Abstract
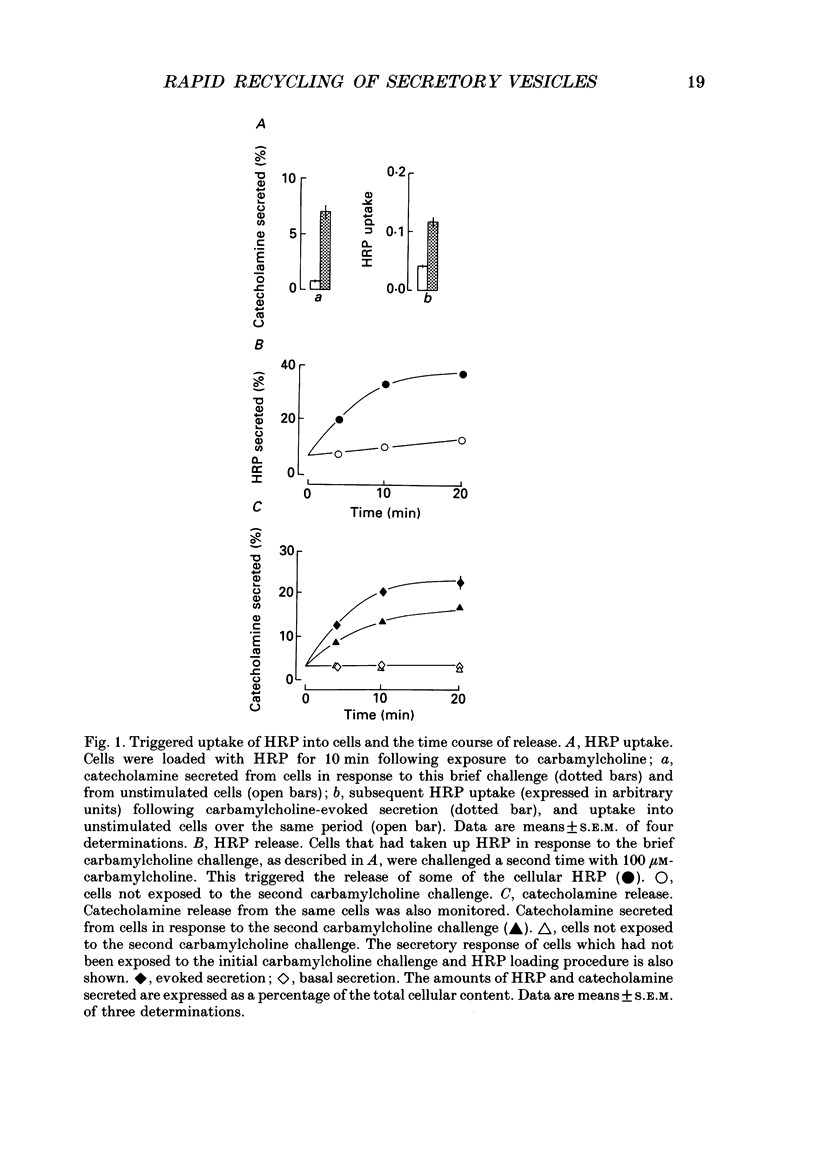
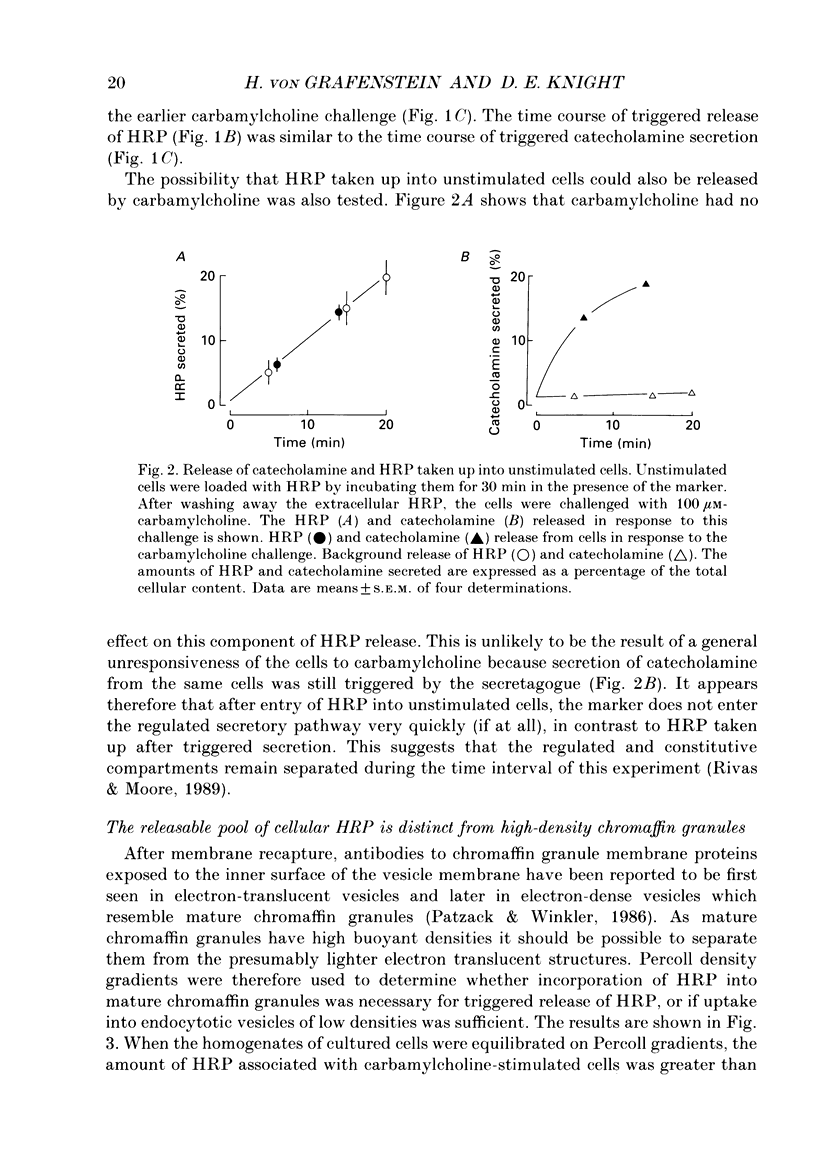
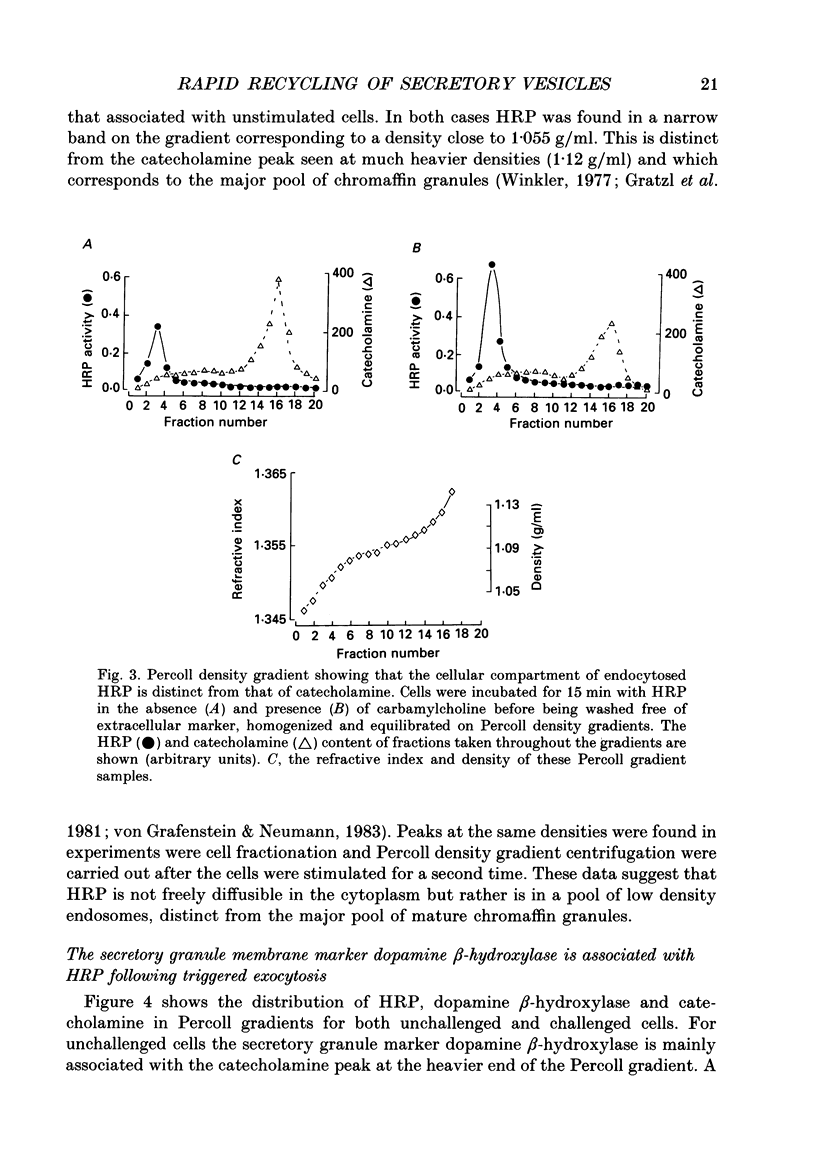
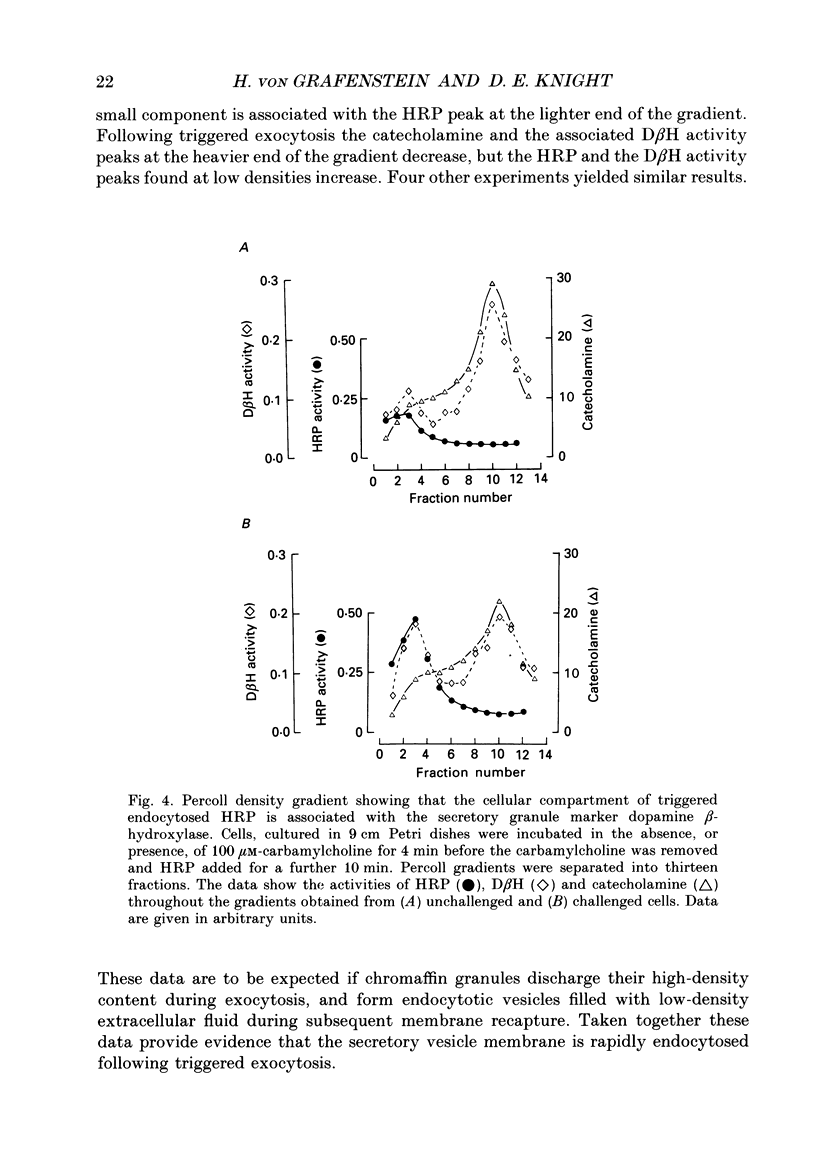
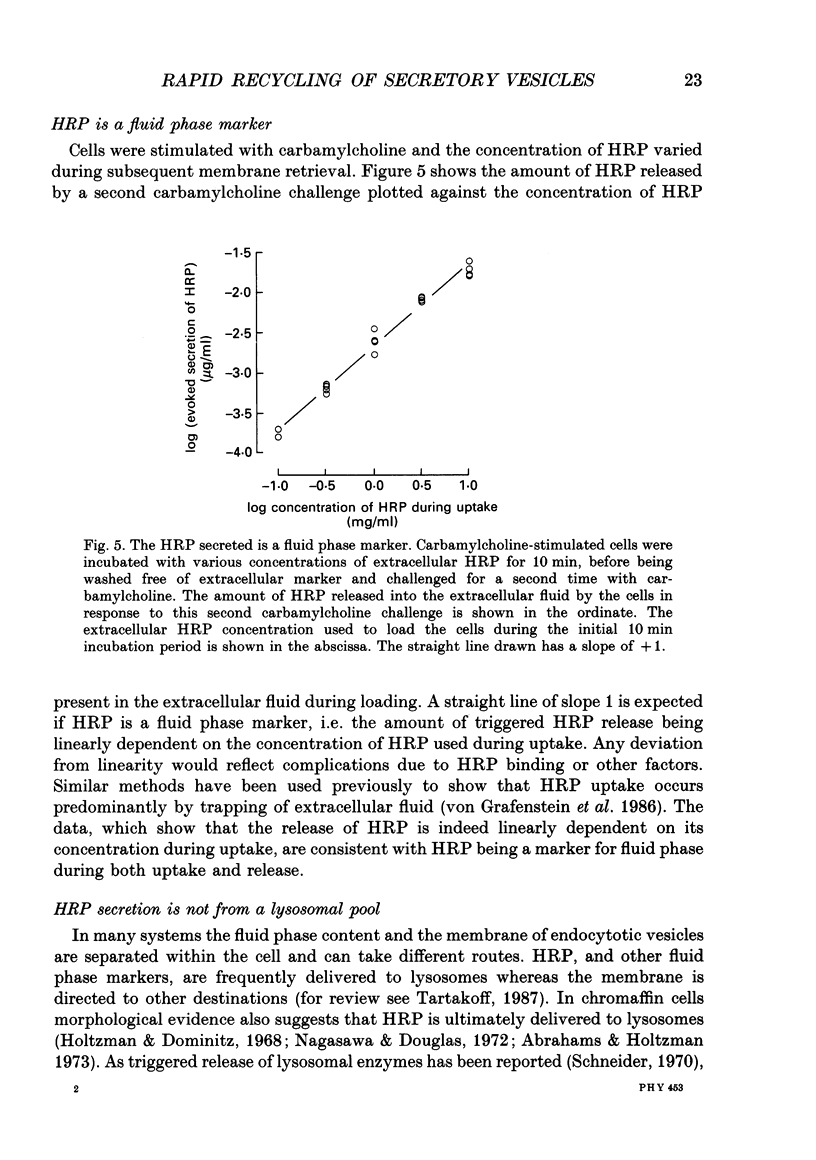
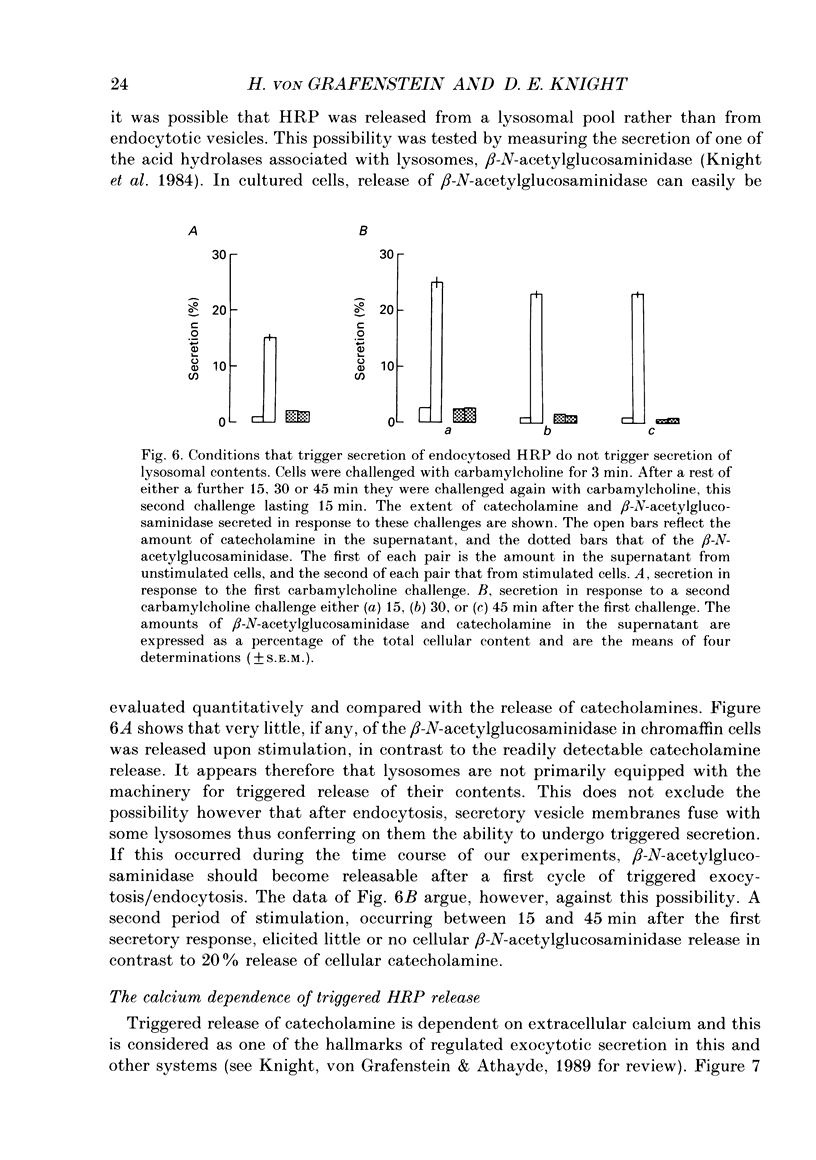
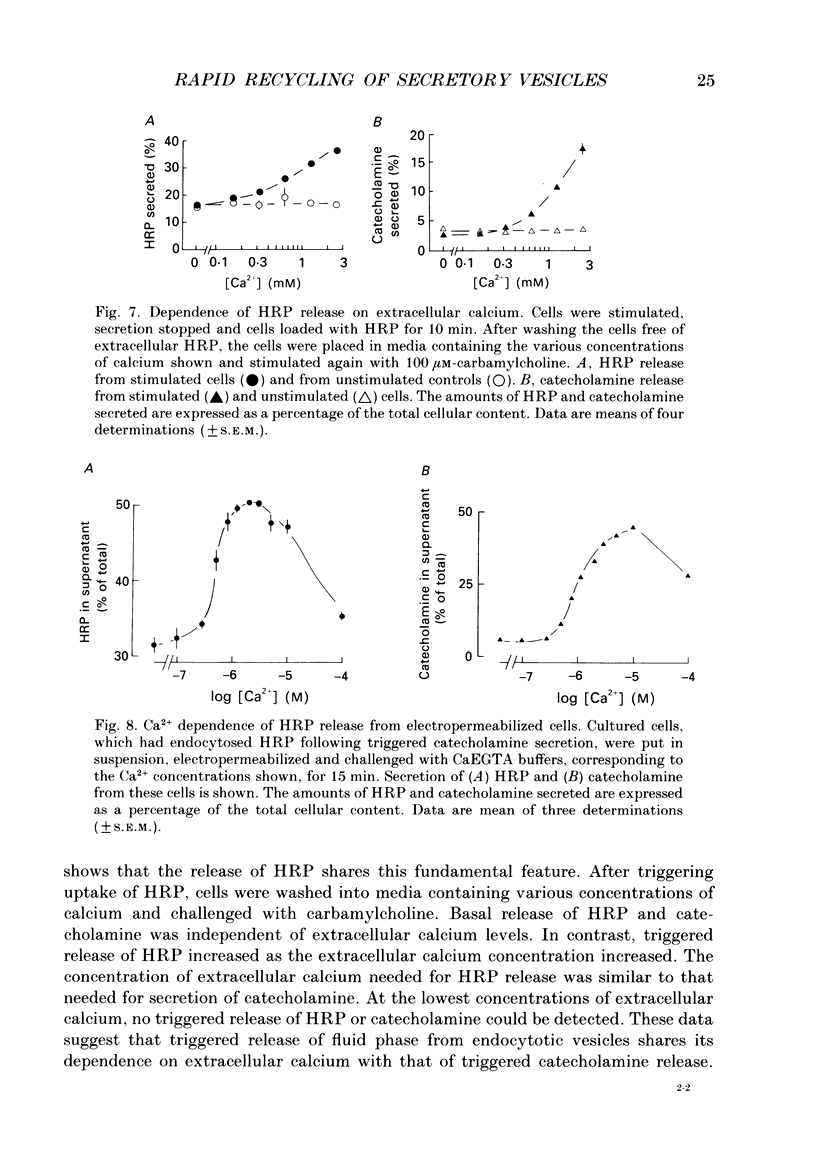
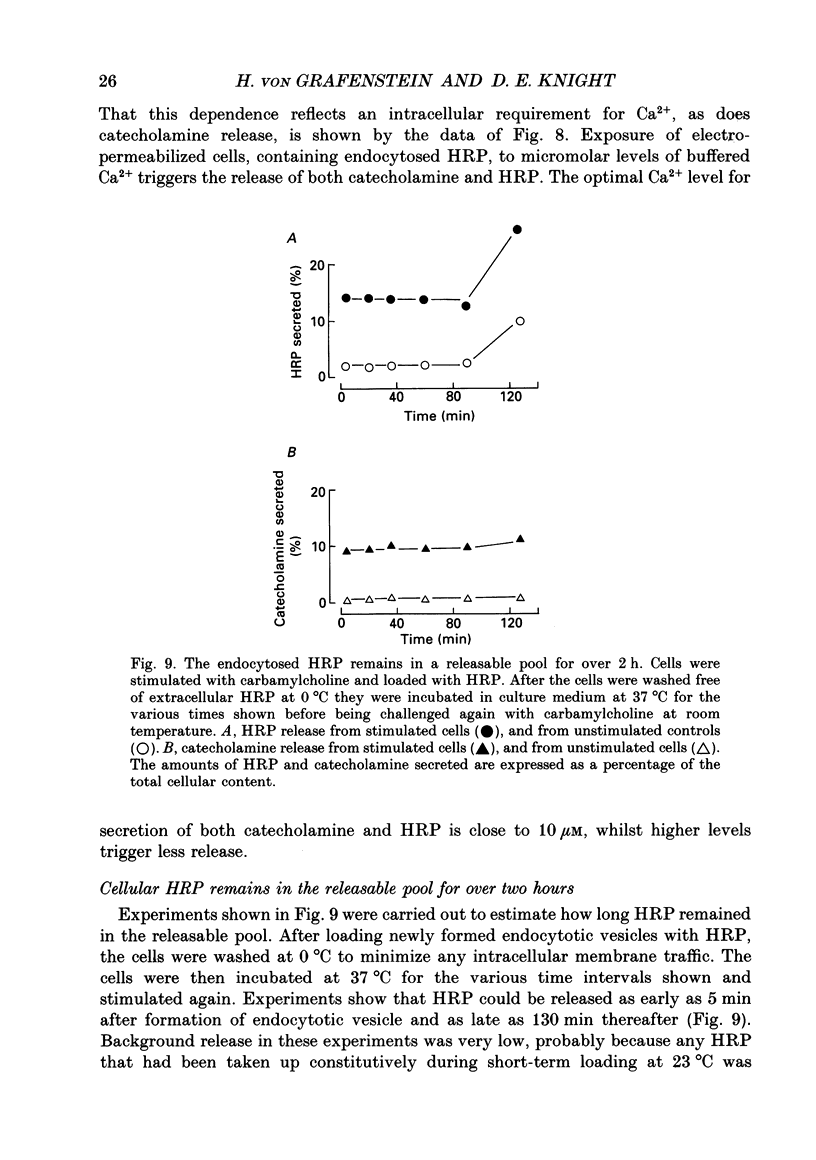
1. Recycling of secretory vesicles in cultured bovine adrenal medullary cells was investigated. 2. Extracellular horseradish peroxidase (HRP), a fluid phase marker, was taken up into cultured adrenal medullary cells following carbamylcholine-induced secretion of catecholamine. 3. The endocytosed HRP remained compartmentalized within the cell, migrating to a low density band on a Percoll density gradient. The endocytotic compartment was distinct from the major pool of catecholamine-containing chromaffin granules, which were found at much higher densities on the Percoll gradient. 4. The chromaffin granule membrane marker dopamine beta-hydroxylase was associated with the endocytosed HRP compartment as well as with the heavier chromaffin granules. 5. A subsequent challenge of the cells with carbamylcholine triggered the release of up to forty per cent of the endocytosed HRP. 6. The time course for secretion of the fluid phase marker was similar to that for catecholamine secretion. 7. Triggered release of HRP was dependent on extracellular calcium. The dependence on the extracellular calcium concentration was similar to that of catecholamine release. 8. Release of HRP could be triggered from electropermeabilized cells by raising the intracellular Ca2+ into the micromolar range. The intracellular Ca2+ dependence of triggered HRP release was similar to that for catecholamine release. 9. HRP could be secreted as early as 5 min, and as late as 2 h after endocytosis. 10. These data provide evidence that endocytotic vesicles can rapidly re-enter the secretory cycle. Endocytosed vesicles may therefore not have to recycle via the trans-Golgi reticulum to form high-density chromaffin granules in order to re-enter the regulated secretory pathway.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams S. J., Holtzman E. Secretion and endocytosis in insulin-stimulated rat adrenal medulla cells. J Cell Biol. 1973 Feb;56(2):540–558. doi: 10.1083/jcb.56.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams C. J., Maurey K. M., Storrie B. Exocytosis of pinocytic contents by Chinese hamster ovary cells. J Cell Biol. 1982 Jun;93(3):632–637. doi: 10.1083/jcb.93.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Airhart J. A., Woodworth R. C., Low R. B. Exocytosis of pinocytosed fluid in cultured cells: kinetic evidence for rapid turnover and compartmentation. J Cell Biol. 1981 Dec;91(3 Pt 1):716–727. doi: 10.1083/jcb.91.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzelius F., Zimmermann H. Recycled synaptic vesicles contain vesicle but not plasma membrane marker, newly synthesized acetylcholine, and a sample of extracellular medium. J Neurochem. 1990 Oct;55(4):1266–1273. doi: 10.1111/j.1471-4159.1990.tb03134.x. [DOI] [PubMed] [Google Scholar]
- Brimijoin S., Helland L. Rapid retrograde transport of dopamine-beta-hydroxylase as examined by the stop-flow technique. Brain Res. 1976 Feb 6;102(2):217–228. doi: 10.1016/0006-8993(76)90878-7. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P., Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980 Apr;60(2):396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Clift-O'Grady L., Linstedt A. D., Lowe A. W., Grote E., Kelly R. B. Biogenesis of synaptic vesicle-like structures in a pheochromocytoma cell line PC-12. J Cell Biol. 1990 May;110(5):1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried R. C., Blaustein M. P. Retrieval and recycling of synaptic vesicle membrane in pinched-off nerve terminals (synaptosomes). J Cell Biol. 1978 Sep;78(3):685–700. doi: 10.1083/jcb.78.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M. Release of noradrenaline from the cat spleen by sodium deprivation. Br J Pharmacol. 1973 Apr;47(4):729–747. doi: 10.1111/j.1476-5381.1973.tb08200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzl M., Krieger-Brauer H., Ekerdt R. Latent acetylcholinesterase in secretory vesicles isolated from adrenal medulla. Biochim Biophys Acta. 1981 Dec 7;649(2):355–366. doi: 10.1016/0005-2736(81)90425-9. [DOI] [PubMed] [Google Scholar]
- Haylett T., Thilo L. Limited and selective transfer of plasma membrane glycoproteins to membrane of secondary lysosomes. J Cell Biol. 1986 Oct;103(4):1249–1256. doi: 10.1083/jcb.103.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog V., Farquhar M. G. Luminal membrane retrieved after exocytosis reaches most golgi cisternae in secretory cells. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5073–5077. doi: 10.1073/pnas.74.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman E., Dominitz R. Cytochemical studies of lysosomes, golgi apparatus and endoplasmic reticulum in secretion and protein uptake by adrenal medulla cells of the rat. J Histochem Cytochem. 1968 May;16(5):320–336. doi: 10.1177/16.5.320. [DOI] [PubMed] [Google Scholar]
- Häggendal J. Noradrenaline and dopamine-beta-hydroxylase levels in rat salivary glands after preganglionic nerve stimulation: evidence for re-use of amine storage granules in transmitter release. J Neural Transm. 1982;53(2-3):147–158. doi: 10.1007/BF01243406. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Stimulus-secretion coupling in isolated bovine adrenal medullary cells. Q J Exp Physiol. 1983 Jan;68(1):123–143. doi: 10.1113/expphysiol.1983.sp002691. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Niggli V., Scrutton M. C. Thrombin and activators of protein kinase C modulate secretory responses of permeabilised human platelets induced by Ca2+. Eur J Biochem. 1984 Sep 3;143(2):437–446. doi: 10.1111/j.1432-1033.1984.tb08391.x. [DOI] [PubMed] [Google Scholar]
- Knight D. E., von Grafenstein H., Athayde C. M. Calcium-dependent and calcium-independent exocytosis. Trends Neurosci. 1989 Nov;12(11):451–458. doi: 10.1016/0166-2236(89)90095-7. [DOI] [PubMed] [Google Scholar]
- Marsh M., Schmid S., Kern H., Harms E., Male P., Mellman I., Helenius A. Rapid analytical and preparative isolation of functional endosomes by free flow electrophoresis. J Cell Biol. 1987 Apr;104(4):875–886. doi: 10.1083/jcb.104.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Ceccarelli B. Exocytosis and membrane recycling. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):55–65. doi: 10.1098/rstb.1981.0171. [DOI] [PubMed] [Google Scholar]
- Nagasawa J., Douglas W. W., Schulz R. A. Micropinocytotic origin of coated and smooth microvesicles ("synaptic vesicles") in neurosecretory terminals of posterior pituitary glands demonstrated by incorporation of horseradish peroxidase. Nature. 1971 Jul 30;232(5309):341–342. doi: 10.1038/232341a0. [DOI] [PubMed] [Google Scholar]
- Nagasawa J., Douglas W. W. Thorium dioxide uptake into adrenal medullary cells and the problem of recapture of granule membrane following exocytosis. Brain Res. 1972 Feb 11;37(1):141–145. doi: 10.1016/0006-8993(72)90356-3. [DOI] [PubMed] [Google Scholar]
- Navone F., Di Gioia G., Jahn R., Browning M., Greengard P., De Camilli P. Microvesicles of the neurohypophysis are biochemically related to small synaptic vesicles of presynaptic nerve terminals. J Cell Biol. 1989 Dec;109(6 Pt 2):3425–3433. doi: 10.1083/jcb.109.6.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navone F., Jahn R., Di Gioia G., Stukenbrok H., Greengard P., De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzak A., Aunis D., Langley K. Membrane recycling after exocytosis: an ultrastructural study of cultured chromaffin cells. Exp Cell Res. 1987 Aug;171(2):346–356. doi: 10.1016/0014-4827(87)90167-4. [DOI] [PubMed] [Google Scholar]
- Patzak A., Winkler H. Exocytotic exposure and recycling of membrane antigens of chromaffin granules: ultrastructural evaluation after immunolabeling. J Cell Biol. 1986 Feb;102(2):510–515. doi: 10.1083/jcb.102.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Burridge K., Wilson S. P., Kirshner N. Visualization of the exocytosis/endocytosis secretory cycle in cultured adrenal chromaffin cells. J Cell Biol. 1983 Dec;97(6):1906–1917. doi: 10.1083/jcb.97.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas R. J., Moore H. P. Spatial segregation of the regulated and constitutive secretory pathways. J Cell Biol. 1989 Jul;109(1):51–60. doi: 10.1083/jcb.109.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Fuchs R., Male P., Mellman I. Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell. 1988 Jan 15;52(1):73–83. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Schneider F. H. Secretion from the bovine adrenal gland. Release of lysosomal enzymes. Biochem Pharmacol. 1970 Mar;19(3):833–847. doi: 10.1016/0006-2952(70)90245-5. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Thoenen H. Mechanism of uptake and retrograde axonal transport of noradrenaline in sympathetic neurons in culture: reserpine-resistant large dense-core vesicles as transport vehicles. J Cell Biol. 1983 Jun;96(6):1538–1547. doi: 10.1083/jcb.96.6.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K. Demonstration of reversible membrane internalization after exocytosis in the rat neurohypophysis. Neurosci Lett. 1989 Oct 9;104(3):292–297. doi: 10.1016/0304-3940(89)90591-0. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchard S. J., Corcoran J. J., Pressman B. C., Rubin R. W. Evidence for secretory granule membrane recycling in cultured adrenal chromaffin cells. Cell Biol Int Rep. 1981 Oct;5(10):953–962. doi: 10.1016/0309-1651(81)90211-3. [DOI] [PubMed] [Google Scholar]
- Torri-Tarelli F., Haimann C., Ceccarelli B. Coated vesicles and pits during enhanced quantal release of acetylcholine at the neuromuscular junction. J Neurocytol. 1987 Apr;16(2):205–214. doi: 10.1007/BF01795304. [DOI] [PubMed] [Google Scholar]
- Valtorta F., Jahn R., Fesce R., Greengard P., Ceccarelli B. Synaptophysin (p38) at the frog neuromuscular junction: its incorporation into the axolemma and recycling after intense quantal secretion. J Cell Biol. 1988 Dec;107(6 Pt 2):2717–2727. doi: 10.1083/jcb.107.6.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade A. R. Recycling of noradrenergic storage vesicles of isolated rat vas deferens. Nature. 1979 Oct 4;281(5730):374–376. doi: 10.1038/281374a0. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D., Malhotra R. K. Restoration of catecholamine content of previously depleted adrenal medulla in vitro: importance of synthesis in maintaining the catecholamine stores. J Neurochem. 1988 Sep;51(3):820–829. doi: 10.1111/j.1471-4159.1988.tb01817.x. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D., Müller C., Schwab M. Epinephrine as a tool to investigate the question of recycling of sympathetic storage vesicles in the heart: chemical and morphological studies. J Pharmacol Exp Ther. 1982 Jun;221(3):820–827. [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Winkler H. The biogenesis of adrenal chromaffin granules. Neuroscience. 1977;2(5):657–683. doi: 10.1016/0306-4522(77)90022-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Vesicle recycling and transmitter release. Neuroscience. 1979;4(12):1773–1804. doi: 10.1016/0306-4522(79)90058-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann H., Volknandt W., Henkel A., Bonzelius F., Janetzko A., Kanaseki T. The synaptic vesicle membrane: origin, axonal distribution, protein components, exocytosis and recycling. Cell Biol Int Rep. 1989 Dec;13(12):993–1006. doi: 10.1016/0309-1651(89)90015-5. [DOI] [PubMed] [Google Scholar]
- von Grafenstein H. R., Neumann E. ATP-stimulated accumulation of calcium by chromaffin granules and mitochondria from the adrenal medulla. Biochem Biophys Res Commun. 1983 Nov 30;117(1):245–251. doi: 10.1016/0006-291x(83)91567-x. [DOI] [PubMed] [Google Scholar]
- von Grafenstein H., Neumann E. Interaction of chromaffin granules with plasma membranes mediated by Ca2+ and Mg2+-ATP using self-generating gradients of Percoll. FEBS Lett. 1981 Jan 26;123(2):238–240. doi: 10.1016/0014-5793(81)80296-7. [DOI] [PubMed] [Google Scholar]
- von Grafenstein H., Roberts C. S., Baker P. F. Kinetic analysis of the triggered exocytosis/endocytosis secretory cycle in cultured bovine adrenal medullary cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2343–2352. doi: 10.1083/jcb.103.6.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]


