DOI] [PubMed] [Google Scholar]
- Krampen, G. , Effertz B., Jostock U., and Müller B.. 1990. “Gender Differences in Personality: Biological and/or Psychological?” European Journal of Personality 4, no. 4: 303–317. 10.1002/per.2410040404. [DOI] [Google Scholar]
- Laakso, M. P. , Vaurio O., Koivisto E., et al. 2001. “Psychopathy and the Posterior Hippocampus.” Behavioural Brain Research 118: 187–193. 10.1016/S0166-4328(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Lapomarda, G. , Grecucci A., Messina I., Pappaianni E., and Dadomo H.. 2021. “Common and Different Gray and White Matter Alterations in Bipolar and Borderline Personality Disorder: A Source‐Based Morphometry Study.” Brain Research 1762: 147401. 10.1016/j.brainres.2021.147401. [DOI] [PubMed] [Google Scholar]
- Lapomarda, G. , Pappaianni E., Siugzdaite R., Sanfey A. G., Rumiati R. I., and Grecucci A.. 2021. “Out of Control: An Altered Parieto‐Occipital‐Cerebellar Network for Impulsivity in Bipolar Disorder.” Behavioural Brain Research 406: 113228. 10.1016/j.bbr.2021.113228. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Zhang T., Li W., Zhang J., Jin Z., and Li L.. 2020. “Linking Brain Structure and Activation in Anterior Insula Cortex to Explain the Trait Empathy for Pain.” Human Brain Mapping 41, no. 4: 1030–1042. 10.1002/hbm.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman, M. D. 2000. “Intuition: A Social Cognitive Neuroscience Approach.” Psychology Bulletin 126: 109–137. 10.1037/0033-2909.126.1.109. [DOI] [PubMed] [Google Scholar]
- Lieberman, M. D. 2007. “Social Cognitive Neuroscience: A Review of Core Processes.” Annual Review of Psychology 58: 259–289. 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Maples, J. L. , Lamkin J., and Miller J. D.. 2014. “A Test of Two Brief Measures of the Dark Triad: The Dirty Dozen and Short Dark Triad.” Psychological Assessment 26, no. 1: 326–331. 10.1037/a0035084. [DOI] [PubMed] [Google Scholar]
- Marques, J. C. , Kober T., Krueger G., Van Der Zwaag W., Van De Moortele P., and Gruetter R.. 2010. “MP2RAGE, a Self Bias‐Field Corrected Sequence for Improved Segmentation and T1‐Mapping at High Field.” NeuroImage 49, no. 2: 1271–1281. 10.1016/j.neuroimage.2009.10.002. [DOI] [PubMed] [Google Scholar]
- McDonald, M. M. , Donnellan M. B., and Navarrete C.. 2012. “A Life History Approach to Understanding the Dark Triad.” Personality and Individual Differences 52, no. 5: 601–605. 10.1016/j.paid.2011.12.003. [DOI] [Google Scholar]
- Mendes, N. , Oligschläger S., Lauckner M. E., et al. 2019. “A Functional Connectome Phenotyping Dataset Including Cognitive State and Personality Measures.” Scientific Data 6, no. 1: 180307. 10.1038/sdata.2018.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, J. L. , Gänssbauer S., Sommer M., et al. 2008. “Gray Matter Changes in Right Superior Temporal Gyrus in Criminal Psychopaths. Evidence From Voxel‐Based Morphometry.” Psychiatry Research 163, no. 3: 213–222. 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Muris, P. , Merckelbach H., Otgaar H., and Meijer E.. 2017. “The Malevolent Side of Human Nature: A Metaanalysis and Critical Review of the Literature on the Dark Triad (Narcissism, Machiavellianism, and Psychopathy).” Perspectives on Psychological Science 12, no. 2: 183–204. 10.1177/1745691616666070. [DOI] [PubMed] [Google Scholar]
- Nestor, P. G. , Nakamura M., Niznikiewicz M., et al. 2013. “In Search of the Functional Neuroanatomy of Sociality: MRI Subdivisions of Orbital Frontal Cortex and Social Cognition.” Social Cognitive and Affective Neuroscience 8, no. 4: 460–467. 10.1093/scan/nss018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls, T. L. , Ogloff J. R., Brink J., and Spidel A.. 2005. “Psychopathy in Women: A Review of Its Clinical Usefulness for Assessing Risk for Aggression and Criminality.” Behavioral Sciences & the Law 23, no. 6: 779–802. 10.1002/bsl.678. [DOI] [PubMed] [Google Scholar]
- Northoff, G. , Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., and Panksepp J.. 2006. “Self‐Referential Processing in Our Brain–A Meta‐Analysis of Imaging Studies on the Self.” NeuroImage 31, no. 1: 440–457. 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Bunge S. A., Gross J. J., and Gabrieli J. D.. 2002. “Rethinking Feelings: An FMRI Study of the Cognitive Regulation of Emotion.” Journal of Cognitive Neuroscience 14: 1215–1229. 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ohira, H. , Nomura M., Ichikawa N., et al. 2006. “Association of Neural and Physiological Responses During Voluntary Emotion Suppression.” NeuroImage 29: 721–733. 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Pappaianni, E. , Siugzdaite R., Vettori S., Venuti P., Job R., and Grecucci A.. 2017. “Three Shades of Grey: Detecting Brain Abnormalities in Children With Autism Using Source‐, Voxel‐ and Surface‐Based Morphometry.” European Journal of Neuroscience 47, no. 6: 690–700. 10.1111/ejn.13704. [DOI] [PubMed] [Google Scholar]
- Patrick, C. J. 2022. “Psychopathy: Current Knowledge and Future Directions.” Annual Review of Clinical Psychology 18: 387–415. 10.1146/annurev-clinpsy-072720-012851. [DOI] [PubMed] [Google Scholar]
- Patrick, C. J. , Fowles D. C., and Krueger R. F.. 2009. “Triarchic Conceptualization of Psychopathy: Developmental Origins of Disinhibition, Boldness, and Meanness.” Development and Psychopathology 21, no. 3: 913–938. 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- Paulhus, D. L. , Neumann C. S., and Hare R. D.. 2009. Manual for the Self‐Report Psychopathy Scale, 4th edition. Toronto: Multi‐Health Systems. https://arc.psych.wisc.edu/self‐report/self‐report‐psychopathy‐srp‐iii/. [Google Scholar]
- Paulhus, D. L. , and Jones D. N.. 2015. “Measures of Dark Personalities.” In Measures of Personality and Social Psychological Constructs, edited by Boyle G. J., Saklofske D. H., and Matthews G., 562–594. Elsevier Academic Press. 10.1016/B978-0-12-386915-9.00020-6. [DOI] [Google Scholar]
- Paulhus, D. L. , and Williams K. L.. 2002. “The Dark Triad of Personality: Narcissism, Machiavellianism, and Psychopathy.” Journal of Research in Personality 36, no. 6: 556–563. 10.1016/s0092-6566(02)00505-6. [DOI] [Google Scholar]
- Pincus, A. L. , Cain N. M., and Wright A. G. C.. 2014. “Narcissistic Grandiosity and Narcissistic Vulnerability in Psychotherapy.” Personality Disorders, Theory, Research, and Treatment 5, no. 4: 439–443. 10.1037/per0000031. [DOI] [PubMed] [Google Scholar]
- Preuschoff, K. , Quartz S. R., and Bossaerts P.. 2008. “Human Insula Activation Reflects Risk Prediction Errors as well as Risk.” Journal of Neuroscience 28: 2745–2752. 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol, J. , Batalla I., Contreras‐Rodríguez O., et al. 2012. “Breakdown in the Brain Network Subserving Moral Judgment in Criminal Psychopathy.” Social Cognitive and Affective Neuroscience 7, no. 8: 917–923. 10.1093/scan/nsr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan, A. M. , Sunaert S., Schilling K., et al. 2022. “An Atlas of White Matter Anatomy, Its Variability, and Reproducibility Based on Constrained Spherical Deconvolution of Diffusion MRI.” NeuroImage 254: 119029. 10.1016/j.neuroimage.2022.119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. , MacLeod A., Snyder A. Z., Powers W. J., Gusnard D. A., and Shulman G. L.. 2001. “A Default Mode of Brain Function.” Proceedings of the National Academy of Sciences of the United States of America 98, no. 2: 676–682. 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine, A. , Portnoy J., Liu J., Mahoomed T., and Hibbeln J. R.. 2015. “Reduction in Behavior Problems With Omega‐3 Supplementation in Children Aged 8–16 Years: A Randomized, Double‐Blind, Placebo‐Controlled, Stratified, Parallel‐Group Trial.” Journal of Child Psychology and Psychiatry, and Allied Disciplines 56, no. 5: 509–520. 10.1111/jcpp.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin, R. , and Terry H.. 1988. “A Principal‐Components Analysis of the Narcissistic Personality Inventory and Further Evidence of Its Construct Validity.” Journal of Personality and Social Psychology 54, no. 5: 890–902. 10.1037/0022-3514.54.5.890. [DOI] [PubMed] [Google Scholar]
- Raskin, R. N. , and Hall C. S.. 1979. “A Narcissistic Personality Inventory.” Psychological Reports 45, no. 2: 590. [DOI] [PubMed] [Google Scholar]
- Rauthmann, J. F. , and Kolar G. P.. 2013. “Positioning the Dark Triad in the Interpersonal Circumplex: The Friendly‐Dominant Narcissist, Hostile‐Submissive Machiavellian, and Hostile‐Dominant Psychopath?” Personality and Individual Differences 54, no. 5: 622–627. 10.1016/j.paid.2012.11.021. [DOI] [Google Scholar]
- Rilling, J. K. , Glenn A. L., Jairam M. R., et al. 2007. “Neural Correlates of Social Cooperation and Non‐Cooperation as a Function of Psychopathy.” Biological Psychiatry 61, no. 11: 1260–1271. 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Rolls, E. T. 2000. “The Orbitofrontal Cortex and Reward.” Cerebral Cortex (New York, N.Y.: 1991) 10, no. 3: 284–294. 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Ruby, P. , and Decety J.. 2004. “How Would You Feel Versus How Do You Think She Would Feel? A Neuroimaging Study of Perspective‐Taking With Social Emotions.” Journal of Cognitive Neuroscience 16, no. 6: 988–999. 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Scarpazza, C. , Baecker L., Vieira S. M. G., and Mechelli A.. 2020. “Chapter 3 ‐ Applications of Machine Learning to Brain Disorders.” In Machine Learning: Methods and Applications to Brain Disorders, edited by Mechelli A. and Vieira S., 45–65. London: Academic Press. 10.1016/b978-0-12-815739-8.00003-1. [DOI] [Google Scholar]
- Schulze, L. , Dziobek I., Vater A., et al. 2013. “Gray Matter Abnormalities in Patients With Narcissistic Personality Disorder.” Journal of Psychiatric Research 47, no. 10: 1363–1369. 10.1016/j.jpsychires.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Sergiou, C. S. , Santarnecchi E., Franken I. H. A., and van Dongen J. D. M.. 2020. “The Effectiveness of Transcranial Direct Current Stimulation as an Intervention to Improve Empathic Abilities and Reduce Violent Behavior: A Literature Review.” Aggression and Violent Behavior 55: 101463. 10.1016/j.avb.2020.101463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay‐Tsoory, S. G. , Tomer R., Berger B. D., Goldsher D., and Aharon‐Peretz J.. 2005. “Impaired “Affective Theory of Mind” Is Associated With Right Ventromedial Prefrontal Damage.” Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology 18, no. 1: 55–67. 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Sheffield, J. M. , and Barch D. M.. 2016. “Cognition and Resting‐State Functional Connectivity in Schizophrenia.” Neuroscience and Biobehavioral Reviews 61: 108–120. 10.1016/j.Neubiorev.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, T. , and Lamm C.. 2009. “The Social Neuroscience of Empathy.” Annals of the New York Academy of Sciences 1156: 81–96. 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Spalletta, G. , Piras F., and Gili T., eds. 2018. Brain Morphometry. Humana Press. [Google Scholar]
- Takahashi, H. , Yahata N., Koeda M., Matsuda T., Asai K., and Okubo Y.. 2004. “Brain Activation Associated With Evaluative Processes of Guilt and Embarrassment: An fMRI Study.” NeuroImage 23, no. 3: 967–974. 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Tiihonen, J. , Rossi R. E., Laakso M. P., et al. 2008. “Brain Anatomy of Persistent Violent Offenders: More Rather Than Less.” Psychiatry Research: Neuroimaging 163, no. 3: 201–212. 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Tremblay, L. , and Schultz W.. 1999. “Relative Reward Preference in Primate Orbitofrontal Cortex.” Nature 398: 704–708. 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Urbonaviciute, G. , and Hepper E. G.. 2020. “When Is Narcissism Associated With Low Empathy? A Meta‐Analytic Review.” Journal of Research in Personality 89: 104036. 10.1016/j.jrp.2020.104036. [DOI] [Google Scholar]
- Vazire, S. , and Funder D. C.. 2006. “Impulsivity and the Self‐Defeating Behavior of Narcissists.” Personality and Social Psychology Review 10, no. 2: 154–165. 10.1207/s15327957pspr1002_4. [DOI] [PubMed] [Google Scholar]
- Verbeke, W. J. M. I. , Rietdijk W. J. R., van den Berg W. E., Dietvorst R. C., Worm L., and Bagozzi R. P.. 2011. “The Making of the Machiavellian Brain: A Structural MRI Analysis.” Journal of Neuroscience, Psychology, and Economics 4, no. 4: 205–216. 10.1037/a0025802. [DOI] [Google Scholar]
- Vieira, S. , Pinaya W. H. L., and Mechelli A.. 2020. “Introduction to Machine Learning.” In Machine Learning: Methods and Applications to Brain Disorders, edited by Mechelli A. and Vieira S., 1–20. London: Academic Press. 10.1016/B978-0-12-815739-8.00001-8. [DOI] [Google Scholar]
- Vize, C. E. , Lynam D. R., Collison K. L., and Miller J. D.. 2018. “Differences Among Dark Triad Components: A Meta‐Analytic Investigation.” Personality Disorders 9, no. 2: 101–111. 10.1037/per0000222. [DOI] [PubMed] [Google Scholar]
- Völlm, B. A. , Taylor A. N., Richardson P., et al. 2006. “Neuronal Correlates of Theory of Mind and Empathy: A Functional Magnetic Resonance Imaging Study in a Nonverbal Task.” NeuroImage 29, no. 1: 90–98. 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wax, M. , and Kailath T.. 1985. “Detection of Signals by Information Theoretic Criteria.” IEEE Transactions on Acoustics, Speech, and Signal Processing 33: 387–392. [Google Scholar]
- Wilson, D. , Near D. C., and Miller R. R.. 1996. “Machiavellianism: A Synthesis of the Evolutionary and Psychological Literatures.” Psychological Bulletin 119, no. 2: 285–299. 10.1037/0033-2909.119.2.285. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Raine A., Colletti P., Toga A. W., and Narr K. L.. 2009. “Abnormal Temporal and Prefrontal Cortical Gray Matter Thinning in Psychopaths.” Molecular Psychiatry 14: 561–562. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Raine A., Lencz T., Bihrle S., Lacasse L., and Colletti P.. 2005. “Volume Reduction in Prefrontal Gray Matter in Unsuccessful Criminal Psychopaths.” Biological Psychiatry 15, no. 57: 1103–1108. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Raine A., Narr K. L., Colletti P., and Toga A. W.. 2009. “Localization of Deformations Within the Amygdala in Individuals With Psychopathy.” Archives of General Psychiatry 66: 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Gao X., Yang Z., et al. 2021. “Shared Gray Matter Alterations in Subtypes of Addiction: A Voxel‐Wise Meta‐Analysis.” Psychopharmacology 238, no. 9: 2365–2379. 10.1007/s00213-021-05920-w. [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Wang F., and Geng Y.. 2021. “Machiavellianism on Quality of Life: The Role of Lifestyle, Age, Gender, Social Support.” Personality and Individual Differences 173: 110609. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Descriptive statistics of the SD3.
Table S2. Normal ranges for the Dark Triad traits.
Table S3. Brain areas for tIVA‐4.
Table S4. Brain areas for tIVA‐5.
Table S5. Brain areas for tIVA‐12.
Table S6. Brain areas for tIVA‐13.
Figure S6. Histograms with kernel density estimate (KDE) plots to visualize the distribution of the data.
Figure S7. QQ plots for assessing normality of SD3 dimensions.
Table S7. Normality test for Dark Triad traits.
Data Availability Statement
The dataset analysed during the current study is available in the MPI‐Leipzig Mind‐Brain‐Body repository (https://openneuro.org/datasets/ds000221/versions/00002). The complete LEMON and N&C Data can be downloaded through this link (https://openneuro.org/datasets/ds000221/versions/00002/download). All data were downloaded using S3: aws s3 sync ‐‐no‐sign‐request s3://openneuro.org/ds000221 ds000221‐download/ (accessed 1 November 2022).


