Abstract
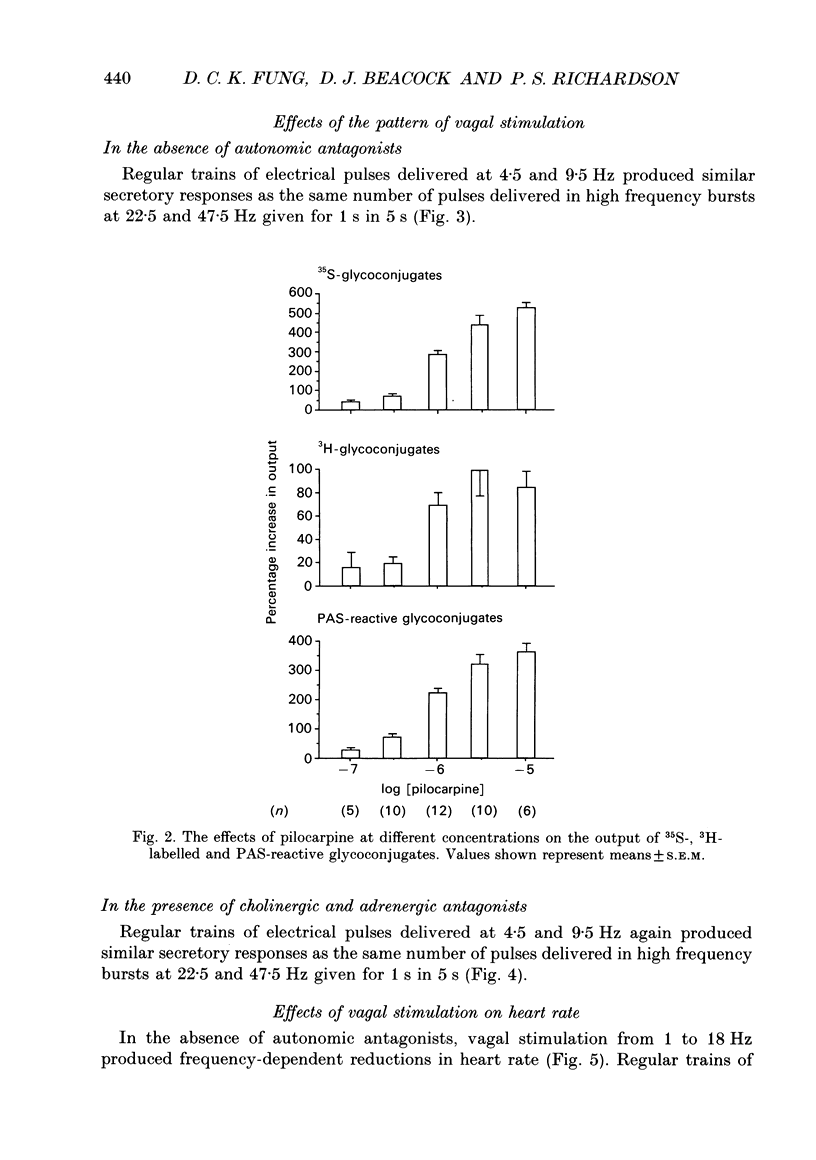
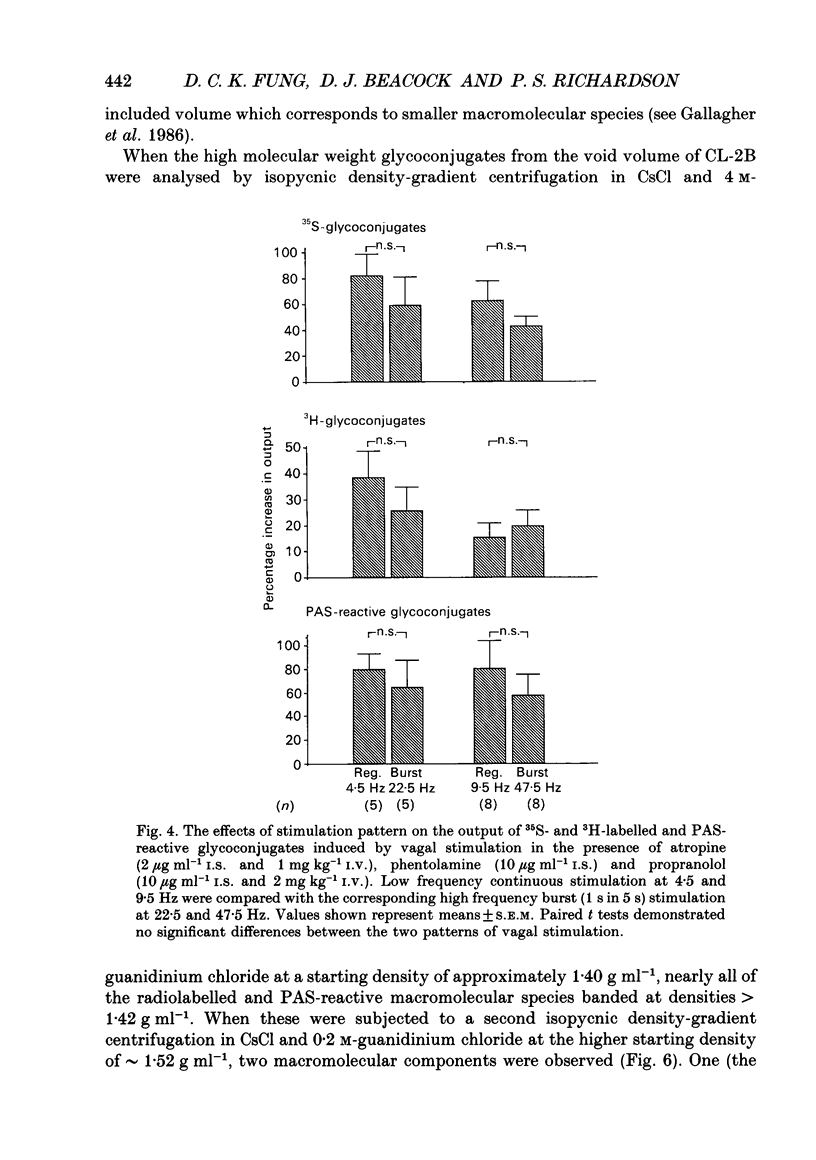
1. We examined the effects of frequencies and patterns of electrical stimulation of the peripheral cut ends of the vagus nerves on the release of mucus glycoconjugates into feline trachea in vivo. Mucus glycoconjugates, radiolabelled biosynthetically with [35S]sulphate and [3H]glucose, were washed from a tracheal segment in situ, and dialysed before being counted and assayed chemically by the periodic acid-Schiff (PAS) method. 2. Vagal stimulation with regular pulses (10 V, 2 ms duration) at 1, 2.25, 4.5, 9 and 18 Hz produced frequency-dependent increases in the output of mucus glycoconjugates. 3. The muscarinic agonist pilocarpine (0.1-10 microM), given intrasegmentally, produced dose-dependent increases in the output of mucus glycoconjugates. 4. Pretreatment with atropine, phentolamine and propranolol reduced but did not abolish the effects of vagal stimulation. Vagus nerve stimulation still caused frequency-dependent increases in the output of mucus glycoconjugates. 5. High frequency stimulations at 22.5 and 47.5 Hz given intermittently (1 s burst then 4 s rest), whether in the absence or presence of cholinergic and adrenergic blockade, produced similar secretory responses as the same number of pulses delivered in regular trains at 4.5 and 9.5 Hz. This suggests that neither cholinergic nor non-adrenergic, non-cholinergic (NANC) nerve mechanisms in this system are potentiated by high frequency, intermittent burst stimulation. 6. In the absence of atropine, regular vagal stimulation had a greater effect on heart rate than did the same number of pulses delivered in bursts. 7. High molecular weight glycoconjugates from secretions were taken from the void volume of a Sepharose CL-2B gel filtration column and separated further by density-gradient centrifugation. Macromolecular components were observed at two densities, a typical mucin at 1.52 g ml-1, and a high density atypical component at 1.63 g ml-1. In secretions collected during vagal stimulation, either in the absence or presence of cholinergic and adrenergic blockade, the ratio of low density to high density macromolecules was higher than in unstimulated secretions. This can be explained if both cholinergic and NANC nervous vagal mechanisms stimulate the output of typical (density = 1.52 g ml-1) mucins into the feline trachea.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson P. O., Bloom S. R., Edwards A. V., Järhult J. Effects of stimulation of the chorda tympani in bursts on submaxillary responses in the cat. J Physiol. 1982 Jan;322:469–483. doi: 10.1113/jphysiol.1982.sp014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson P. O., Bloom S. R., Edwards A. V. Parotid responses to stimulation of the parasympathetic innervation in bursts in weaned lambs. J Physiol. 1982 Sep;330:163–174. doi: 10.1113/jphysiol.1982.sp014335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. Regulatory peptides in the respiratory system. Experientia. 1987 Jul 15;43(7):832–839. doi: 10.1007/BF01945361. [DOI] [PubMed] [Google Scholar]
- Bartfai T., Iverfeldt K., Fisone G., Serfözö P. Regulation of the release of coexisting neurotransmitters. Annu Rev Pharmacol Toxicol. 1988;28:285–310. doi: 10.1146/annurev.pa.28.040188.001441. [DOI] [PubMed] [Google Scholar]
- Borson D. B., Charlin M., Gold B. D., Nadel J. A. Neural regulation of 35SO4-macromolecule secretion from tracheal glands of ferrets. J Appl Physiol Respir Environ Exerc Physiol. 1984 Aug;57(2):457–466. doi: 10.1152/jappl.1984.57.2.457. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K., Ulmsten U., Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983 Apr 1;211(1):13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K. Macromolecular properties and polymeric structure of mucus glycoproteins. Ciba Found Symp. 1984;109:157–172. doi: 10.1002/9780470720905.ch11. [DOI] [PubMed] [Google Scholar]
- Davies J. R., Gallagher J. T., Richardson P. S., Sheehan J. K., Carlstedt I. Mucins in cat airway secretions. Biochem J. 1991 May 1;275(Pt 3):663–669. doi: 10.1042/bj2750663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B., Chinn R., Gold J., Popovac D., Widdicombe J. G., Nadel J. A. Hypoxemia reflexly increases secretion from tracheal submucosal glands in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jun;52(6):1416–1419. doi: 10.1152/jappl.1982.52.6.1416. [DOI] [PubMed] [Google Scholar]
- Edwards A. V., Andersson P. O., Järhult J., Bloom S. R. Studies of the importance of the pattern of autonomic stimulation in relation to alimentary effectors. Q J Exp Physiol. 1984 Jul;69(3):607–614. doi: 10.1113/expphysiol.1984.sp002847. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Hall R. L., Phipps R. J., Jeffery P. K., Kent P. W., Richardson P. S. Mucus-glycoproteins (mucins) of the cat trachea: characterisation and control of secretion. Biochim Biophys Acta. 1986 Apr 29;886(2):243–254. doi: 10.1016/0167-4889(86)90142-4. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Kent P. W., Passatore M., Phipps R. J., Richardson P. S. The composition of tracheal mucus and the nervous control of its secretion in the cat. Proc R Soc Lond B Biol Sci. 1975 Dec 31;192(1106):49–76. doi: 10.1098/rspb.1975.0151. [DOI] [PubMed] [Google Scholar]
- Mantle M., Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans. 1978;6(3):607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- Martling C. R., Gazelius B., Lundberg J. M. Nervous control of tracheal blood flow in the cat measured by the laser Doppler technique. Acta Physiol Scand. 1987 Jul;130(3):409–417. doi: 10.1111/j.1748-1716.1987.tb08156.x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki Y., Hamasaki Y., Said S. I. Vasoactive intestinal peptide: a possible transmitter of nonadrenergic relaxation of guinea pig airways. Science. 1980 Dec 12;210(4475):1252–1253. doi: 10.1126/science.6254154. [DOI] [PubMed] [Google Scholar]
- Ollerenshaw S., Jarvis D., Woolcock A., Sullivan C., Scheibner T. Absence of immunoreactive vasoactive intestinal polypeptide in tissue from the lungs of patients with asthma. N Engl J Med. 1989 May 11;320(19):1244–1248. doi: 10.1056/NEJM198905113201904. [DOI] [PubMed] [Google Scholar]
- Peatfield A. C., Richardson P. S. Evidence for non-cholinergic, non-adrenergic nervous control of mucus secretion into the cat trachea. J Physiol. 1983 Sep;342:335–345. doi: 10.1113/jphysiol.1983.sp014854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps R. J., Richardson P. S. The effects of irritation at various levels of the airway upon tracheal mucus secretion in the cat. J Physiol. 1976 Oct;261(3):563–581. doi: 10.1113/jphysiol.1976.sp011574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. F., Barnes P. J. Opioid inhibition of neurally mediated mucus secretion in human bronchi. Lancet. 1989 Apr 29;1(8644):930–932. doi: 10.1016/s0140-6736(89)92509-9. [DOI] [PubMed] [Google Scholar]
- Thornton D. J., Davies J. R., Kraayenbrink M., Richardson P. S., Sheehan J. K., Carlstedt I. Mucus glycoproteins from 'normal' human tracheobronchial secretion. Biochem J. 1990 Jan 1;265(1):179–186. doi: 10.1042/bj2650179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton D. J., Holmes D. F., Sheehan J. K., Carlstedt I. Quantitation of mucus glycoproteins blotted onto nitrocellulose membranes. Anal Biochem. 1989 Oct;182(1):160–164. doi: 10.1016/0003-2697(89)90735-5. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. G. Action potentials in parasympathetic and sympathetic efferent fibres to the trachea and lungs of dogs and cats. J Physiol. 1966 Sep;186(1):56–88. doi: 10.1113/jphysiol.1966.sp008020. [DOI] [PMC free article] [PubMed] [Google Scholar]


