Abstract
1. Activation of gastric sensory afferents alters gastric motor and secretory function via the gastric vago-vagal reflex. In this report, we investigated in the rat the impact of gastric mechanoreceptor activation on the brain stem components of the reflex, which are located in the dorsal vagal complex (DVC), i.e. the nucleus of the solitary tract (NTS) and the subjacent dorsal motor nucleus (DMN). 2. In our extracellular recordings of single-cell activity in the DVC, we observed a relation between the response to antral distention and the location of the cell in the DVC. Specifically, cells that were excited by antral distention (ON cells) were located dorsal to those that were inhibited (OFF cells) by the same stimulus (mean depth = 536 +/- 15 and 627 +/- 14 microns for ON and OFF cells, respectively). 3. For a subset of DVC cells, the location was marked by ionophoretic ejection of Pontamine Blue from the recording barrel. Histological analysis indicated that ON cells were located in the NTS, and OFF cells were located in the ventral NTS or within the boundaries of the DMN. Together, these data led to the hypothesis that ON and OFF cells are functionally different groups of neurones, i.e. ON cells may be NTS neurones, and OFF cells may be DMN neurones. We tested this directly by employing both an intragastric balloon and a non-traumatic vagal stimulating electrode to determine whether inflation-related cells were NTS or DMN cells via orthodromic and antidromic activation, respectively. 4. Almost all ON cells (12/13) were orthodromically activated by vagal stimulation, i.e. they were NTS neurones. One ON cell was antidromically activated, and therefore was a DMN neurone. Of the twenty-eight OFF cells that were encountered, ten were classified as NTS neurones because they were orthodromically inhibited by vagal stimulation. The remaining eighteen OFF cells were orthodromically inhibited and antidromically activated (i.e. DMN neurones). Thus, our results support the hypothesis that ON and OFF cells can be functionally distinct populations of neurones, in that almost all ON cells are NTS cells and approximately 2/3 of the OFF cells are DMN neurones. 5. The response to mechanoreceptor activation was different for NTS and DMN neurones. NTS cells were activated (55%) or inhibited (45%) by balloon distention of the stomach, whereas DMN cells were almost exclusively inhibited (95%) by this stimulus. This information provides insight into the organization of excitatory and inhibitory connections of the brain stem components that mediate gastric vago-vagal reflexes.
Full text
PDF



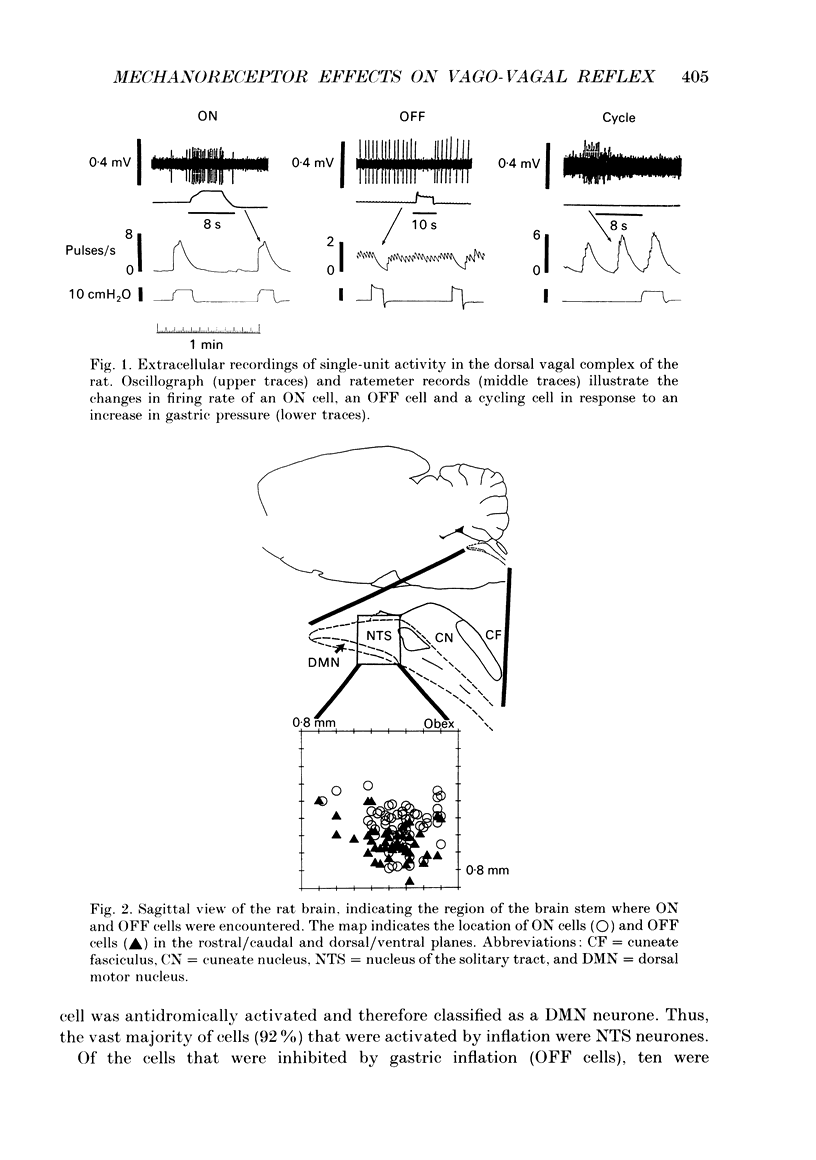
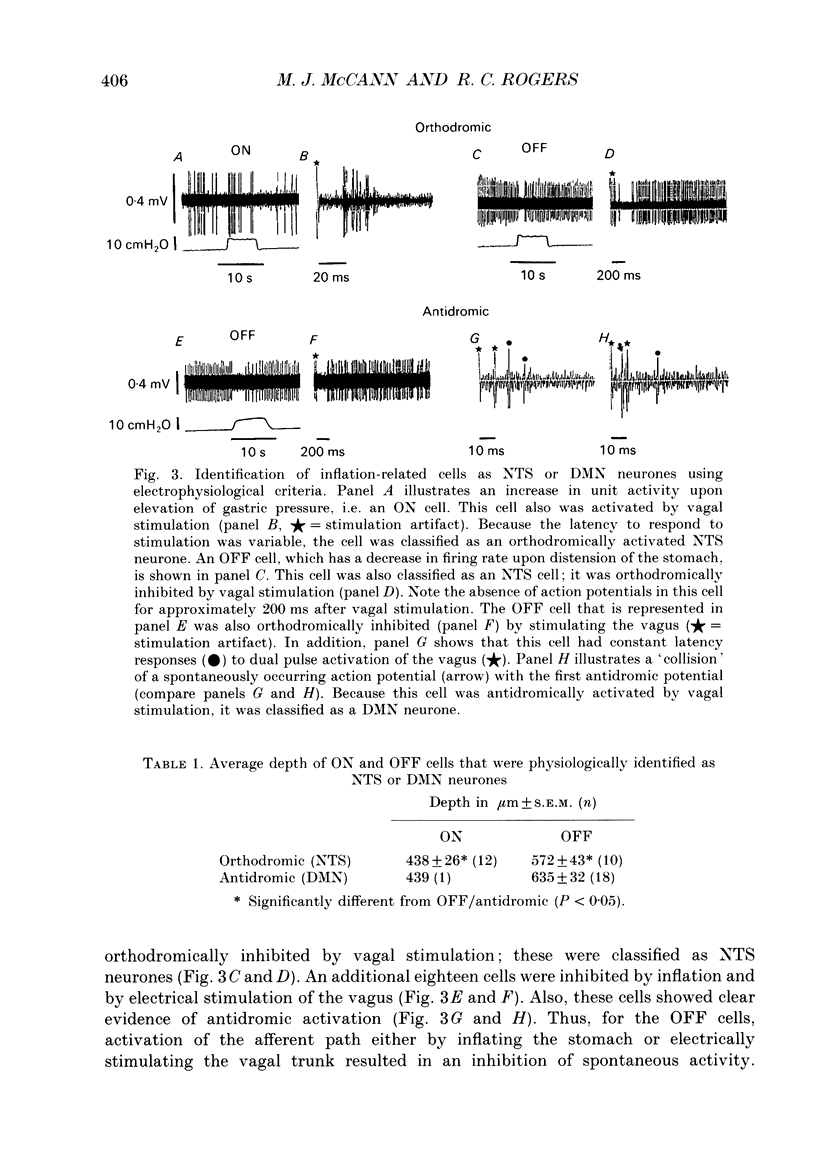





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson H. Vagal relaxation of the stomach induced from the gastric antrum. Acta Physiol Scand. 1973 Nov;89(3):406–414. doi: 10.1111/j.1748-1716.1973.tb05535.x. [DOI] [PubMed] [Google Scholar]
- Bieger D., Hopkins D. A. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987 Aug 22;262(4):546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- Fox E. A., Powley T. L. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 1985 Aug 26;341(2):269–282. doi: 10.1016/0006-8993(85)91066-2. [DOI] [PubMed] [Google Scholar]
- Fuller J. H., Schlag J. D. Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res. 1976 Aug 13;112(2):283–298. doi: 10.1016/0006-8993(76)90284-5. [DOI] [PubMed] [Google Scholar]
- GROSSMAN M. I. Secretion of acid and pepsin in response to distention of vagally innervated fundic gland area in dogs. Gastroenterology. 1962 Jun;42:718–721. [PubMed] [Google Scholar]
- Grundy D., Salih A. A., Scratcherd T. Modulation of vagal efferent fibre discharge by mechanoreceptors in the stomach, duodenum and colon of the ferret. J Physiol. 1981;319:43–52. doi: 10.1113/jphysiol.1981.sp013890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARPER A. A., KIDD C., SCRATCHERD T. Vago-vagal reflex effects on gastric and pancreatic secretion and gastrointestinal motility. J Physiol. 1959 Oct;148:417–436. doi: 10.1113/jphysiol.1959.sp006297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R., Leek B. F. Central projections of gastric afferent vagal inputs. J Physiol. 1973 Jan;228(1):73–90. doi: 10.1113/jphysiol.1973.sp010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson G. Vago-vagal reflex relaxation of the stomach in the cat. Acta Physiol Scand. 1969 Jan-Feb;75(1):245–252. doi: 10.1111/j.1748-1716.1969.tb04376.x. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980 Sep 15;193(2):435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Mantyh P. W., Hunt S. P. Neuropeptides are present in projection neurones at all levels in visceral and taste pathways: from periphery to sensory cortex. Brain Res. 1984 May 14;299(2):297–312. doi: 10.1016/0006-8993(84)90711-x. [DOI] [PubMed] [Google Scholar]
- McCann M. J., Rogers R. C. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol. 1990 Sep;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeley M. P., Underwood M. D., Talman W. T., Reis D. J. Content and in vitro release of endogenous amino acids in the area of the nucleus of the solitary tract of the rat. J Neurochem. 1989 Dec;53(6):1807–1817. doi: 10.1111/j.1471-4159.1989.tb09247.x. [DOI] [PubMed] [Google Scholar]
- Miolan J. P., Roman C. Discharge of efferent vagal fibers supplying gastric antrum: indirect study by nerve suture technique. Am J Physiol. 1978 Oct;235(4):E366–E373. doi: 10.1152/ajpendo.1978.235.4.E366. [DOI] [PubMed] [Google Scholar]
- Perrone M. H. Biochemical evidence that L-glutamate is a neurotransmitter of primary vagal afferent nerve fibers. Brain Res. 1981 Dec 28;230(1-2):283–293. doi: 10.1016/0006-8993(81)90407-8. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Chan J., Park D. H., Joh T. H., Milner T. A. Ultrastructural localization of phenylethanolamine N-methyltransferase in sensory and motor nuclei of the vagus nerve. J Neurosci Res. 1986;15(4):439–455. doi: 10.1002/jnr.490150402. [DOI] [PubMed] [Google Scholar]
- Rinaman L., Card J. P., Schwaber J. S., Miselis R. R. Ultrastructural demonstration of a gastric monosynaptic vagal circuit in the nucleus of the solitary tract in rat. J Neurosci. 1989 Jun;9(6):1985–1996. doi: 10.1523/JNEUROSCI.09-06-01985.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R. C., Nelson D. O. Neurons of the vagal division of the solitary nucleus activated by the paraventricular nucleus of the hypothalamus. J Auton Nerv Syst. 1984 Apr;10(2):193–197. doi: 10.1016/0165-1838(84)90057-2. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E. Central connections of the sensory and motor nuclei of the vagus nerve. J Auton Nerv Syst. 1983 Oct;9(1):13–26. doi: 10.1016/0165-1838(83)90129-7. [DOI] [PubMed] [Google Scholar]
- Siaud P., Denoroy L., Assenmacher I., Alonso G. Comparative immunocytochemical study of the catecholaminergic and peptidergic afferent innervation to the dorsal vagal complex in rat and guinea pig. J Comp Neurol. 1989 Dec 15;290(3):323–335. doi: 10.1002/cne.902900302. [DOI] [PubMed] [Google Scholar]
- Spencer S. E., Talman W. T. Central modulation of gastric pressure by substance P: a comparison with glutamate and acetylcholine. Brain Res. 1986 Oct 22;385(2):371–374. doi: 10.1016/0006-8993(86)91085-1. [DOI] [PubMed] [Google Scholar]
- Takayama K., Ishikawa N., Miura M. Sites of origin and termination of gastric vagus preganglionic neurons: an HRP study in the rat. J Auton Nerv Syst. 1982 Sep;6(2):211–223. doi: 10.1016/0165-1838(82)90052-2. [DOI] [PubMed] [Google Scholar]


