Abstract
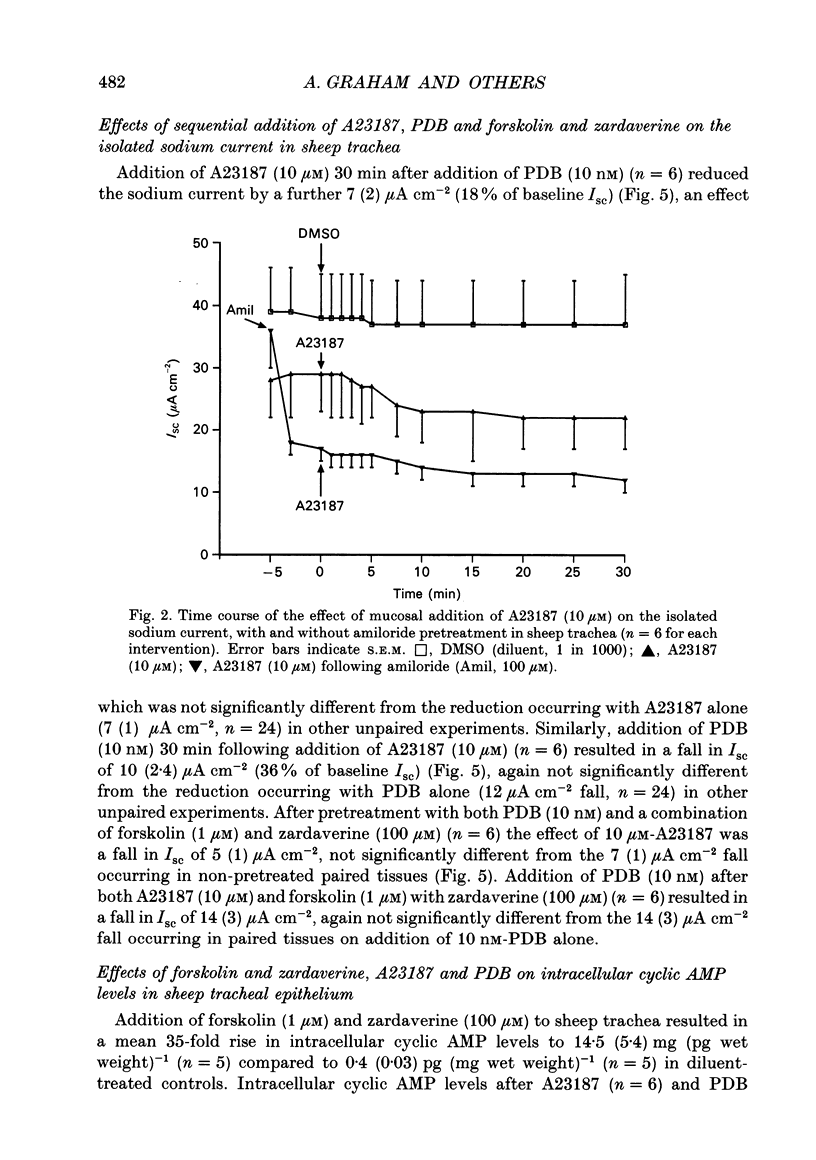
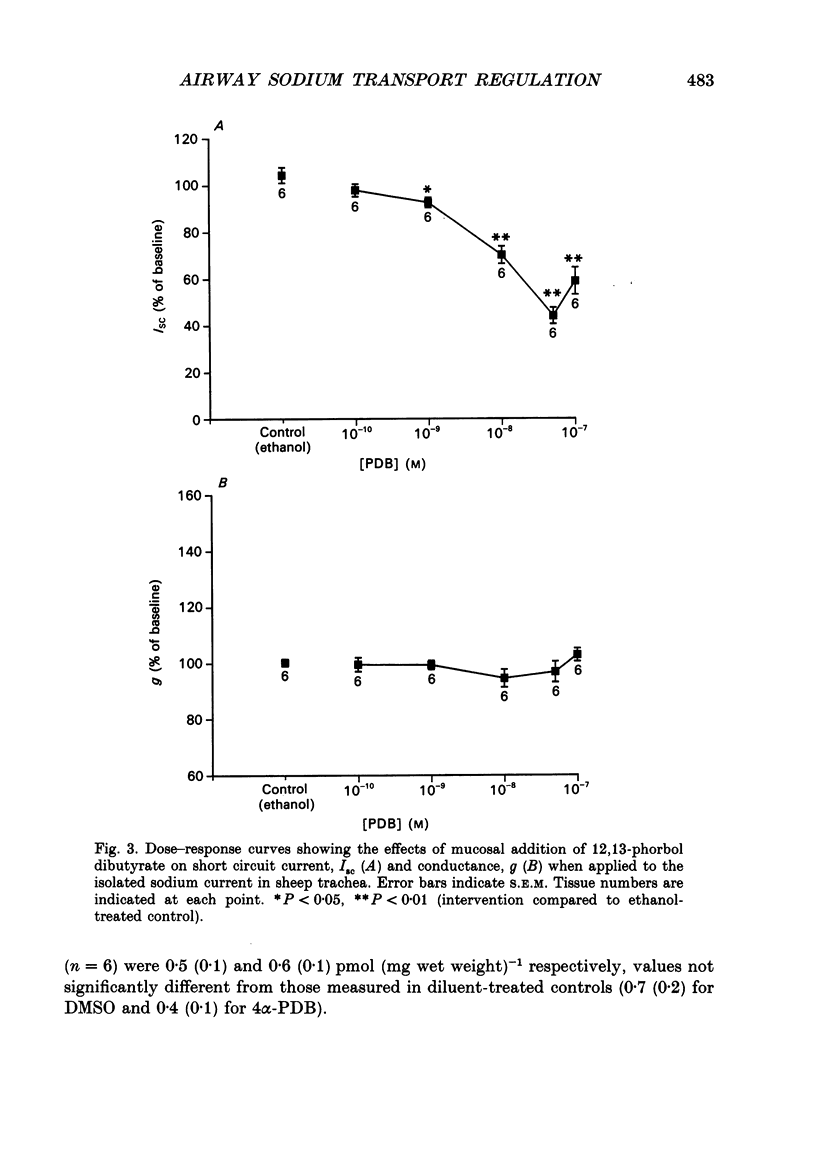
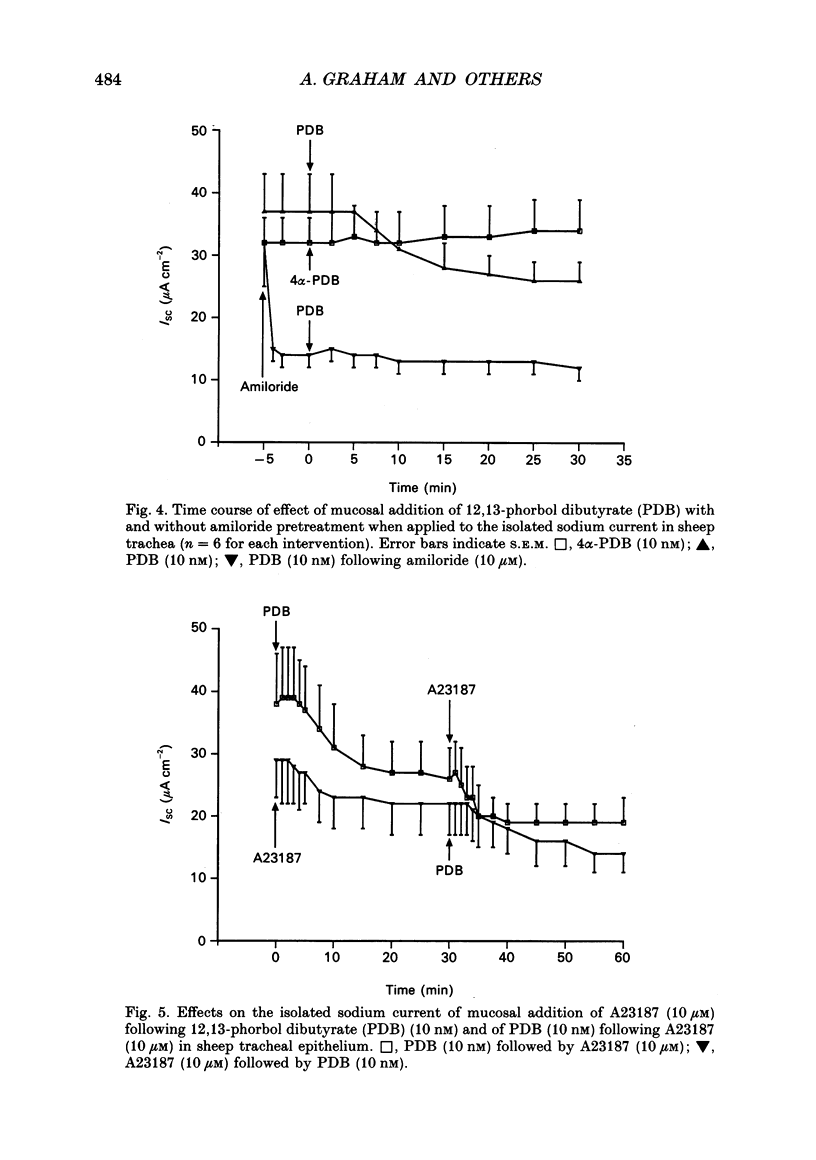
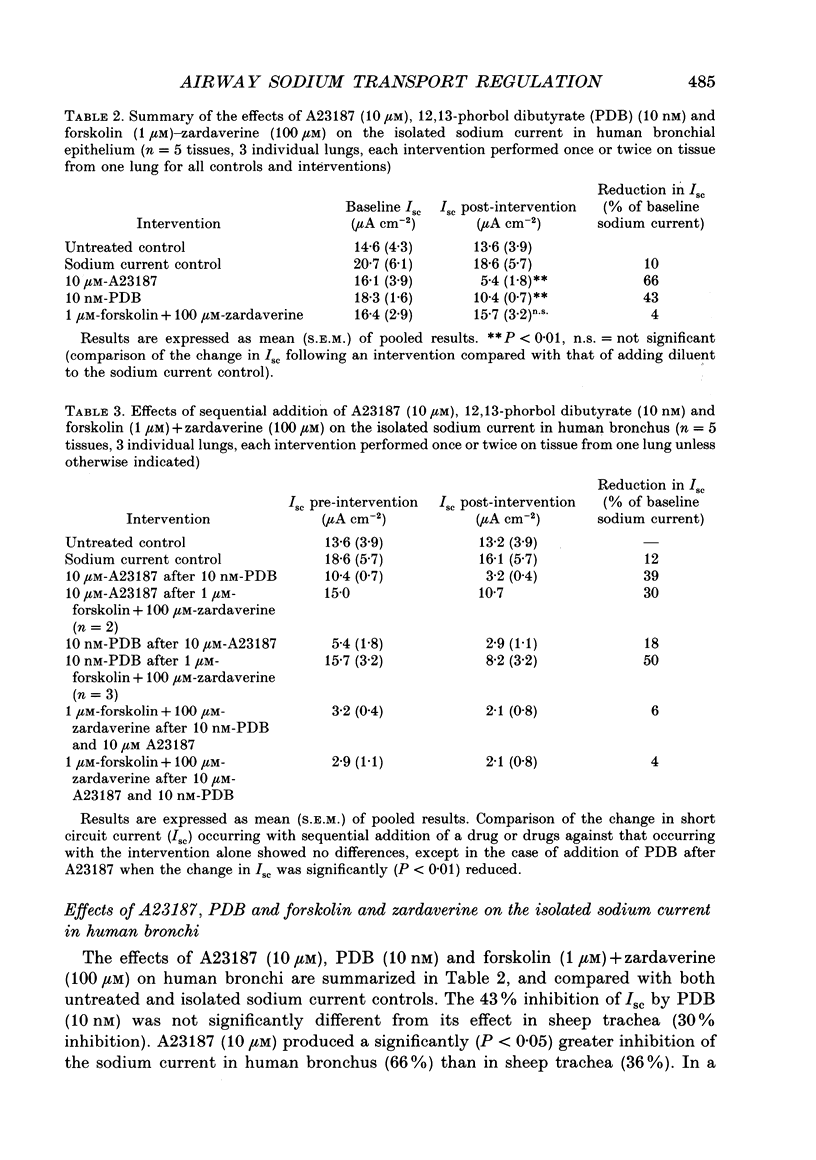
1. Sodium absorption is the dominant ion transport process in conducting airways and is a major factor regulating the composition of airway surface liquid. However, little is known about the control of airway sodium transport by intracellular regulatory pathways. 2. In sheep tracheae and human bronchi mounted in Ussing chambers under short circuit conditions, the sodium current can be isolated by pretreating tissues with acetazolamide (100 microM) to inhibit bicarbonate secretion, bumetanide (100 microM) to inhibit chloride secretion and phloridzin (200 microM) to inhibit sodium-glucose cotransport. This sodium current consists of amiloride-sensitive (57%) and amiloride-insensitive (43%) components. 3. The regulation of the isolated sodium current by three second messenger pathways was studied using the calcium ionophore A23187 to elevate intracellular calcium, a combination of forskolin and the phosphodiesterase inhibitor zardaverine to elevate intracellular cyclic AMP, and the phorbol ester 12,13-phorbol dibutyrate (PDB) to stimulate protein kinase C. 4. In sheep trachea, A23187 produces a dose-related inhibition of the sodium current with maximal effect (38% of ISC) at 10 microM and IC50 1 microM. This response affects both the amiloride-sensitive and insensitive components of the sodium current and is not altered by prior stimulation of protein kinase C or elevation of intracellular cyclic AMP. In human bronchi, A23187 (10 microM) produced a significantly greater inhibition of ISC (68%), a response which was unaffected by prior treatment with PDB or forskolin-zardaverine. 5. In sheep trachea, stimulation of protein kinase C with PDB produced a dose-related inhibition of ISC maximal (56% of ISC) at 50 nM (IC50 7 nM). This response was abolished by amiloride (100 microM) pretreatment suggesting a selective effect on the amiloride-sensitive component of the sodium current. The response was not altered by prior elevation of intracellular calcium or cyclic AMP. PDB (10 nM) caused a similar inhibition of ISC in human bronchi (43%). The effect of PKC stimulation following pretreatment with A23187 was diminished in human bronchi. Elevating intracellular cyclic AMP did not alter this response. 6. Addition of forskolin (1 microM) together with the phosphodiesterase inhibitor zardaverine (100 microM) produced a mean 35-fold increase in intracellular cyclic AMP in sheep trachea. This was associated with a small, but significant, 6% transient increase in ISC followed by a significant 4% fall. Neither effect could be abolished by amiloride pretreatment. In human bronchi, a small decrease in ISC which could not be distinguished from that occurring in controls was observed.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acevedo M., Olver R. E., Ward M. R. Ionic permeabilities of the cell membranes of sheep tracheal epithelium. J Physiol. 1990 Mar;422:67–81. doi: 10.1113/jphysiol.1990.sp017973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando Y., Jacobson H. R., Breyer M. D. Phorbol myristate acetate, dioctanoylglycerol, and phosphatidic acid inhibit the hydroosmotic effect of vasopressin on rabbit cortical collecting tubule. J Clin Invest. 1987 Aug;80(2):590–593. doi: 10.1172/JCI113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- App E. M., King M., Helfesrieder R., Köhler D., Matthys H. Acute and long-term amiloride inhalation in cystic fibrosis lung disease. A rational approach to cystic fibrosis therapy. Am Rev Respir Dis. 1990 Mar;141(3):605–612. doi: 10.1164/ajrccm/141.3.605. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Cotton C. U., Gatzy J. T., Knowles M. R., Yankaskas J. R. Evidence for reduced Cl- and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol. 1988 Nov;405:77–103. doi: 10.1113/jphysiol.1988.sp017322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Cao Y. Z., Reddy C. C., Mastro A. M. Evidence for protein kinase C independent activation of phospholipase D by phorbol esters in lymphocytes. Biochem Biophys Res Commun. 1990 Sep 28;171(3):955–962. doi: 10.1016/0006-291x(90)90777-k. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Civan M. M., Peterson-Yantorno K., O'Brien T. G. Diacylglycerols stimulate short-circuit current across frog skin by increasing apical Na+ permeability. J Membr Biol. 1987;97(3):193–204. doi: 10.1007/BF01869222. [DOI] [PubMed] [Google Scholar]
- Civan M. M., Rubenstein D., Mauro T., O'Brien T. G. Effects of tumor promoters on sodium ion transport across frog skin. Am J Physiol. 1985 May;248(5 Pt 1):C457–C465. doi: 10.1152/ajpcell.1985.248.5.C457. [DOI] [PubMed] [Google Scholar]
- Cook P. F., Neville M. E., Jr, Vrana K. E., Hartl F. T., Roskoski R., Jr Adenosine cyclic 3',5'-monophosphate dependent protein kinase: kinetic mechanism for the bovine skeletal muscle catalytic subunit. Biochemistry. 1982 Nov 9;21(23):5794–5799. doi: 10.1021/bi00266a011. [DOI] [PubMed] [Google Scholar]
- Cotton C. U., Lawson E. E., Boucher R. C., Gatzy J. T. Bioelectric properties and ion transport of airways excised from adult and fetal sheep. J Appl Physiol Respir Environ Exerc Physiol. 1983 Nov;55(5):1542–1549. doi: 10.1152/jappl.1983.55.5.1542. [DOI] [PubMed] [Google Scholar]
- Cullen J. J., Welsh M. J. Regulation of sodium absorption by canine tracheal epithelium. J Clin Invest. 1987 Jan;79(1):73–79. doi: 10.1172/JCI112811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duszyk M., French A. S., Man S. F. Cystic fibrosis affects chloride and sodium channels in human airway epithelia. Can J Physiol Pharmacol. 1989 Oct;67(10):1362–1365. doi: 10.1139/y89-217. [DOI] [PubMed] [Google Scholar]
- Frindt G., Windhager E. E. Ca2(+)-dependent inhibition of sodium transport in rabbit cortical collecting tubules. Am J Physiol. 1990 Mar;258(3 Pt 2):F568–F582. doi: 10.1152/ajprenal.1990.258.3.F568. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Halm D. R., Rechkemmer G., Shoemaker R. L. Chloride channel regulation in secretory epithelia. Fed Proc. 1986 Nov;45(12):2727–2731. [PubMed] [Google Scholar]
- Garty H., Benos D. J. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev. 1988 Apr;68(2):309–373. doi: 10.1152/physrev.1988.68.2.309. [DOI] [PubMed] [Google Scholar]
- Jarnigan F., Davis J. D., Bromberg P. A., Gatzy J. T., Boucher R. C. Bioelectric properties and ion transport of excised rabbit trachea. J Appl Physiol Respir Environ Exerc Physiol. 1983 Dec;55(6):1884–1892. doi: 10.1152/jappl.1983.55.6.1884. [DOI] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Knowles M., Murray G., Shallal J., Askin F., Ranga V., Gatzy J., Boucher R. Bioelectric properties and ion flow across excised human bronchi. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):868–877. doi: 10.1152/jappl.1984.56.4.868. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landry D. W., Reitman M., Cragoe E. J., Jr, Al-Awqati Q. Epithelial chloride channel. Development of inhibitory ligands. J Gen Physiol. 1987 Dec;90(6):779–798. doi: 10.1085/jgp.90.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., McCann J. D., Anderson M. P., Clancy J. P., Liedtke C. M., Nairn A. C., Greengard P., Welsch M. J. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989 Jun 16;244(4910):1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- Ludens J. H. Studies on the inhibition of Na+ transport in toad bladder by the ionophore A23187. J Pharmacol Exp Ther. 1978 Aug;206(2):414–422. [PubMed] [Google Scholar]
- Maren T. H. Use of inhibitors in physiological studies of carbonic anhydrase. Am J Physiol. 1977 Apr;232(4):F291–F297. doi: 10.1152/ajprenal.1977.232.4.F291. [DOI] [PubMed] [Google Scholar]
- Murphy E., Cheng E., Yankaskas J., Stutts M. J., Boucher R. C. Cell calcium levels of normal and cystic fibrosis nasal epithelium. Pediatr Res. 1988 Jul;24(1):79–84. doi: 10.1203/00006450-198807000-00019. [DOI] [PubMed] [Google Scholar]
- Nathanson I., Widdicombe J. H., Nadel J. A. Effects of amphotericin B on ion and fluid movement across dog tracheal epithelium. J Appl Physiol Respir Environ Exerc Physiol. 1983 Oct;55(4):1257–1261. doi: 10.1152/jappl.1983.55.4.1257. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- O'Brodovich H. When the alveolus is flooding, it's time to man the pumps. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1247–1248. doi: 10.1164/ajrccm/142.6_Pt_1.1247. [DOI] [PubMed] [Google Scholar]
- O'Grady S. M., Palfrey H. C., Field M. Characteristics and functions of Na-K-Cl cotransport in epithelial tissues. Am J Physiol. 1987 Aug;253(2 Pt 1):C177–C192. doi: 10.1152/ajpcell.1987.253.2.C177. [DOI] [PubMed] [Google Scholar]
- Olver R. E., Ramsden C. A., Strang L. B., Walters D. V. The role of amiloride-blockable sodium transport in adrenaline-induced lung liquid reabsorption in the fetal lamb. J Physiol. 1986 Jul;376:321–340. doi: 10.1113/jphysiol.1986.sp016156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Frindt G. Effects of cell Ca and pH on Na channels from rat cortical collecting tubule. Am J Physiol. 1987 Aug;253(2 Pt 2):F333–F339. doi: 10.1152/ajprenal.1987.253.2.F333. [DOI] [PubMed] [Google Scholar]
- Satoh T., Endou H. Inhibitory effect of phorbol ester on sodium transport in frog urinary bladder. Am J Physiol. 1990 Sep;259(3 Pt 2):F425–F431. doi: 10.1152/ajprenal.1990.259.3.F425. [DOI] [PubMed] [Google Scholar]
- Schudt C., Winder S., Müller B., Ukena D. Zardaverine as a selective inhibitor of phosphodiesterase isozymes. Biochem Pharmacol. 1991 Jun 21;42(1):153–162. doi: 10.1016/0006-2952(91)90694-z. [DOI] [PubMed] [Google Scholar]
- Tousson A., Alley C. D., Sorscher E. J., Brinkley B. R., Benos D. J. Immunochemical localization of amiloride-sensitive sodium channels in sodium-transporting epithelia. J Cell Sci. 1989 Jun;93(Pt 2):349–362. doi: 10.1242/jcs.93.2.349. [DOI] [PubMed] [Google Scholar]
- Turnheim K. Intrinsic regulation of apical sodium entry in epithelia. Physiol Rev. 1991 Apr;71(2):429–445. doi: 10.1152/physrev.1991.71.2.429. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., McCann J. D. Intracellular calcium regulates basolateral potassium channels in a chloride-secreting epithelium. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8823–8826. doi: 10.1073/pnas.82.24.8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J. H., Gashi A. A., Basbaum C. B., Nathanson I. T. Structural changes associated with fluid absorption by dog tracheal epithelium. Exp Lung Res. 1986;10(1):57–69. doi: 10.3109/01902148609057503. [DOI] [PubMed] [Google Scholar]
- Willumsen N. J., Boucher R. C. Activation of an apical Cl- conductance by Ca2+ ionophores in cystic fibrosis airway epithelia. Am J Physiol. 1989 Feb;256(2 Pt 1):C226–C233. doi: 10.1152/ajpcell.1989.256.2.C226. [DOI] [PubMed] [Google Scholar]
- Witt J. J., Roskoski R., Jr Rapid protein kinase assay using phosphocellulose-paper absorption. Anal Biochem. 1975 May 26;66(1):253–258. doi: 10.1016/0003-2697(75)90743-5. [DOI] [PubMed] [Google Scholar]
- Yanase M., Handler J. S. Activators of protein kinase C inhibit sodium transport in A6 epithelia. Am J Physiol. 1986 Mar;250(3 Pt 1):C517–C522. doi: 10.1152/ajpcell.1986.250.3.C517. [DOI] [PubMed] [Google Scholar]


