Abstract
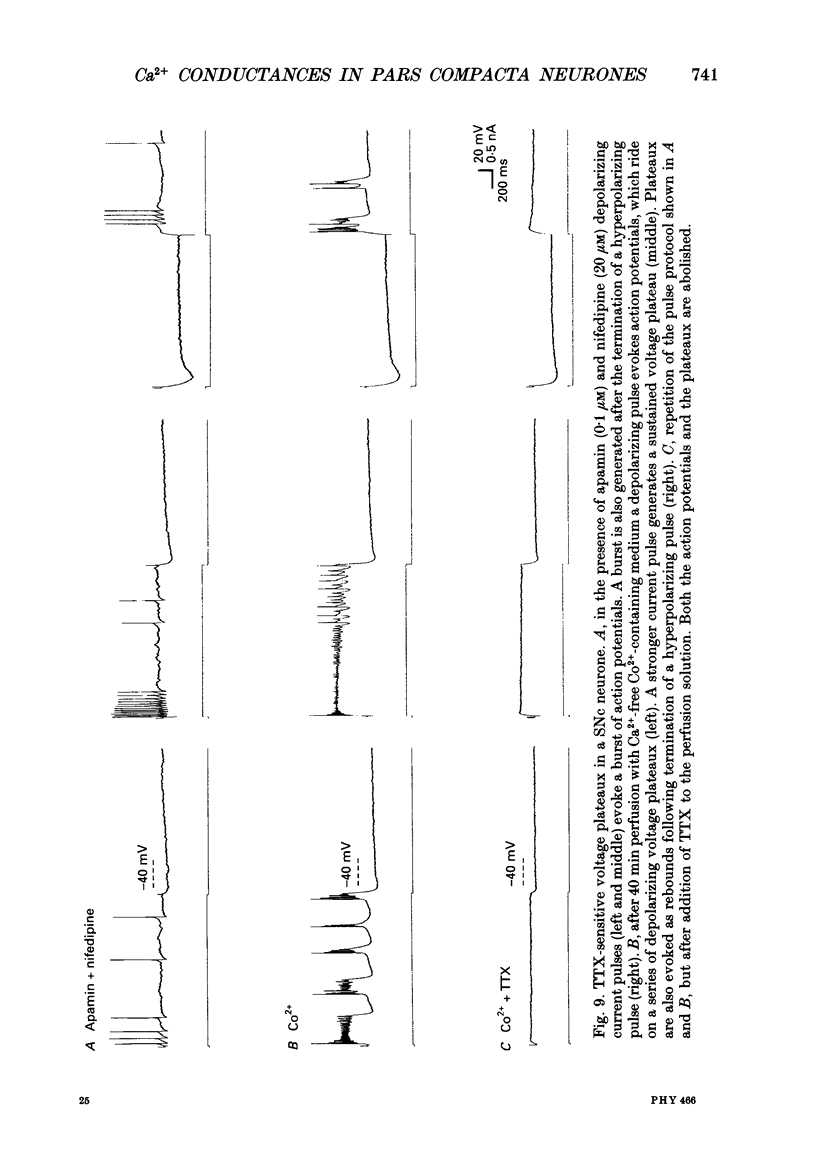
1. The membrane properties of substantia nigra pars compacta (SNc) neurones were recorded in guinea-pig in vitro brain slices. 2. In the presence of tetrodotoxin (TTX) a Ca(2+)-dependent slow oscillatory potential (SOP) was generated. Application of 0.5-20 microM nifedipine abolished both spontaneous and evoked SOPs. A tetraethylammonium chloride (TEA)-promoted high-threshold Ca2+ spike (HTS) was little affected by nifedipine. On the other hand, omega-conotoxin applied either locally or via the perfusion medium (1-10 microM) blocked a part of the HTS, but it did not alter the SOP. 3. In normal medium nifedipine blocked the spontaneous discharge, decreased the interspike interval (ISI) recorded during depolarizing current injections and selectively reduced the slow component of the spike after-hyperpolarization (AHP). omega-Conotoxin decreased both the rising and falling slopes of the normal action potential, reduced the peak amplitude of the spike AHP, and, in some of the neurones, reduced the ISI during depolarization. The Na+ spikes recorded in Ca(2+)-free medium were not altered by omega-conotoxin. 4. The SOP was not blocked by octanol (100-200 microM), amiloride (100-250 microM), or Ni2+ (100-300 microM). However, at 500 microM Ni2+ attenuated the SOP. 5. Application of apamin (0.5-2.0 microM) induced irregular firing or bursting, abolished the slow component of the spike AHP and reduced its peak amplitude. In the presence of TTX and apamin long-duration plateau potentials occurred, which were subsequently blocked by nifedipine. 6. In Ca(2+)-free, Co(2+)-containing medium TTX-sensitive spikes and voltage plateaux were generated by depolarizing current pulses. It is suggested that a persistent Na+ conductance underlies the plateaux, which may be co-activated during the SOP. 7. The results suggest that the Ca2+ currents underlying the SOP and the HTS are different and that they activate at least two Ca(2+)-dependent K+ conductances. These conductances play major roles in the maintenance of spontaneous discharge and in control of membrane excitability.
Full text
PDF


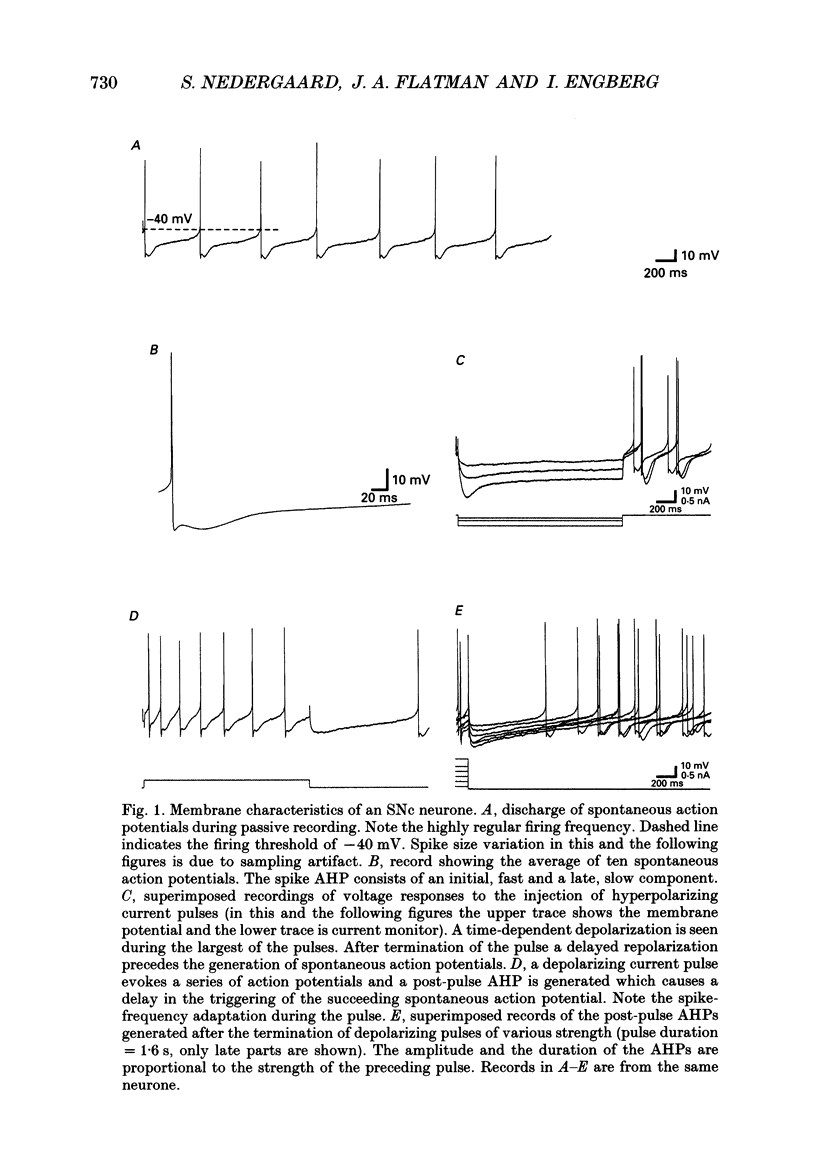
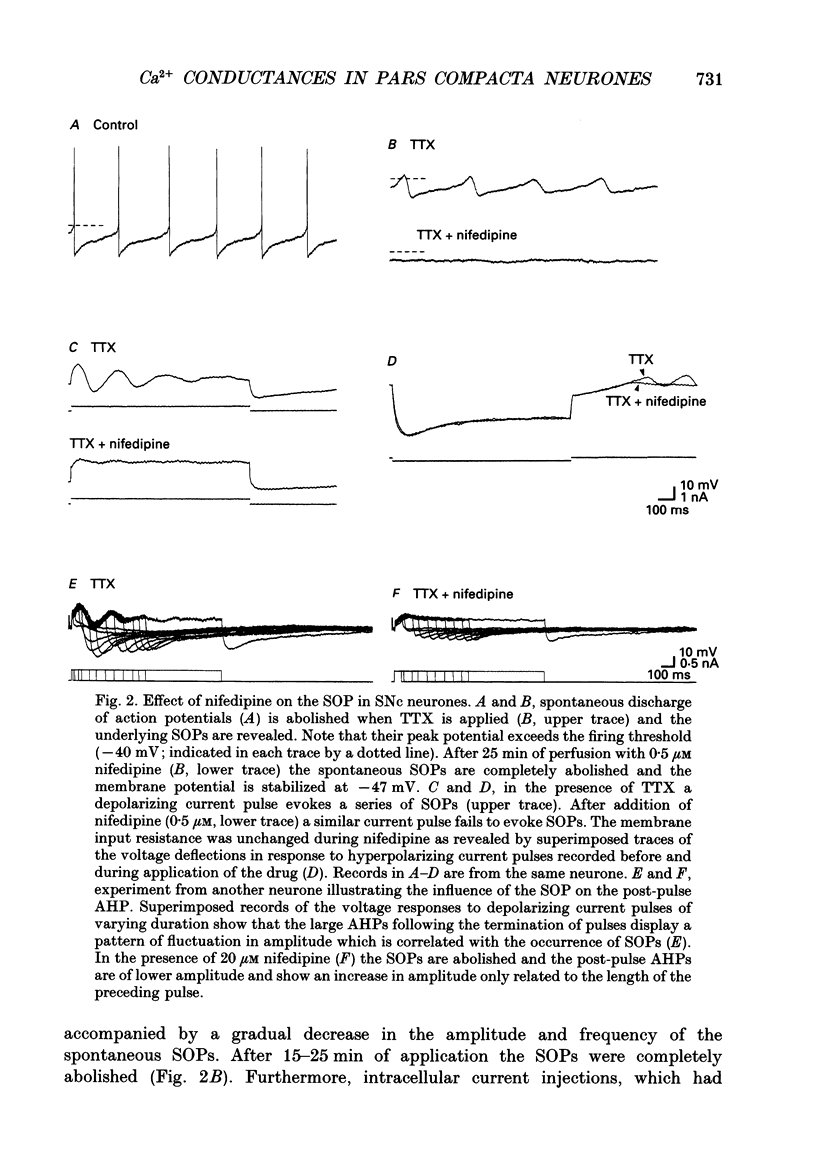
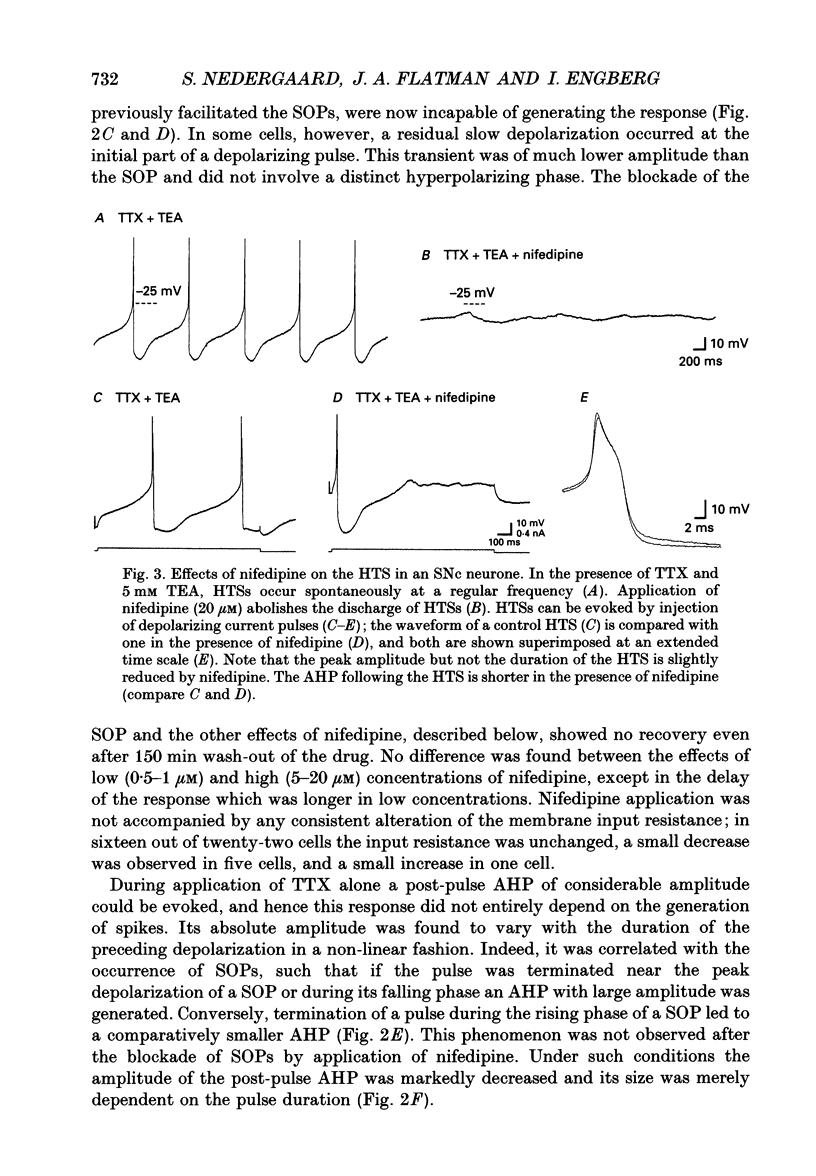
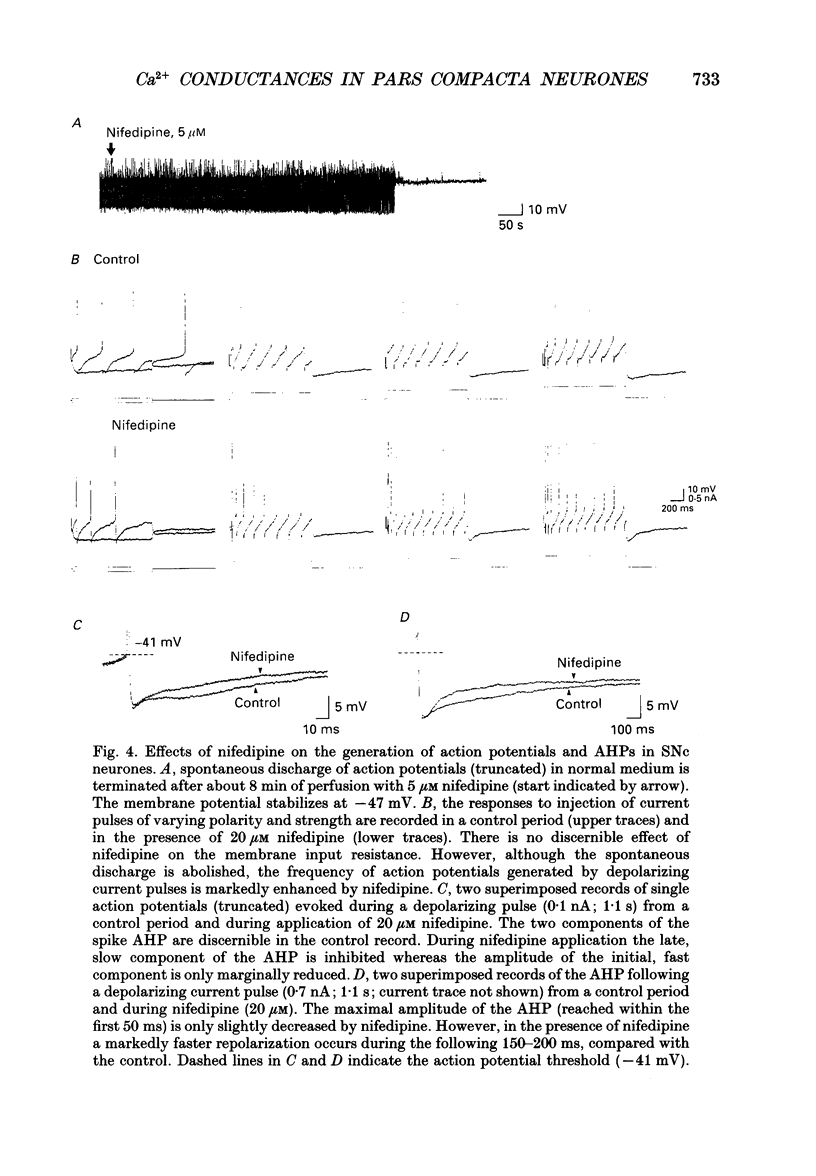
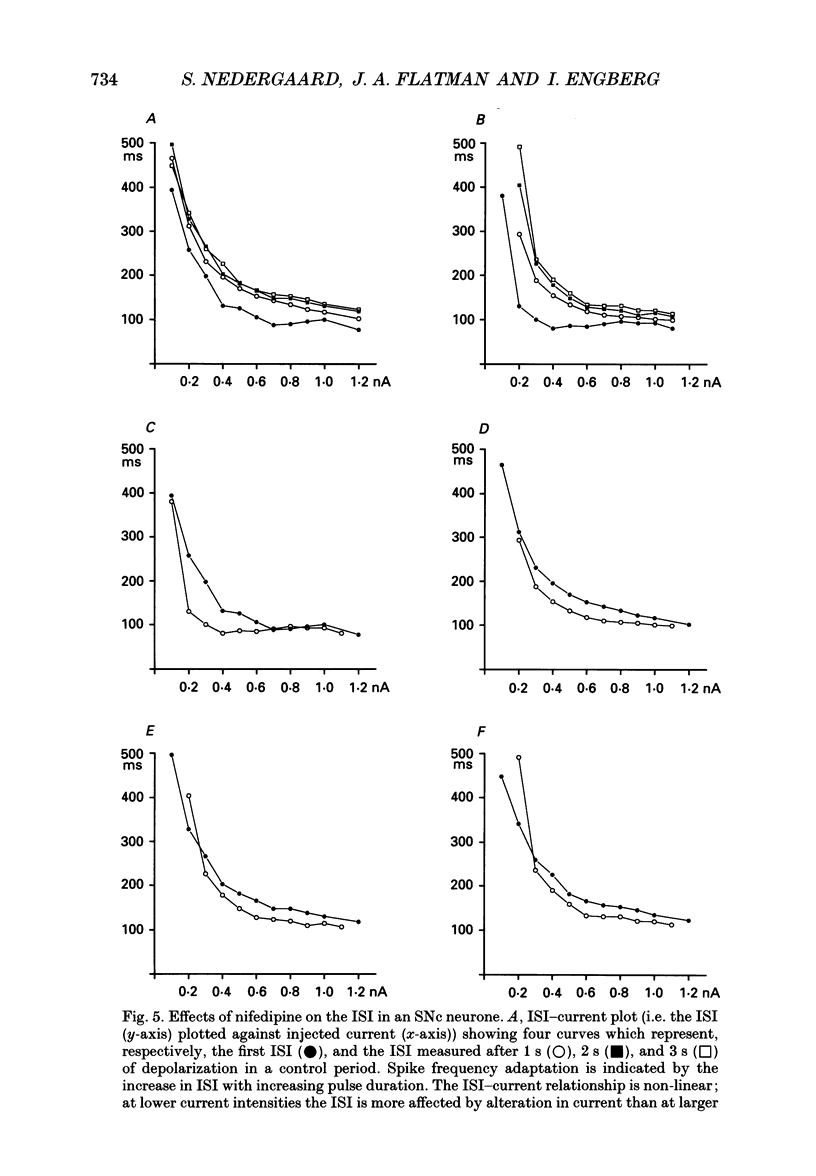

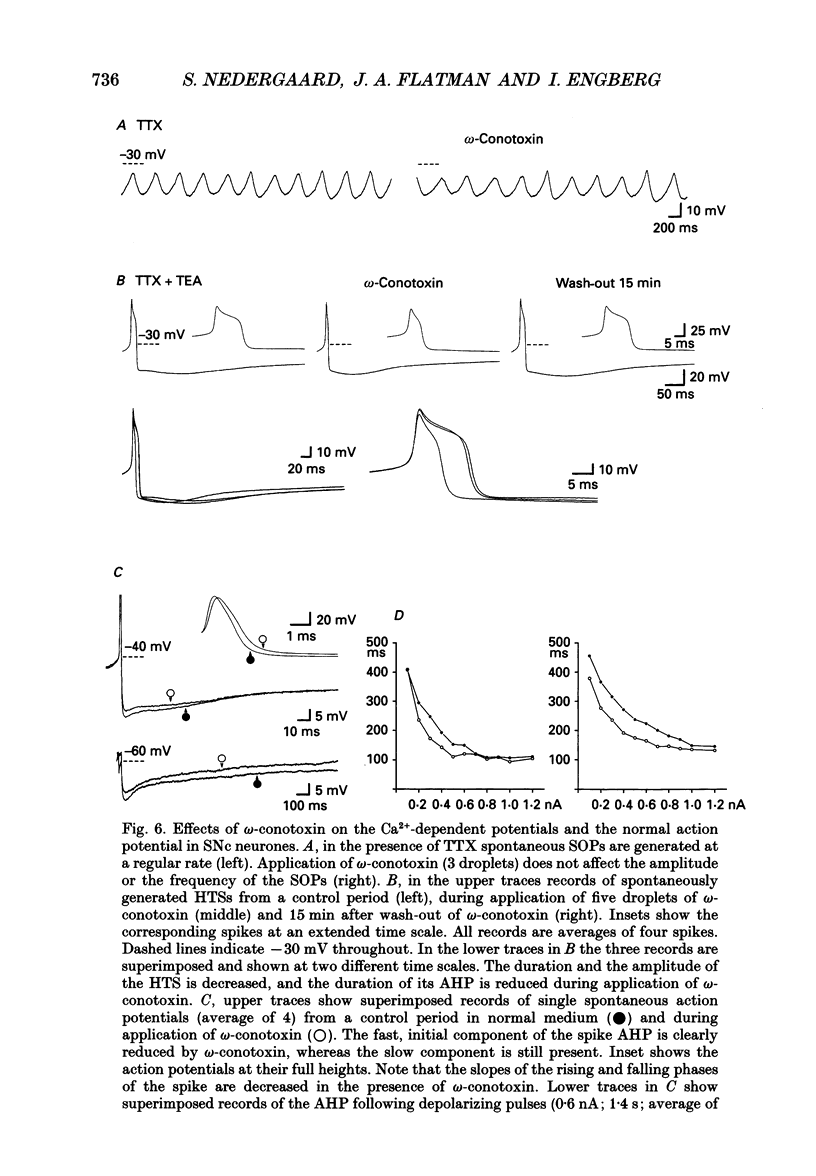

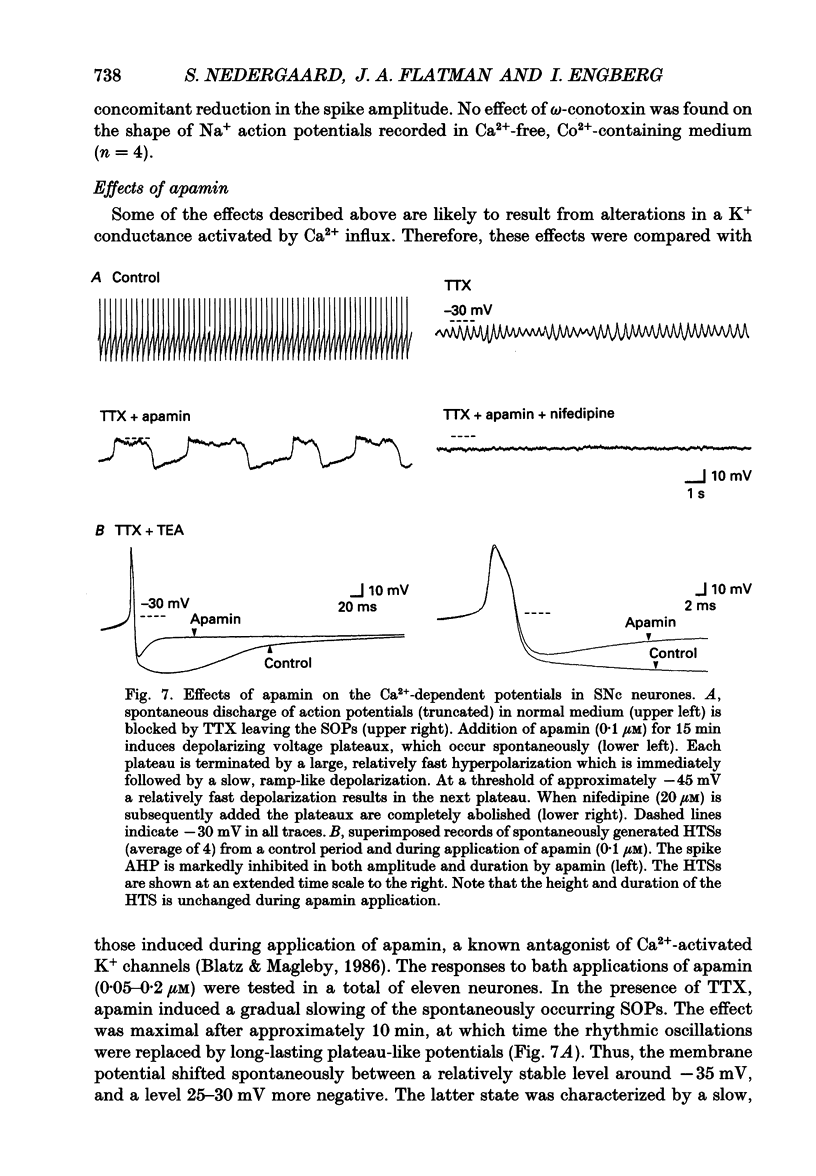
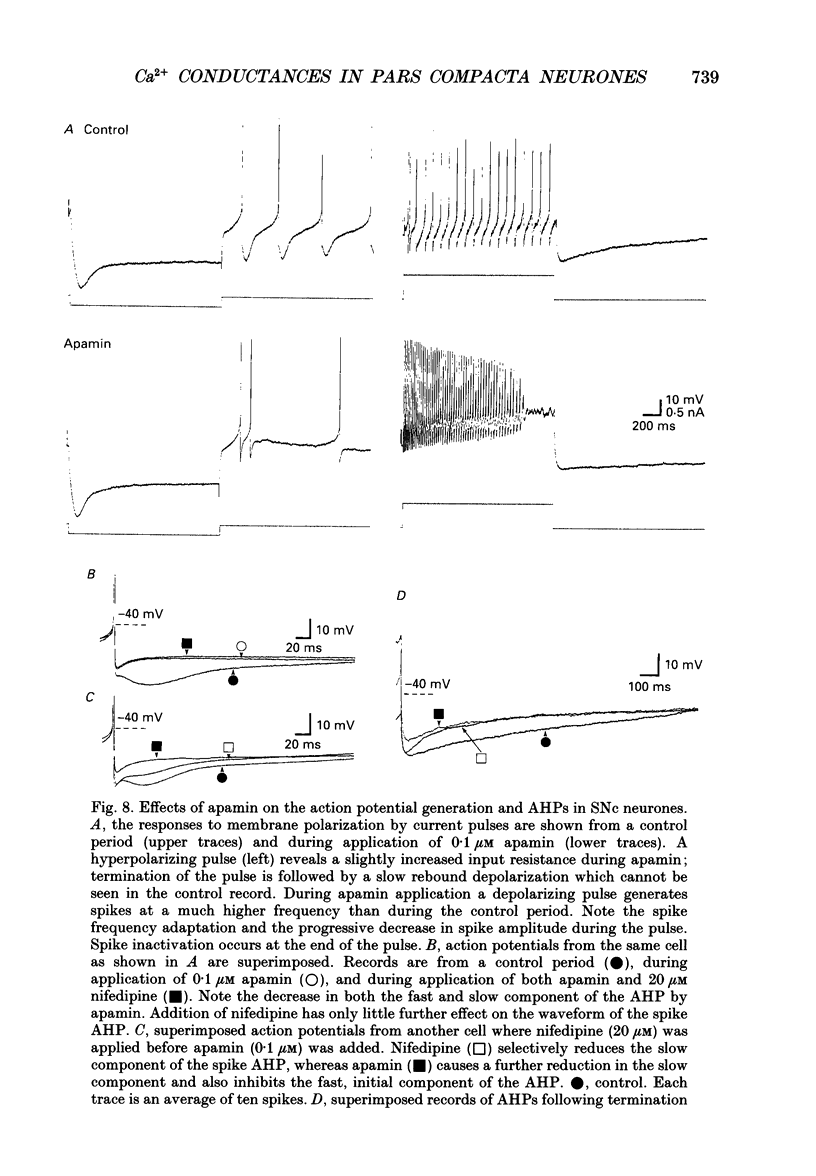







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aosaki T., Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and omega-conotoxin GVIA. Pflugers Arch. 1989 Jun;414(2):150–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A., Adams P. R. Ca-activated potassium current in vertebrate sympathetic neurons. Cell Calcium. 1983 Dec;4(5-6):407–420. doi: 10.1016/0143-4160(83)90017-9. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Lightowler S., Pollard C. E. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol. 1989 Jun;413:543–561. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura K., Matsuda Y. Autogenous oscillatory potentials in neurons of the guinea pig substantia nigra pars compacta in vitro. Neurosci Lett. 1989 Sep 25;104(1-2):53–57. doi: 10.1016/0304-3940(89)90328-5. [DOI] [PubMed] [Google Scholar]
- Grace A. A. Evidence for the functional compartmentalization of spike generating regions of rat midbrain dopamine neurons recorded in vitro. Brain Res. 1990 Jul 30;524(1):31–41. doi: 10.1016/0006-8993(90)90488-w. [DOI] [PubMed] [Google Scholar]
- Grace A. A., Onn S. P. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989 Oct;9(10):3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A. A. Regulation of spontaneous activity and oscillatory spike firing in rat midbrain dopamine neurons recorded in vitro. Synapse. 1991 Mar;7(3):221–234. doi: 10.1002/syn.890070307. [DOI] [PubMed] [Google Scholar]
- Hainsworth A. H., Röper J., Kapoor R., Ashcroft F. M. Identification and electrophysiology of isolated pars compacta neurons from guinea-pig substantia nigra. Neuroscience. 1991;43(1):81–93. doi: 10.1016/0306-4522(91)90419-o. [DOI] [PubMed] [Google Scholar]
- Harris N. C., Webb C., Greenfield S. A. A possible pacemaker mechanism in pars compacta neurons of the guinea-pig substantia nigra revealed by various ion channel blocking agents. Neuroscience. 1989;31(2):355–362. doi: 10.1016/0306-4522(89)90379-5. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Jacobs L. S. Dihydropyridine actions on calcium currents of frog sympathetic neurons. J Neurosci. 1990 Jul;10(7):2261–2267. doi: 10.1523/JNEUROSCI.10-07-02261.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Aosaki T., Fukuda J. Presynaptic Ca-antagonist omega-conotoxin irreversibly blocks N-type Ca-channels in chick sensory neurons. Neurosci Res. 1987 Feb;4(3):228–235. doi: 10.1016/0168-0102(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Kasai H., Neher E. Dihydropyridine-sensitive and omega-conotoxin-sensitive calcium channels in a mammalian neuroblastoma-glioma cell line. J Physiol. 1992 Mar;448:161–188. doi: 10.1113/jphysiol.1992.sp019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Blockade of Ca-activated K conductance by apamin in rat sympathetic neurones. Br J Pharmacol. 1986 Jan;87(1):225–232. doi: 10.1111/j.1476-5381.1986.tb10175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Kita H., Kitai S. T. Electrical membrane properties of rat substantia nigra compacta neurons in an in vitro slice preparation. Brain Res. 1986 Apr 30;372(1):21–30. doi: 10.1016/0006-8993(86)91454-x. [DOI] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989 Apr;9(4):1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B., Adams P. R. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986 Jun;55(6):1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lazdunski M. Apamin, a neurotoxin specific for one class of Ca2+-dependent K+ channels. Cell Calcium. 1983 Dec;4(5-6):421–428. doi: 10.1016/0143-4160(83)90018-0. [DOI] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llinás R., Greenfield S. A., Jahnsen H. Electrophysiology of pars compacta cells in the in vitro substantia nigra--a possible mechanism for dendritic release. Brain Res. 1984 Feb 27;294(1):127–132. doi: 10.1016/0006-8993(84)91316-7. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Fujimura K., Yoshida S. Two types of neurons in the substantia nigra pars compacta studied in a slice preparation. Neurosci Res. 1987 Dec;5(2):172–179. doi: 10.1016/0168-0102(87)90033-2. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogul D. J., Fox A. P. Evidence for multiple types of Ca2+ channels in acutely isolated hippocampal CA3 neurones of the guinea-pig. J Physiol. 1991 Feb;433:259–281. doi: 10.1113/jphysiol.1991.sp018425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard S., Flatman J. A., Engberg I. Excitation of substantia nigra pars compacta neurones by 5-hydroxy-tryptamine in-vitro. Neuroreport. 1991 Jun;2(6):329–332. doi: 10.1097/00001756-199106000-00007. [DOI] [PubMed] [Google Scholar]
- Nedergaard S., Greenfield S. A. Sub-populations of pars compacta neurons in the substantia nigra: the significance of qualitatively and quantitatively distinct conductances. Neuroscience. 1992;48(2):423–437. doi: 10.1016/0306-4522(92)90502-s. [DOI] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W. J., Foehring R. C., Stafstrom C. E., Chubb M. C., Crill W. E. Multiple potassium conductances and their functions in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1988 Feb;59(2):424–449. doi: 10.1152/jn.1988.59.2.424. [DOI] [PubMed] [Google Scholar]
- Shepard P. D., Bunney B. S. Repetitive firing properties of putative dopamine-containing neurons in vitro: regulation by an apamin-sensitive Ca(2+)-activated K+ conductance. Exp Brain Res. 1991;86(1):141–150. doi: 10.1007/BF00231048. [DOI] [PubMed] [Google Scholar]
- Silva N. L., Pechura C. M., Barker J. L. Postnatal rat nigrostriatal dopaminergic neurons exhibit five types of potassium conductances. J Neurophysiol. 1990 Jul;64(1):262–272. doi: 10.1152/jn.1990.64.1.262. [DOI] [PubMed] [Google Scholar]
- Sánchez D., Ribas J. Properties and ionic basis of the action potentials in the periaqueductal grey neurones of the guinea-pig. J Physiol. 1991;440:167–187. doi: 10.1113/jphysiol.1991.sp018702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung W. H., Häusser M. A., Jack J. J. Electrophysiology of dopaminergic and non-dopaminergic neurones of the guinea-pig substantia nigra pars compacta in vitro. J Physiol. 1991 May;436:643–667. doi: 10.1113/jphysiol.1991.sp018571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Krnjević K. Apamin depresses selectively the after-hyperpolarization of cat spinal motoneurons. Neurosci Lett. 1987 Feb 10;74(1):58–62. doi: 10.1016/0304-3940(87)90051-6. [DOI] [PubMed] [Google Scholar]


