Abstract
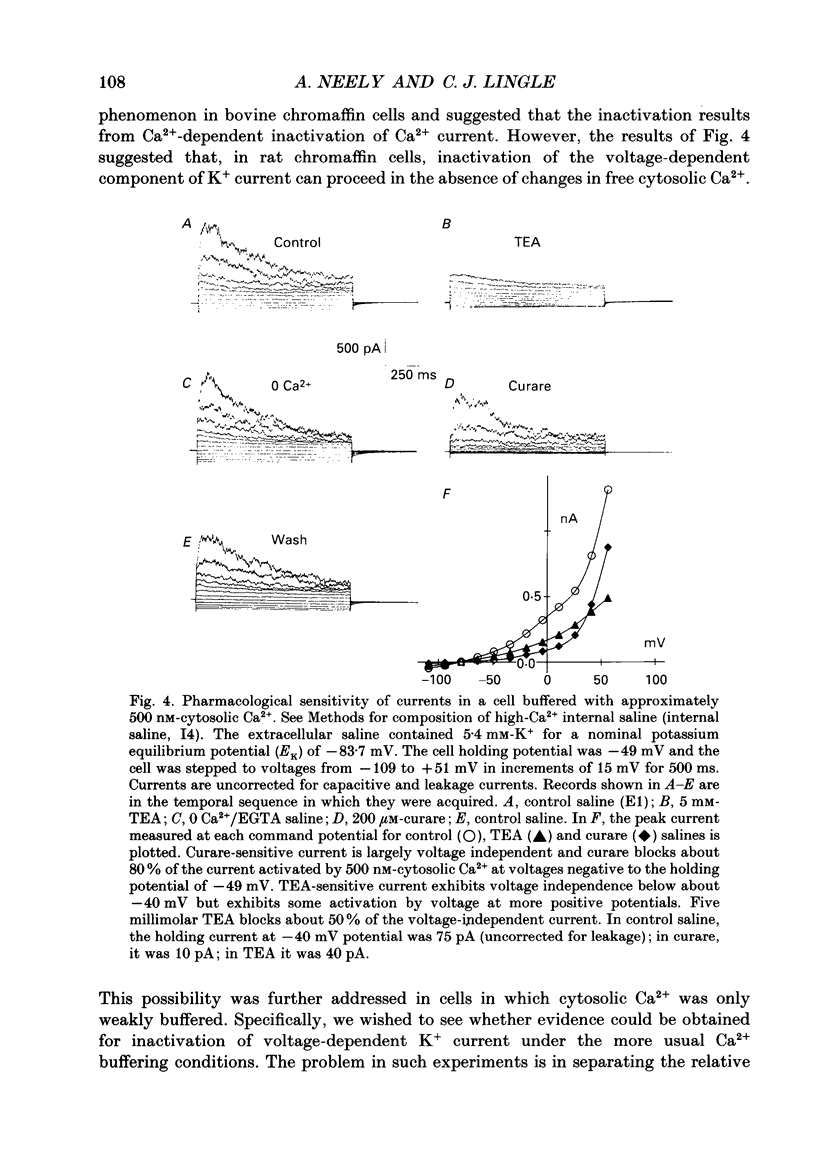
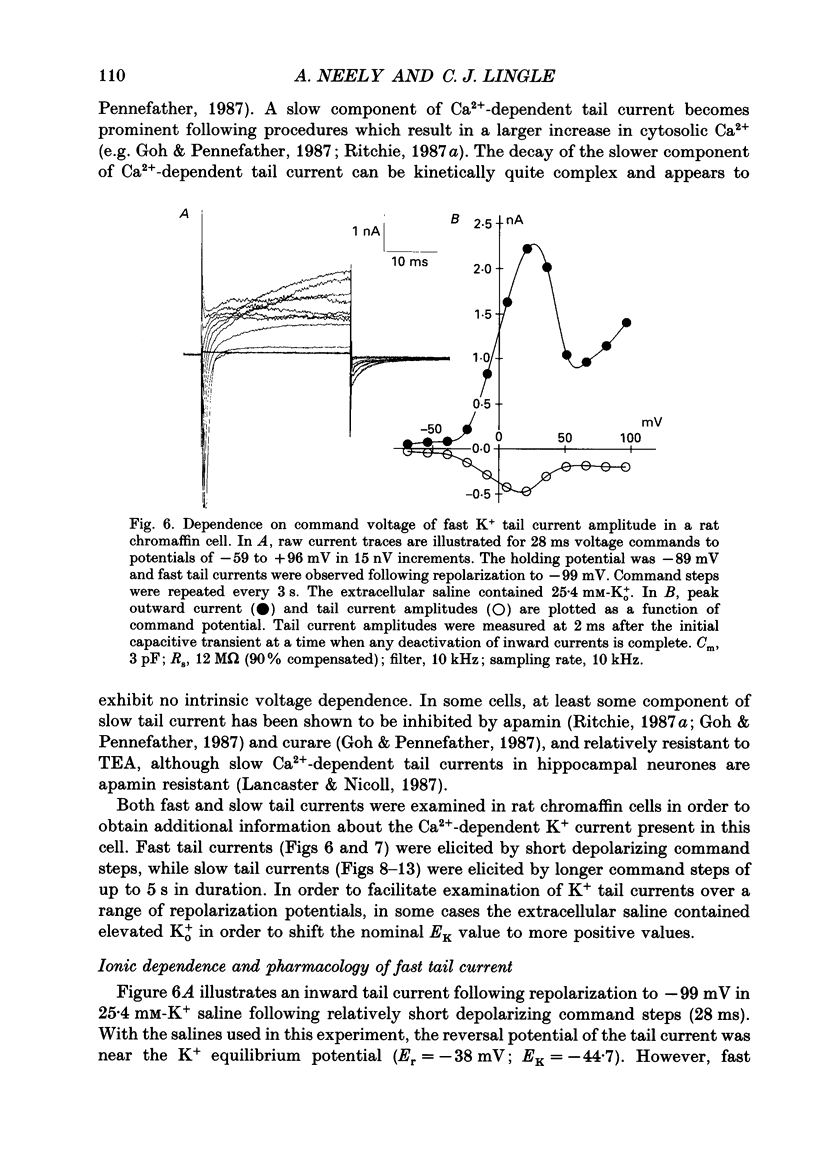
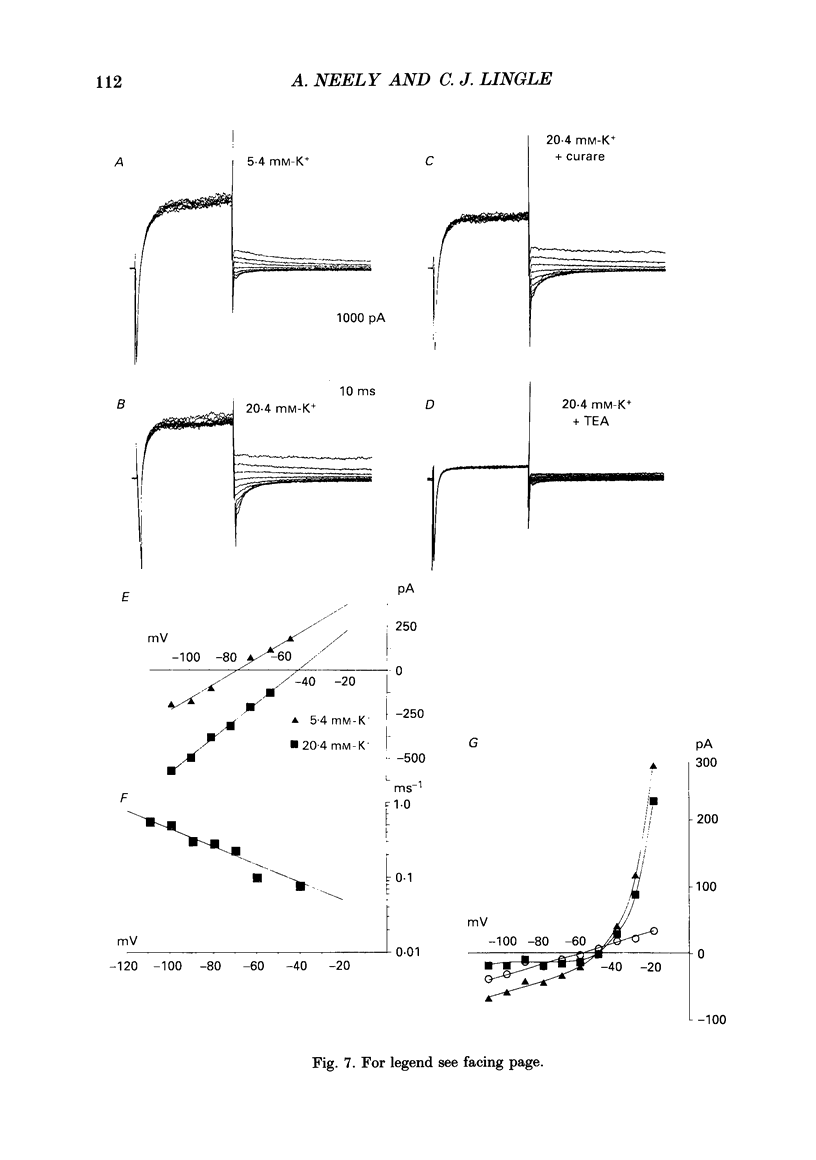
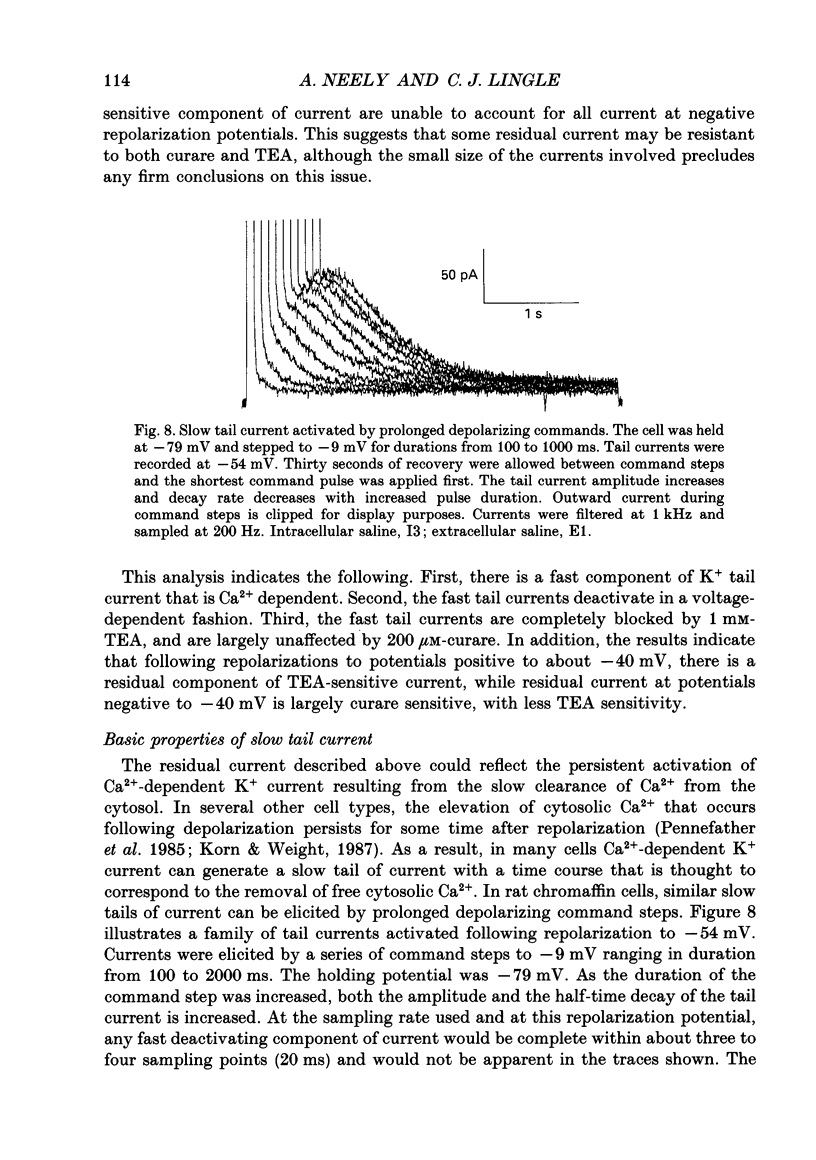
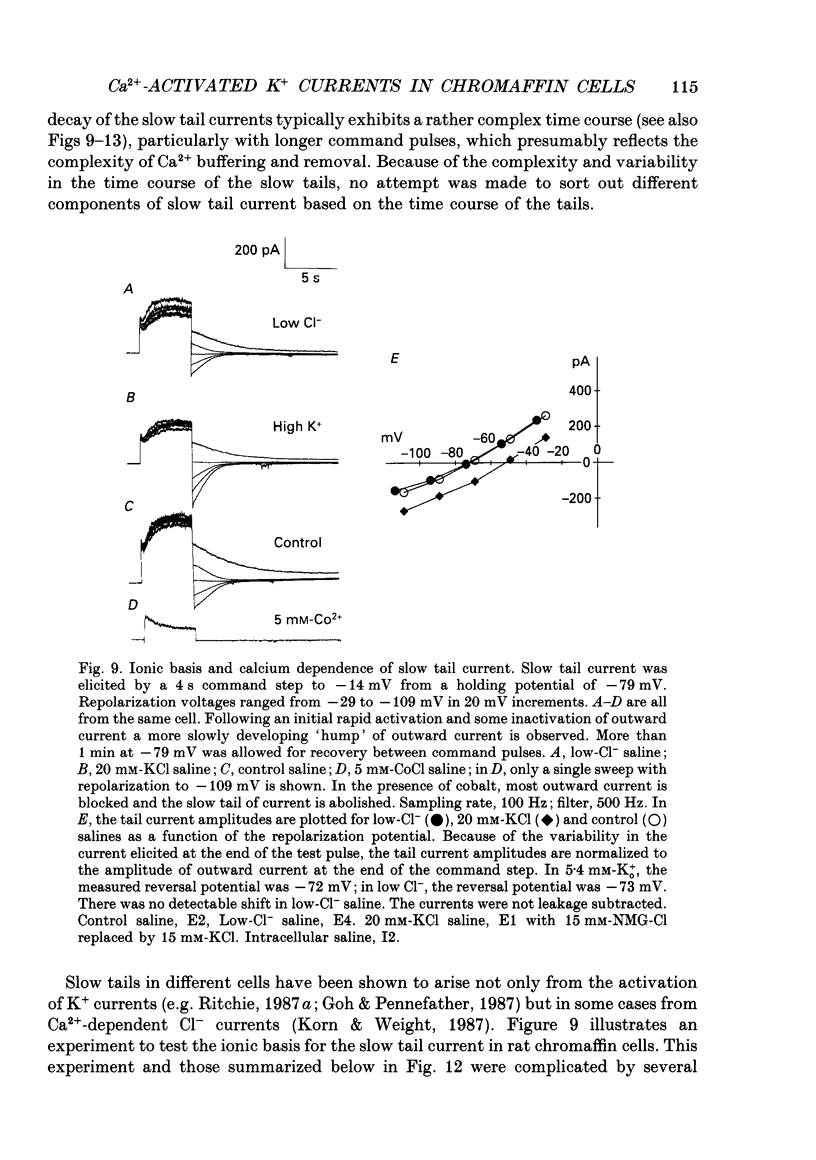
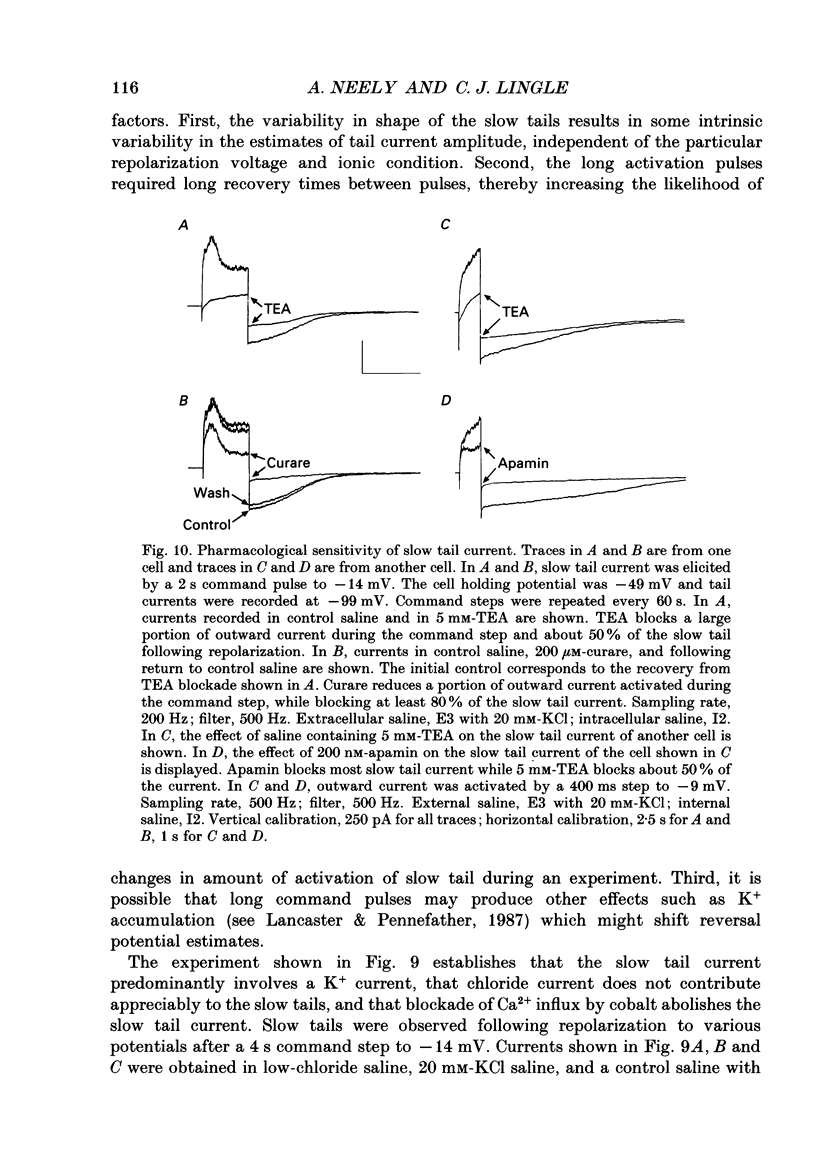
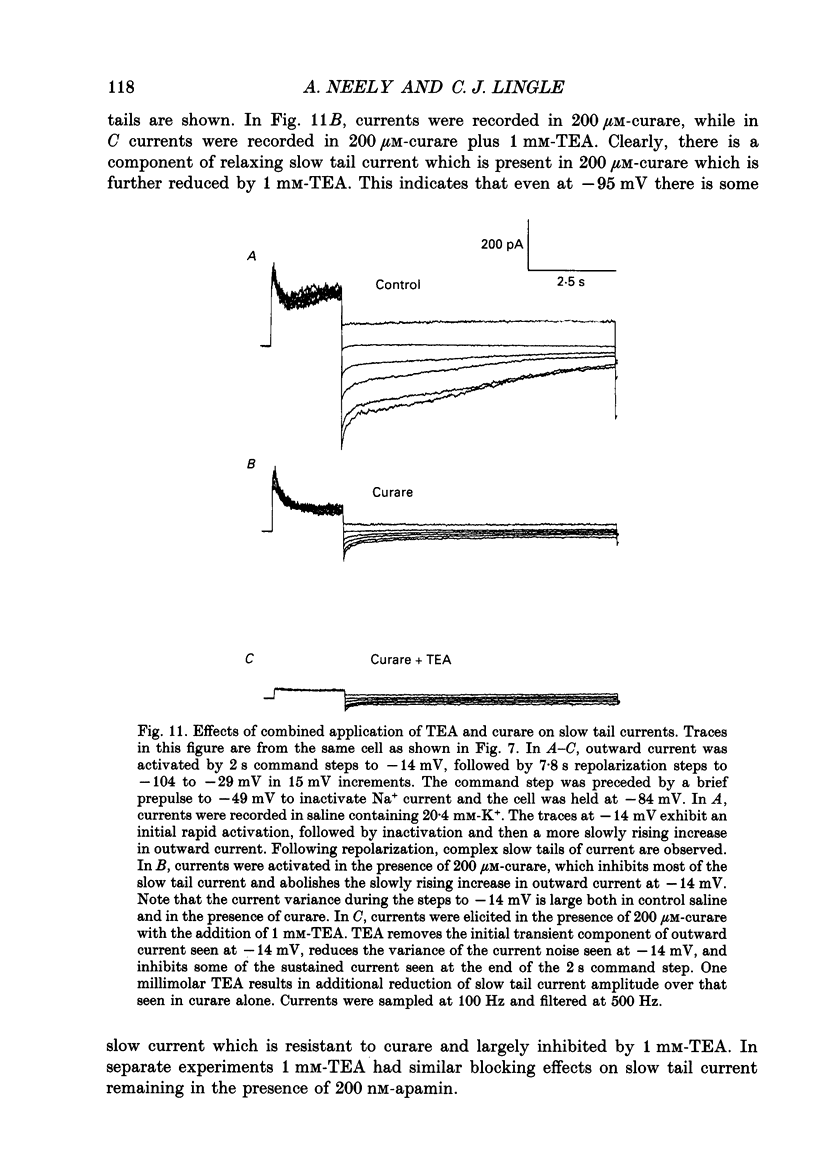
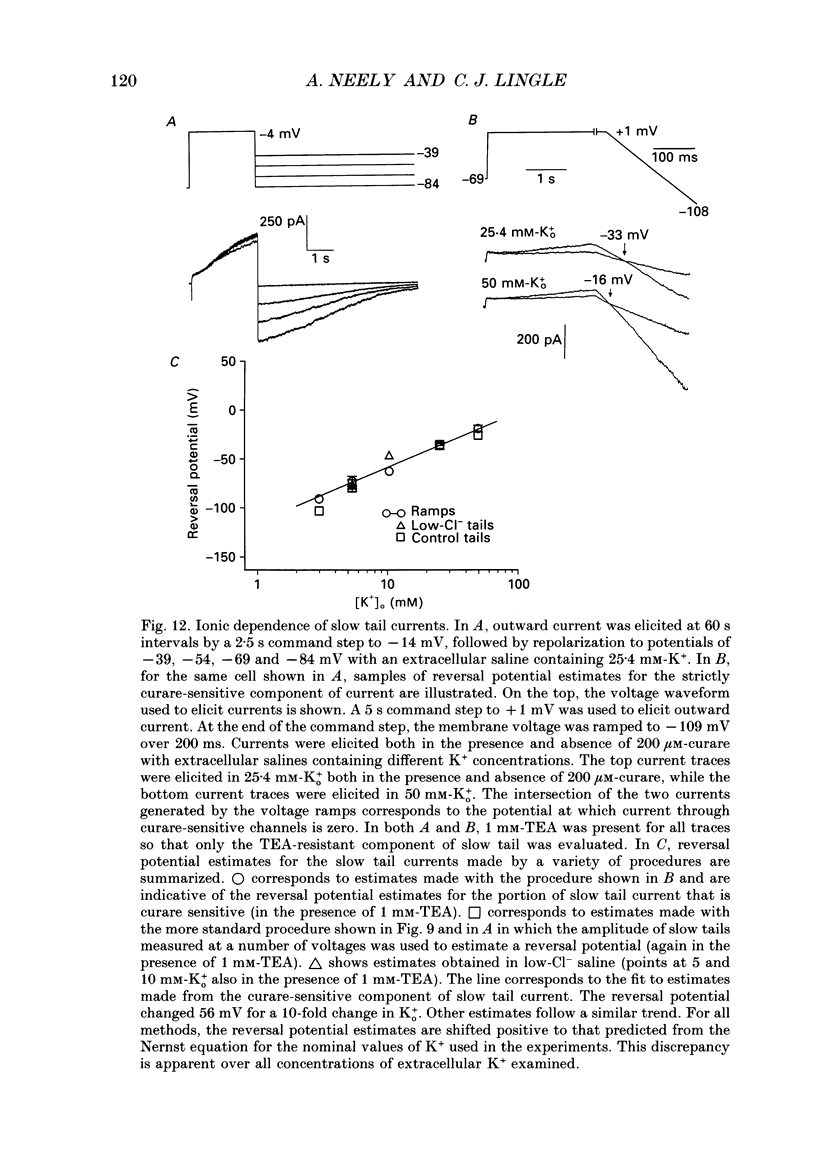
1. The activation of calcium (Ca2+)-dependent potassium (K+) currents in dissociated rat adrenal chromaffin cells was investigated using the dialysed cell recording technique. 2. Ca(2+)-dependent K+ current was the major component of outward current at command potentials from -30 mV to about +50 mV. 3. Two components of Ca(2+)-dependent outward current could be distinguished based on the voltage dependence of activation, the properties of tail currents following repolarization, and pharmacological properties. 4. One Ca(2+)-dependent current was similar to an after-hyperpolarization current (often termed IAHP) observed in other cell types. This current was largely blocked by 200 nM-apamin or 200 microM-curare, was associated with slow Ca(2+)-dependent tail current, and exhibited little dependence on voltage. In cells with cytosolic Ca2+ buffered to 500 nM-1 microM, curare-sensitive current accounted for most of the membrane current at potentials negative to about -40 mV. 5. A second component of Ca(2+)-activated K+ current exhibited voltage-dependent activation, was completely blocked by 1 mM-TEA, and turned off rapidly following repolarization. An unusual aspect of the TEA-sensitive currents was that they appeared to inactivate under conditions of constant cytosolic Ca2+. 6. A novel observation during these experiments was a slow hump of outward current which appears to result from a non-monotonic elevation in cytosolic Ca2+ during prolonged voltage jumps.
Full text
PDF



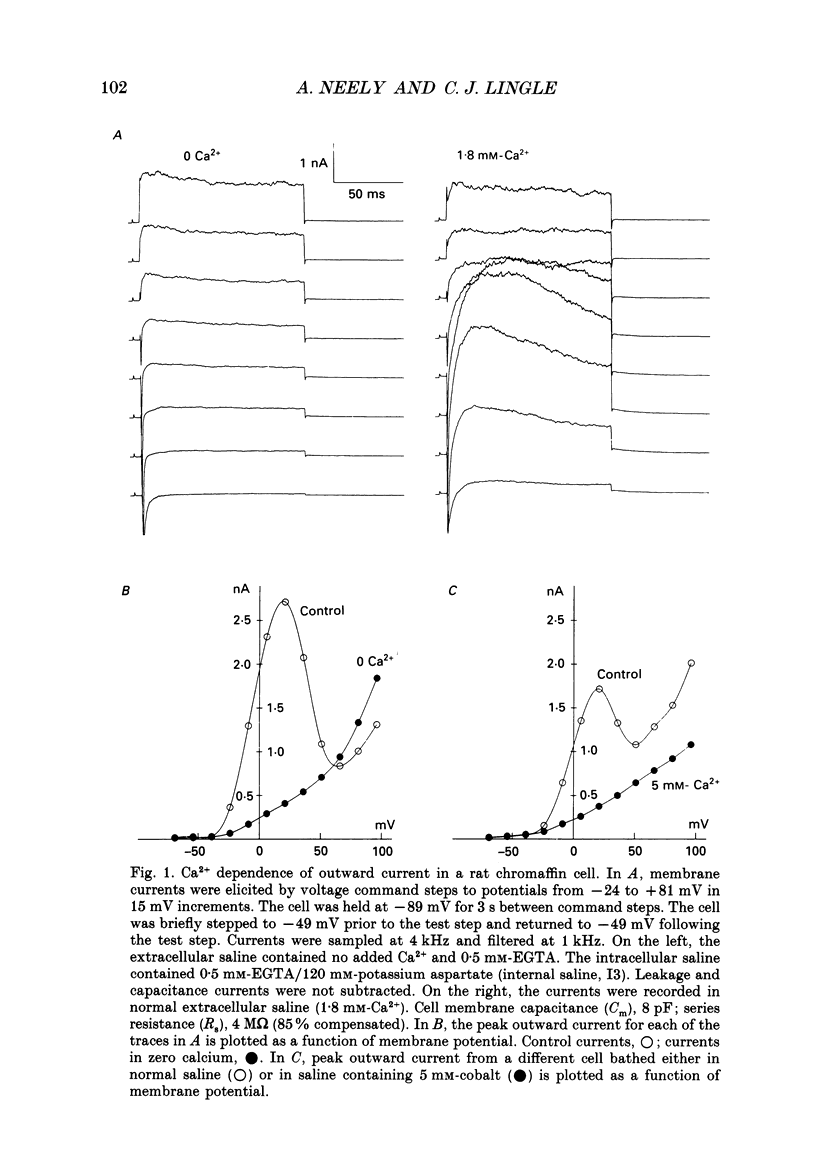

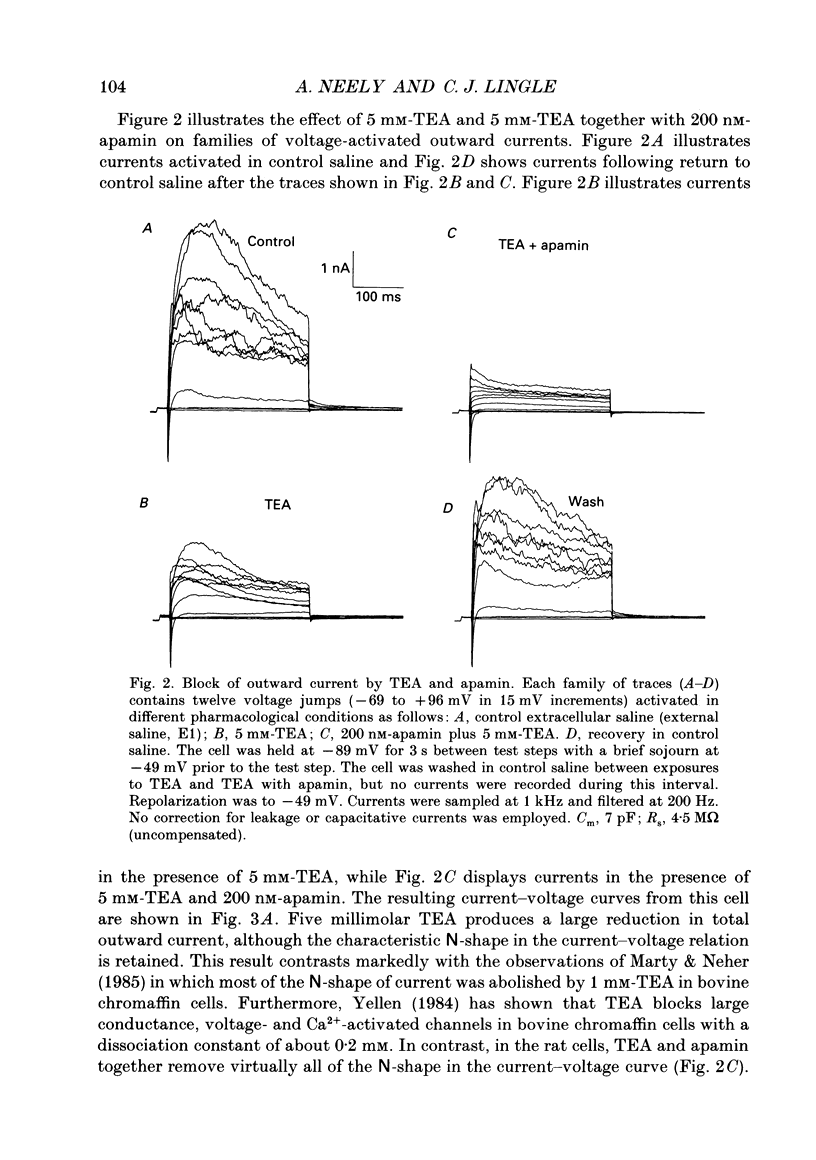
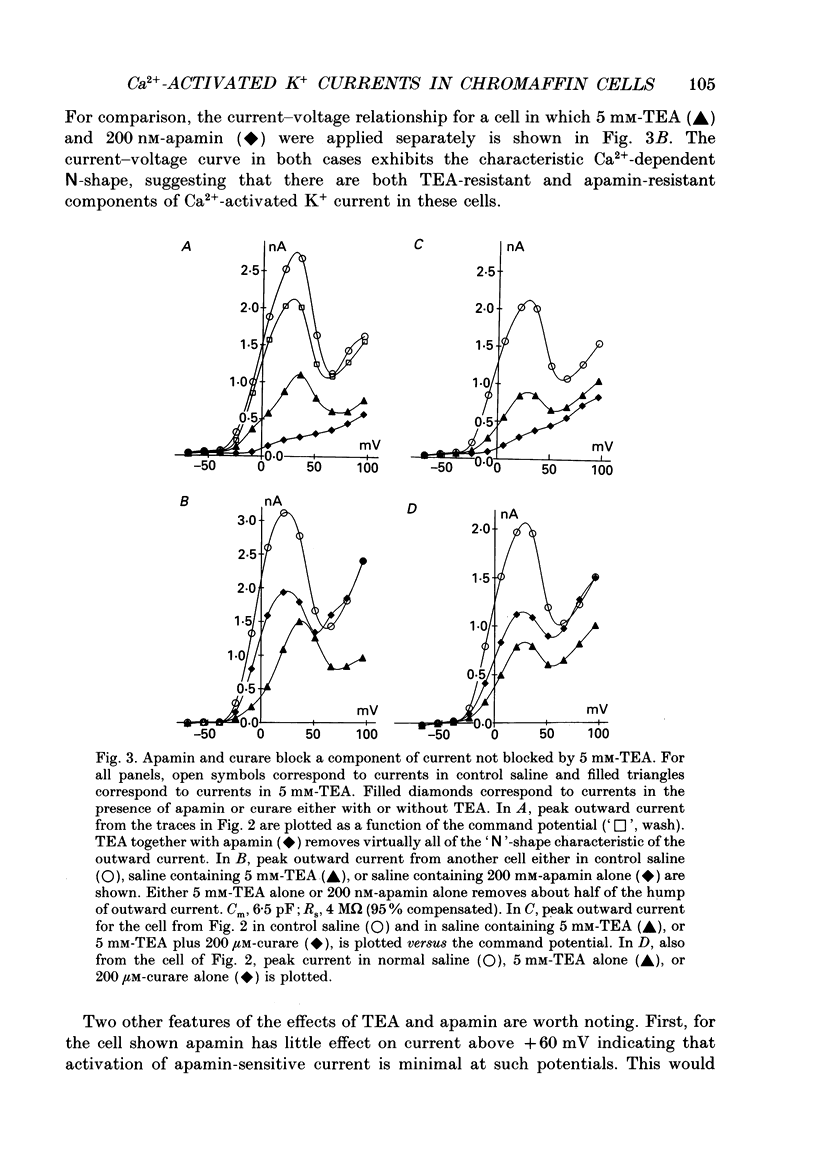

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Akaike A., Mine Y., Sasa M., Takaori S. A patch clamp study of muscarinic excitation of the rat adrenal chromaffin cells. J Pharmacol Exp Ther. 1990 Oct;255(1):340–345. [PubMed] [Google Scholar]
- Akaike A., Mine Y., Sasa M., Takaori S. Voltage and current clamp studies of muscarinic and nicotinic excitation of the rat adrenal chromaffin cells. J Pharmacol Exp Ther. 1990 Oct;255(1):333–339. [PubMed] [Google Scholar]
- Almers W., McCleskey E. W., Palade P. T. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol. 1984 Aug;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo C. R., Dahmer M. K., Perlman R. L., Fox A. P. Two types of Ca2+ currents are found in bovine chromaffin cells: facilitation is due to the recruitment of one type. J Physiol. 1991 Jan;432:681–707. doi: 10.1113/jphysiol.1991.sp018406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biales B., Dichter M., Tischler A. Electrical excitability of cultured adrenal chromaffin cells. J Physiol. 1976 Nov;262(3):743–753. doi: 10.1113/jphysiol.1976.sp011618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., De Waard M., Feltz A. Inactivation characteristics reveal two calcium currents in adult bovine chromaffin cells. J Physiol. 1991 Jun;437:603–620. doi: 10.1113/jphysiol.1991.sp018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Voltage- and calcium-activated potassium currents in mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:149–165. doi: 10.1113/jphysiol.1988.sp016993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. S., Haylett D. G. Effects of apamin, quinine and neuromuscular blockers on calcium-activated potassium channels in guinea-pig hepatocytes. J Physiol. 1985 Jan;358:373–394. doi: 10.1113/jphysiol.1985.sp015556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Evidence that the secreting adrenal chromaffin cell releases catecholamines directly from ATP-rich granules. J Physiol. 1966 Mar;183(1):236–248. doi: 10.1113/jphysiol.1966.sp007863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Jiang Z. G., Mo N. Tubocurarine suppresses slow calcium-dependent after-hyperpolarization in guinea-pig inferior mesenteric ganglion cells. J Physiol. 1986 Jun;375:499–514. doi: 10.1113/jphysiol.1986.sp016130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard D. A., Holz R. W. Intracellular Ca2+ activates phospholipase C. Trends Neurosci. 1988 Dec;11(12):517–520. doi: 10.1016/0166-2236(88)90174-9. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Fajdiga P. B., Howe N. B., Livett B. G. Functional and morphological characterization of isolated bovine adrenal medullary cells. J Cell Biol. 1978 Jan;76(1):12–30. doi: 10.1083/jcb.76.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschi J. E. Membrane currents of cultured rat sympathetic neurons under voltage clamp. J Neurophysiol. 1983 Dec;50(6):1460–1478. doi: 10.1152/jn.1983.50.6.1460. [DOI] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J. W., Pennefather P. S. Pharmacological and physiological properties of the after-hyperpolarization current of bullfrog ganglion neurones. J Physiol. 1987 Dec;394:315–330. doi: 10.1113/jphysiol.1987.sp016872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Aldrich R. W. Gating kinetics of four classes of voltage-dependent K+ channels in pheochromocytoma cells. J Gen Physiol. 1988 Jan;91(1):107–131. doi: 10.1085/jgp.91.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Aldrich R. W. Voltage-dependent K+ currents and underlying single K+ channels in pheochromocytoma cells. J Gen Physiol. 1988 Jan;91(1):73–106. doi: 10.1085/jgp.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao L. S., Schneider A. S. Muscarinic receptors on bovine chromaffin cells mediate a rise in cytosolic calcium that is independent of extracellular calcium. J Biol Chem. 1985 Feb 25;260(4):2019–2022. [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Blockade of Ca-activated K conductance by apamin in rat sympathetic neurones. Br J Pharmacol. 1986 Jan;87(1):225–232. doi: 10.1111/j.1476-5381.1986.tb10175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Ritchie A. K. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 1980 Oct;307:199–216. doi: 10.1113/jphysiol.1980.sp013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Ritchie A. K., Hagiwara S. Effect of tetrodotoxin on adrenaline secretion in the perfused rat adrenal medulla. Nature. 1979 Mar 1;278(5699):63–65. doi: 10.1038/278063a0. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Ledbetter F. H., Carson K. A., Kirshner A. G., Slepetis R., Kirshner N. Stability of bovine adrenal medulla cells in culture. J Neurochem. 1980 Sep;35(3):679–692. doi: 10.1111/j.1471-4159.1980.tb03707.x. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Stimulus-secretion coupling in isolated bovine adrenal medullary cells. Q J Exp Physiol. 1983 Jan;68(1):123–143. doi: 10.1113/expphysiol.1983.sp002691. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Influence of sodium-calcium exchange on calcium current rundown and the duration of calcium-dependent chloride currents in pituitary cells, studied with whole cell and perforated patch recording. J Gen Physiol. 1989 Nov;94(5):789–812. doi: 10.1085/jgp.94.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Weight F. F. Patch-clamp study of the calcium-dependent chloride current in AtT-20 pituitary cells. J Neurophysiol. 1987 Dec;58(6):1431–1451. doi: 10.1152/jn.1987.58.6.1431. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Nicoll R. A., Perkel D. J. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. J Neurosci. 1991 Jan;11(1):23–30. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B., Nicoll R. A. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987 Aug;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B., Pennefather P. Potassium currents evoked by brief depolarizations in bull-frog sympathetic ganglion cells. J Physiol. 1987 Jun;387:519–548. doi: 10.1113/jphysiol.1987.sp016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. G., Ritchie A. K. Large and small conductance calcium-activated potassium channels in the GH3 anterior pituitary cell line. Pflugers Arch. 1987 Dec;410(6):614–622. doi: 10.1007/BF00581321. [DOI] [PubMed] [Google Scholar]
- Lang D. G., Ritchie A. K. Tetraethylammonium blockade of apamin-sensitive and insensitive Ca2(+)-activated K+ channels in a pituitary cell line. J Physiol. 1990 Jun;425:117–132. doi: 10.1113/jphysiol.1990.sp018095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. G., Ritchie A. K. Tetraethylammonium ion sensitivity of a 35-pS CA2(+)-activated K+ channel in GH3 cells that is activated by thyrotropin-releasing hormone. Pflugers Arch. 1990 Aug;416(6):704–709. doi: 10.1007/BF00370618. [DOI] [PubMed] [Google Scholar]
- Livett B. G. Adrenal medullary chromaffin cells in vitro. Physiol Rev. 1984 Oct;64(4):1103–1161. doi: 10.1152/physrev.1984.64.4.1103. [DOI] [PubMed] [Google Scholar]
- Malhotra R. K., Wakade T. D., Wakade A. R. Comparison of secretion of catecholamines from the rat adrenal medulla during continuous exposure to nicotine, muscarine or excess K. Neuroscience. 1988 Jul;26(1):313–320. doi: 10.1016/0306-4522(88)90147-9. [DOI] [PubMed] [Google Scholar]
- Malhotra R. K., Wakade T. D., Wakade A. R. Vasoactive intestinal polypeptide and muscarine mobilize intracellular Ca2+ through breakdown of phosphoinositides to induce catecholamine secretion. Role of IP3 in exocytosis. J Biol Chem. 1988 Feb 15;263(5):2123–2126. [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P. The initiation of calcium release following muscarinic stimulation in rat lacrimal glands. J Physiol. 1989 Dec;419:665–687. doi: 10.1113/jphysiol.1989.sp017892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misbahuddin M., Oka M. Muscarinic stimulation of guinea pig adrenal chromaffin cells stimulates catecholamine secretion without significant increase in Ca2+ uptake. Neurosci Lett. 1988 May 3;87(3):266–270. doi: 10.1016/0304-3940(88)90459-4. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Latorre R. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. J Gen Physiol. 1983 Oct;82(4):511–542. doi: 10.1085/jgp.82.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely A., Lingle C. J. Effects of muscarine on single rat adrenal chromaffin cells. J Physiol. 1992;453:133–166. doi: 10.1113/jphysiol.1992.sp019221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohmi M., Kuba K. (+)-Tubocurarine blocks the Ca2+-dependent K+-channel of the bullfrog sympathetic ganglion cell. Brain Res. 1984 May 28;301(1):146–148. doi: 10.1016/0006-8993(84)90412-8. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S. Calcium-activated potassium channels in rat muscle inactivate from a short-duration open state. J Physiol. 1985 Jun;363:501–516. doi: 10.1113/jphysiol.1985.sp015724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A. K. Thyrotropin-releasing hormone stimulates a calcium-activated potassium current in a rat anterior pituitary cell line. J Physiol. 1987 Apr;385:611–625. doi: 10.1113/jphysiol.1987.sp016510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A. K. Two distinct calcium-activated potassium currents in a rat anterior pituitary cell line. J Physiol. 1987 Apr;385:591–609. doi: 10.1113/jphysiol.1987.sp016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role L. W., Perlman R. L. Purification of adrenal medullary chromaffin cells by density gradient centrifugation. J Neurosci Methods. 1980 Jun;2(3):253–265. doi: 10.1016/0165-0270(80)90014-x. [DOI] [PubMed] [Google Scholar]
- Romey G., Lazdunski M. The coexistence in rat muscle cells of two distinct classes of Ca2+-dependent K+ channels with different pharmacological properties and different physiological functions. Biochem Biophys Res Commun. 1984 Jan 30;118(2):669–674. doi: 10.1016/0006-291x(84)91355-x. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., Hugues M., Lazdunski M. Properties of the apamin-sensitive Ca2+-activated K+ channel in PC12 pheochromocytoma cells which hyper-produce the apamin receptor. J Biol Chem. 1986 Jul 5;261(19):8633–8637. [PubMed] [Google Scholar]
- Smart T. G. Single calcium-activated potassium channels recorded from cultured rat sympathetic neurones. J Physiol. 1987 Aug;389:337–360. doi: 10.1113/jphysiol.1987.sp016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoehr S. J., Smolen J. E., Holz R. W., Agranoff B. W. Inositol trisphosphate mobilizes intracellular calcium in permeabilized adrenal chromaffin cells. J Neurochem. 1986 Feb;46(2):637–640. doi: 10.1111/j.1471-4159.1986.tb13014.x. [DOI] [PubMed] [Google Scholar]
- Wakade A. R. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B. S., Lecar H., Adler M. Single calcium-dependent potassium channels in clonal anterior pituitary cells. Biophys J. 1982 Sep;39(3):313–317. doi: 10.1016/S0006-3495(82)84522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]


