Abstract
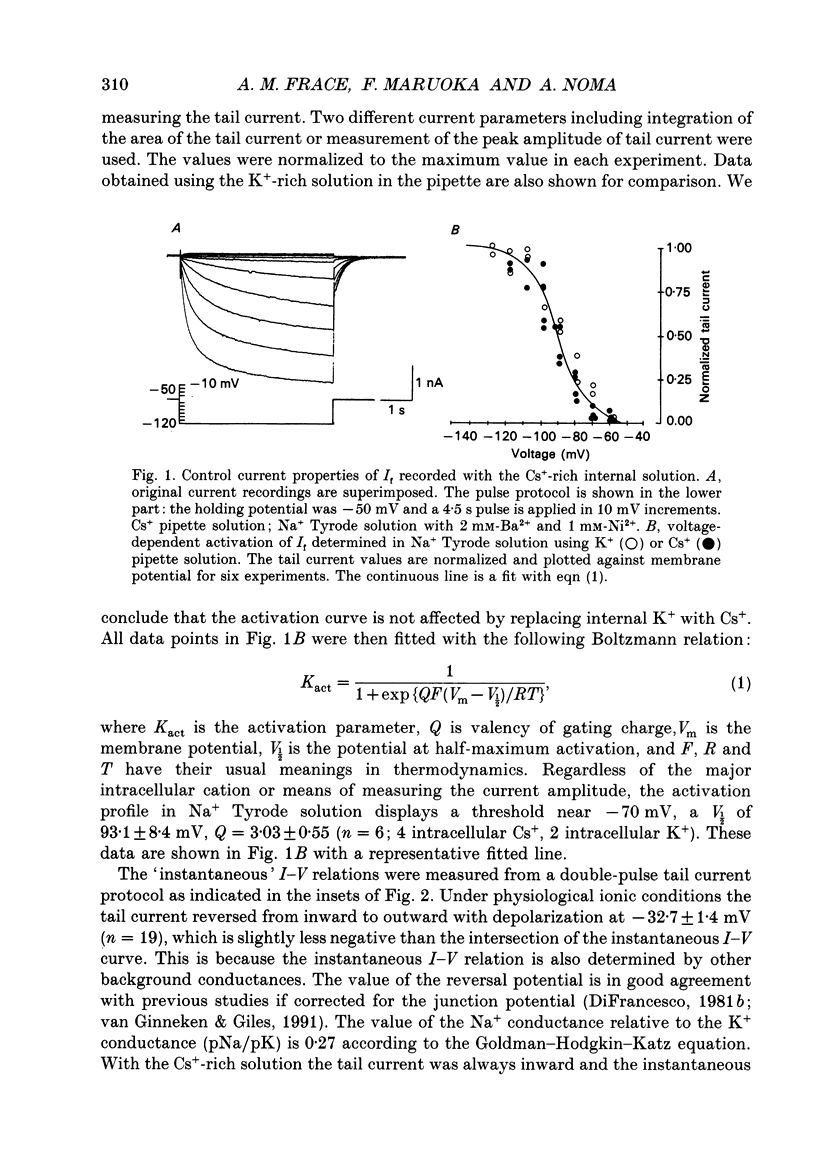
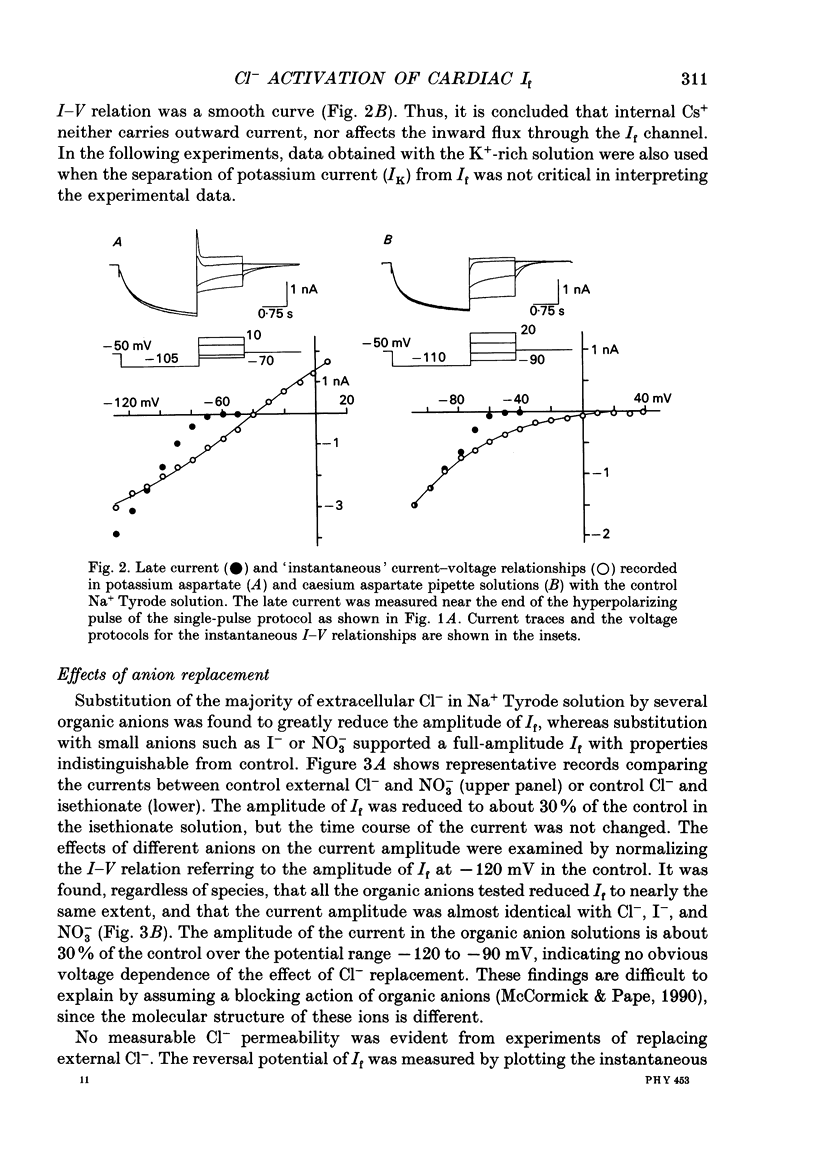
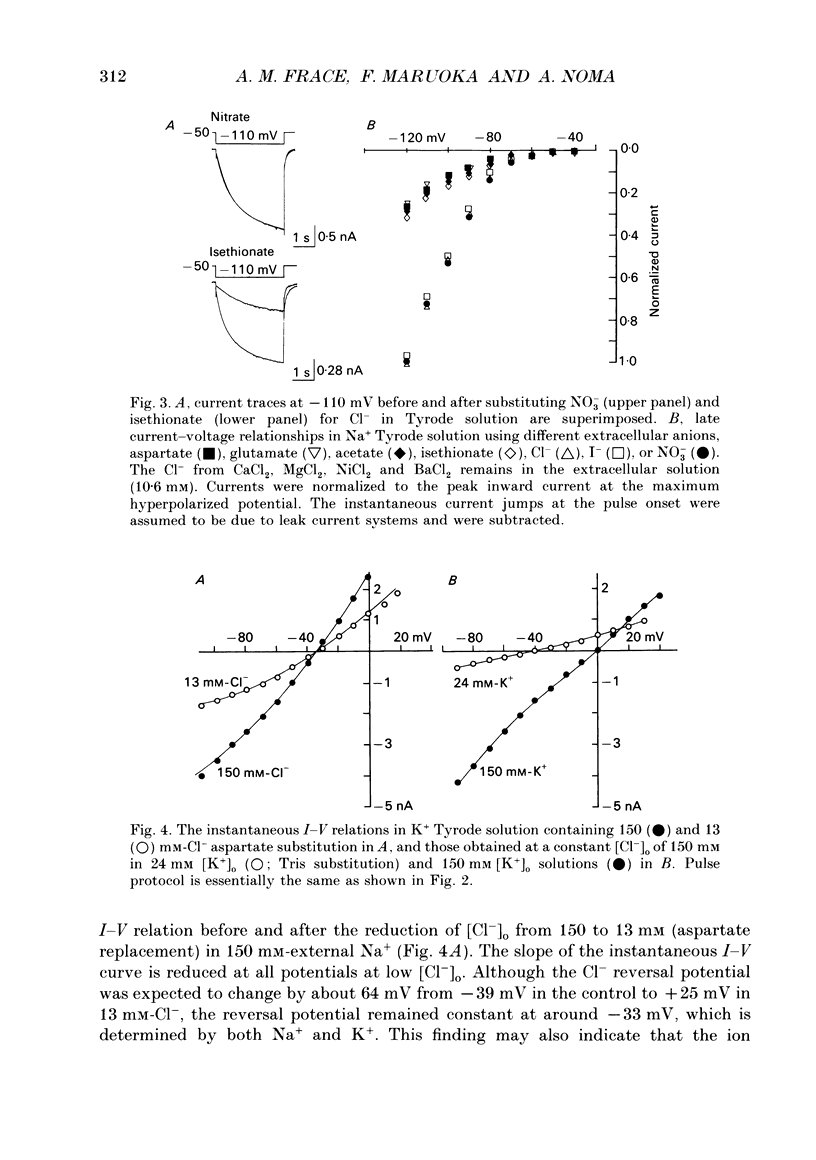
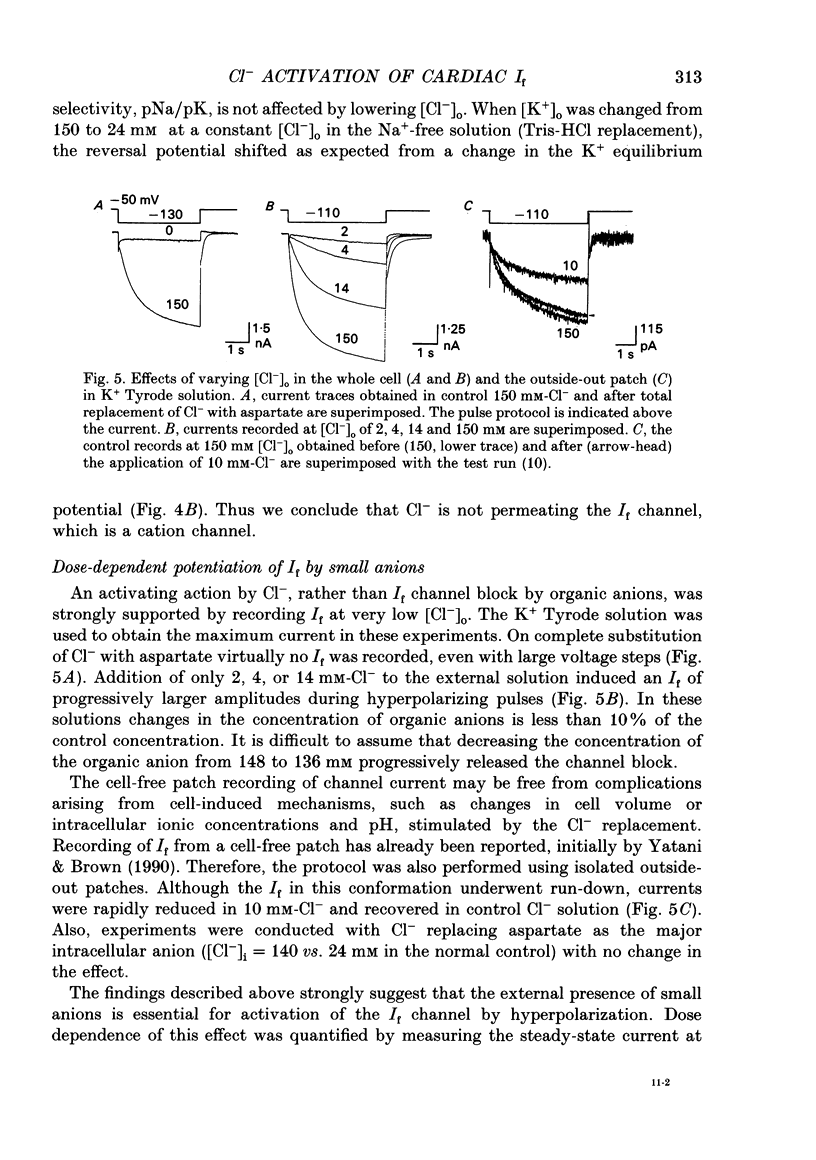
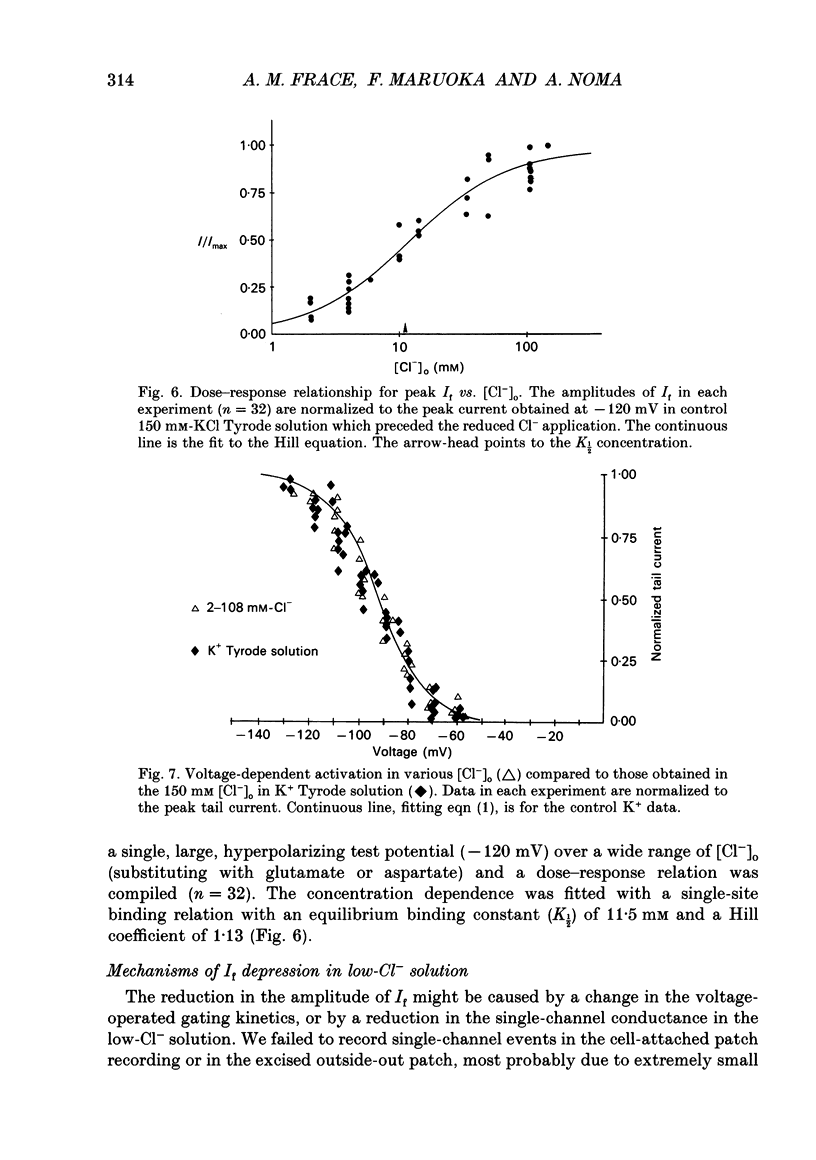
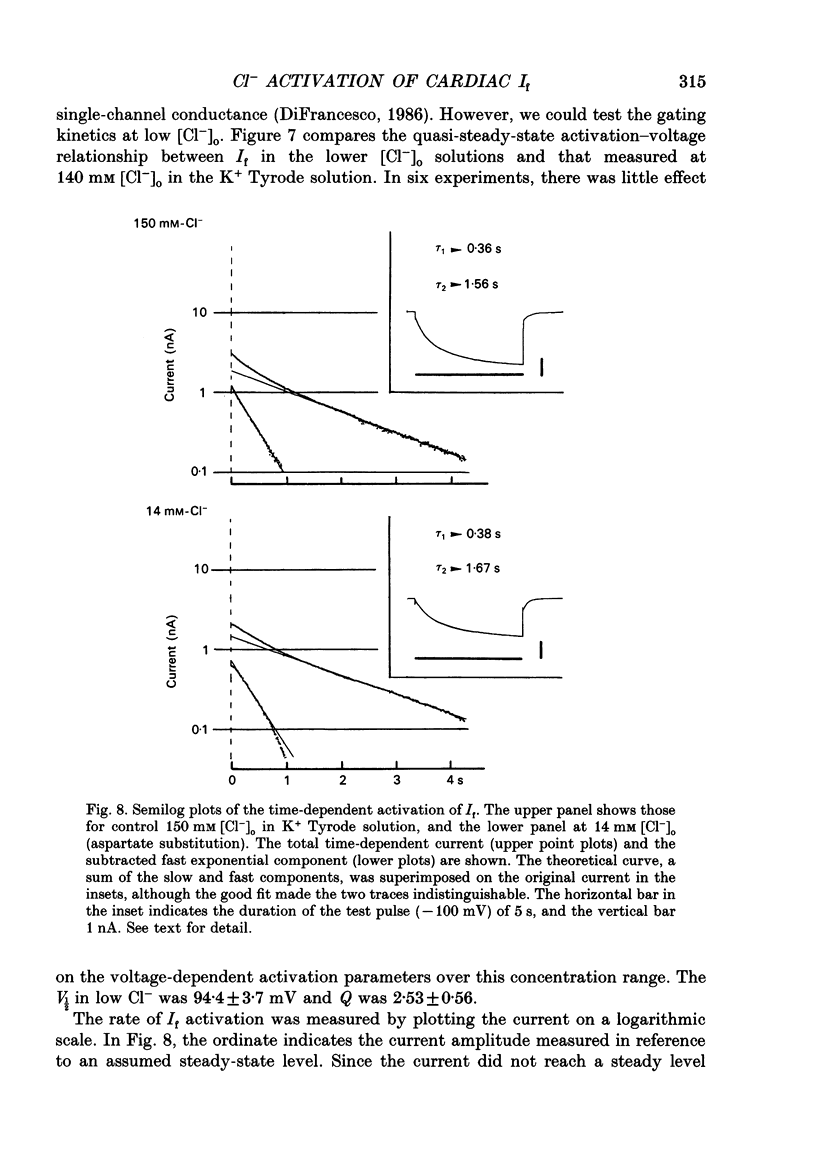
1. Effects of varying concentrations of anions on the hyperpolarization-activated current (I(f)) were studied in myocytes isolated from the rabbit sino-atrial node. Substituting Cs+ for the intracellular K+ clearly separated I(f) from the delayed rectifier K+ current. Control properties, including gating kinetics and ion selectivity, similar to previous studies were obtained. 2. Substitution of extracellular Cl- with larger anions including isethionate, glutamate, acetate, and aspartate, reduced the amplitude of I(f) without changing the reversal potential. Substitution with small anions such as iodide or nitrate supported an intact I(f). These effects were reproduced in the excised outside-out patch conformation. 3. The conductance for I(f) was a saturating function of the extracellular Cl- concentration ([Cl-]o) with an equilibrium binding constant (K1/2) of 11 mM and a slope factor of about 1 when substituted with large anions. Total removal of small anions completely abolished I(f). 4. The voltage-dependent gating of I(f) was not affected by changing ([Cl-]o), suggesting that Cl- modulates conductance properties of I(f). 5. The results indicate that I(f) conductance is unique in that it is dependent on an extracellular anion (Cl-), yet it is carried exclusively by cations, K+ and Na+. These effects are independent of any measurable voltage-dependent gating parameters.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Bertrand D. Effect of changes in intra- and extracellular sodium on the inward (anomalous) rectification in salamander photoreceptors. J Physiol. 1984 Feb;347:611–631. doi: 10.1113/jphysiol.1984.sp015086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer J. C., Brown H. F. Rabbit sino-atrial node cells: isolation and electrophysiological properties. J Physiol. 1990 Sep;428:405–424. doi: 10.1113/jphysiol.1990.sp018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Block and activation of the pace-maker channel in calf purkinje fibres: effects of potassium, caesium and rubidium. J Physiol. 1982 Aug;329:485–507. doi: 10.1113/jphysiol.1982.sp014315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature. 1986 Dec 4;324(6096):470–473. doi: 10.1038/324470a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Sandblom J., Neher E. Interactions in cation permeation through the gramicidin channel. Cs, Rb, K, Na, Li, Tl, H, and effects of anion binding. Biophys J. 1978 May;22(2):307–340. doi: 10.1016/S0006-3495(78)85491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciolini F., Nonner W. Anion and cation permeability of a chloride channel in rat hippocampal neurons. J Gen Physiol. 1987 Oct;90(4):453–478. doi: 10.1085/jgp.90.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989 Feb;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Irisawa H., Kokobun S., Kotake H., Nishimura M., Watanabe Y. Slow current systems in the A-V node of the rabbit heart. Nature. 1980 May 22;285(5762):228–229. doi: 10.1038/285228a0. [DOI] [PubMed] [Google Scholar]
- Noma A., Morad M., Irisawa H. Does the "pacemaker current" generate the diastolic depolarization in the rabbit SA node cells? Pflugers Arch. 1983 May;397(3):190–194. doi: 10.1007/BF00584356. [DOI] [PubMed] [Google Scholar]
- Seyama I. Characteristics of the anion channel in the sino-atrial node cell of the rabbit. J Physiol. 1979 Sep;294:447–460. doi: 10.1113/jphysiol.1979.sp012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. Regulation of cardiac pacemaker current If in excised membranes from sinoatrial node cells. Am J Physiol. 1990 Jun;258(6 Pt 2):H1947–H1951. doi: 10.1152/ajpheart.1990.258.6.H1947. [DOI] [PubMed] [Google Scholar]
- van Ginneken A. C., Giles W. Voltage clamp measurements of the hyperpolarization-activated inward current I(f) in single cells from rabbit sino-atrial node. J Physiol. 1991 Mar;434:57–83. doi: 10.1113/jphysiol.1991.sp018459. [DOI] [PMC free article] [PubMed] [Google Scholar]


