Abstract
Nanozymes with specific catalytic activity inhibit inflammation and promote wound healing efficiently and safely. In this work, multifunctional manganese-based nanozymes (MnGA) with antioxidant properties were successfully constructed via a simple coordination reaction in which manganese chloride was used as the manganese source and gallic acid (GA) was used as the ligand solution. MnGA possesses both catalase-like (CAT-like) and superoxide dismutase-like (SOD-like) activities and a reactive nitrogen species (RNS) scavenging capacity, which enables it to efficiently inhibit the inflammatory response. Specifically, MnGA scavenges superoxide anions and produces H2O2 via SOD-like activity and then consumes H2O2 to convert it to nontoxic H2O and O2 via CAT-like activity, resulting in a cascade of catalytic reactions to scavenge reactive oxygen species (ROS). Moreover, the scavenging of RNS by MnGA can amplify the anti-inflammatory effect in combination with the scavenging of ROS. RNA sequencing of mouse skin tissue further revealed that MnGA significantly reduces inflammation by modulating the nuclear factor kappa-B (NF-κB), Toll-like receptor (TLR), and NOD-like receptor (NLR) signaling pathways and promotes skin regeneration. In summary, MnGA nanocatalysts possess excellent antioxidative and anti-inflammatory properties, highlighting their potential applications in wound healing and inflammation treatment.
Keywords: MnGA, Catalase-like, Superoxide dismutase-like, Inflammatory response
Graphical abstract
Highlights
-
•
MnGA exhibites high SOD/CAT activity and demonstrates excellent in vitro antioxidant capacity.
-
•
MnGA can clear ROS generated by oxidative stress in cells, inhibit inflammation, and promote skin regeneration.
-
•
MnGA reduces inflammation by modulating the NF-κB, Toll-like receptor, and NOD-like receptor signaling pathways.
1. Introduction
The inflammatory phase is an early stage of wound healing characterized by the activation of inflammatory responses [[1], [2], [3], [4]]. During this phase, the immune system in the body responds to injury by releasing inflammatory mediators and free radicals to clear damaged tissues and potential infections [[5], [6], [7], [8]]. However, excessive free radicals and reactive oxygen species (ROS) can lead to oxidative stress, exacerbating tissue damage and inflammation, which in turn impairs the efficiency of wound healing [[9], [10], [11]]. Oxidative stress not only directly damages cell membranes, proteins, and DNA but also promotes the release of inflammatory mediators, increasing the intensity of the inflammatory response [[12], [13], [14]]. This delay in wound healing may lead to chronic inflammation and other complications [10,15,16]. Notably, nanozymes with antioxidant properties play crucial roles in this process [[17], [18], [19], [20]]. Nanozymes refer to a class of nanomaterials with enzyme-like properties and catalytic activity. Compared with natural enzymes, nanozymes have the advantages of low cost, high stability, simple preparation, long service life and adjustable activity. Owing to these advantages, nanozymes have broad potential for biomedical and other applications [21]. By mimicking natural antioxidant enzymes (such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px)), these nanozymes effectively neutralize free radicals and ROS in the body, thereby alleviating oxidative stress and related inflammatory responses [[22], [23], [24], [25]]. The high specific surface area and optimized catalytic activity of nanozymes enable them to exert strong antioxidant effects at relatively low concentrations [[26], [27], [28]]. Additionally, modern nanozymes are designed with an emphasis on biocompatibility, reducing potential toxicity to wounds and surrounding tissues [[29], [30], [31], [32]].
Manganese (Mn), a transition metal, has multiple oxidation states, with Mn(Ⅱ) and Mn(Ⅲ) being particularly significant [[33], [34], [35]]. Specifically, manganese ions can mimic the functions of two key biological antioxidant enzymes: CAT and SOD [36,37]. These enzymes catalyze redox reactions to help eliminate free radicals and ROS, thus protecting cells from oxidative stress [38,39]. In recent years, manganese salts (such as manganese chloride) have attracted considerable attention as sources of manganese for the synthesis of manganese-based nanomaterials when they react with organic ligands [[40], [41], [42]]. Yao et al. prepared Mn3O4 NPs with synergistic SOD-like and CAT-like activities. The multivalent mixing of Mn(Ⅱ) and Mn(Ⅲ) resulted in the strong O2.- elimination ability of the Mn3O4 NPs. Moreover, the Mn3O4 NPs have CAT-like activity and can catalyze H2O2 disproportionation. These nanozymes have the ability to scavenge ROS and can be used to eliminate ear inflammation [43]. Wang et al. synthesized temperature-sensitive hydrogels of Mn3O4 nanozymes, which possessed multienzymatic activities (e.g., CAT, GSH-Px and SOD) and antioxidant effects. This nanozyme composite hydrogel can directly target enteritis lesions and alleviate the symptoms of DSS-induced colitis by directly scavenging ROS, which can be used for interventional treatment of DSS-induced colitis in mice [44]. Wu et al. reported the triple enzyme-like activity of bimetallic oxidized Cu1.5Mn1.5O4 CFNSs. The Cu1.5Mn1.5O4 CFNSs possessed OXD-like, POD-like, and GSH-Px-like activities and effectively treated bacterium-infected wounds [45]. These materials not only exhibit excellent catalytic performance but also demonstrate good biocompatibility and safety, highlighting their broad potential applications in biomedicine and environmental protection. Among these organic ligands, gallic acid (GA) stands out as an ideal choice for synthesizing manganese-based nanomaterials because of its excellent coordination ability and biocompatibility [46,47]. GA, with its multiple hydroxyl groups, can effectively coordinate with manganese ions to form stable complexes [48,49]. Reacting manganese salts with gallic acid results in manganese-based nanomaterials with specific morphologies and properties. Notably, GA, a potent antioxidant, can scavenge free radicals and ROS, thereby alleviating oxidative stress and reducing inflammation [50,51]. Additionally, GA exerts anti-inflammatory effects by inhibiting the release of inflammatory mediators such as COX-2 and LOX and modulating the NF-κB signaling pathway [52,53]. It also regulates immune cell activity, reduces excessive inflammation, and provides cellular protection and repair [54,55]. The low solubility, stability and bioavailability of GA limit its application. The coordination of Mn not only improved the solubility and stability of GA but also increased the specific surface area and number of active sites while conferring catalytic activity to GA-like enzymes and improving free radical scavenging efficiency. These multifaceted effects render MnGA valuable for treating inflammation-related diseases, including skin inflammation, arthritis, cardiovascular diseases, and neurodegenerative disorders, although its clinical efficacy still requires further validation.
Therefore, MnGA nanozymes were successfully constructed in this work via the use of manganese chloride as the manganese source through a coordination reaction between metal ions and ligands and were formulated with GA to form a ligand solution. The constructed MnGA nanozymes had a spindle-shaped structure without obvious aggregation, indicating good dispersion. The MnGA nanozymes possessed CAT-like activity, SOD-like activity and excellent RNS-scavenging ability. MnGA scavenges ROS to alleviate inflammatory responses by scavenging O2.- and generating H2O2 through SOD-like activity, and then uses CAT-like compounds to consume H2O2 to generate H2O and water. Moreover, MnGA scavenges reactive nitrogen species (RNS) while reducing oxidative stress-promoting inflammation. Eukaryotic transcriptome RNA sequencing analysis revealed that MnGA regulated several signaling pathways related to cell proliferation, skin regeneration, and inflammation, including the NF-κB signaling pathway, Toll-like receptor signaling pathway, and NOD-like receptor signaling pathway (Scheme 1). In summary, MnGA nanozymes show significant potential in catalysis, antioxidation, and anti-inflammatory therapy. The excellent performance and biocompatibility of these materials suggest their broad range of applications in medical and biological fields.
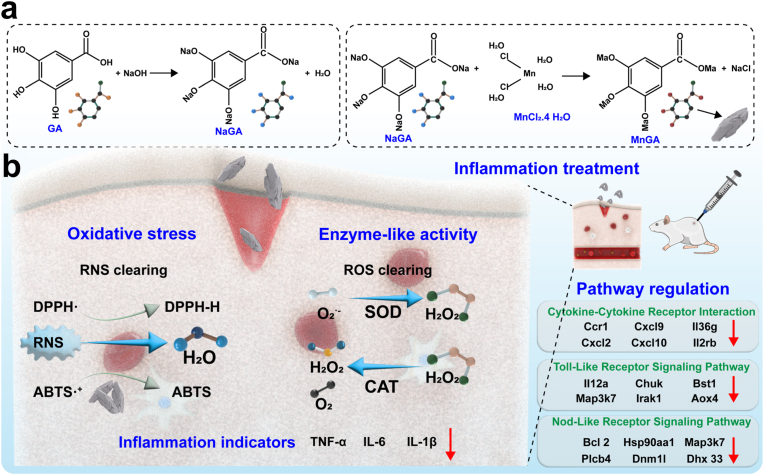
Scheme 1.
Schematic diagram of the use of MnGA for acute wound treatment. (a) Diagram of the synthesis of MnGA nanozymes. (b) MnGA inhibits inflammation to promote wound healing.
2. Methods
2.1. Preparation of MnGA
First, 2 mmol of GA was dissolved in a NaOH solution (0.05 M) to prepare the ligand solution. Next, under stirring conditions, 10 mM manganese chloride (MnCl₂) was slowly added to the GA solution and stirred continuously for 1 h to ensure that the reaction was complete. The pH of the mixed solution was then adjusted to 7.0, and the mixture was stirred at room temperature for 12 h. Following the reaction, a yellow-brown precipitate formed and was separated by centrifugation (5000 rpm). The separated precipitate was washed three times with deionized water to remove impurities and then dried. Finally, the structure and properties of the obtained MnGA product were characterized via techniques such as transmission electron microscopy (TEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS).
2.2. Evaluation of antioxidant properties
First, groups of MnGA at concentrations of 0, 12.5, 25, 40, 60, and 80 μg/mL were established to evaluate SOD-like activity via a total superoxide anion assay kit. Next, 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL MnGA were used to verify the CAT-like activity via a hydrogen peroxide assay kit. The DPPH· radical scavenging assay was used to assess the antioxidant capacity. A 0.1 mM DPPH· solution was first prepared. The samples were dissolved in PBS. The DPPH· solution and the samples were mixed, typically at a 2:1 vol ratio, and allowed to react in the dark for 30 min to 1 h. The absorbance was then measured at 517 nm via a spectrophotometer. The scavenging rate (%) was calculated via the following formula:
where A0 is the absorbance of the solution without the sample and A is the absorbance of the solution with the sample.
The ABTS·⁺ radical scavenging assay was used to evaluate the antioxidant capacity of the samples. First, ABTS was reacted with hydrogen peroxide to generate ABTS·⁺ radicals, which were then diluted with anhydrous ethanol to a specific absorbance (0.70 ± 0.05) at 734 nm. The samples were then mixed with the ABTS·⁺ solution and allowed to stand for 5–10 min before the absorbance of the mixture was measured via a spectrophotometer. The ABTS·⁺ scavenging rate was calculated via the following formula:
where A0 is the absorbance of the solution without the sample and A is the absorbance of the solution with the sample.
2.3. Ability of intracellular MnGA to scavenge ROS
RAW264.7 macrophages were obtained from the Chinese Academy of Sciences. Macrophages were cultured in 6-well plates and treated with LPS or high (100 μg/mL) or low (50 μg/mL) concentrations of MnGA. After 12 h of incubation, the samples were stained with DCFH-DA fluorescent dye and photographed via flow cytometry analysis and laser confocal microscopy.
2.4. In vitro cytotoxicity assay
For the CCK-8 assay, HUVECs were first seeded into a 96-well plate and cultured for 24 h to allow for attachment. The cells were subsequently treated with different concentrations of MnGA (0, 25, 50, 75, 100, 125, and 150 μg/mL). After treatment, CCK-8 reagent was added, and the cells were incubated for 4 h. The absorbance (OD value) was measured at 450 nm via a microplate reader. The relative cell viability of the treated groups compared with that of the control group was calculated to assess cytotoxicity. The results were used to generate graphs and perform statistical analysis.
2.5. Anti-inflammation assay
In vitro anti-inflammatory assessments were performed using RAW264.7 cells, a mouse macrophage line. After the cells were treated with different concentrations of MnGA, the supernatants were collected, and the concentrations of inflammatory cytokines (IL-6, IL-1β and TNF-α) were determined via appropriate enzyme-linked immunosorbent assay (ELISA) quantification kits according to the manufacturer's instructions.
2.6. In vivo use of MnGA for wound treatment
The animal experiments were conducted in accordance with the guidelines established by the Animal Health and Use Committee of Anhui Medical University (Approval No. LLSC 20231268). BALB/c mice were randomly divided into three groups, with three mice per group. First, the hair on the backs of the mice was shaved, and a wound approximately 8 mm in diameter was created via sterile surgical scissors. The wounds were subsequently treated with PBS, 50 μg/mL MnGA, or 100 μg/mL MnGA, which were applied directly onto the wound surface. Wound healing was monitored and recorded with a camera over an 8-day period. The wound areas in the images were quantified via ImageJ for statistical analysis. On day 8, skin tissues and major internal organs (heart, liver, spleen, lung, and kidney) were collected from the mice. Skin tissues were examined for tissue structure and pathological changes via H&E and Masson staining. The internal organs were assessed for pathological changes via H&E staining. Additionally, the distributions of ROS, IL-6, and TNF-α in skin tissues were observed via fluorescent dyes. Blood samples were obtained via the ocular vein for blood tests to verify the safety of MnGA in vivo.
2.7. Eukaryotic transcriptome sequencing of skin tissues
To further investigate the mechanism by which MnGA inhibits inflammation and promotes wound healing, transcriptomic analysis was performed on mouse tissues collected on day 8. The transcriptomic analysis was conducted by Panosen Biotechnology Co., Ltd. (Shanghai, China).
2.8. Statistical analysis
The data were statistically analyzed via Student's t-test. When it was necessary to compare the means of more than two groups, one-way analysis of variance (ANOVA) was used for data that conformed to a normal distribution. The data are presented as the means ± standard errors of the means (SEMs); the level of statistical significance for differences was set at P < 0.05, labeled ∗ for P < 0.05, ∗∗ for P < 0.01, ∗∗∗ for P < 0.001, and ∗∗∗∗for P < 0.0001.
3. Results and discussion
In this study, MnGA nanozymes were synthesized via a simple and efficient coordination reaction between manganese salt (manganese chloride) and GA to form a ligand solution (Fig. 1a). TEM images revealed that MnGA has an elongated shape with no aggregation (Fig. 1b). Fourier transform infrared spectroscopy (FTIR) revealed the disappearance of hydroxyl (-OH) peaks at approximately 3200–3500 cm⁻1 and carboxyl (-COOH) peaks at approximately 1700–1750 cm⁻1, indicating the successful coordination of phenolic hydroxyl and carboxyl groups with metal ions. The peak at 1600–1650 cm⁻1 corresponds to the C=C stretching vibration of the benzene ring in GA (Fig. 1c). Dynamic light scattering (DLS) revealed a single, narrow peak with an average size of 181.64 nm, suggesting that the elongated shape was relatively uniform (Fig. 1d). The zeta potentials of GA and MnGA were +2.36 and −28.94 mV, respectively, indicating that the MnGA nanozymes possess a strong negative surface charge, which aids in their stability in solution (Fig. 1e). Moreover, in vitro stability experiments revealed that MnGA dissolved in different solutions caused no significant particle precipitation or color change on day 7 (Fig. S1). DLS analysis of MnGA dispersed in different solvents on day 7 revealed little change in the hydrated particle size of MnGA (Fig. S2). These results also demonstrated the stability of MnGA at room temperature. Additionally, the XRD results revealed no distinct sharp diffraction peaks for MnGA but rather a weak and broad characteristic diffraction peak, indicating the absence of a long-range ordered crystalline structure and confirming its oligocrystalline nature (Fig. 1f). The XPS spectrum of Mn 2p showed significant Mn2+ signals (641.32 eV and 653.12 eV), which, in combination with the presence of satellite peaks (Sat.), indicating that the Mn ions were involved in coordination, mainly in the oxidation state of Mn2+ (Fig. 1g). XPS contrast analysis revealed that the C 1s and O 1s binding energies of GA changed significantly upon coordination with Mn2+, indicating the involvement of phenolic hydroxyl (-OH) and carboxyl (-COOH) groups in coordination (Fig. S3). After coordination with Mn2⁺, the binding energies of C 1s and O 1s significantly significantly changed, indicating that phenolic hydroxyl (-OH) and carboxyl (-COOH) groups were involved in the coordination. Specifically, the binding energy of C-O in C 1s shifted from 286.29 eV to 286.61 eV, indicating that the oxygen atom in the phenolic hydroxyl group lost part of its electron density due to coordination; meanwhile, the binding energy of C=O decreased from 289.04 eV to 288.59 eV, indicating that the oxygen atom in the carboxyl group formed a coordination bond with manganese ions through electron transfer. and O=C in O 1s shifted from 532.88 eV to 531.83 eV–532.98 eV and 531.29 eV, respectively, further confirming the roles of the hydroxyl and carboxyl groups in the coordination process (Fig. 1h and i). In summary, multiple characterization techniques demonstrate that this straightforward synthesis method successfully produces MnGA nanozymes.
Fig. 1.
Synthesis and characterization of MnGA. (a) Schematic diagram of MnGA synthesis. (b) TEM images of MnGA at different magnifications. (c) FTIR image of MnGA. (d) DLS image of MnGA. (e) Zeta potential of MnGA. (f) XRD pattern of MnGA. (g–i) XPS patterns of the Mn 2p region, C 1s region, and O 1s region of MnGA.
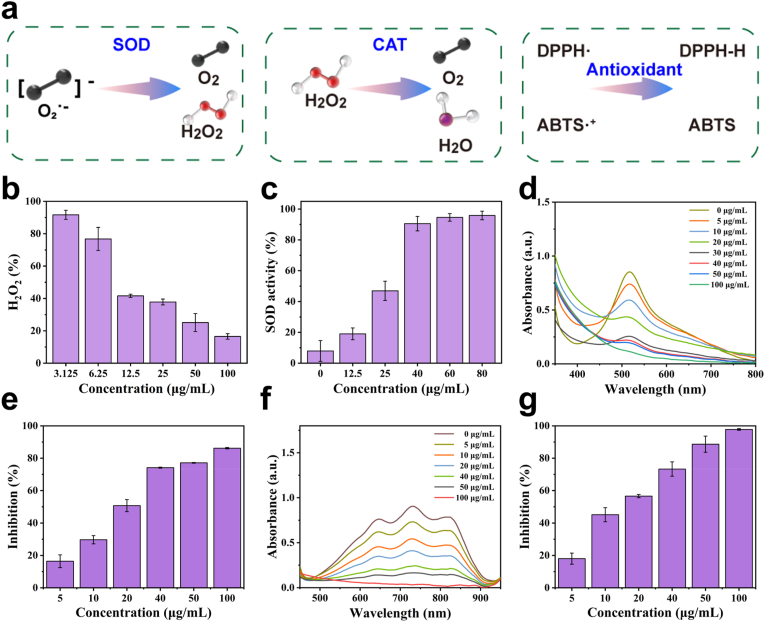
The activity of MnGA nanozymes is crucial because it affects their efficiency in catalytic reactions and their performance in wound applications. In inflammation treatment, the antioxidant function of nanozymes can effectively scavenge oxidative free radicals and peroxides in the body, significantly reducing oxidative stress and inflammatory responses and thereby alleviating tissue damage caused by inflammation (Fig. 2a). Therefore, MnGA nanozymes were systematically evaluated and demonstrated CAT-like, SOD-like, and reactive nitrogen species scavenging activities. First, CAT-like activity involves catalyzing the decomposition of H₂O₂ into H2O and O2, maintaining the intracellular redox balance and antioxidant defense systems. At a MnGA concentration of 100 μg/mL, the H2O2 scavenging rate was 16.54 %, indicating that MnGA exhibited CAT-like activity (Fig. 2b). Additionally, SOD-like activity is a key antioxidant enzyme that primarily converts intracellular superoxide radicals (O2.-) into oxygen and hydrogen peroxide. SOD-like activity reached over 90 % at MnGA concentrations above 40 μg/mL (Fig. 2c). The dual enzyme-like activities involve SOD-like activity to scavenge O2.- and produce H2O2, followed by CAT-like activity to eliminate excess H2O2 produced during the inflammatory phase. For further comparison, an enzyme reaction kinetics study was carried out to analyze the relationship between the substrate concentration and reaction rate. The results revealed that the Michaelis-Menten constant (Km) of the catalase (CAT) of MnGA was 3.512 mM, indicating high substrate affinity. In addition, the maximum reaction rate (Vmax) of MnGA CAT was 22.09 μM/s, which indicated high catalytic activity (Fig. S4, Table S4) [10,[56], [57], [58]]. The enzyme activity units (U values) of SOD and CAT are shown in Fig. S5. On the other hand, the reactive nitrogen species scavenging ability of the MnGA nanozymes was assessed via DPPH· and ABTS·+ radical scavenging assays. The peak at 519 nm for DPPH· decreased with increasing MnGA nanozyme concentration, indicating that the ability of MnGA to scavenge reactive nitrogen species improved with increasing concentration (Fig. 2d). The scavenging rate for reactive nitrogen species reached 86.21 % at 100 μg/mL MnGA in the DPPH· assay (Fig. 2e). Furthermore, in the ABTS·+ radical scavenging assay, a decreasing trend in the characteristic peak at 734 nm was observed with increasing concentration, and the scavenging rate for ABTS·+ radicals reached 97.79 % at 100 μg/mL MnGA, which was consistent with the results of the DPPH· assay (Fig. 2f and g). In addition, the scavenging activities of GA and MnGA against CAT-like, SOD-like and RNS were compared. The results revealed that the antioxidant capacity of MnGA was much greater than that of GA (Figs. S6 and S7). Interestingly, when different ratios of MnGA were tested for their antioxidant activity, the MnGA synthesized in this study exhibited the greatest antioxidant effect. This may be attributed to the ability of this ratio to form structurally stable coordination complexes while maintaining a uniform distribution of catalytically active sites. The antioxidant capacity of GA was able to synergize with the redox activity of Mn2⁺ to enhance the overall antioxidant effect (Fig. S8). In summary, the ability of MnGA to scavenge SOD-like, CAT-like, and RNS has significant potential for application in the treatment of inflammation.
Fig. 2.
Evaluation of the antioxidant capacity of MnGA. (a) Schematic diagram of the MnGA antioxidant. (b) Results for CAT-like activity. (c) Results for SOD-like activity. (d) DPPH· free radical detection assay and (e) scavenging rate of MnGA. (f) ABTS·+ radical detection assay and (g) scavenging rate of MnGA.
Persistent ROS are a significant feature of the inflammatory response. They can cause cellular damage and apoptosis, exacerbating inflammation. ROS also activate proinflammatory signaling pathways, promote the release of inflammatory cytokines, and lead to oxidative stress, further intensifying inflammation (Fig. 3a). Therefore, controlling ROS levels is crucial for managing inflammation and related diseases. After treatment with MnGA, the flow cytometry results revealed a significant decrease in the ROS levels in the macrophages, with 57.56 % in the low-concentration group (50 μg/mL) and 52.35 % in the high-concentration group (100 μg/mL) (Fig. 3c and d). Additionally, fluorescence imaging via the DCFH-DA probe revealed a significant reduction in green fluorescence intensity in the MnGA treatment groups, with the high-concentration group showing slightly greater fluorescence intensity than the low-concentration group did (Fig. 3h). Furthermore, during inflammation, TNF-α, IL-6, and IL-1β are key proinflammatory cytokines that regulate the inflammatory response through interactions; dysregulation or excessive production of these cytokines can lead to chronic inflammation and tissue damage. Compared with those in the LPS-induced group, the expression levels of TNF-α, IL-6, and IL-1β were significantly lower in the groups treated with 50 μg/mL and 100 μg/mL MnGA. After treatment with MnGA at a concentration of 100 μg/mL, the levels of inflammatory factors decreased even more significantly. Specifically, the concentration of TNF-α decreased from 270.56 pg/mL to 105.63 pg/mL, the concentration of IL-6 decreased from 396.65 pg/mL to 210.45 pg/mL, and the concentration of IL-1β decreased from 65.65 pg/mL to 23.45 pg/mL (Fig. 3e–g). These changes indicated that the treatment effect of MnGA was positively correlated with the concentration, and as the concentration increased, the inhibitory effect of inflammatory factors became more obvious. Interestingly, the Cell Counting Kit-8 (CCK-8) assay revealed no significant change in absorbance at 450 nm, even at MnGA concentrations as high as 150 μg/mL, indicating that MnGA has no noticeable toxicity within the 0–150 μg/mL range (Fig. 3b). In addition, hemolysis experiments revealed that MnGA has good blood biocompatibility, with a hemolysis rate of <5 % (Fig. S9).
Fig. 3.
In vitro assessment of the ability to inhibit inflammation. (a) Schematic diagram of the reduction in inflammation caused by MnGA. (b) Cell safety assay of MnGA. (c) Flow cytometric analysis of intracellular ROS and (d) quantification of ROS via flow cytometric analysis. (e–g) ELISA results for TNF-α, IL-1β, and IL-6. (h) ROS fluorescence images obtained via laser confocal microscopy.
In vitro experiments demonstrated that MnGA exhibited excellent ROS scavenging and antioxidant capabilities, which are beneficial for treating the inflammatory phase of wound healing in mice. To investigate the actual healing effect of MnGA on wounds, an acute wound mouse model was established (Fig. 4a). BALB/c mice were randomly divided into three groups, with three mice in each group, and treated with PBS, MnGA (50 μg/mL), or MnGA (100 μg/mL). Wound photographs revealed that the healing rate of wounds treated with MnGA in both treatment groups was significantly faster than that in the PBS group (Fig. 4b). Additionally, the relative wound area of the mice from days 0–8 indicated that, compared with the PBS group, the treatment groups presented a smaller increase in the wound area over time, with MnGA (100 μg/mL) showing a better healing effect than MnGA (50 μg/mL) (Fig. 4c). On day 8, the relative wound area in the treatment groups was significantly different from that in the PBS group (Fig. 4d). Moreover, the body weights of the mice did not change significantly within 8 days of treatment (Fig. S10). H&E staining and Masson's trichrome staining on day 8 revealed that the skin in the treatment groups had more intact cellular morphology and tissue structure (Fig. 4e). Furthermore, the staining images revealed that the MnGA (100 μg/mL) group exhibited a uniform collagen network, evenly distributed blood vessels, and an intact epidermis. Compared with those in the PBS group, the number of collagen fibers in the treatment groups was significantly different (Fig. 4g). Moreover, the thickness of the sarcomeres significantly increased in the high-concentration treatment group (Fig. S11). These results indicate that high concentrations of MnGA significantly promote acute wound healing. To further validate the anti-inflammatory capacity of MnGA in vivo, relevant inflammatory marker and ROS expression levels were assessed. The level of ROS in mouse skin is the most direct measure of the anti-inflammatory ability of MnGA. Fluorescence images of skin tissue revealed that ROS levels were significantly reduced in the MnGA (100 μg/mL) group, indicating that high-concentration MnGA results in increased SOD enzyme activity and effectively scavenges ROS during the inflammatory phase (Fig. 4f–h). Additionally, IL-6 and TNF-α are two typical markers of inflammation. After treatment, the IL-6 and TNF-α levels in the mouse skin were significantly reduced, with almost complete disappearance in the MnGA (100 μg/mL) group (Fig. 4f–i, j). These results demonstrate that 100 μg/mL MnGA effectively inhibits inflammation, thereby promoting wound healing.
Fig. 4.
MnGA promotes acute wound healing. (a) Schematic of mouse model construction and treatment. (b) Photographs of mouse wounds within 8 days. (c) Wound healing area within 8 days. (d) Significance analysis of the trauma area in mice on day 8. (e) H&E and Masson staining. (f) ROS, IL-6, and TNF-α staining. (g) Quantitative analysis of collagen fibers via Masson's trichrome staining. (h–j) ROS, IL-6, and TNF-α fluorescence quantitative analysis.
To investigate the mechanism by which MnGA promotes wound healing through the inhibition of inflammation, the MnGA (100 μg/mL) group and the PBS group were further analyzed via high-throughput RNA sequencing. Volcano plots revealed a total of 237 differentially expressed genes (DEGs) between the MnGA (100 μg/mL) group and the PBS group (Fig. 5a). The heatmaps provided a visual representation of the DEG distribution (Fig. 5b). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses revealed significant regulation of processes related to cell proliferation, skin regeneration, and inflammation (Fig. 5c and d). Protein interaction analysis revealed significant associations in the GO biological process (BP) network, with a p value less than 0.001 (Fig. 5e). Additionally, gene set enrichment analysis (GESA) revealed significant downregulation of genes related to the NF-κB signaling pathway, Toll-like receptor signaling pathway, NOD-like receptor signaling pathway, and cytokine-cytokine receptor interaction (Fig. 5f). The NF-κB signaling pathway, which involves core transcription factors that regulate immune responses and inflammation, initiates and maintains inflammation by modulating the production of various cytokines and chemokines. The Toll-like receptor (TLR) signaling pathway triggers innate immune responses and inflammatory signals by recognizing pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). The NOD-like receptor (NLR) signaling pathway, which is located in the cytoplasm, detects pathogens and damage signals, activating inflammasomes that promote the maturation and release of inflammatory cytokines such as IL-1β and IL-18. Finally, cytokine-cytokine receptor interactions are crucial for cell communication during inflammation, with cytokines such as TNF-α, IL-1, and IL-6 playing key roles in the initiation and maintenance of inflammation. These results suggest that MnGA modulates relevant pathways and interactions during the inflammatory process, thereby promoting wound healing.
Fig. 5.
The mechanism by which MnGA inhibits the inflammatory response to promote wound healing. (a) Volcano plot of differentially expressed genes. (b) Heatmap of differentially expressed genes. (c) GO and (d) KEGG enrichment analyses. (f) Protein-protein interaction (PPI) network map. (f) GO enrichment in GSEA.
The biocompatibility of MnGA nanozymes directly affects their safety for in vivo use. Evaluating their long-term impact on tissues and organs, as well as potential immune responses, is crucial for ensuring their nontoxicity. On day 8, histological examination of the heart, liver, spleen, lungs, and kidneys via H&E staining revealed that MnGA treatment resulted in normal cell morphology and basic structure (Fig. 6a). Additionally, routine blood and biochemical tests are important for assessing the safety of nanozymes. There were no significant abnormalities in blood parameters, such as BUN, AST, ALT, CRE, PLT, HGB, HCT, PCT, MCH, WBC, RBC, or MCV, in the experimental group compared with those in the control group (Fig. 6b). In short, MnGA is safe in mice and has no apparent side effects.
Fig. 6.
In vivo biocompatibility assessment of MnGA. (a) H&E sections of major organs (heart, liver, spleen, lung, and kidney) of mice in different groups on day 8. (b) Routine blood and biochemical parameters of normal mice and other treatment groups on day 8.
4. Conclusions
In this study, chlorinated manganese was used as the manganese source and mixed with GA to prepare the ligand solution. Through the coordination reaction between metal ions and ligands, spindle-shaped MnGA nanozymes were successfully synthesized. The MnGA nanozymes exhibited significant antioxidant activity, with CAT-like and SOD-like activities, indicating their ability to effectively catalyze the decomposition of hydrogen peroxide and scavenge superoxide radicals. Furthermore, MnGA demonstrated strong radical scavenging ability in the DPPH· and ABTS·+ assays. MnGA significantly reduced the level of intracellular ROS and decreased the expression of the inflammatory markers TNF-α, IL-6, and IL-1β. Both in vitro and in vivo experiments revealed that MnGA is effective at promoting wound healing, with a more pronounced effect observed at higher concentrations. Additionally, MnGA showed no apparent toxicity in mice, and tissue and blood tests indicated good biocompatibility. Eukaryotic transcriptome RNA sequencing analysis further revealed that MnGA regulates inflammation-related pathways, promoting wound healing by modulating NF-κB, TLR, NLR, and cytokine-cytokine receptor interactions.
CRediT authorship contribution statement
Xueting Guo: Writing – original draft, Formal analysis, Data curation, Conceptualization. Wenqi Wang: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Liting Lin: Formal analysis, Data curation, Conceptualization. Jie Shan: Methodology, Formal analysis, Data curation. Junyao Zhu: Formal analysis, Data curation. Shipeng Ning: Formal analysis, Data curation. Hanmei Li: Writing – review & editing, Visualization, Software, Resources. Xianwen Wang: Writing – review & editing, Visualization, Software, Resources, Project administration, Methodology. Decheng Lu: Writing – review & editing, Validation, Software, Resources, Methodology.
Declaration of competing interest
All authors declare that No conflict of interest exists.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82372552), the Anhui Provincial Natural Science Foundation (2408085Y016), the Excellent Youth of Natural Science Research Projects in Anhui Province Universities (2023AH030060), Anhui Province Excellent Research and Innovation Team Project (2024AH010013), Anhui Province Excellent Research and Innovation Team Project (2024AH010013), and the Guangxi Provincial Natural Science Foundation (2024JJA141518). The authors would like to thank the Shiyan laboratory (www.shiyanjia.com) for their help in language polishing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2024.101435.
Contributor Information
Xianwen Wang, Email: xianwenwang@ahmu.edu.cn.
Decheng Lu, Email: ludecheng@gxmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Deng Z., Zhang M. Liposozyme for wound healing and inflammation resolution. Nat. Nanotechnol. 2024;19(8):1083–1084. doi: 10.1038/s41565-024-01656-8. [DOI] [PubMed] [Google Scholar]
- 2.Shi S., Jiang Y., Yu Y., Liang M., Bai Q., Wang L., Yang D., Sui N., Zhu Z. Piezo-augmented and photocatalytic nanozyme integrated microneedles for antibacterial and anti-inflammatory combination therapy. Adv. Funct. Mater. 2023;33(10) [Google Scholar]
- 3.Ma L., Zheng J.-J., Zhou N., Zhang R., Fang L., Yang Y., Gao X., Chen C., Yan X., Fan K. A natural biogenic nanozyme for scavenging superoxide radicals. Nat. Commun. 2024;15(1):233. doi: 10.1038/s41467-023-44463-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng X., Fan H., Chen L., He J., Hong C., Xie J., Hou Y., Wang K., Gao X., Gao L., Yan X., Fan K. Ultrasmall metal alloy nanozymes mimicking neutrophil enzymatic cascades for tumor catalytic therapy. Nat. Commun. 2024;15(1):1626. doi: 10.1038/s41467-024-45668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang F., Shu R., Li B., Dai W., Chen W., Sun J., Bai D., Yang W., Deng Y. Anti-inflammasome bioheterojunction (AI-bioHJ): revolutionizing diabetic wound healing with in situ self-transformation and programmed gas therapy. Chem. Eng. J. 2024;482 [Google Scholar]
- 6.Yu Y., Zhao X., Xu X., Cai C., Tang X., Zhang Q., Zhong L., Zhou F., Yang D., Zhu Z. Rational design of orally administered cascade nanozyme for inflammatory bowel disease therapy. Adv. Mater. 2023;35(44) doi: 10.1002/adma.202304967. [DOI] [PubMed] [Google Scholar]
- 7.Li J., Song J., Deng Z., Yang J., Wang X., Gao B., Zhu Y., Yang M., Long D., Luo X., Zhang M., Zhang M., Li R. Robust reactive oxygen species modulator hitchhiking yeast microcapsules for colitis alleviation by trilogically intestinal microenvironment renovation. Bioact. Mater. 2024;36:203–220. doi: 10.1016/j.bioactmat.2024.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., Wang Y., Yang J., Liu W. Bacteria activated-macrophage membrane-coated tough nanocomposite hydrogel with targeted photothermal antibacterial ability for infected wound healing. Chem. Eng. J. 2021;420 [Google Scholar]
- 9.Li X.L., Liu Y., Qi X.W., Xiao S.L., Xu Z.S., Yuan Z.X., Liu Q., Li H.S., Ma S.Y., Liu T.F., Huang Y., Zhang X.R., Zhang X., Mao Z.W., Luo G.X., Deng J. Sensitive activatable nanoprobes for real-time ratiometric magnetic resonance imaging of reactive oxygen species and ameliorating inflammation in vivo. Adv. Mater. 2022;34(19) doi: 10.1002/adma.202109004. [DOI] [PubMed] [Google Scholar]
- 10.Liu T., Xiao B., Xiang F., Tan J., Chen Z., Zhang X., Wu C., Mao Z., Luo G., Chen X., Deng J. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 2020;11(1):2788. doi: 10.1038/s41467-020-16544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu C., Lu H., Zhou T., Zhang W., Deng L., Cao W., Yang Z., Wang Z., Wu X., Ding J., Xu F., Gao C. Promoting the healing of infected diabetic wound by an antibacterial and nanoenzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. 2022;286 doi: 10.1016/j.biomaterials.2022.121597. [DOI] [PubMed] [Google Scholar]
- 12.Emami M.H., Sereshki N., Malakoutikhah Z., Dehkordi S.A.E., Fahim A., Mohammadzadeh S., Maghool F. Nrf2 signaling pathway in trace metal carcinogenesis: a cross-talk between oxidative stress and angiogenesis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022;254 doi: 10.1016/j.cbpc.2022.109266. [DOI] [PubMed] [Google Scholar]
- 13.Lugrin J., Rosenblatt-Velin N., Parapanov R., Liaudet L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014;395(2):203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- 14.Schäfer M., Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008;58(2):165–171. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Nie W., Huang Y., Wang Y., Kengla C., Scott Copus J., Sun J., Shao Z., Dai X., Shen Y. Temperature sensitive polyMOF hydrogel formed by in situ open-ring polymerization for infected chronic wound treatment. Chem. Eng. J. 2022;446 [Google Scholar]
- 16.Wang W., Gao Y., Xu W., Xu Y., Zhou N., Li Y., Zhang M., Tang B.Z. The one-stop integrated nanoagent based on photothermal therapy for deep infection healing and inflammation inhibition. Adv. Mater. 2024;36(3) doi: 10.1002/adma.202307785. [DOI] [PubMed] [Google Scholar]
- 17.Shang L., Yu Y., Jiang Y., Liu X., Sui N., Yang D., Zhu Z. Ultrasound-augmented multienzyme-like nanozyme hydrogel spray for promoting diabetic wound healing. ACS Nano. 2023;17(16):15962–15977. doi: 10.1021/acsnano.3c04134. [DOI] [PubMed] [Google Scholar]
- 18.Wang D., Wang J., Gao X.J., Ding H., Yang M., He Z., Xie J., Zhang Z., Huang H., Nie G., Yan X., Fan K. Employing noble metal–porphyrins to engineer robust and highly active single-atom nanozymes for targeted catalytic therapy in nasopharyngeal carcinoma. Adv. Mater. 2024;36(7) doi: 10.1002/adma.202310033. [DOI] [PubMed] [Google Scholar]
- 19.Yang F., Shu R., Dai W., Li B., Liu C., Yang H., Johnson H.M., Yu S., Bai D., Yang W., Deng Y. H2Se-evolving bioheterojunctions promote cutaneous regeneration in infected wounds by inhibiting excessive cellular senescence. Biomaterials. 2024;311 doi: 10.1016/j.biomaterials.2024.122659. [DOI] [PubMed] [Google Scholar]
- 20.Yao P., Bao Q., Yao Y., Xiao M., Xu Z., Yang J., Liu W. Environmentally stable, robust, adhesive, and conductive supramolecular deep eutectic gels as ultrasensitive flexible temperature sensor. Adv. Mater. 2023;35(21) doi: 10.1002/adma.202300114. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y., Ren J., Qu X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019;119(6):4357–4412. doi: 10.1021/acs.chemrev.8b00672. [DOI] [PubMed] [Google Scholar]
- 22.Jin X., Zhang W., Shan J., He J., Qian H., Chen X., Wang X. Thermosensitive hydrogel loaded with nickel–copper bimetallic hollow nanospheres with SOD and CAT enzymatic-like activity promotes acute wound healing. ACS Appl. Mater. Interfaces. 2022;14(45):50677–50691. doi: 10.1021/acsami.2c17242. [DOI] [PubMed] [Google Scholar]
- 23.Wang W., Gao P., Gui H., Wei X., Zhang H., Wang X. Copper-based nanomaterials for the treatment of bacterium-infected wounds: material classification, strategies and mechanisms. Coord. Chem. Rev. 2024;522 [Google Scholar]
- 24.Jiang W., Li Q., Zhang R., Li J., Lin Q., Li J., Zhou X., Yan X., Fan K. Chiral metal-organic frameworks incorporating nanozymes as neuroinflammation inhibitors for managing Parkinson's disease. Nat. Commun. 2023;14(1):8137. doi: 10.1038/s41467-023-43870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B., Yang W., Shu R., Yang H., Yang F., Dai W., Chen W., Chan Y.K., Bai D., Deng Y. Antibacterial and angiogenic (2A) bio-heterojunctions facilitate infectious ischemic wound regeneration via an endogenous–exogenous bistimulatory strategy. Adv. Mater. 2024;36(6) doi: 10.1002/adma.202307613. [DOI] [PubMed] [Google Scholar]
- 26.Bai Q., Liang M., Wu W., Zhang C., Li X., Liu M., Yang D., Yu W.W., Hu Q., Wang L., Du F., Sui N., Zhu Z. Plasmonic nanozyme of graphdiyne nanowalls wrapped hollow copper sulfide nanocubes for rapid bacteria-killing. Adv. Funct. Mater. 2022;32(20) [Google Scholar]
- 27.Gao W., He J., Chen L., Meng X., Ma Y., Cheng L., Tu K., Gao X., Liu C., Zhang M., Fan K., Pang D.-W., Yan X. Deciphering the catalytic mechanism of superoxide dismutase activity of carbon dot nanozyme. Nat. Commun. 2023;14(1):160. doi: 10.1038/s41467-023-35828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y., Chen Z., Sui N., Zhu Z. Data-driven evolutionary design of multienzyme-like nanozymes. J. Am. Chem. Soc. 2024;146(11):7565–7574. doi: 10.1021/jacs.3c13588. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z., Zhang R., Yan X., Fan K. Structure and activity of nanozymes: Inspirations for de novo design of nanozymes. Mater. Today. 2020;41:81–119. [Google Scholar]
- 30.Wang W., Cui Y., Wei X., Zang Y., Chen X., Cheng L., Wang X. CuCo2O4 nanoflowers with multiple enzyme activities for treating bacterium-infected wounds via cuproptosis-like death. ACS Nano. 2024;18(24):15845–15863. doi: 10.1021/acsnano.4c02825. [DOI] [PubMed] [Google Scholar]
- 31.Dai W., Shu R., Yang F., Li B., Johnson H.M., Yu S., Yang H., Chan Y.K., Yang W., Bai D., Deng Y. Engineered bio-heterojunction confers extra and intracellular bacterial ferroptosis and hunger-triggered cell protection for diabetic wound repair. Adv. Mater. 2024;36(9) doi: 10.1002/adma.202305277. [DOI] [PubMed] [Google Scholar]
- 32.Deng Y., Ouyang X., Sun J., Shi X., Li Y., Chan Y.K., Yang W., Peng S. Rapid sterilization and diabetic cutaneous regeneration using cascade bioheterojunctions through glucose oxidase-primed therapy. Bioact. Mater. 2023;25:748–765. doi: 10.1016/j.bioactmat.2022.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye J., Lv W., Li C., Liu S., Yang X., Zhang J., Wang C., Xu J., Jin G., Li B., Fu Y., Liang X. Tumor response and NIR-II photonic thermal Co-enhanced catalytic therapy based on single-atom manganese nanozyme. Adv. Funct. Mater. 2022;32(47) [Google Scholar]
- 34.Chen Y., Tian Q., Wang H., Ma R., Han R., Wang Y., Ge H., Ren Y., Yang R., Yang H., Chen Y., Duan X., Zhang L., Gao J., Gao L., Yan X., Qin Y. A manganese-based metal–organic framework as a cold-adapted nanozyme. Adv. Mater. 2024;36(10) doi: 10.1002/adma.202206421. [DOI] [PubMed] [Google Scholar]
- 35.Qiao W., Chen J., Zhou H., Hu C., Dalangood S., Li H., Yang D., Yang Y., Gui J. A single-atom manganese nanozyme Mn-N/C promotes anti-tumor immune response via eliciting type I interferon signaling. Adv. Sci. 2024;11(14) doi: 10.1002/advs.202305979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan F., Jia Q., Liang G., Wang M., Zhu L., McHugh K.J., Jing L., Du M., Zhang Z. Schottky junction nanozyme based on Mn-bridged Co-phthalocyanines and Ti3C2Tx nanosheets boosts integrative type I and II photosensitization for multimodal cancer therapy. ACS Nano. 2023;17(12):11290–11308. doi: 10.1021/acsnano.2c12270. [DOI] [PubMed] [Google Scholar]
- 37.Liu S., Bai Q., Jiang Y., Gao Y., Chen Z., Shang L., Zhang S., Yu L., Yang D., Sui N., Zhu Z. Multienzyme-like nanozyme encapsulated ocular microneedles for keratitis treatment. Small. 2024;20(21) doi: 10.1002/smll.202308403. [DOI] [PubMed] [Google Scholar]
- 38.Wang J., Wang Y., Xiaohalati X., Su Q., Liu J., Cai B., Yang W., Wang Z., Wang L. A bioinspired manganese-organic framework ameliorates ischemic stroke through its intrinsic nanozyme activity and upregulating endogenous antioxidant enzymes. Adv. Sci. 2023;10(20) doi: 10.1002/advs.202206854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong S., Dong Y., Liu B., Liu J., Liu S., Zhao Z., Li W., Tian B., Zhao R., He F., Gai S., Xie Y., Yang P., Zhao Y. Guiding transition metal-doped hollow cerium tandem nanozymes with elaborately regulated multi-enzymatic activities for intensive chemodynamic therapy. Adv. Mater. 2022;34(7) doi: 10.1002/adma.202107054. [DOI] [PubMed] [Google Scholar]
- 40.Sun W., Sun Q. Bioinspired manganese and iron complexes for enantioselective oxidation reactions: ligand design, catalytic activity, and beyond. Accounts Chem. Res. 2019;52(8):2370–2381. doi: 10.1021/acs.accounts.9b00285. [DOI] [PubMed] [Google Scholar]
- 41.Cozzolino A.F., Brozek C.K., Palmer R.D., Yano J., Li M., Dincă M. Ligand redox noninnocence in the stoichiometric oxidation of Mn2(2,5-dioxidoterephthalate) (Mn-MOF-74) J. Am. Chem. Soc. 2014;136(9):3334–3337. doi: 10.1021/ja411808r. [DOI] [PubMed] [Google Scholar]
- 42.Yin C., Bao Z., Tan H., Zhou H., Li J. Metal-organic framework-mediated synthesis of LiNi0.5Mn1.5O4: tuning the Mn3+ content and electrochemical performance by organic ligands. Chem. Eng. J. 2019;372:408–419. [Google Scholar]
- 43.Yao J., Cheng Y., Zhou M., Zhao S., Lin S., Wang X., Wu J., Li S., Wei H. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem. Sci. 2018;9(11):2927–2933. doi: 10.1039/c7sc05476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Z., Zhou X., Wang L., Guo H., Han M., Guo H., Chen Y., Wu A., Li H., Chen S., Xie Y., Wang X. Mn3O4 nanozyme loaded thermosensitive PDLLA-PEG-PDLLA hydrogels for the treatment of inflammatory bowel disease. ACS Appl. Mater. Interfaces. 2023;15(28):33273–33287. doi: 10.1021/acsami.3c03332. [DOI] [PubMed] [Google Scholar]
- 45.Wu K., Zhu D., Dai X., Wang W., Zhong X., Fang Z., Peng C., Wei X., Qian H., Chen X., Wang X., Zha Z., Cheng L. Bimetallic oxide Cu1.5Mn1.5O4 cage-like frame nanospheres with triple enzyme-like activities for bacterial-infected wound therapy. Nano Today. 2022;43 [Google Scholar]
- 46.Yang J., Huang Z., Tan J., Pan J., Chen S., Wan W. Copper ion/gallic acid MOFs-laden adhesive pomelo peel sponge effectively treats biofilm-infected skin wounds and improves healing quality. Bioact. Mater. 2024;32:260–276. doi: 10.1016/j.bioactmat.2023.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang G., Fan R., Yang J., Yi L., Chen S., Wan W. Magnesium/gallic acid bioMOFs laden carbonized mushroom aerogel effectively heals biofilm-infected skin wounds. Biomaterials. 2023;302 doi: 10.1016/j.biomaterials.2023.122347. [DOI] [PubMed] [Google Scholar]
- 48.Park H.-H., Ko S.-C., Oh G.-W., Jang Y.-M., Kim Y.-M., Park W.S., Choi I.-W., Jung W.-K. Characterization and biological activity of PVA hydrogel containing chitooligosaccharides conjugated with gallic acid. Carbohydr. Polym. 2018;198:197–205. doi: 10.1016/j.carbpol.2018.06.070. [DOI] [PubMed] [Google Scholar]
- 49.Yao Y., Xu Z., Liu B., Xiao M., Yang J., Liu W. Multiple H-bonding chain extender-based ultrastiff thermoplastic polyurethanes with autonomous self-healability, solvent-free adhesiveness, and AIE fluorescence. Adv. Funct. Mater. 2021;31(4) [Google Scholar]
- 50.Huang H., Gong W., Wang X., He W., Hou Y., Hu J. Self-assembly of naturally, small molecules into supramolecular fibrillar networks for wound healing. Adv. Healthcare Mater. 2022;11(12) doi: 10.1002/adhm.202102476. [DOI] [PubMed] [Google Scholar]
- 51.Yu J., Wang K., Fan C., Zhao X., Gao J., Jing W., Zhang X., Li J., Li Y., Yang J., Liu W. An ultrasoft self-fused supramolecular polymer hydrogel for completely preventing postoperative tissue adhesion. Adv. Mater. 2021;33(16) doi: 10.1002/adma.202008395. [DOI] [PubMed] [Google Scholar]
- 52.Park S.G., Li M.-X., Cho W.K., Joung Y.K., Huh K.M. Thermosensitive gallic acid-conjugated hexanoyl glycol chitosan as a novel wound healing biomaterial. Carbohydr. Polym. 2021;260 doi: 10.1016/j.carbpol.2021.117808. [DOI] [PubMed] [Google Scholar]
- 53.Zeng H., Ying Z.-R., Luo X., Tan S., Liu X.-H., Zhao X.-Y., He S.-S., Chen F., Kulak A.I., Lu B.-Q. Gallic acid-modified bioglass with combined photothermal and antibacterial effects for the regeneration of infected diabetic wound. Compos. B Eng. 2023;257 [Google Scholar]
- 54.Feng M., Zeng X., Lin Q., Wang Y., Wei H., Yang S., Wang G., Chen X., Guo M., Yang X., Hu J., Zhang Y., Yang X., Du Y., Zhao Y. Characterization of chitosan-gallic acid graft copolymer for periodontal dressing hydrogel application. Adv. Healthcare Mater. 2024;13(7) doi: 10.1002/adhm.202302877. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M., Xu C., Liu D., Han M.K., Wang L., Merlin D. Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. Journal of Crohn's and Colitis. 2018;12(2):217–229. doi: 10.1093/ecco-jcc/jjx115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bai M., Wang T., Xing Z., Huang H., Wu X., Adeli M., Wang M., Han X., Ye L., Cheng C. Electron-donable heterojunctions with synergetic Ru-Cu pair sites for biocatalytic microenvironment modulations in inflammatory mandible defects. Nat. Commun. 2024;15(1):9592. doi: 10.1038/s41467-024-53824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong S., Dong Y., Liu B., Liu J., Liu S., Zhao Z., Li W., Tian B., Zhao R., He F., Gai S., Xie Y., Yang P., Zhao Y. Guiding transition metal-doped hollow cerium tandem nanozymes with elaborately regulated multi-enzymatic activities for intensive chemodynamic therapy. Adv. Mater. 2022;34(7) doi: 10.1002/adma.202107054. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y., Jiang B., Hao H., Li H., Qiu C., Liang X., Qu Q., Zhang Z., Gao R., Duan D., Ji S., Wang D., Liang M. Atomic-level regulation of cobalt single-atom nanozymes: engineering high-efficiency catalase mimics. Angew. Chem. Int. Ed. 2023;62(19) doi: 10.1002/anie.202301879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.