Abstract
Neonicotinoid insecticides are widely used in both urban and agricultural settings around the world. Historically, neonicotinoid insecticides have been viewed as ideal replacements for more toxic compounds, like organophosphates, due in part to their perceived limited potential to affect the environment and human health. This critical review investigates the environmental fate and toxicity of neonicotinoids and their metabolites and the potential risks associated with exposure. Neonicotinoids are found to be ubiquitous in the environment, drinking water, and food, with low-level exposure commonly documented below acceptable daily intake standards. Available toxicological data from animal studies indicate possible genotoxicity, cytotoxicity, impaired immune function, and reduced growth and reproductive success at low concentrations, while limited data from ecological or cross-sectional epidemiological studies have identified acute and chronic health effects ranging from acute respiratory, cardiovascular, and neurological symptoms to oxidative genetic damage and birth defects. Due to the heavy use of neonicotinoids and potential for cumulative chronic exposure, these insecticides represent novel risks and necessitate further study to fully understood their risks to humans.
1. Introduction
Neonicotinoids represent a relatively new class of insecticides that have quickly become the most widely used class1 in the world for a variety of urban and agricultural uses.2–4 Industry crop scientists consider the discovery of neonicotinoid insecticides a milestone in agrochemical research that resulted in the most rapidly-growing class of insecticides since the commercialization of pyrethroids.5 The word neonicotinoid means “new nicotine-like insecticide”.6, 7 Historically, neonicotinoid insecticides were viewed as ideal replacements for some insecticides (e.g., organophosphates and carbamates) due in part to both their perceived low risk to the environment and to non-target organisms.5 Within the agricultural sector, neonicotinoids are preferred over other insecticides for several reasons including their: (1) flexibility of application (e.g., spray, injections, or seed treatments)2, 8–13; (2) broad-spectrum insect toxicity; (3) perceived low acute toxicity to non-target aquatic and terrestrial organisms14–17, and (4) high potency for insects.3, 8, 13, 18–22 In the United States, neonicotinoids are commonly applied as seed treatments1, 23 and neonicotinoid use has become particularly prevalent in the Midwest.24
Few studies have characterized human exposure to neonicotinoids or the insecticides’ potential adverse human health risks. An editorial entitled, “Catching Up with Popular Pesticides: More Human Health Studies Are Needed on Neonicotinoids”25, pointed to accumulating evidence that neonicotinoids are “contributing to devastating losses of honeybees” and while there is widespread exposure, “little research had been conducted assessing potential effects on human health.” In addition, a systematic literature review on neonicotinoid studies published between 2005 and 2015 identified only eight studies that addressed human health effects of neonicotinoids.26 Four of these studies focused on acute exposures (e.g., intentional self-poisoning) and four studies reviewed chronic environmental exposures. The findings from the four chronic exposure studies, which primarily used surrogate exposure data, indicated causal associations between chronic low-level neonicotinoid exposure and various adverse developmental outcomes and neurological effects.26 In 2013, the European Union (EU) identified two neonicotinoids, acetamiprid and imidacloprid, as potential neurodevelopmental toxins.27 The U.S. Environmental Protection Agency (EPA) is currently finalizing a human health risk assessment for acetamiprid, imidacloprid, clothianidin, thiamethoxam, and dinotefuran.28–32 Preliminary human health assessments for these compounds were completed and released for comment in 2017 and 2018. The USEPA registration for thiacloprid was voluntarily cancelled by the registrant in 2014.33
The purpose of this critical review is to bring together current literature to understand the environmental fate of neonicotinoids and their potential consequences for human health. The review followed a structured approach, starting with a general search of PubMed and Google Scholar databases for a combination of the key words – “neonicotinoids”, “neonicotinoids AND health” “neonicotinoids AND nicotine”, “neonicotinoids AND metabolism” and “neonicotinoids AND environment”. Over 500 publications were reviewed, with over 300 cited here to provide a comprehensive overview of the risks posed to human health.
2. Structure, Mode of Action & Receptor Binding Mechanisms
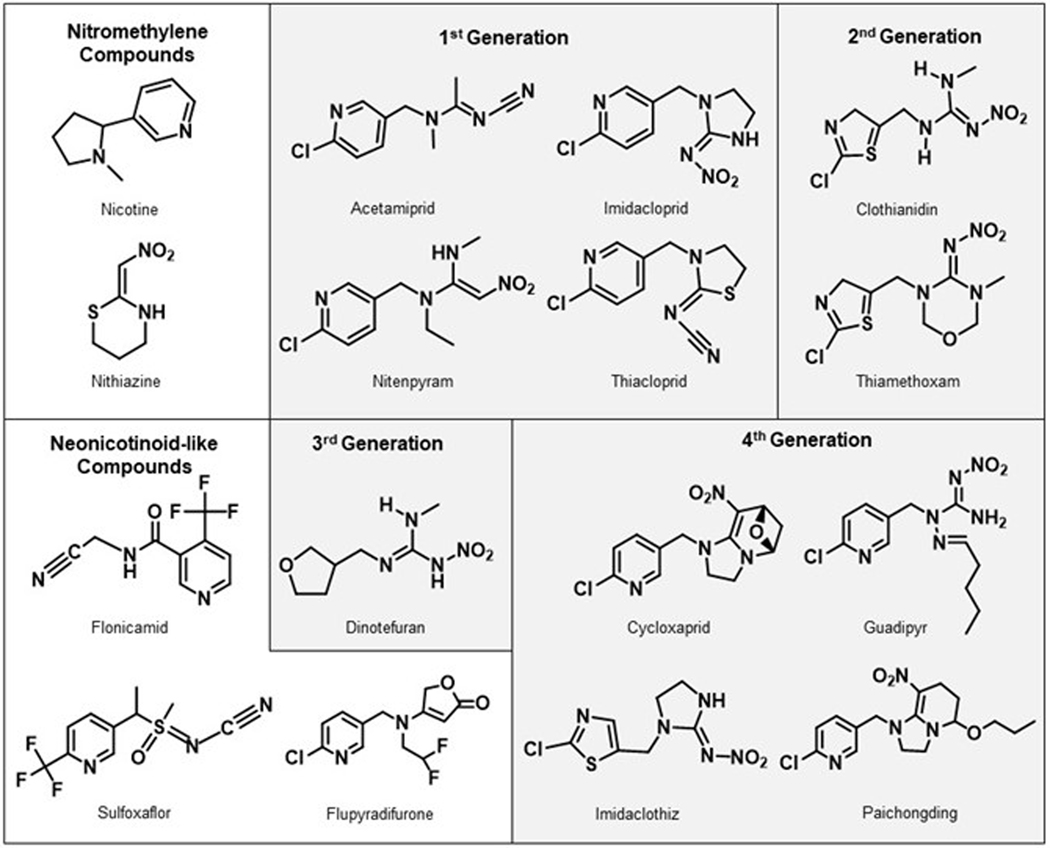
Seven synthetic commercially available neonicotinoid insecticides (Figure 1, Table 1) have been introduced into the marketplace including imidacloprid (Bayer CropScience) in 1991, nitenpyram (Sumitomo Chemical Takeda Agro Co.) and acetamiprid (Nippon Soda) in 1995, thiamethoxam (Syngenta) in 1998, clothianidin (Sumitomo Chemical Takeda Agro Co./Bayer CropScience) and thiacloprid (Bayer CropScience) in 2001, and dinotefuran (Mitsui Chemicals) in 2002.1, 8, 24, 34–36
Figure 1:
Chemical structures of common neonicotinoids and related compounds. Figure adapted from C. Giorio, et al. Environmental Science and Pollution Research, 2017, 1–33.35
Table 1:
Neonicotinoid background information
| Name | Year Introduced | EPA PC Code | CAS Number | CAS Name | Chemical Formula | Molar Mass |
|---|---|---|---|---|---|---|
| Acetamiprid | 1995 | 099050 | 135410–20-7 | (1E)-N-[(6-chloro-3-pyridinyl)methyl]-N'-cyano-N-methylethanimidamide | C10H11ClN4 | 222.7 |
| Clothianidin | 2001 | 044309 | 210880–92-5 | (C(E))-N-((2-chloro-5-thiazolyl)methyl)-N'-methyl-N'-nitroguanidine | C6H8ClN5O2S | 249.7 |
| Dinotefuran | 2002 | 044312 | 165252–70-0 | N-methyl-N'-nitro-N''-((tetrahydro-3-furanyl)methyl)guanidine | C7H14N4O3 | 202.2 |
| Imidacloprid | 1991 | 129099 | 138261–41-3 | (2E)-1-((6-chloro-3-pyridinyl)methyl)-N-nitro-2-imidazolidinimine | C9H10ClN5O2 | 255.7 |
| Nitenpyram | 1995 | - | 150824–47-8 | (1E)-N-[(6-chloro-3-pyridinyl)methyl]-N-ethyl-N'-methyl-2-nitro-1,1-ethenediamine | C11H15ClN4O2 | 270.7 |
| Thiacloprid | 2001 | 014019 | 111988–49-9 | (Z)-(3-((6-chloro-3-pyridinyl)methyl)-2-thiazolidinylidene)cyanamide | C10H9ClN4S | 252.7 |
| Thiamethoxam | 1998 | 060109 | 153719–23-4 | 3-[(2-chloro-5-thiazolyl)methyl]tetrahydro-5-methyl-N-nitro-4H-1,3,5-oxadiazin-4-imine | C8H10ClN5O3S | 291.7 |
Table adapted from Lewis, et al., Human and Ecological Risk Assessment, 2016. 22(4): p. 1050–1064;17 Jeschke, et al., Journal of Agriculture and Food Chemistry, 2010. 59: p. 2897–29081; Simon-Delso, et al., Environmental Science and Pollution Research, 2015. 22: p. 5–348; Bass, et al., Pestic. Biochem. Physiol., 2015, 121. P. 78–8734; Abou-Donia, et al., Toxicology and Environmental Health Impact, Part A, 2008, 71: p. 119–13035; and U.S. Environmental Protection Agency, 2017.28–32, 57 This article contains CAS Registry Numbers®, which is a Registered Trademark of the American Chemical Society. CAS recommends the verification of the CASRNs through CAS Client ServicesSM.
Imidacloprid, nitenpyram, thiacloprid, and acetamiprid share a chloropyridine moeity, thiamethoxam and clothianidin each have a chlorothiazole group, and the structure of dinotefuran includes a tetrahydrofuran functionality. Neonicotinoids can be further classified as either N-nitroguanidines (i.e., imidacloprid, thiamethoxam, clothianidin, and dinotefuran), nitromethylenes (i.e., nitenpyram), or N-cyanoamidines (i.e., acetamiprid).1 The electron-rich nitromethylene, nitroimine, or cyanoimine group enables the neonicotinoid to selectively target insects by binding more strongly to insect nicotinic acetylcholine receptors (nAChRs) compared to mammalian nAChRs.3, 18, 37
Several other compounds have been identified as neonicotinoids or neonicotinoid-like, including nithiazine, flonicamid, flupyradifurone, sulfoxaflor, guadipyr, cycloxaprid, paichongding, and imidaclothiz.5, 8, 38–40 Nithiazine was one of the first neonicotinoids, but due to its limited efficacy in field trials was not commercialized until 1997 as an active ingredient against flies.5 Flonicamid, flupyradifurone, and sulfoxaflor are classified as pyridine, butenolid, and sulfoximine compounds respectively.5, 17, 39, 41 Flupyradifurone and sulfoxaflor are sometimes included as neonicotinoids because they have similar neonicotinoid-like modes of action.39, 42 Flonicamid is often classified as a neonicotinoid, but has a different mode of action compared to other neonicotinoids.38, 41 New neonicotinoid-like compounds are also being produced. In China, over 600 neonicotinoid compounds have been synthesized.43 Cycloxaprid, guadipyr, paichongding, and imidaclothiz are four compounds registered for use in China, but not in the United States or EU.44, 45 Cycloxaprid and paichongding are cis-neonicotinoids (in which the nitro or cyano group is located in a cis orientation instead of the typical trans arrangement)43, and imidaclothiz is a nitroguanidine thiazole neonicotinoid.43 This review will primarily focus on the first, second, and third generation neonicotinoids shown in Figure 1.
Neonicotinoids work systemically in plants. Upon application, the insecticides are absorbed by plants and translocated throughout the roots, leaves, and tissue.46, 47 Once ingested by insects, neonicotinoids bind with nAChRs.1, 3, 48, 49 This bond is irreversible and triggers nerve signaling in a manner similar to how acetylcholine operates. Acetylcholine esterase, the enzyme that breaks down acetylcholine, is unable to breakdown neonicotinoids, leading to nerve stimulation at low concentrations and receptor blockage, paralysis, and death at higher concentrations.3, 50
Nicotinic acetylcholine receptors are not exclusive to targeted pests but are expressed broadly in both vertebrates and invertebrates. In humans, nAChRs are found in both the peripheral and central nervous systems.37 They transmit signals within the nervous system direct to skeletal muscles to contract.7 In insects, the function of nAChRs is not as clearly understood as humans, but they are also responsible for post-synaptic neurotransmission.51 The receptors are found in greater numbers in insects than mammals, but are expressed primarily in the insects’ central nervous system.3, 37
All neonicotinoids are structurally similar to nicotine.3 The binding behavior of neonicotinoids on the insect nAChRs is similar to those of nicotine and nicotinoids in humans.1, 48 Nicotine and other nicotinoids, are both agonists of nAChRs.37, 52 In humans, nicotine remains at synapses longer than acetylcholine, which is hydrolyzed by acetylcholine esterase. This results in modifications to neural signaling.53–56 A key difference is that nicotine is protonated at physiological pH whereas the neonicotinoids are not protonated but possess an electronegative nitro- or cyano- functional group.3, 37
The weaker interaction between the insecticides and vertebrates relative to insects is due to the abundance of nAChRs in insects and differences in nAChR subtypes and binding sites at the molecular level between insects and mammals.3, 19–22 On this basis, neonicotinoids are estimated to be 5 to 10 times more selective for insects versus mammals compared to organophosphates, methylcarbamates, and organochlorines.3
Neonicotinoids are high affinity agonists of insect nAChRs resulting in paralysis and death in insects.3, 37 Because neonicotinoids do not have a high affinity for mammalian and other non-insect nAChRs,58–64 they are thought to have limited toxicity in non-target organisms, including humans. For example, the half maximal effective concentration (EC50) values for imidacloprid are 0.86–1 μM against insect nAChRs compared to an EC50 of 70 μM against mammalian nAChRs.65 Neonicotinoids produce moderate to minimal toxicity in animal studies irrespective of the route of exposure (Lethal Dose, 50% (LD50) values > 200 mg/kg body weight) by mechanisms that may involve nicotinic stimulation or represent non-specific toxic effects.66–69
3. Use
Neonicotinoid insecticides have rapidly gained popularity, in the United States and globally, for both urban and agricultural use since their introduction in the 1990s.1, 5, 8, 24 Neonicotinoids were registered in over 120 counties as insecticides marketed for protection against chewing insects (e.g., plant hoppers, thrips, some micro-Lepidoptera, and other coleopteran pests1), capturing 25% of the total global insecticide sales34 in 2014 and a global market value of approximately $3.7 billion U.S. Dollars (USD).70 In 2012, thiamethoxam, imidacloprid, and clothianidin represented 85% of all neonicotinoids sales in the world.34 From a global perspective, imidacloprid trails only the herbicide glyphosate71 as the second most widely used pesticide in the world.72 While neonicotinoid use has led to a decrease in some older pesticides, such as pyrethroids and carbamates, overall use of insecticides has not declined.1, 4, 8, 24, 73 Use of neonicotinoids has led to an expansion of treatment on new acres.74 This increase has not corresponded with an increased risk from target pest species, meaning some applications may be unnecessary.74
Neonicotinoids are registered globally for both agricultural and non-agricultural uses.33 Neonicotinoids can be applied using several application methods including foliar application by aerial or ground spray equipment, soil drench, chemigation, tree injection, and as seed treatments.2, 8–13 In the United States, for example, there are over 1,000 EPA primary and supplementary registered neonicotinoid-containing products on the market. 75 They are used in products ranging from oral formulations (i.e. tablet) for dogs and cats as a flea adulticide to agricultural products including cucurbit vegetables, fruiting vegetables, grapes, leafy brassica, leafy vegetables, and nut trees (Table S-1). 33 In 2014, the U.S. Geological Survey (USGS) estimated that over 3.5 million kg of neonicotinoids were applied annually to all crops in the United States.24
Neonicotinoids have a variety of formulations and application methods that are effective against a wide spectrum of insects.9 For example, professional application treatment options for the emerald ash borer Agrilus planipennis include soil injection or drench using either imidacloprid or dinotefuran (Merit®, Safari™, Transtect™, Xylam® Liquid Systemic Insecticide, and Xytect™), trunk injection using imidacloprid (Imicide®), or systemic basal bark spray using dinotefuran (Safari™, Transtect™, and Xylam® Liquid Systemic Insecticide).76 Homeowner applications include soil drench with a mixture of dinotefuran and imidacloprid (Bayer Advanced™ Protect and Feed II), soil drench using imidacloprid (Bayer Advanced™ Tree & Shrub Insect Control or Optrol™), and use of dinotefuran containing granules (Ortho Tree and Shrub Insect Control Ready to Use Granules®).76
Neonicotinoids are most commonly used as seed treatments.77 In 2008, the insecticides comprised 80% of the global treated seed market.1 By 2025, the seed treatment market is expected to grow to as much as $10 billion USD per year, with use increasing as much as 14% in China.78 North America represents the largest market presently for seed treatments1 with clothianidin or thiamethoxam coated seeds applied to over 80% of corn (maize) seed grown on the continent.77 Over the past decade there has been a three-fold increase in the use of treated seeds in the United States 8, 24, 79 with a particularly rapid increase in use between 2003 and 2011 as a pre-emptive insecticide applied as a seed coating for row crops such as corn (maize), cotton, soybeans, and wheat.74 Presently, 50% of soybeans (18.2 million hectares),23 nearly 100% of corn (>36.4 million hectares) and 95% of cotton (15 million hectares) are treated with neonicotinoids.74, 80 By comparison, <50% of corn and <10% of soybean acreage was treated with any type of insecticide prior to their popular use as seed treatments.81 Seed treatments also contain multiple active ingredients - fungicides, herbicide safeners, nematicides, and plant growth regulators and surfactants/adjuvants.4 They are also often applied with other pesticides and can co-occur in the environment with fertilizers, metals and pharmaceuticals.4
Concerns about the potential detrimental effects of neonicotinoid use have been growing in recent years due to their potential negative effects on pollinators.82 The European Food Safety Authority (EFSA) stated that neonicotinoids pose an unacceptably high risk to bees.72 In 2013, the EU halted the use of imidacloprid, clothianidin, and thiamethoxam on flowering field crops such as corn because of evidence that the pesticides harm domesticated honeybees.83 In 2018, this ban was made permanent and extended to include use on all outdoor field crops.84 Health Canada's Pest Management Regulatory Agency (PMRA) is planning to phase-out the use of some neonicotinoids over the next 3–5 years,85–88 while the EPA cancelled 12 products containing thiamethoxam and clothianidin in 2019.89 The EPA is scheduled to release updated pollinator risk assessments on five neonicotinoids by the end of 2019.33 As of 2019, six states in the United States have enacted legislation intended to limit neonicotinoids and protect pollinators, and an additional seven states had bills introduced that added restrictions to neonicotinoid usage.84–90
4. Occurrence in the Environment, Water and Food
The persistence of neonicotinoids in water, soil, and biota presents a potential environmental health concern that has been previously highlighted by numerous researchers and public health agencies.4, 5, 8, 40, 46, 47, 91–94 Neonicotinoids have relatively long half-lives in soil, high water solubility, and low sorption in soil (Table 2); these factors contribute to the persistence and transport of these insecticides in the environment.46, 92, 93, 95–97
Table 2:
Solubility and half-life of neonicotinoids
| Neonicotinoid | Half-Life (DT50) in Soil (Days) | Solubility in Water at 20ºC, pH =7 (mg/L) | Leaching Potentiala | Vapor Pressure 20oC (mPa) | Volatilityb |
|---|---|---|---|---|---|
| Acetamiprid | 31–450 | 2,950 | Low | 1.73 X 10-04 | Low |
| Clothianidin | 148 – 6,931 | 340 | High | 2.8 X 10-08 | Low |
| Dinotefuran | 75–82 | 39,830 | High | 0.0017 | Low |
| Imidacloprid | 100 – 1,250 | 610 | High | 4.0 X 10-07 | Low |
| Nitenpyram | 8 days | 590,000 | Moderate | 0.0011 | Low |
| Thiacloprid | 3.4–1,000 days | 184 | Low | 3.00 X 10-07 | Low |
| Thiamethoxam | 7–335 days | 4,100 | High | 6.60 X 10-06 | Low |
Leaching Potential = > 2.8 = High leachability 2.8 – 1.8 = Transition state < 1.8 = Low leachability
Volatility = Vapor pressure at 25oC (mPa) < 5.0 = Low volatility 5.0 – 10.0 = Moderately volatile > 10 = Highly volatile
Soil degradation reported at DT50 for aerobic environment at typical for field conditions
Table adapted from Kathleen A. Lewis, et al., Human and Ecological Risk Assessment, 2016. 22(4): p. 1050–1064.17; Bonmatin, Environ. Sci. Pollut. Res. Int., 2015, 22, 35–67.46; Wood and Goulson, Environmental Science and Pollution Research, 2017, 24, 17285–1732591; and Goulson, J. Appl. Ecol., 2013, 50, 977–98792.
4.1 Soil
Neonicotinoids can persist in soils; the reported half-lives for neonicotinoids in soil range from as little as 1 day to almost 19 years (Table 2).46, 92 Half-lives can also be affected by local soil type, ultraviolet radiation, moisture, temperature, and pH.46 For example, neonicotinoids typically biodegrade rapidly, but in dry soils with high organic matter content and low temperatures, they can persist and potentially accumulate.46 These values include results from both field and laboratory studies with different soil types, including clay, elder, loam, and sand and silt soils.92
For neonicotinoids used as seed treatments or granules, only 2 to 20% of the active ingredient in the coating is absorbed by the crop.47 This leaves between 80 to 98% of the active ingredient remaining in the environment and able to accumulate in soil, be lost as dust during planting, or be transported to surface and/or groundwater.98, 99 The risk of dust drift is affected by several factors including: design and setting of the seed drill, mass and size of particles, quality of seed coating, meteorological conditions, and morphological properties of dust particles.100 Depending on the crop, individual seeds can contain from 1−17 mg/kg of neonicotinoids.47 Planting treated seeds results in dust clouds around the operating tractor, with neonicotinoid concentrations as high as 30 μg/m3.98, 101–104 Atmospheric emissions of neonicotinoid seed treatment as particulate matter are also caused by tillage and wind events. With passive and active air samplers, the average clothianidin and thiamethoxam concentrations in total suspended particulates ranged from trace to 1.91 ng/m3 during tillage and wind compared to a peak of 16.22 ng/m3 during planting.105
Several studies have documented that neonicotinoids persist in soils several years after the planting of treated seeds and can accumulate in soils after repeat application.106, 107 A study investigating imidacloprid seed treatments on winter wheat in the United Kingdom documented soil concentrations increasing from 6 to 8 ng/g after the first year of planting to 18 to 60 ng/g 6 years later.92 Randomly sampled soil from 74 farms in France documented that 84% of the fields contained detectable levels of imidacloprid (>0.1 ng/g) and 59% contained >1 ng/g; farms with 2 years of imidacloprid treatment had higher soil concentrations than those soils that received only 1 year of treatment.106 In areas of seed treatment use in North America, clothianidin and thiamethoxam have commonly been detected with average soil concentrations of <10 ng/g.108–111 Studies have also shown that soil concentrations increase with repeated applications, plateauing around 4–6 years after repeated use of seed treatments, and may remain in the soil several years after treated seed have stopped being used, with average concentrations below 6 ng/g.4, 92, 106, 109–112 Similarly, studies examining spray applications of imidacloprid in Europe have also shown accumulation in soil with concentrations ranging from 6 to 18 ng/g one year after sowing compared to 18 to 60 ng/g after 5 years of repeat application.92, 113
4.2 Water
As highly water-soluble compounds, neonicotinoids are frequently detected in surface and groundwater around the world.99, 107, 111, 114–125 Neonicotinoids are resistant to hydrolysis at neutral or acidic pH under anaerobic conditions, but may undergo rapid photodegradation if there is sufficient light penetration.93, 126–128 Increasing turbidity in water can reduce photodegradation.129 Additionally, differences in light intensity and temperature with latitude affect half-lives by region, with mid and higher latitudes having longer half-lives compared to tropical regions.46
Contamination of water can take place through multiple pathways and mechanisms, including overspray, drift, spread of talcum and graphite powder from treated seeds, surface runoff, leaching into groundwater, discharge into wetlands, and via snowmelt.13, 108, 109, 114, 117, 118, 121, 124, 130–133 In China, six neonicotinoids have been detected in 100% of samples from the Han and Yangtze Rivers, where median concentrations ranged from 13–186 ng/L.134 In Canada, imidacloprid, clothianidin, and thiamethoxam were detected in over 90% of streams sampled, with two locations exceeding Canadian freshwater guidelines with concentrations in excess of 230 ng/L in 75% of samples.135 In streams within the Midwestern United States, Hladik et al. detected clothianidin, thiamethoxam, and imidacloprid in 75%, 47%, and 23%, respectively, of samples during growing season with maximum individual concentrations between 42.7 – 257 ng/L.117 Currently, neonicotinoids are not regulated under the U.S. Safe Drinking Water Act’s National Primary Drinking Water Regulations or Canada’s Guidelines for Drinking Water Quality136.137 In Europe, pesticides are regulated through several directives, with maximum limits set at 100 ng/L for individual pesticides and their degradates and 500 ng/L for total pesticide residues.138–142 For comparison, the maximum contaminant levels established for lindane, methoxychlor and oxamyl, insecticides regulated by the EPA in public drinking water, range between 200 and 200,000 ng/L.137 The limits for glyphosate and atrazine, two commonly used herbicides, are 700,000 ng/L and 3,000 ng/L, respectively. The EPA does not regulate total pesticide residues.137
Recent planting of treated seeds and subsequent precipitation events can drive neonicotinoid pulses to streams.117 In one study, clothianidin and thiamethoxam concentrations were positively correlated with the percentage of the land used for cultivated crop production, while imidacloprid was linked to the percentage of urban area within the basin.143 Across studies, average concentrations for imidacloprid in surface waters were generally in the tens of ng/L, with a maximum concentration of 320,000 ng/L reported in the Netherlands.99 The EPA estimates peak surface water contamination to be between 40 and 269,000 ng/L for acetamiprid, clothianidin, dinotefuran, imidacloprid and thiamethoxam.28–32, 144
Groundwater concentrations as high as 140 ng/L have been measured beneath areas planted with treated seeds 108, 118 and concentrations of up to 10,000 ng/L with in-furrow applications for potatoes.124 Groundwater concentrations in fields with coated seeds exhibited less seasonable variability compared to surface water, which tends to have higher concentrations occurring later in the summer.108 The EPA estimates that the peak concentrations of neonicotinoids in groundwater range from 58,000 to 211,000 ng/L for acetamiprid, clothianidin, dinotefuran, imidacloprid and thiamethoxam.28–32, 144
Despite the ubiquity of their occurrence in water sources, limited research has been conducted to assess human exposure risk from neonicotinoids in drinking water. Only six studies, all published within the past 3 years, have focused on municipal systems. A cross-sectional study of 100 sites across the United States detected imidacloprid in untreated and treated water samples from 0.008 – 0.202 ng/L between 1999 and 2015, with detection frequency increasing between 2004 and 2011.145 Detections peaked in 2011, with imidacloprid found in 36.7% of untreated and 29.7% of treated water. 145 In 2016, Klarich et al. found clothianidin, imidacloprid, and thiamethoxam at concentrations up to 57 ng/L in finished water samples at the University of Iowa water treatment facility in Iowa City, Iowa, USA, over a 7-week period following corn and soybean planting.118 While conventional water treatment processes, like rapid sand filtration, did not remove clothianidin or imidacloprid, thiamethoxam decreased by 40–60% following lime softening due to hydrolysis at the high pH values used for this process. In comparison, treatment using granular activated carbon was able to remove >80% of all three neonicotinoids.118 A follow-up study detected two metabolites of imidacloprid, desnitro-imidacloprid and imidacloprid-urea, in finished drinking water with concentrations up to 0.60 ng/L.146 In China, multiple neonicotinoids have been detected in 100% of tap water samples, with median concentrations between 2.6 and 138 ng/L.134
Water treatment was most effective at removing acetamiprid (40% reduction) and thiacloprid (20%).134 In Canada, thiamethoxam, clothianidin, and imidacloprid have been detected in water systems.147, 148 In Ontario, the mean concentrations were below the limits of detection but had a peak value of 91.7 ng/L for thiamethoxam.147 In Quebec, tap water from four samples had maximum concentrations for these three neonicotinoids ranging from 1.0 – 10 ng/L.148
No studies were identified to date that looked at private well water as a potential source of exposure. Globally, 435 million people rely on drinking water from unregulated wells and springs.149 These water sources are generally vulnerable to contamination from a variety of sources with treatment left to individual households.149 150, 151 The World Health Organization estimates that groundwater constitutes 97% of global freshwater and is one of the most important supplies of drinking water.152 Groundwater can also be contaminated by chemical hazards from the land surface, with contaminated groundwater sources reported in countries from all levels of economic development.152 For example, in the United States, a study of 2,100 wells nationwide found that 23% of domestic wells contained one or more contaminants at concentrations greater than human-health benchmarks and 73% of wells contained multiple contaminants greater than human-health benchmarks.153 Based on these earlier works, it is likely that many rural wells are similarly contaminated with neonicotinoids, and further research is needed to characterize well water contamination with these pesticides. Several studies have hypothesized that long-term consumption of private well-water is associated with increased risks for adverse health effects due to exposure to contaminants.154–158
4.3 Food
The small molecular weight and high water solubility of neonicotinoids provide the systemic property to enable the insecticides to enter plant tissues.159 Several neonicotinoids have been shown to translocate into pollen, vegetables, and fruits and therefore represent a potential human exposure route as washing does not completely remove neonicotinoid insecticides in fruits and vegetables being consumed.160–166
Neonicotinoids have been frequently detected in food including rice, tea, honey, and fruit and vegetables.38, 145, 167–197 A “market basket” study was conducted to assess levels of neonicotinoids in fresh fruits, vegetables, and honey available in a supermarket in Massachusetts, USA.38 All foods, except nectarines and tomatoes, tested positive for one or more neonicotinoids. Imidacloprid was detected at the highest singular concentration and most frequently (70% overall). Seventy-two percent of fruits and 45% of vegetables contained multiple neonicotinoids.38 A review analyzing neonicotinoid pesticides from the U.S. Department of Agriculture Pesticide Data Program’s between 1999 and 2015 found the insecticides in both domestic and imported commodities.145 The study found that neonicotinoids were detected in commonly consumed fruits and vegetables, and trends indicated an increase in use of acetamiprid, clothianidin, and thiamethoxam. Imidacloprid was the most commonly detected neonicotinoid with an overall detection frequency of 12%. The overall detection frequency for neonicotinoids was 5%. Higher detection frequencies were observed for specific foods: cherries (46%), apples (30%), pears (24%), and strawberries (21%) for acetamiprid; and cauliflower (58%), lettuce (46%), spinach (39%), kale (31%), potatoes (31%), cilantro (31%), grapes (29%), cherries (26%), collard greens (25%), and celery (21%) for imidacloprid.145 In the Pesticide Data Program’s 2015 report, clothianidin was detected in 31% of spinach, 23% of potatoes, and 10% of tomato samples.38, 186 Thiamethoxam was detected in 30% of cherries, 22% of lettuce, and 14% of watermelons. Imidacloprid was detected in 46% of potatoes, 43% of spinach, and 35% of cherries.38, 186
Two cross-sectional studies (the U.S. Congressional Cafeteria Study and Hangzhou China Study) provide further evidence that neonicotinoids have become ubiquitous in the global food supply.184 The results showed that most of the tested fruits and vegetables contained neonicotinoids. The United States study found at least one neonicotinoid in 79% of fruits and 65% of vegetables, and 3 or more in 62% and 38% of fruits and vegetables, respectively. In comparison, the Hangzhou Study detected one or more neonicotinoid in 57% of fruits and 63% of vegetables, and three or more in 30% and 42% of commodities. Thiamethoxam (U.S.-53%; China-51%) and imidacloprid (U.S.-52%; China-66%) were the most frequently detected neonicotinoids in both studies. A 2011 survey conducted by the Japanese Ministry of Agriculture, Forestry, and Fisheries found dinotefuran to be the most frequently detected neonicotinoid in fruits and vegetables with a frequency rate of 63.3%.187 In Turkey, acetamiprid, imidacloprid, and thiamethoxam have been detected in 71%, 36%, and 7%, respectively, of fruit, and 83%, 67%, and 33%, respectively of vegetables. 176
Neonicotinoid residues may be reduced, but not eliminated through processing and washing. One study examined processed foods (tomato products) in addition to raw fruits, reporting that food processing altered the concentration of neonicotinoid residuals.171 This research also reported that the concentrations of imidacloprid were significantly lower (i.e., 7–30%) on washed tomatoes, indicating that at least some portion of neonicotinoids can be mechanically removed. The intake of imidacloprid through direct ingestion of tomatoes has been found to vary between 10−2 and 10−6 (kgingested/kgapplied) depending on whether the tomatoes were unwashed, washed, or washed and peeled.188
Neonicotinoids have also been frequently detected in honey.38, 189–197 A global survey found five neonicotinoids present in honey190 with 75% of all honey samples testing positive for at least one neonicotinoid.190 North America, Asia, and Europe had the greatest proportion of detections.190 Results from eight studies report that the detection of individual neonicotinoids ranged from non-detect to as high as 192.8 μg/kg (clothianidin).38, 191, 192 192–197 Metabolites such as imidacloprid-olefin (5.6 ng/g) and 5-hydroxy imidacloprid (21.1 ng/g) have also been detected, indicating that an increased emphasis is warranted on metabolites in terms of potential health risks and exposure through food.197
Despite the frequency of detection, estimates of daily intake indicate consumption typically does not exceed established tolerance levels.38, 145, 174, 183, 184, 198, 199 Maximum residue levels have been established in several countries for tea, grains, fruits, vegetables, dairy, and meat. Values range from 10 to 600,000 μg/kg.31, 45, 200–209 In Japan, it is estimated the average adult consumes from 206 to 1,050 μg/day of individual neonicotinoids (reviewed in 198). Harada et al. estimated that the average daily intake of neonicotinoids amongst Japanese adults was between 0.53 and 3.66 μg/day, with a high of 64.5 μg/day for dinotefuran.198 This peak value was <1% of the acceptable daily intake set by the Japanese government.198 Studies have estimated the total daily dietary intake of neonicotinoids in the United States and China to be 10.1 and 37.9 μg/day, respectively.184 These concentrations are below the acceptable daily intake levels established by the World Health Organization.210 These levels indicate that between 10 and 200 μg per kilogram of body weight of various neonicotinoids can be consumed daily for a lifetime without appreciable risk to health (Table S-2).210
The EPA estimates that exposure to neonicotinoids through acute and chronic dietary exposure is generally not a health concern for the U.S. population. Risk estimates ranged from 2% to 38% of the acceptable daily intake overall for the general population.28–32 Children 1–2 years old are considered the most vulnerable, with risk estimates ranging from 8% to 93% for acute and 6% to 52% for chronic population adjusted dose. The EPA considers exposure a concern when risk estimates exceed 100% of the reference dose.28–32 Similarly, a Chinese study assessed the cumulative risk of total neonicotinoids from fruit and vegetable intake school-aged children between 8 and 12 years old.199 Although the study detected at least one neonicotinoid in each of the fruits and vegetables tested, the acceptable daily intake was found to be below the chronic reference doses established by the EPA and the Chinese government.199
4.4. Knowledge Gaps and Research Needs
Neonicotinoids are known to occur in numerous environmental compartments (e.g., soil, water, food) but to date there has been little emphasis on how these residues relate to human exposure. More information is needed on the exposure of neonicotinoids in matrices directly consumed by humans. This includes more information on the occurrence of neonicotinoids in drinking water sources and potential removal of neonicotinoids during drinking water treatment. Drinking water data are especially needed for rural areas with high-intensity agriculture where neonicotinoid use is particularly prevalent. While current food resides indicate daily consumption rates of neonicotinoids are well below daily intake levels, the addition of metabolites to the analysis of water and food would provide a more comprehensive exposure of humans to neonicotinoids.
5. Transformation Pathways
5.1 Transformation Products of Altered Toxicity
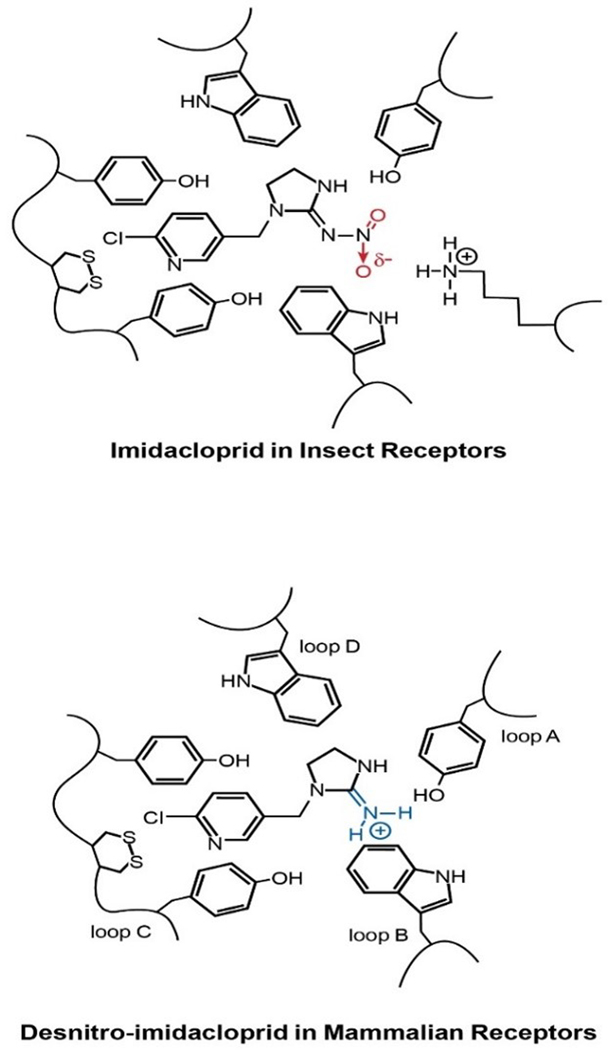
Understanding the environmentally relevant transformation products and pathways of neonicotinoids is critical to characterizing potential health risks. Some transformation products are biologically active, sometimes even increasing toxicity to either insects or vertebrate organisms.211 In most cases, altered bioactivity is due to transformation of the electron-rich nitro- or cyano- functional groups (pharmacophore) on the parent neonicotinoid. The nitro group can transform to the desnitro guanidine metabolite, then to the urea metabolite. The desnitro form is substantially more toxic to vertebrates due to positive charge distribution at the guanidine that favorably interacts with the mammalian nAChRs.212 The cyano functional group appears more stable than the nitro group, with more limited reports of transformation.213, 214 Major structural alteration of metabolites likely decreases toxicity to vertebrates. Exceptions to nitro- or cyano-transformation are the formation of imidacloprid olefin, which is significantly more insecticidal215 and the demethylation of acetamiprid, which results in a less toxic product.216
Several reviews46, 94 have summarized physiochemical characteristics of neonicotinoids, reporting degradation and persistence in soil and water matrices, and degradation by abiotic and microbial transformations, as well as plant uptake and sorption to/leaching from soils. Current knowledge is summarized here.
5.2 Abiotic Transformation Mechanisms in the Environment
5.2.1 Photolysis.
Photolysis studies under environmentally relevant conditions are limited. Nitro-based neonicotinoids thiamethoxam, clothianidin, and imidacloprid can undergo direct photodegradation. The cyano-neonicotinoids acetamiprid and thiacloprid are largely stable in sunlight. Although laboratory reports of direct photolysis half-lives can be on the order of minutes to hours,217,218 the authors acknowledge that these rates are only applicable under ideal, near-surface conditions; environmental rates are presumed much lower and help explain neonicotinoid persistence.218 A laboratory study with simulated natural light (> 290 nm) estimated neonicotinoid photolysis rate constants and half-lives for direct photolysis during different seasons.217 Acetamiprid can undergo indirect photolysis with hydroxyl radical, but is not expected to contribute significantly to degradation in the environment.218 Multiple photoproducts were observed, with limited information on their structures.
Imidacloprid photo transformation on thin films revealed transformation to desnitro-imidacloprid (16%) and imidacloprid urea (84%), indicating a mechanism involving photodissociation of the nitro group.219 Another study220 reported the main products of imidacloprid following irradiation at 290 nm were221 6-chloronicotinaldehyde, N-methulnicotinacidamide, 1-(6-chloronicotinyl) imidazolidone and 6-chloro-3-pyridyl-methylethylendiamine.
Maximum absorbance of clothianidin222 occurs at about 267 nm (t1/2= 3.6 h), yielding eight major products via multiple mechanisms (e.g., denitration, nucleophilic substitution, ring opening/closure), including several stable products. Advanced oxidation processes yielded half-lives between 5 hours and 19 days for imidacloprid, thiacloprid, and acetamiprid.223
5.2.2. Hydrolysis and Chlorination.
Under most environmentally relevant conditions and time scales, the only neonicotinoid to meaningfully transform via hydrolysis is thiamethoxam.118 In experiments up to 150 days, other neonicotinoids representing diverse structural groups (i.e., nitenpyram, imidacloprid, acetamiprid, clothianidin) underwent hydrolysis only at pH 10 conditions (not pH 4, 6, 7, 8) and in all cases appears base-catalyzed, forming urea hydrolysis products.218 A thiamethoxam carbonyl product under alkaline pH soil conditions has been reported,224 (t1/2= 11–26 days). Indeed, elevated pH conditions can be relevant to human exposure through some drinking water treatment processes. In the lime softening basin of a drinking water treatment plant in Iowa, base-catalyzed hydrolysis occurred.118 Batch tests using the softening revealed a t1/2 of 0.75 day (0.9 d-1).118 Another study225 determined thiamethoxam was largely stable at neutral pH (t1/2 =29.2 days) as compared to acidic (t1/2 =13.9 days) or alkaline (t1/2 =2.1 days) conditions. Hydrolysis reaction rates changed with temperature but were similar at environmentally relevant conditions.226 Base-catalyzed hydrolysis of thiamethoxam yields two products (Figure 3).146 Notably, thiamethoxam-H 237 is reactive toward chlorine; chemical disinfection typically occurs subsequent to softening. At a chlorine residual of 5 mg/L as Cl2, the half-life of thiamethoxam-H 237 was 4.8 hours, yielding a single species referred to as clothianidin-thiamethoxam-H 270. Neither thiamethoxam nor thiamethoxam-H 248 reacted with chlorine. The proposed products of hydrolysis146 are shown in Figure 3. Clothianidin, imidacloprid, and two imidacloprid metabolites (desnitro-imidacloprid and imidacloprid-urea), also react during chlorination,146 indicating the potential for formation of novel disinfection by-products from neonicotinoids during treatment and distribution (Figure 4).
Figure 3:
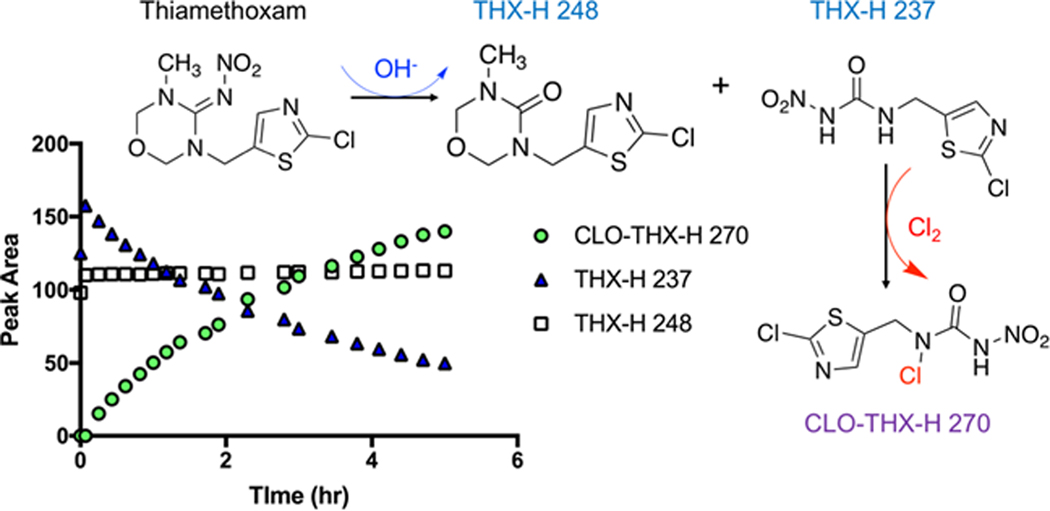
Chlorination of the hydrolysis products of thiamethoxam (THX-H 237 and THX-H 248) to form novel chlorinated product CLO-THX-H 270. Figure 3 taken with permission from Klarich-Wong, et al., Environmental Science & Technology Letters, 2019. 6(2): p. 98–105.146 Copyright 2019 American Chemical Society.
Figure 4:

Chlorination of desnitro-imidacloprid and imidacloprid-urea to form chlorinated products desnitro-IMI 245, desnitro-IMI 279, and IMI-urea 246.
Figure 4 taken with permission from Klarich-Wong, et al., Environmental Science & Technology Letters, 2019. 6(2): p. 98–105.146 Copyright 2019 American Chemical Society.
5.3 Biotransformation
5.3.1 Microbial Processes
Biotransformation is often implicated in neonicotinoid loss in soil degradation studies, even when not specifically examined.227 Microbial transformation of neonicotinoids generates a suite of metabolites. Nitro-based neonicotinoids most commonly used (e.g., imidacloprid, clothianidin, thiamethoxam) have been best studied in terms of microbial metabolites. For example, isolated soil bacteria were capable of degrading imidacloprid and thiamethoxam when not the sole carbon source.228,229 Both neonicotinoids transformed to nitrosoguanidine (=N-NO), desnitro (=NH), and urea (=O) metabolites (Figure 5) in a pathway similar to the metabolization of neonictinoids by the liver in mammals.228 Soil microbes also transformed imidacloprid to 5-hydoxyl imidacloprid and imidacloprid olefin; the latter is significantly more insecticidal than imidacloprid.215 Transformation of imidacloprid to 5-hydroxyl imidacloprid occurred with cultures enriched with imidacloprid as the sole nitrogen source, but not sole carbon source; addition of sucrose promoted hydroxylation.215
Figure 5:
Main transformation of the nitro-functional group neonicotinoids from the electronegative functional group to the positive guanidine (blue) metabolites of enhanced vertebrate toxicity. Image created by LeFevre; Data and information adapted from Dai et al., Appl. Microbiol. Biotechnol., 2006, 71, 927–934215; Pandey et al., Biochem. Biophys. Res. Commun., 2009, 380, 710–714.228; and Zhou et al., Appl. Microbiol. Biotechnol., 2013, 97, 4065–4074.229
Microbial transformation of cyano-based neonicotinoids results in a different suite of metabolites, mainly the result of demethylation but also formation of amide products. Activated sludge bacteria were capable of dechlorinating and demethylating acetamiprid as the sole carbon source (>99% in 3 days).230
Bacteria degraded acetamiprid via an N-deacetylation reaction that removes the electronegative functional group.231,232 N-demethylation also occurs,233 with the metabolite retaining the cyano functional group, but with lowered insecticidal activity.
Thiacloprid and acetamiprid undergo similar transformations at the cyano group (Figure 6). Microbially facilitated hydrolysis of thiacloprid (via nitrile hydratase) to thiacloprid amide represents 98% of the final product214, 234 and is presumed the main sink in soils.213 Similar to thiacloprid, acetamiprid is transformed via nitrile hydratase to a N-carbamoylimine derivative, impacting the cyano group.235 This metabolite also had significantly lower insecticidal effects and was further degraded to acetamiprid-NH and acetamiprid-NH2 products.
Figure 6:
Microbial transformation of neonicotinoids containing a cyano functional group. The electronegative cyano pharmacophore can be transformed via nitrile hydratase enzymes to amide metabolites, with subsequent decomposition to guanidine metabolites. Various products are also formed via the demethylation pathway. Image created by LeFevre; Data and information adapted from Chen et al., Biodegradation, 2008, 19, 651–658.233; Dai et al., J. Agric. Food Chem., 2010, 58, 2419–2425.213; Tang et al., Process Biochem., 2012, 47, 1820–1825.232; Wang et al., Bioresour. Technol., 2013, 138, 359–368.231; Yang et al., Int. Biodeterior. Biodegrad. 85, 2013, 95–102.230; Zhang et al., J. Agric. Food Chem., 2012, 60, 153–159.214; and Zhou et al., Int. Biodeterior. Biodegrad., 2014, 93, 10–17.235
Neonicotinoids can alter microbial communities when applied at high levels. For example, imidacloprid treatment altered soil respiration and soil biomass carbon content, the total number/diversity of bacteria, and enzyme activities, with nitrifying and nitrogen fixing bacteria being the most sensitive.236, 237,238 Over application of neonicotinoids has also been linked to alteration of the biocatalytic capacity of soils, including loss of nitrite and nitrate reductase enzyme activity.239
5.3.2 Fungal Transformation
There is limited specific information on fungal transformation of neonicotinoids. Fungal biotransformation differs from microbial processes due to the presence of both extra and intracellular enzymes. Extra-cellular enzymes do not degrade neonicotinoids; rather, cytochrome P450 enzymes are involved.216,240 One study focused on clothianidin transformation by the white-rot fungi Phanerochaete sordida240 reported 37% degraded in 20 days in a liquid culture incubation. The metabolite N-(2-chlorothiazol-5-yl-methyl)-N′-methylurea was identified and determined to no longer be neurotoxic to mouse neuroblastoma cells. This product represents the same major transformation pathway as described for bacteria (Figure 5).
Acetamiprid216 was transformed via a demethylation reaction, yielding in the same metabolite shown in Figure 6. This product was detected in honeybees, mice, spinach, and soil bacteria,241–244 and has a 10-fold lower insecticidal activity than the parent compound.233
5.3.3 Plant Processes
Because neonicotinoids are designed to be applied to plants and act systemically, a substantial amount of research has focused on plant uptake and translocation for insecticidal effects and is outside the scope of this review.5, 245 Much less work has focused on the transformation of neonicotinoids by plants to yield novel products of ecological or human exposure significance. One such study246 examined in substantial detail the plant transformation products and pathways of a suite of neonicotinoids in spinach plants, comparing the products to metabolites discovered in mice. Metabolic reactions occurred including nitro reduction, cyano hydrolysis, demethylation, sulfoxidation, hydroxylation and olefin formation, hydroxylation and ring opening, and dechlorination. The phase I plant metabolites were similar to those observed in other transformation processes, but phase II plant metabolites were different, and included N-glucosides and amino acid conjugates. Some plant metabolites remain metabolically active for vertebrates (e.g., desnitro- and decynanoguanidines and olefins) and insects. Specifically, in spinach,243 the =N-NO2 group was reduced to =N-NO and =N-NH2.
Neonicotinoids also affect plants. Beyond the insecticidal mode of action, imidacloprid and clothianidin increase plant vigor against abiotic stress by inducing salicylic acid-associated responses via their in-planta metabolites.247 Neonicotinoids can alter the plant hormone systems, as demonstrated by geno and phenotypical evidence.
The microbial community associated with plants may facilitate neonicotinoid transformation. Duckweed Lemna and its microbial community synergistically transformed nitro-based imidacloprid and cyano-based thiacloprid, but neither the duckweed nor bacteria from the duckweed degraded the neonicotinoids on their own.248 Formation of metabolites with increased toxicity was very small or absent, indicating duckweed-microbial transformation likely decreased overall aquatic toxicity.248
5.4 Mammalian Species
In mammals, neonicotinoids are metabolized by enzymes in the liver249, 250 (e.g., cytochrome P450 enzymes, aldehyde oxidase), as well as anywhere these enzymes are expressed250–252 (i.e., including red blood cells, plasma, lung, skin, brain, kidney, spleen, endocrine tissue, adipose tissue, placenta, testis, and ovaries). Metabolism of neonicotinoids in vertebrates occurs via several reactions: reduction, demethylation, hydroxylation, and olefin formation.8, 211, 243, 253 Imidacloprid, nitenpyram, thiacloprid, and acetamiprid share a chloropyridinyl chemical structure that degrades into 6-chloronicotinic acid. Thiamethoxam and clothianidin both have a chlorothiazole structure that is metabolized into 2-chloro-1,3-thiazole-5-carboxylic acid. Dinotefuran is metabolized into 3-furoic acid. Each of these metabolites are then conjugated with either glycine or glucuronic acid and excreted in the urine.49, 242 These metabolites have been observed in both mouse and human urine. 49, 187, 198, 242, 253–258
Imidacloprid and thiamethoxam are metabolized to produce nitrosoguanidine metabolites and later to less toxic urea metabolites. As in bacterial transformation, intermediate formation of desnitro/guanidine intermediates (i.e., desnitro-imidacloprid) occurs. This metabolite is also formed in metabolism studies with human liver microsomes.259–261 Similar metabolism occurs in other vertebrates, including mice, rats, goats, and hens.49, 228
Neonicotinoids and their metabolites are primarily excreted in the urine due to their low molecular weights and water solubility.250 Pharmacokinetic models for acetamiprid, clothianidin, dinotefuran, and imidacloprid describe fates in urine.198 In this study, 12 healthy adults orally ingested micro doses of deuterated neonicotinoids. Increased urinary concentrations of the labelled neonicotinoids followed dosing, with 64% of the clothianidin and 93% of dinotefuran recovered unchanged in 3 days. In comparison, imidacloprid and acetamiprid were readily metabolized with 13% of imidacloprid and 3% of acetamiprid recovered unchanged. Acetamiprid, mainly (31%) transformed to desmethyl-acetamiprid, which was eliminated more slowly.198. Imidacloprid, clothianidin, and dinotefuran are also excreted in urine with short biological half-lives in animal studies.49, 242 Indeed, 46% of nitenpyram, 27% of thiamethoxam, and 1% of thiacloprid were recovered unchanged in mice urine within 24 hours.49, 242 Thiacloprid was converted to 6-chloropyridine carboxylic acid (12%) and thiamethoxam was converted into clothianidin (11%).49, 242
5.5 Knowledge Gaps and Research Needs
Bonmatin et al. specifically commented on the lack of information regarding the concentrations and dynamics of neonicotinoid degradation products and metabolites46, highlighting this as a research need. This distinct paucity could have profound effects on human and ecosystem health. For example, neonicotinoid metabolites were substantially more reactive towards chlorine than parent compounds,146 highly relevant to human exposure during drinking water disinfection. Neonicotinoid metabolite toxicity profiles for vertebrates is also distinct from parent pesticides as a result of slight structural alteration to the insecticidal pharmacophore. Additional research and monitoring (including availability of commercial standards) are needed to elucidate novel neonicotinoid metabolites / transformation products in the environment, as well as measure levels in media relevant to human exposure (i.e., plants for food, drinking water).
6. Toxicity in Mammals
The ecotoxicology of neonicotinoids has been reviewed previously. For example, imidacloprid and clothianidin exerted sub-lethal effects in vertebrate wildlife—mammals, birds, fish, amphibians, and reptiles.262 The effects observed included genotoxicity, cytotoxicity, impaired immune function, reduced growth and reproductive success at concentrations well below those expected to cause mortality.262, 263 A comparison of the toxicology data in rats, birds, and fish shows that most of the neonicotinoids have a moderate to high acute oral LD5017 Comparable insecticides like chlorpyrifos, diazinon, and malathion (organophosphates) and carbofuran and aldicarb (carbamates) typically have higher acute toxicity in mammals and birds compared to neonicotinoids.17 Organophosphates are typically rated as having a high potential to bioconcentrate in tissue, like fat, whereas neonicotinoids are believed to have a low potential to concentrate in tissue.264–266 The following section briefly describes proprietary and peer-reviewed toxicity studies in mammalian models. A brief summary of toxicity data for neonicotinoids is presented in Table 3.
Table 3:
Summary of toxicity data for neonicotinoids
| Health Issue | Acetamiprid | Clothianidin | Dinotefuran | Imidacloprid | Nitenpyram | Thiacloprid | Thiamethoxam |
|---|---|---|---|---|---|---|---|
| Carcinogen | No | No | No | No | No Data | Possible | No |
| Genotoxic | |||||||
| Chromosome Aberration | No Data | No Data | No Data | Negative | No Data | Negative | Negative |
| DNA Damage/Repair | No Data | No Data | No Data | Negative | No Data | No Data | Negative |
| Gene Mutation | No Data | No Data | No Data | Negative | No Data | No Data | Negative |
| Genome Mutation | No Data | No Data | No Data | Negative | No Data | No Data | No Data |
| Unspecified Genotoxicity | Negative | Negative | No Data | Mixed | No Data | Negative | No Data |
|
| |||||||
| Endocrine Disruption | No Data | Possible | No Data | No Data | No Data | Yes | No |
|
| |||||||
| Reproduction/ Development Effects | No | Possible | Possible | Yes | No | Yes | No |
|
| |||||||
| Cholinesterase Inhibition | No | No | No | No | No Data | No | No |
|
| |||||||
| Neurotoxicant | No | Yes | No | Possible | No Data | Yes | No |
|
| |||||||
| Respiratory Tract Irritant | No | No | No Data | No | Yes | No | No |
|
| |||||||
| Skin Irritant | Yes | No | Possible | Possible | No | Yes | Possible |
|
| |||||||
| Skin Sensitizer | No Data | No Data | No Data | No Data | No Data | No | No Data |
|
| |||||||
| Eye Irritant | Possible | No | Yes | Possible | Yes | Yes | No |
|
| |||||||
| Phototoxicant | No Data | No Data | No Data | No Data | No Data | No | No Data |
|
| |||||||
| General Health Comments | N/A | Effects consistent with endocrine disruption noted in rodents / dogs. May cause low blood pressure, hypothermia, and impaired pupillary function |
N/A | Moderately toxic. Potential liver, kidney, thyroid, heart and spleen toxicant | N/A | Possible liver and thyroid toxicant. Probable human carcinogen | Increased incidence of liver cell adenoma and adenocarcinoma in mice |
Yes = Known to cause a problem; No = Known to not cause a problem; Possible = Status has not been identified
Table adapted from Kathleen A. Lewis, et al., Human and Ecological Risk Assessment, 2016. 22(4): p. 1050–1064.17
6.1. Oxidative Stress
Exposure to neonicotinoids frequently causes oxidative stress, as shown by increased lipid peroxidation, decreased glutathione levels and altered activity of key antioxidant enzymes (e.g., catalase, superoxide dismutase, and glutathione peroxidase).267–272 Imidacloprid exposure increases both nitric oxide production and transcript levels of nitric oxide synthases in the brain and liver of female rats.270 Similarly, thiacloprid, increases nitric oxide levels in polymorphonuclear leukocytes and the plasma of thiacloprid-exposed rats.271, 273 Antioxidants, such as curcumin and vitamin C, can protect tissues from neonicotinoid-induced oxidative damage.269, 272 Based on the available evidence, oxidative stress caused by exposure to neonicotinoid pesticides has been proposed to play an important role in their toxicity in non-target species.274
6.2 Reproductive Toxicity
Multiple studies report adverse reproductive and developmental effects in mammals exposed to neonicotinoids, including higher rates of embryo death, premature birth, reduced pregnancy rate, reduced sperm production and function, reduced weight of offspring, and stillbirth.35, 262, 275, 276 Experimental evidence indicates that neonicotinoids affect the fertilization rate in rodent models.275, 277, 278 A study investigating in vitro fertilization reported that direct exposure to acetamiprid or imidacloprid adversely affect the fertilization ability of mouse spermatozoa.275 Moreover, exposure of male rats to imidacloprid significantly decreased serum levels of testosterone.278 Similarly, exposure to imidacloprid at no observed adverse effect level (NOAEL) dose-levels, causes suppression of testicular function in adult male rats.279 Clothianidin exposure in male rats significantly increased levels of thiobarbituric acid-reactive substances, cholesterol, and palmitic, linoleic and arachidonic acids in testis, but did not cause sperm DNA fragmentation.280 Exposure to acetamiprid in 3-week-old mice for 180 days led to a decrease in body weight, mildly affected spermatogenesis, and decreased expression of testosterone-metabolism genes, nAChR subunit genes, and proliferation-associated genes in the testes.281 In contrast, imidacloprid is not a primary embryotoxicant, is not teratogenic, and does not affect reproduction or development following exposure via the maternal diet.66, 273
6.3 Hepatotoxicity
Hepatotoxicity from a neonicotinoid exposure is typically apparent only at high doses and frequently accompanied by a decrease in food consumption and a reduced bodyweight. Serum activities of alanine aminotransferase, alkaline phosphatase, and aspartate aminotransferase, markers of hepatotoxicity, are typically elevated in rats exposed to imidacloprid.66, 269, 282 A study examined liver histological changes and enzyme activity in female albino rats exposed to a high (1/10th LD50) and a low (1/50th LD50) dose of imidacloprid for 4 weeks.283 Histological alterations involving a rise in liver enzymes, degeneration of hepatocytes, and dilations of the central vein were observed in the high dose group.283 Dietary exposure to imidacloprid also reduced cholesterol levels in serum in rats66 and caused fatty degeneration in the liver of female BALB/c mice.284 Antioxidants, such as curcumin, can protect animals from liver injury caused by neonicotinoids such as imidacloprid.269 Liver pathology is fully reversible in rats allowed to recover after exposure to imidacloprid.66 Similar to imidacloprid, chronic thiamethoxam exposure causes liver injury in mice, as indicated by elevated serum activities of AST and ALT.285 In contrast, exposures to thiacloprid results in a significant decrease in AST and ALT levels in serum compared to control rats.271
6.4 Genotoxicity and Carcinogenicity
Neonicotinoids are not mutagenic based on in vitro and in vivo studies performed as part of the registration process.66 The Ames test generally reveals that neonicotinoids are not mutagenic;66, 286 however, a imidacloprid pesticide formulation induces G-C base pair mutations.287 Neonicotinoids, tested either as pure insecticides or as the corresponding formulations, cause dose dependent DNA damage in human peripheral blood lymphocytes (PBLs)288, 289, human lymphocytes290, and HepG2286 cells, as assessed using the comet assay. Neonicotinoids also have a significant effect in the micronucleus assay in bone-marrow cells287, 291, 292, human lymphocytes289, 293, and HepG2 cells286, whereas no induction of micronuclei was observed in other studies using polychromatic erythrocytes for imidacloprid-exposed mice294 and human lymphocytes.291 Some, but not all studies291 report that neonicotinoids significantly induced chromosome aberrations 287, 290, 292, 293, 295, 296 and sister chromatide exchanges.293, 295 Commercial formulations with neonicotinoids as active ingredients cause DNA damage in human peripheral blood lymphocytes in vitro.288
Carcinogenicity studies with imidacloprid66 and clothianidin273 showed no carcinogenic effects of exposure in rodents. In contrast, dietary exposure to thiamethoxam increased the incidence of liver tumors in mice285, 297, but not in rats.297, 298 The mode of action that results in tumor formation in mice is not relevant to rats and humans, indicating that thiamethoxam does not pose a cancer risk in humans.297 Based upon available research, acetamiprid, clothianidin, dinotefuran, imidacloprid, and thiamethoxam are classified as “Not Likely to be Carcinogenic to Humans” by the EPA.28–32
6.5 Neurotoxicity
In acute neurotoxicity studies performed according to EPA guidelines, no persistent or delayed neurotoxic effects were observed.66, 299 Tremors occurred in mice exposed to neonicotinoids.59 The results from subchronic studies performed following EPA guidelines indicate that sustained dietary exposure to neonicotinoids does not produce the principal toxicities observed in acute studies and causes little-to-no neurotoxicity. These findings are consistent with the rapid elimination of neonicotinoids in rats67–69, their poor penetration across the blood-brain barrier,60, 66 and their poor affinity for non-insect nAChRs.58–64 Some studies, however, indicate that imidacloprid and thiamethoxam affect motor activity in rats.48, 269, 300 Thiamethoxam appears to cause an anxiogenic effect in rats48 and clothianidin elevates anxiety-like behavior in mice.301
A critical review of in vitro, in vivo, and epidemiology studies by Sheets et al. in 2016302 found systemic toxicity was common at high doses; however, no developmental neurotoxicity was observed consistent with the effects of nicotine exposure. A few studies indicate that exposure to neonicotinoids may cause developmental neurotoxicity in rodents. Gestational exposure to imidacloprid results in sensorimotor deficits in the offspring.35 Developmental exposure to clothianidin adversely affected neurobehavioral parameters in mice 273 and cognitive function in rats.303 Low-concentrations (> 1μM) of acetamiprid and imidacloprid exerted similar excitatory effects on neonatal rat cerebellar neurons.304 Other studies in rats observed decreased sensorimotor performance following imidacloprid exposure, increased dopamine release following thiamethoxam and clothianidin administration, and altered behavioral and biochemical processes related to the rat cholinergic systems following thiamethoxam exposure.35, 48, 305 Because these studies did not follow the recommendations for rigor and reproducibility outlined by Landis et al.306, their findings need to be interpreted with caution.
6.6 Endocrine Effects
In vitro testing has characterized the effects of neonicotinoids on endocrine disruption.307 Thiacloprid, thiamethoxam, and imidacloprid affect aromatase (CYP19) activity in a model of fetoplacental steroidogenesis. Increases in estrone and estradiol production were observed, while estriol production was inhibited. Estrogens are important during pregnancy, and disruption of the biosynthesis of estrogens may affect the fetus along with potential effects to the mother’s health. Evidence indicates that neonicotinoids may be metabolized by CYP3A7, which affects the conversion of dehydroepiandrosterone sulfate into estriol and this may be the reason for the observed decrease in estriol production. Other studies have also demonstrated that thiacloprid and imidacloprid may affect aromatase activity through promoters for aromatase, which may lead to excess estrogen production in breast tissue.308–310 This finding is of concern because an increased estrogen production in tumors has been shown to promote cancer cell growth, with aromatase being a key regulator of this process.311, 312
6.7 Metabolite Toxicity
Neonicotinoid metabolites can be more toxic than the parent compounds.253, 313, 314 In particular, metabolites formed by the removal of the nitro- or cyano-functional groups are potentially more selective260 and bind more strongly with mammalian nAChRs.246 For example, desnitro-imidacloprid has an affinity for mammalian nAChRs that is comparable to nicotine.19, 22, 59, 261 The selectivity ratio for insects compared to vertebrates changes from 565 to 0.005 when the neonicotinoid is metabolized to the desnitro metabolite.3, 18 Studies with mice found that imidacloprid and desnitro-imidacloprid activate the extracellular signal-regulated kinase135 cascade via the nicotinic receptor and induce the mobilization of intracellular calcium in rat PC12h cells similar to nicotine.261, 315 Desnitro-imidacloprid, a metabolite of imidacloprid, and a descyano and an olefin derivative of thiacloprid up-regulated α4β2 nAChR sites at EC50s of 870 nM; 500 nM and 22 nM respectively. In comparison, nicotine up-regulated α4β;2 nAChR at EC50s of 760 nM and imidacloprid and thiacloprid at EC50s of 70,000 and 19,000 nM.22
6.8. Knowledge Gaps and Research Needs
A considerable body of evidence, most as proprietary studies performed for regulatory purposes, indicate that neonicotinoid insecticides are safer than other insecticides currently on the market. In contrast, the peer-reviewed literature provides conflicting results with regards to toxic endpoints affected by neonicotinoid exposure. Several factors likely contribute to the conflicting findings, including different model systems, use of pure neonicotinoids vs. technical formulations, high concentrations or doses that do not reflect current environmental exposure levels, and lack of scientific rigor. The mammalian toxicity of neonicotinoids, especially at environmentally relevant concentrations, is most likely not mediated by nAChRs. While adverse outcome pathways (AOPs) have been proposed for honeybees,316 AOPs for the mammalian toxicity of neonicotinoids and their bio-transformation products have not been established to date. Based on the evidence summarized above, there remains a need to further characterize the biological plausibility of adverse outcomes associated with environmental neonicotinoid exposures using robust, reproducible, and transparent studies.
7. Human Exposure and Health
In humans, documented adverse health effects from neonicotinoid exposure have generally been considered limited.18 However, rigorous scientific studies examining the risk are lacking, demonstrating the need for additional human health-based research.26 In 2013, the EFSA Panel on Plant Protection Products and their Residues stated that there is good evidence that two neonicotinoids, acetamiprid and imidacloprid, can damage the developing human nervous system, including the brain, and exhibit harmful effects similar to those caused by nicotine.27, 72 The insecticides may alter development of neurons and brain structures associated with functions such as learning and memory.27, 72 An important aspect of the PMRA and the EPA re-evaluation is the potential for effects in non-target organisms.13, 33
7.1 Usage Risks
Neonicotinoids are generally considered safe for public and occupational uses, with minimal human health risks due to dermal, inhalation, and oral exposure routes. Draft human health risk assessment published by the EPA in 2017 for acetamiprid, clothianidin, dinotefuran, imidacloprid, and thiamethoxam indicated that exposure occurs in both residential and occupational settings.28–32, 144 Overall the assessment findings indicate that the human exposure risks associated with neonicotinoid use were limited.
One way to evaluate the human risk of exposure to neonicotinoids is to evaluate the margin of exposure, which is a ratio of its NOAEL to its theoretical, predicted, or estimated dose or concentration of human intake. The margins-of-exposure (MOE) for residential scenarios were generally greater than the EPA’s level of concern. MOEs for the assessed scenarios ranged between 42 and 80,000,000,000.28–32, 144 A MOE greater or equal to 100, the level of concern, is considered protective of human health. The only residential scenario that found an estimate of concern below the MOE of 100 focused on children aged 1–2 years old exposed through a combined dermal and incidental oral routes to pets treated with pet collars containing imidacloprid.31 The MOE for this scenario ranged from 42 –110 and assumed that pet collars contained a 1% liquid / 99% dust formulation for small and large dogs and cats. Children 1–2 years old exposed to indoor bed bug treatments and pet spot-on treatments with acetamiprid had margins of exposure of 120 and 110, respectively.28 Post application non-occupational exposure to imidacloprid used for controlling burrowing shrimp in Washington State had the highest margins of exposure. Estimates ranged from 3,300 (dermal) to 80,000,000,000 (inhalation) for children and adult exposed through these routes.31 None of the examined scenarios for thiamethoxam, dinotefuran, or clothianidin resulted in risk estimates of concern.29, 30, 32 Residential exposure scenarios were generally assumed to be short-term, with the exception of pet products, which due to their preventative nature are used longer term and presented longer-term potential exposure risks.31
The EPA evaluated several occupational scenarios, as part of their risk assessment, including direct mixers, loaders, applicators, and other handlers for neonicotinoid exposure.28–32, 144 Most of these risk assessment scenarios assumed handlers used a baseline layer of protection including long-sleeved shirt, long pants, shoes plus socks, no protective gloves, and no respirator. Most of the combined dermal and inhalation risk estimates were not of concern, using baseline clothing, with total margins of exposure between 2 to 1,500,000. The highest MOEs were typically found for clothianidin, imidacloprid, and thiamethoxam in on-farm seed treatment and planting exposures (2 – 350).29, 31, 32 Mixing, loading, applying dry flowable or wettable powders and liquid formulations using manual and pressurized handguns also had elevated MOEs for acetamiprid, imidacloprid, and thiamethoxam (3.8 – 3,000).28, 31, 32 The highest reported MOE of 1,500,000 was for mixing or loading acetamiprid tree injections.28 With additional personal protective equipment, such as double layer of clothing and/or a respirator (PF5 or PF10), most of these exposure scenarios reach acceptable MOEs greater than 100. However, this extra protection was still insufficient in some scenarios where thiamethoxam and acetamiprid exposures occurred through seed treatments, planting, and dry flowable and wettable powders with handguns and backpacks.28, 32 Dinotefuran was not assessed for occupational handler exposures because no dermal or inhalation hazards were identified.30
7.2 Biomonitoring
Although it is unknown what the dominant routes of exposure to neonicotinoids are for humans, exposure via consumption (residue or in plant tissue), drinking water, or aerosols/dust associated with application are the most cited modes of exposure.38, 184, 187, 317–319 Over the past decade, several studies have been conducted to assess human exposure to neonicotinoids and their metabolites.187, 198, 250, 254, 258, 318–333 These studies indicate that neonicotinoid exposure is commonplace.
Studies indicate that neonicotinoids exposure may vary by geographic region and age. For example, in Japan, several studies assessed exposure to neonicotinoids. In 2013, low concentrations of 6-chloronicotinic acid, 2-chloro-1,3-thiazole-5-carboxylic acid, and 3-furoic acid were detected in urine from 10 farmers in Japan.258 In 2016, neonicotinoid exposure was reported to be common amongst a cohort of 223 children aged 3 years old.318 Urine samples collected during two seasons, summer and winter, showed detection rates of 58% for dinotefuran, 25% for thiamethoxam, 21% for nitenpyram, and <16% for other neonicotinoids (acetamiprid, clothianidin, imidacloprid, thiacloprid, and thiamethoxam). Higher concentrations in urine were detected in the summer compared to winter. The study also evaluated exposure to pyrethroid and organophosphate pesticides, finding exposure to all three classes. The detection rates for neonicotinoids and organophosphate and pyrethroid metabolites all were above 80%. Interestingly, this study found the detection of neonicotinoids to be higher in children exposed to organophosphate pesticides. The study was unable to confirm whether exposure was due to diet, household/agricultural application, or a combination of the two.318 Similarly, a study investigating exposure to sprayed thiacloprid found that young children, aged 3–6 years old, were exposed to multiple neonicotinoids on a daily basis, with inhaled thiacloprid accounting for <1% of the daily intake. Diet was believed to be the primary source of exposure.330
A study of 373 Japanese adults, found exposure to clothianidin (96.5%), dinotefuran (93.3%), imidacloprid (76.7%) thiamethoxam (92.0%) and desmethyl-acetamiprid (100%) were common.198 Positive correlations were reported between clothianidin, desmethyl-acetamiprid, dinotefuran, and imidacloprid concentrations and fruit intake; dinotefuran and imidacloprid with vegetable intake; and the dinotefuran concentration with cereal intake.198 Clothianidin, dinotefuran, and imidacloprid concentrations were also associated with drinking or smoking.198 Two other studies conducted in 2014 and 2015 also found neonicotinoid exposure in Japanese adults.187, 319 The first study evaluated exposure in 52 adults that reported no occupational exposure to neonicotinoids.187, 319 The detection rate was >96% for four neonicotinoids - dinotefuran (100%), thiamethoxam (100%), clothianidin (96%), imidacloprid (96%), acetamiprid (>50%), thiacloprid (>50%), and nitenpyram (29%). The researchers suggested that this exposure came mainly from diet and drinking water.319 The second study examined exposure among 95 women in Kyoto, Japan and surrounding areas between the ages of 45–75 from 1994–2011.187 Like the other studies, this research found that exposure was commonplace and increased with time. Detection rates increased from the mid-1990s to 2011, with few detections in the 1990s and detection rates between >5% and >70% for seven different neonicotinoids in 2010 and 2011. Thiamethoxam and dinotefuran were the most common neonicotinoids found with detection rates above 70%. The geometric mean for total urinary neonicotinoids also increased over this time period with values increasing from 0.05 nmol/g creatinine in 1994 to 12.83 nmol/g creatinine in 2011.187
In the United States, more recent studies using National Health and Nutrition Examination Survey (NHANES) data reported exposure to neonicotinoids. In 2015–2016 NHANES found that nearly half of the United States’ population 3 years of age and older may be exposed to neonicotinoids. Out of 3,038 total samples, researchers commonly detected two metabolites N-desmethyl-acetamiprid (35%) and 5-hydroxy imidacloprid (20%), followed by clothianidin (8%), and imidacloprid (4%). Acetamiprid and thiacloprid were detected in <0.5% samples. When compared to other age ranges and ethnicities young children (3–5 years old) and Asians had higher exposures to the two metabolites. The cause of the reported differences between age and ethnic groups was not known.331 A 2017 NHANES study conducted in Atlanta, Georgia, USA, detected two metabolites — N-desmethyl-acetamiprid (90%) and 5-hydroxy-imidacloprid (42%) — and three neonicotinoids: clothianidin (37%), imidacloprid (30%), and acetamiprid (2%).328 The study was conducted among anonymous male and female donors with no documented exposure to neonicotinoids.328 In a 2019 analytical methods development paper, researchers detected imidaclothiz, a neonicotinoid manufactured and registered for use in China, in 100% of 20 spot urine samples collected from healthy adults in Albany, New York.329 N-desmethyl acetamiprid (90%), 6-chloronicotinic acid (90%), clothianidin (85%), imidacloprid (70%), and thiamethoxam (55%) were also commonly detected.
Studies have also indicated that people working on or living near farms that use neonicotinoids have high exposure to these compounds. A 2007 cross-sectional study comparing 25 non-spraying control farmers and 89 pesticide sprayers who used neonicotinoids in southeastern Spain reported a suggestive relation between neonicotinoid application and lung dysfunction.334 The lung dysfunctions included lower total lung capacity, residual volume, and functional residual capacity.334 Exposed farmers were also more likely to report lung irritation. Regression analyses were used to compare relations between sociodemographic factors, occupational exposure, and clinical symptoms. These analyses showed a relation between short-term exposure (>25% drop in serum cholinesterase from baseline levels) with reduced forced expired volume, and long-term exposure with reduced forced expiratory flow rate. Long-term exposure was estimated based upon a lifelong cumulative index for each worker that multiplied the average number of hours working with pesticides on a weekly basis by the average number of weeks per year, and by the lifetime number of years working with pesticides.334 In another Spanish study from 2017, urine samples from a cohort of 36 pregnant women living in agricultural areas contained imidacloprid, acetamiprid and acetamiprid-n-desmethyl at concentrations between 0.2 and 1.6 μg/L.326 Dinotefuran was detected at trace levels and acetamiprid-n-desmethyl was the compound most widely detected. In total, at least one of these compounds was found in 16% of subjects.326
Additionally, evidence that proximity to farms increases exposure to neonicotinoids has also been highlighted by studies that compared humans living in urban versus rural landscapes. In China and Greece, neonicotinoid levels in urine have been compared between urban and rural subjects.323, 324, 332 In general, neonicotinoid concentrations increased following insecticide application. In 2015, a Chinese study found frequently detected imidacloprid and 6-chloronicotinic acid in 295 spot urine samples from rural (100% and 32%, respectively) and urban (95% and 23%, respectively) subjects.324 The geometric mean concentration of imidacloprid increased significantly among rural adults following pesticide spraying (pre-application: 0.18 ng/ml vs post-application: 0.62 ng/mL).324 In Henan Province, pre- and post-application spot urine sampling amongst 43 randomly selected neonicotinoid applicators showed that the urinary concentration of imidacloprid increased significantly following pesticide application.332 Imidacloprid and 6-chloronicotinic acid were detected in 100% of 43 spot urine samples collected from pesticide applicators.332 A three-fold increase in urine concentration was observed for both analytes following field application of imidacloprid.332 In Greece, a study of imidacloprid exposure in urine and hair also compared concentrations between urban and rural populations.323 The study found that rural residents engaged in agriculture were more likely (66%) than their urban counterparts (0%) to have a positive detection for imidacloprid in their hair. The median and maximum concentrations of imidacloprid in hair were 0.03 ng/mg and 27 ng/mg, respectively.323
7.3 Health Effects
Recently, several epidemiological studies have highlighted concerns for human health, including the effects of acute poisonings and possible chronic effects. In humans, acute poisonings with neonicotinoids resulted in respiratory, cardiovascular, and neurological symptoms, including death.335–350 Subacute intoxication from food consumption, specifically fruit, vegetables, and tea, has been documented in Japan.255 Six patients that consumed greater than 500g/day of either domestic fruits/vegetables and/or tea reported symptoms including finger tremor, impaired short-term memory, fever, general fatigue, headache, palpitation/chest pain, abdominal pain, muscle pain/muscle weakness/muscle spasm, and cough.254, 255 Similar results were found by Marfo et al.250 who found an association between N-desmethyl-acetamiprid concentrations in urine and increased prevalence of neurologic symptoms among symptomatic patients versus 50-non-symptomatic volunteers {Odds ratio: 14, 95% Confidence Interval (CI): 3.5–57}. Symptoms included memory loss, finger tremor, headache, general fatigue, palpitation/chest pain, abdominal pain, muscle pain/weakness/spasm, and cough.250 Other studies have reported a variety of respiratory, cardiovascular and neurological symptoms such as shortness of breath, coma, and irregular heart beat (slow and rapid), low blood pressure, and dilated pupils following an acute exposure.337, 339, 341, 346, 347, 351–356 Several case reports have also detailed fatalities due to acute exposure, although mortality is generally considered uncommon.339, 341, 346, 347, 351–354 Studies reviewing incidents of acute neonicotinoid poisoning indicate that death occurred in less than 5% of cases.337, 355, 356 Abnormal electrocardiograms post neonicotinoid exposure have also been observed.254
Researchers have also examined the potential health effects on workers who apply pesticides. Koureas et al. found that neonicotinoid application was related to the induction of oxidative damage to DNA in the whole blood of 80 pesticide sprayers.357 Seasonal exposure to neonicotinoids {Risk Ratio: 2.22 (95% CI:1.07–4.63)} had greater effects on 8-OHdG levels.357 In Sri Lanka, neonicotinoid use among rice paddy farmers was not associated with higher risk of chronic kidney disease.325 As discussed above, neonicotinoid spraying has also been associated with impaired lung function.334
Links have also been found between maternal exposure to neonicotinoids during pregnancy and adverse birth outcomes. Associations between imidacloprid exposure and an increased risk for tetralogy of Fallot {Adjusted Odds Ratio (AOR) = 2.4, (95% CI: 1.1–5.1)}, a type of congenital heart defect, autism {AOR = 2.0, (95% CI 1.0–3.9)} and anencephaly in newborns {AOR = 2.9, (95% CI: 1.0–8.2)}.335, 358–360 Exposure was estimated in each of these studies through self-report on pesticide use or residential proximity to fields where neonicotinoids had been applied. A 2019 study assessing exposure of very low birth weight infants with gestational age between 23–34 weeks, 48 hours following delivery, found a significant association between birth weight and exposure to one metabolite, N-desmethyl-acetamiprid.361 Low birth-weight infants weighing below the 10th percentile for gestational age had a higher detection rate {43% vs. 15%, p<0.05} and higher mean concentration {0.04 vs. 0.02 ng/g, p<0.05] compared to infants with an appropriate gestational age (10th – 90th percentile for gestational age).361
7.4. Knowledge Gaps and Research Needs
Although human exposure to neonicotinoids has been observed to occur through diet and occupational exposure, there are insufficient data to definitively link neonicotinoids with potential health risks. While generally considered to be safe for humans at low concentrations, the limited research that has been conducted appears to indicate long-term potential for genotoxicity, cytotoxicity, impaired immune function and reproduction, and birth defects; and acute health effects ranging from respiratory, cardiovascular and neurological symptoms. Published epidemiological studies primarily used ecological or cross-sectional designs. These designs are limited because they are unable to assess temporal relations between exposure and outcome. In addition, exposure was primarily estimated by either using proximity to application sites as a proxy. Biomonitoring would be necessary to confirm exposure has taken place. Scientifically rigorous epidemiological studies have not been conducted to examine potential adverse health risks; case-control and cohort studies are needed to examine potential adverse health outcome related to chronic neonicotinoid exposure.
8. Conclusions
Despite widespread use of neonicotinoid insecticides, there have been relatively few published studies addressing the risks of human exposure, and some critical gaps remain in our understanding of their environmental fate and occurrence necessary to best characterize exposure. Future research studies could include the following priorities:
8.1 Use
Research has found effects when neonicotinoids are applied in combination with pyrethroids and fungicides. A combination of neonicotinoids and pyrethroids was found to lead to impaired foraging, increased worker mortality, and increased colony failure, as well as a significant enhancement in the toxicity of thiacloprid to honeybees.191, 362 Only one study was found to have assessed co-exposure to neonicotinoids and other pesticides,318 and no studies described the potential health risks of these combinations. It is important that mixture studies consider the risks of exposure to neonicotinoids combined with other contaminants including pesticides, co-formulants, inert ingredients, fertilizers, metals, and pharmaceuticals that are used in parallel or may co-occur in the environment.4
Research is needed on approaches for minimizing exposure to neonicotinoids, their known metabolites, and other breakdown products. For the control of neonicotinoids in water, this includes identification of best practices that can be deployed at the edge of field, as well as engineered treatment systems for use in centralized community water systems and in home (e.g., point of use).
Risks associated with occupational exposure to neonicotinoids also warrant further study. Limited research has been published that assess human exposure risks from dust. Exposure scenarios evaluated by the EPA concluded that even with additional protection, such as PF5 or PF10 respirators, exposures to neonicotinoids from seed treatments, planting, and dry flowable and wettable powders still exceeded the MOEs.28–32, 144
8.2 Transformation products
Breakdown products for neonicotinoids are potentially more toxic than the parent compounds, but their concentrations are rarely measured complicating risk assessment.49, 246, 253, 313, 314 Assessment of these compounds is complicated by the fact that many transformation products are not available commercially as standards for analytical analyses, further limiting the ability to effectively confirm their occurrence and measure their abundance and concentration in the environment. More research is needed to identify novel transformation products of neonicotinoids and their known metabolites and the processes responsible for their formation. Improved understanding of their toxicology and health risks would complement improved understanding of their formation. In assessing formation of breakdown products, there remains a need to consider biological metabolites, as well as transformation products generated during environmental processing and engineered treatment (e.g., chemical disinfection and chemical oxidation processes in drinking water treatment).
8.3 Human Exposure, Toxicity and Health Effects
Human exposure to neonicotinoids has been shown to occur through different routes, including ingestion (e.g., water, food), inhalation (e.g., dust), and dermal exposure, and during occupational and residential use. Although these exposures appear to take place at very low concentrations, it is currently unknown which route serves as the primary exposure pathway to humans or whether major exposure routes vary across different neonicotinoid species. While neonicotinoid analyses of food and water indicate that exposure may take place simultaneously through multiple exposure routes (e.g., ingestion, inhalation, dermal contact), further studies are needed to provide a detailed evaluation of the relative contribution of neonicotinoid exposure from multiple exposure pathways.28–32, 38, 118, 134, 144–148, 167–197
In order to evaluate the relative contribution of neonicotinoid exposure from multiple exposure pathways, the magnitude and variability of neonicotinoids in dietary sources needs to be assessed. For example, research is needed to understand the actual potential for neonicotinoid exposure and the various factors (e.g., land use, private versus public water supply) that affect the occurrence and temporal variability of neonicotinoids in tap water. While reported concentrations in food and juice appear to be below currently acceptable daily intake levels, numerous surveys have documented that chronic neonicotinoid exposure may occur regularly through these routes.38, 145, 167–197 Several studies have measured the concentrations of these insecticides in foods, but few attempts have been made to estimate daily intake.38, 145, 172, 174, 176, 183, 184, 199, 317, 363 No case-control or cohort studies have been conducted that include an estimate of the retrospective intake of neonicotinoids via food. In addition, EPA exposure estimates indicate that the greatest acute and chronic exposures may occur amongst young children. More studies are needed to assess exposure at different stages of life and to identify factors that may influence risk.
Supplementary Material
Figure 2:
The electronegative pharmacophore (either nitro- or cyano-group) of the neonicotinoid confers selective binding to the insect nicotinic acetylcholine receptor (nAChR). Insecticidal target specificity is lost when the metabolite desnitro-imidacloprid without the nitro-group binds to the the vertebrate nAChR. Republished with permission of Annual Reviews, Inc, from Neonicotinoid Insecticide Toxicology: Mechanisms of Selective Action, Motohiro Tomizawa, John E. Casida, vol 45, 2005; permission conveyed through Copyright Clearance Center, Inc.
Acknowledgements
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the authors or the U.S. Government.
This work was supported by University of Iowa’s Center for Health Effects of Environmental Contamination, Iowa Institute of Public Health Research and Policy, NIOSH funded Heartland Center for Occupational Health and Safety (Training Grant No. T42OH008491), NIEHS funded Environmental Health Sciences Research Center (Grant No. P30 ES005605), the State Hygienic Laboratory at the University of Iowa, and the U.S. Geological Survey Toxics Substances Hydrology Program. Gregory H. LeFevre and David M. Cwiertny were supported by a grant from the National Science Foundation (CBET Environmental Engineering 1803197). The authors would also like to acknowledge the contributions of members of the University of Iowa’s Neonicotinoid Collaboratory.
Footnotes
Conflicts of interest
There are no conflicts of interest to declare.
Notes and references
‡ County level estimates from the U.S. Geological Survey (USGS) that include seed treatments are only available through 2014. In 2015, the provider of the surveyed pesticide use data stopped making estimates to derive the county-level use estimates of seed treatment use because of complexity and uncertainty.
- 1.Jeschke P, Nauen R, Schindler M. and Elbert A, Overview of the status and global strategy for neonicotinoids, Journal of Agriculture and Food Chemistry, 2010, 59, 2897–2908. [DOI] [PubMed] [Google Scholar]
- 2.Myers C. and Hill E, Benefits of Neonicotinoid Seed Treatments to Soybean Production, U.S. Environmental Protection Agency, 2014. [Google Scholar]
- 3.Tomizawa M. and Casida JE, Neonicotinoid insecticide toxicology: mechanisms of selective action, Annu. Rev. Pharmacol. Toxicol, 2005, 45, 247–268. [DOI] [PubMed] [Google Scholar]
- 4.Hladik ML, Main AR and Goulson D, Environmental risks and challenges associated with neonicotinoid insecticides, Environ. Sci. Technol, 2018. [DOI] [PubMed] [Google Scholar]
- 5.Jeschke P. and Nauen R, Neonicotinoids—from zero to hero in insecticide chemistry, Pest Manage. Sci, 2008, 64, 1084–1098. [DOI] [PubMed] [Google Scholar]
- 6.Shivanandappa T. and Rajashekar Y, in Advances in plant biopesticides, Springer, 2014, pp. 323–345. [Google Scholar]
- 7.Yamamoto I. and Casida JE, in Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor, ed. Izuru JCY, Springer-Verlag, Tokyo, 1999, pp. 3–27. [Google Scholar]
- 8.Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke CH, Liess M, Long E, McField M, Mineau P, Mitchell EAD, Morrissey CA, Noome DA, Pisa L, Settele J, Stark JD, Tapparo A, Van Dyck H, Van Praagh J, Van der Sluijs JP, Whitehorn PR and Wiemers M, Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites, Environmental Science and Pollution Research, 2015, 22, 5–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uneme H , Chemistry of Clothianidin and related compounds, J. Agric. Food Chem, 2011, 59, 2932–2937. [DOI] [PubMed] [Google Scholar]
- 10.Cappaert D, McCullough DG, Poland TM and Siegert NW, Emerald ash borer in North America: a research and regulatory challenge, Am. Entomol, 2005, 51, 152–165. [Google Scholar]
- 11.Struger J. and Fletcher T, Occurrence of lawn care and agricultural pesticides in the Don River and Humber River watersheds (1998–2002), J. Great Lakes Res, 2007, 33, 887–905. [Google Scholar]
- 12.McCurdy JD, Held DW, Gunn JM and Barickman TC, Dew from Warm-Season Turfgrasses as a Possible Route for Pollinator Exposure to Lawn-Applied Imidacloprid, Crop, Forage & Turfgrass Management, 2017, 3. [Google Scholar]
- 13.Anderson JC, Dubetz CP and Palace VP, Neonicotinoids in the Canadian Aquatic Environment: A Literature Review on Current use of products with a focus on fate, exposure and biological effects, Sci. Total Environ, 2015, 1, 409–422. [DOI] [PubMed] [Google Scholar]
- 14.Fishel FM, Pesticide Toxicity Profile: Neonicotinoid Pesticides, http://edis.ifas.ufl.edu/pdffiles/PI/PI11700.pdf, (accessed October 30, 2016).
- 15.Fishel FM, Pesticide toxicity profile: carbamate pesticides, http://edis.ifas.ufl.edu/pdffiles/PI/PI11700.pdf, (accessed October 30, 2016).
- 16.Fishel FM, Pesticide Toxicity Profile: Organophosphate Pesticides, http://edis.ifas.ufl.edu/pi087, (accessed October 30, 2016).
- 17.Lewis KA, Tzilivakis J, Warner DJ and Green A, An international database for pesticide risk assessments and management, Hum. Ecol. Risk Assess, 2016, 22, 1050–1064. [Google Scholar]
- 18.Tomizawa M. and Casida JE, Selective toxicity of neonicotinoids attributable to specificity to insect and mammalian nicotinic receptors, Annu. Rev. Entomol, 2003, 48, 339–364. [DOI] [PubMed] [Google Scholar]
- 19.Tomizawa M. and Casida JE, Minor structural changes in nicotinoid insecticides confer differential subtype selectivity for mammalian nicotinic acetylcholine receptors, Br. J. Pharmacol, 1999, 127, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lansdell SJ and Millar NS, The influence of nicotinic receptor subunit composition upon agonist, alpha-bungarotoxin and insecticide (imidacloprid) binding affinity, Neuropharmacology, 2000, 39, 671–679. [DOI] [PubMed] [Google Scholar]
- 21.Okazawa Y, Nakagawa Y, Akamatsu M, Ueno T. and Nishimura S, Comparison of the binding activities of chloronicotinyl insecticides toward the nicotinic acetylcholine receptors from rats and houseflies, J. Pestic. Sci, 2000, 25, 40–43. [Google Scholar]
- 22.Tomizawa M. and Casida JE, Imidacloprid, thiacloprid, and their imine derivatives up-regulate the alpha 4 beta 2 nicotinic acetylcholine receptor in M10 cells., Toxicol. Appl. Pharmacol, 2000, 169, 114–120. [DOI] [PubMed] [Google Scholar]
- 23.Hurley T. and Mitchell P, Value of neonicotinoid seed treatments to US soybean farmers, Pest Manage. Sci, 2017, 73, 102–112. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Department of the Interior and U.S. Geological Survey, Pesticide National Synthesis Project, Estimated Annual Agricultural Pesticide Use, https://water.usgs.gov/nawqa/pnsp/usage/maps/index.php). [Google Scholar]
- 25.Seltenrich N, Catching Up with Popular Pesticides: More Human Health Studies Are Needed on Neonicotinoids, Environ. Health Perspect, 2017, 125, A41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cimino AM, Boyles AL, Thayer KA and Perry MJ, Effects of neonicotinoid pesticide exposure on human health: a systematic review, Environ. Health Perspect, 2016, 125, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Food Safety Authority, Scientific Opinion on the developmental neurotoxicity potential of acetamiprid and imidacloprid, EFSA Journal, 2013, DOI: 10.2903/j.efsa.2013.3471. [DOI] [Google Scholar]
- 28.U.S. Environmental Protection Agency, Acetamiprid. Draft Human Health Risk Assessment for Registration Review Journal, 2017. [Google Scholar]
- 29.U.S. Environmental Protection Agency, Clothianidin: Draft Human Health Risk Assessment in Support of Registration, https://www.regulations.gov/docket?D=EPA-HQ-OPP-2011-0865). [Google Scholar]
- 30.U.S. Environmental Protection Agency, Dinotefuran: Human Health Draft Risk Assessment for Registration Review., https://www.regulations.gov/docket?D=EPA-HQ-OPP-2011-0920). [Google Scholar]
- 31.U.S. Environmental Protection Agency, Imidacloprid: Human Health Draft Risk Assessment for Registration Review, https://www.regulations.gov/docket?D=EPA-HQ-OPP-2008-0844). [Google Scholar]
- 32.U.S. Environmental Protection Agency, Thiamethoxam: Draft Human Health Risk Assessment for Registration Review, https://www.regulations.gov/docket?D=EPA-HQ-OPP-2011-0581). [Google Scholar]
- 33.U.S. Environmental Protection Agency, Pollinator Protection: Schedule for Review of Neonicotinoid Pesticides, https://www.epa.gov/pollinator-protection/schedule-review-neonicotinoid-pesticides). [Google Scholar]
- 34.Bass C, Denholm I, Williamson MS and Nauen R, The global status of insect resistance to neonicotinoid insecticides, Pestic. Biochem. Physiol, 2015, 121, 78–87. [DOI] [PubMed] [Google Scholar]
- 35.Abou-Donia MB, Goldstein LB, Bullman S, Tu T, Khan WA, Dechkovskaia AM and Abdel-Rahman AA, Imidacloprid induces neurobehavioral deficits and increases expression of glial fibrillary acidic protein in the motor cortex and hippocampus in offspring rats following in utero exposure, Journal of Toxicology and Environmental Health Impact, Part A, 2008, 71, 119–130. [DOI] [PubMed] [Google Scholar]
- 36.Van der Sluijs JP, Simon-Delso N, Goulson D, Maxim L, Bonmatin J-M and Belzunces LP, Neonicotinoids, bee disorders and the sustainability of pollinator services, Current Opinion in Environmental Sustainability, 2013, 5, 293–305. [Google Scholar]
- 37.Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M. and Sattelle DB, Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors., Trends Pharmacol. Sci, 2001, 22, 573–580. [DOI] [PubMed] [Google Scholar]
- 38.Chen M, Tao L, McLean J. and Lu C, Quantitative analysis of neonicotinoid insecticide residues in foods: implication for dietary exposures, J. Agric. Food Chem, 2014, 62, 6082–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nauen R, Jeschke P, Velten R, Beck ME, Ebbinghaus-Kintscher U, Thielert W, Wölfel K, Haas M, Kunz K. and Raupach G, Flupyradifurone: a brief profile of a new butenolide insecticide, Pest Manage. Sci, 2015, 71, 850–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giorio C, Safer A, Sánchez-Bayo F, Tapparo A, Lentola A, Girolami V, Van Lexmond MB and Bonmatin J-M, An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 1: new molecules, metabolism, fate, and transport, Environmental Science and Pollution Research, 2017, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morita M, Yoneda T. and Akiyoshi N, Research and development of a novel insecticide, flonicamid, J. Pestic. Sci, 2014, 39, 179–180. [Google Scholar]
- 42.Watson GB, Loso MR, Babcock JM, Hasler JM, Letherer TJ, Young CD, Zhu Y, Casida JE and Sparks TC, Novel nicotinic action of the sulfoximine insecticide sulfoxaflor, Insect Biochem. Mol. Biol, 2011, 41, 432–439. [DOI] [PubMed] [Google Scholar]
- 43.Shao X, Liu Z, Xu X, Li Z. and Qian X, Overall status of neonicotinoid insecticides in China: production, application and innovation, J. Pestic. Sci, 2012, D12–037. [Google Scholar]
- 44.U.S. Environmental Protection Agency, Pesticide Chemical Search, https://iaspub.epa.gov/apex/pesticides/f?p=chemicalsearch:1, (accessed September 12, 2019).
- 45.European Commission, EU - Pesticides database, https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN, (accessed September 9, 2019).
- 46.Bonmatin JM, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, Marzaro M, Mitchell EA, Noome DA, Simon-Delso N. and Tapparo A, Environmental fate and exposure; neonicotinoids and fipronil, Environ. Sci. Pollut. Res. Int, 2015, 22, 35–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sánchez-Bayo F, The trouble with neonicotinoids, Science, 2014, 346, 806–807. [DOI] [PubMed] [Google Scholar]
- 48.Rodrigues KJA, Santana MB, Do Nascimento JLM, Picanco-Diniz DLW, Maues LAL, Santos SN, Ferreira VMM, Alfonso M, Duran R. and Faro LRF, Behavioral and biochemical effects on neonicotinoid thiamethoxam on the cholinergic system in rats, Ecotoxicol. Environ. Saf, 2010, 73, 101–107. [DOI] [PubMed] [Google Scholar]
- 49.Ford KA and Casida JE, Unique and common metabolites of thiamethoxam, clothianidin, and dinotefuran in mice, Chem. Res. Toxicol, 2006, 19, 1549–1556. [DOI] [PubMed] [Google Scholar]
- 50.Gervais JA, Luukinen B, Buhl K. and Stone D, Imidacloprid General Fact Sheet, http://npic.orst.edu/factsheets/imidagen.html, (accessed October 30, 2016).
- 51.Gundelfinger ED and Schulz R, in Handbook of Experimental Pharmacology. Vol. 144: Neuronal Nicotinic Receptors, Springer, Berlin, 2000, pp. 497–521. [Google Scholar]
- 52.Matsuda K, Kanaoka S, Akamatsu M. and Sattelle DB, Diverse Actions and Target-Ste Selectivity of Neonicotinoids: Structural Insights, Mol. Pharmacol, 2009, 76, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arneric SP, Holladay M. and Williams M, Neuronal nicotinic receptors: A perspective on two decades of drug discovery research, Biochem. Pharmacol, 2007, 74, 1092–1101. [DOI] [PubMed] [Google Scholar]
- 54.Dani JA and Bertrand D, Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system., Annu. Rev. Pharmacol. Toxicol, 2007, 47, 699–729. [DOI] [PubMed] [Google Scholar]
- 55.Levin ED and Rezvani AH, Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function, Biochem. Pharmacol, 2007, 74, 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Changeux J-P and Tally A, Nicotinic receptors, allosteric proteins and medicine., Trends Mol. Med, 2008, 14, 93–102. [DOI] [PubMed] [Google Scholar]
- 57.U.S. Environmental Protection Agency, Thiacloprid - Notice of Registration Review Case Closure, https://www.regulations.gov/document?D=EPA-HQ-OPP-2012-0218-0018). [Google Scholar]
- 58.Liu M-Y, Lanford J. and Casida JE, Relevance of [3H]Imidacloprid Binding Site in House Fly Head Acetylcholine Receptor to Insecticidal Activity of 2-Nitromethylene- and 2-Nitroimino-imidazolidines, Pestic. Biochem. Physiol, 1993, 46, 200–206. [Google Scholar]
- 59.Chao SL and Casida JE, Interaction of imidacloprid metabolites and analogs with the nicotinic acetylcholine receptor of mouse brain in relation to toxicity, Pestic. Biochem. Physiol, 1997, 58, 77–88. [Google Scholar]
- 60.Yamamoto I, Yabuta G, Tomizawa M, Saito T, Miyamoto T. and Kagabu S, Molecular Mechanism for Selective Toxicity of Nicotinoids and Neonicotinoids, J. Pestic. Sci, 1995, 20, 33–40. [Google Scholar]
- 61.Zwart R, Oortgiesen M. and Vijverberg HPM, The nitromethylene heterocycle 1-(pyridin-3-yl-methyl)-2-nitromethylene-imidazolidine distinguishes mammalian from insect nicotinic receptor subtypes, Eur. J. Pharmacol, 1992, 228, 165–169. [DOI] [PubMed] [Google Scholar]
- 62.Zwart R, Oortgiesen M. and Vijverberg HPM, Nitromethylene Heterocycles: Selective Agonists of Nicotinic Receptors in Locust Neurons Compared to Mouse N1E-115 and BC3H1 Cells, Pestic. Biochem. Physiol, 1994, 48, 202–213. [Google Scholar]
- 63.Yamamoto I, Tomizawa M, Saito T, Miyamoto T, Walcott EC and Sumikawa K, Structural factors contributing to insecticidal and selective actions of neonicotinoids, Arch. Insect Biochem. Physiol, 1998, 37, 24–32. [DOI] [PubMed] [Google Scholar]
- 64.Matsuda K, Buckingham SD, Freeman JC, Squire MD, Baylis HA and Sattelle DB, Effects of the alpha subunit on imidacloprid sensitivity of recombinant nicotinic acetylcholine receptors, Br. J. Pharmacol, 1998, 123, 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bal R, Erdogan S, Theophilidis G, Baydas G. and Naziroglu M, Assessing the effects of the neonicotinoid insecticide imidacloprid in the cholinergic synapses of the stellate cells of the mouse cochlear nucleus using whole-cell patch-clamp recording, Neurotoxicology, 2010, 31, 113–120. [DOI] [PubMed] [Google Scholar]
- 66.Sheets LP, Imidacloprid: A Neonicotinoid Insecticide in Hayes' Handbook of Pesticide Toxicology (Third Edition).Journal, 2010, 2055–2064. [Google Scholar]
- 67.Thyssen J. and Machemer L, in Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor, Springer, 1999, pp. 213–222. [Google Scholar]
- 68.Akayama A. and Minamida I, in Nicotinoid insecticides and the nicotinic acetylcholine receptor, Springer, Tokyo, 1999, pp. 127–148. [Google Scholar]
- 69.Yamamoto I. and Casida JE, Nicotinoid insecticides and the nicotinic acetylcholine receptor, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerwick BC and Sparks TC, Natural products for pest control: an analysis of their role, value and future, Pest Manage. Sci, 2014, 70, 1169–1185. [DOI] [PubMed] [Google Scholar]
- 71.Benbrook CM, Trends in glyphosate herbicide use in the United States and globally, Environmental Sciences Europe, 2016, 28, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.European Food Safety Authority, Conclusion on the peer review of the pesticide risk assessment for bees for the active substance imidacloprid, European Food Safety Authority Journal, 2013, 11, 3068 [3055 pp]. [Google Scholar]
- 73.Douglas MR and Tooker JF, Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops, Environ. Sci. Technol, 2015, 49, 5088–5097. [DOI] [PubMed] [Google Scholar]
- 74.Tooker JF, Douglas MR and Krupke CH, Neonicotinoid seed treatments: limitations and compatibility with integrated pest management, Agricultural & Environmental Letters, 2017, 2. [Google Scholar]
- 75.National Pesticide Information Center and Oregon State University, NPIC Product Research Online, http://npic.orst.edu/NPRO/, (accessed September 4, 2019).
- 76.Herms D, McCullough D, Clifford C, Smitley D, Miller F. and Cranshaw W, Insecticide options for protecting ash trees from emerald ash borer, http://www.emeraldashborer.info/documents/Multistate_EAB_Insecticide_Fact_Sheet.pdf,3rdEdition). [Google Scholar]
- 77.Alford A. and Krupke CH, Translocation of the neonicotinoid seed treatment clothianidin in maize, PLoS One, 2017, 12, e0173836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.CISION PR Newswire, Global Seed Treatment Industry, https://www.prnewswire.com/news-releases/global-seed-treatment-industry-300896380.html, (accessed February 13, 2020).
- 79.Haire B, Are seed treatments worth the investment?, http://southeastfarmpress.com/soybeans/are-seed-treatments-worth-investment). [Google Scholar]
- 80.Douglas MR and Tooker JF, Large scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops, Environ. Sci. Technol, 2015, 49, 5088–5097. [DOI] [PubMed] [Google Scholar]
- 81.Osteen CD and Fernandez-Cornejo J, Economic and policy issues of US agricultural pesticide use trends, Pest Manage. Sci, 2013, 69, 1001–1025. [DOI] [PubMed] [Google Scholar]
- 82.Henry M, Beguin M, Requier F, Rollin O, Odoux J-F, Aupinel P, Aptel J, Tchamitchian S. and Decourtye A, A common pesticide decreases foraging success and survival in honey bees, Science, 2012, 336, 348–350. [DOI] [PubMed] [Google Scholar]
- 83.European Food Safety Authority, Bee Health: EU takes additional measures on pesticides to better protect Europe’s bees, http://europa.eu/rapid/press-release_IP-13-708_en.htm). [Google Scholar]
- 84.European Commission, Neonicotinoids: Current status of the neonicotinoids in the EU, https://ec.europa.eu/food/plant/pesticides/approval_active_substances/approval_renewal/neonicotinoids_en).
- 85.Pest Management Regulatory Agency, Re-evaluation Decision RVD2019–04, Thiamethoxam and Its Associated End-use Products: Pollinator Re-evaluation, https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/reevaluation-decision/2019/thiamethoxam.html). [Google Scholar]
- 86.Pest Management Regulatory Agency, Re-evaluation Decision RVD2019–05, Clothianidin and Its Associated End-use Products: Pollinator Re-evaluation, https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/reevaluation-decision/2019/clothianidin.html). [Google Scholar]
- 87.Pest Management Regulatory Agency, Re-evaluation Decision RVD2019–06, Imidacloprid and Its Associated End-use Products: Pollinator Re-evaluation, https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/decisions-updates/reevaluation-decision/2019/imidacloprid.html). [Google Scholar]
- 88.Health Canada, Health Canada to consult on plan to phase out most uses of the neonicotinoids clothianidin and thiamethoxam, https://www.canada.ca/en/health-canada/news/2018/08/health-canada-to-consult-on-plan-to-phase-out-most-uses-of-the-neonicotinoids-clothianidin-and-thiamethoxam.html). [Google Scholar]
- 89.U.S. Environmental Protection Agency, Product Cancellation Order for Certain Pesticide Registrations, Fed. Regist, May 20, 2019. [Google Scholar]
- 90.National Caucus of Environmental Legislators, 2019. Neonicotinoid Legislation, https://www.quorum.us/spreadsheet/external/IcBmFYETcsZMXEBUXfaH/). [Google Scholar]
- 91.Wood TJ and Goulson D, The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013, Environmental Science and Pollution Research, 2017, 24, 17285–17325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goulson D, Review: An overview of the environmental risks posed by neonicotinoid insecticides, J. Appl. Ecol, 2013, 50, 977–987. [Google Scholar]
- 93.Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC and Liber K, Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review, Environ. Int, 2015, 74, 291–303. [DOI] [PubMed] [Google Scholar]
- 94.van Lexmond MB, Bonmatin J-M, Goulson D. and Noome DA, Worldwide integrated assessment on systemic pesticides, Environmental Science and Pollution Research, 2015, 22, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.European Food Safety Authority, Conclusion regarding the peer review of the pesticide risk assessment of the active substance imidacloprid, European Food Safety Authority Journal, 2008, 6, 148r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Canadian Council of Ministers of the Environment, Canadian Water Quality Guidelines: Imidacloprid. Scientific Supporting Document, http://www.ccme.ca/files/Resources/supporting_scientific_documents/imidacloprid_ssd_1388.pdf). [Google Scholar]
- 97.Kurwadkar ST, Dewinne D, Wheat R, McGahan DG and Mitchell FL, Time dependent sorption behavior of dinotefuran, imidacloprid and thiamethoxam, J. Environ. Sci. Health, Pt. B: Pestic., Food Contam., Agric. Wastes, 2013, 48, 237–242. [DOI] [PubMed] [Google Scholar]
- 98.Tapparo A, Marton D, Giorio C, Zanella A, Solda L, Marzaro M, Vivan L. and Girolami V, Assessment of the environmental exposure of honeybees to particulate matter containing neonicotinoid insecticides coming from corn coated seeds, Environ. Sci. Technol, 2012, 46, 2592–2599. [DOI] [PubMed] [Google Scholar]
- 99.Van Dijk TC, Van Staalduinen MA and Van der Sluijs JP, Macro-invertebrate decline in surface water polluted with imidacloprid, PLoS One, 2013, 8, e62374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nuyttens D. and Verboven P, Dust Emission from Pesticide Treated Seeds During Seed Drilling, Outlooks on Pest Management, 2015, 26, 215–219. [DOI] [PubMed] [Google Scholar]
- 101.Girolami V, Marzaro M, Vivan L, Mazzon L, Greatti M, Giorio C, Marton D. and Tapparo A, Fatal powdering of bees in flight with particulates of neonicotinoids seed coating and humidity implication, J. Appl. Entomol, 2012, 136, 17–26. [Google Scholar]
- 102.Pochi D, Biocca M, Fanigliulo R, Gallo P. and Pulcini P, Sowing of seed dressed with thiacloprid using a pneumatic drill modified for reducing abrasion dust emissions, Bull. Insectol, 2015, 68, 273–279. [Google Scholar]
- 103.Biocca M, Conte E, Pulcini P, Marinelli E. and Pochi D, Sowing simulation tests of a pneumatic drill equipped with systems aimed at reducing the emission of abrasion dust from maize dressed seed, J. Environ. Sci. Health, Pt. B: Pestic., Food Contam., Agric. Wastes, 2011, 46, 438–448. [DOI] [PubMed] [Google Scholar]
- 104.Girolami V, Marzaro M, Vivan L, Mazzon L, Giorio C, Marton D. and Tapparo A, Aerial powdering of bees inside mobile cages and the extent of neonicotinoid cloud surrounding corn drillers, J. Appl. Entomol, 2013, 137, 35–44. [Google Scholar]
- 105.Forero LG, Limay-Rios V, Xue Y. and Schaafsma A, Concentration and movement of neonicotinoids as particulate matter downwind during agricultural practices using air samplers in southwestern Ontario, Canada, Chemosphere, 2017, 188, 130–138. [DOI] [PubMed] [Google Scholar]
- 106.Bonmatin JM, Moineau I, Charvet R, Colin ME, Fleche C. and Bengsch ER, in Environmental Chemistry, Springer, 2005, pp. 483–494. [Google Scholar]
- 107.Hladik ML, Bradbury S, Schulte LA, Helmers M, Witte C, Kolpin DW, Garrett JD and Harris M, Neonicotinoid insecticide removal by prairie strips in row-cropped watersheds with historical seed coating use, Agric., Ecosyst. Environ, 2017, 241, 160–167. [Google Scholar]
- 108.de Perre C, Murphy TM and Lydy MJ, Fate and effects of clothianidin in fields using conservation practices, Environ. Toxicol. Chem, 2015, 34, 258–265. [DOI] [PubMed] [Google Scholar]
- 109.Schaafsma A, Limay-Rios V, Baute T, Smith J. and Xue Y, Neonicotinoid Insecticide Residues in Surface Water and Soil Associated with Commercial Maize (Corn) Fields in Southwestern Ontario, PLoS One, 2015, 10, e0118139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu T, Dyer DG, McConnell LL, Bondarenko S, Allen R. and Heinemann O, Clothianidin in agricultural soils and uptake into corn pollen and canola nectar after multiyear seed treatment applications, Environ. Toxicol. Chem, 2016, 35, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hladik ML and Kolpin DW, First national-scale reconnaissance of neonicotinoid insecticides in streams across the USA, Environmental Chemistry, 2015, 13, 12–20. [Google Scholar]
- 112.Schaafsma A, Limay-Rios V, Xue Y, Smith J. and Baute T, Field-scale examination of neonicotinoid insecticide persistence in soil as a result of seed treatment use in commercial maize (corn) fields in southwestern Ontario, Environ. Toxicol. Chem, 2016, 35, 295–302. [DOI] [PubMed] [Google Scholar]
- 113.European Commission, Draft assessment Report (DAR) – Public Version – Initial risk assessment provided by the rapporteur Member State Germany for the existing active substance imidacloprid of the Third Stage (Part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC.Journal, 2006. [Google Scholar]
- 114.Lamers M, Anyusheva M, La N, Nguyen VV and Streck T, Pesticide Pollution in Surface-and Groundwater by Paddy Rice Cultivation: A Case Study from Northern Vietnam, Clean–Soil, Air, Water, 2011, 39, 356–361. [Google Scholar]
- 115.Hladik ML and Calhoun DL, Analysis of the herbicide diuron, three diuron degradates, and six neonicotinoid insecticides in water-Method details and application to two Georgia streams, Report 2012–5206, Reston, VA, 2012. [Google Scholar]
- 116.Hladik ML, Corsi SR, Kolpin DW, Baldwin AK, Blackwell BR and Cavallin JE, Year-round presence of neonicotinoid insecticides in tributaries to the Great Lakes, USA, Environ. Pollut, 2018, 235, 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hladik ML, Kolpin DW and Kuivila KM, Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA, Environ. Pollut, 2014, 193, 189–196. [DOI] [PubMed] [Google Scholar]
- 118.Klarich KL, Pflug NC, DeWald EM, Hladik ML, Kolpin DW, Cwiertny DM and LeFevre GH, Occurrence of Neonicotinoid Insecticides in Finished Drinking Water and Fate during Drinking Water Treatment, Environmental Science and Technology Letters, 2017, 4, 168–173. [Google Scholar]
- 119.Zhang C, Tian D, Yi X, Zhang T, Ruan J, Wu R, Chen C, Huang M. and Ying G, Occurrence, distribution and seasonal variation of five neonicotinoid insecticides in surface water and sediment of the Pearl Rivers, South China, Chemosphere, 2019, 217, 437–446. [DOI] [PubMed] [Google Scholar]
- 120.Bradford BZ, Huseth AS and Groves RL, Widespread detections of neonicotinoid contaminants in central Wisconsin groundwater, PLoS One, 2018, 13, e0201753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sanchez-Bayo F. and Hyne RV, Detection and analysis of neonicotinoids in river waters--development of a passive sampler for three commonly used insecticides, Chemosphere, 2014, 99, 143–151. [DOI] [PubMed] [Google Scholar]
- 122.Starner K. and Goh KS, Detections of the neonicotinoid insecticide imidacloprid in surface waters of three agricultural regions of California, USA, 2010–2011, Bull. Environ. Contam. Toxicol, 2012, 88, 316–321. [DOI] [PubMed] [Google Scholar]
- 123.Kreuger J, Graaf S, Patring J. and Adielsson S, Pesticides in surface water in areas with open ground and greenhouse horticultural crops in Sweden 2008, 2010. [Google Scholar]
- 124.Huseth AS and Groves RL, Environmental Fate of Soil Applied Neonicotinoid Insecticides in an Irrigated Potato Agroecosystem, PLoS One, 2014, 9, e97081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Johnson H, Juenemann M, MacDonald M, Matteson S, Paddock J, Rassumussen K, Ribikawskis M, Schaefer B, Timm D. and Tollefson D, 2016. Water Quality Monitoring Report: January - December 2016, http://www.mda.state.mn.us/sites/default/files/inline-files/2016wqmrpt.pdf). [Google Scholar]
- 126.Guzsvány V, Csanádi J. and Gaál F, NMR study of the influence of pH on the persistence of some neonicotinoids in water, Acta Chimica Slovenica, 2006, 53, 52. [Google Scholar]
- 127.Mulligan RA, Tomco PL, Howard MW, Schempp TT, Stewart DJ, Stacey PM, Ball DB and Tjeerdema RS, Aerobic versus anaerobic microbial degradation of clothianidin under simulated california rice field conditions, J. Agric. Food Chem, 2016, 64, 7059–7067. [DOI] [PubMed] [Google Scholar]
- 128.Liu Z, Dai Y, Huang G, Gu Y, Ni J, Wei H. and Yuan S, Soil microbial degradation of neonicotinoid insecticides imidacloprid, acetamiprid, thiacloprid and imidaclothiz and its effect on the persistence of bioefficacy against horsebean aphid Aphis craccivora Koch after soil application, Pest Manage. Sci, 2011, 67, 1245–1252. [DOI] [PubMed] [Google Scholar]
- 129.Sarkar MA, Roy S, Kole RK and Chowdhury A, Persistence and metabolism of imidacloprid in different soils of West Bengal, Pest Manage. Sci, 2001, 57, 598–602. [DOI] [PubMed] [Google Scholar]
- 130.Main AR, Headley JV, Peru KM, Michel NL, Cessna AJ and Morrissey CA, Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada's Prairie Pothole Region, PLoS One, 2014, 9, e92821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krupke CH, Hunt GJ, Eitzer BD, Andino G. and Given K, Multiple routes of pesticide exposure for honey bees living near agricultural fields, PLoS One, 2012, 7, e29268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nuyttens D, Devarrewaere W, Verboven P. and Foqué D, Pesticide-laden dust emission and drift from treated seeds during seed drilling: a review, Pest Manage. Sci, 2013, 69, 564–575. [DOI] [PubMed] [Google Scholar]
- 133.Main AR, Michel NL, Cavallaro MC, Headley JV, Peru KM and Morrissey CA, Snowmelt transport of neonicotinoid insecticides to Canadian Prairie wetlands, 2016. [Google Scholar]
- 134.Wan Y, Wang Y, Xia W, He Z. and Xu S, Neonicotinoids in raw, finished, and tap water from Wuhan, Central China: Assessment of human exposure potential, Sci. Total Environ, 2019, 675, 513–519. [DOI] [PubMed] [Google Scholar]
- 135.Struger J, Grabuski J, Cagampan S, Sverko E, McGoldrick D. and Marvin CH, Factors influencing the occurrence and distribution of neonicotinoid insecticides in surface waters of southern Ontario, Canada, Chemosphere, 2017, 169, 516–523. [DOI] [PubMed] [Google Scholar]
- 136.Health Canada, Guidelines for Canadian Drinking Water Quality - Summary Table, https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewh-semt/alt_formats/pdf/pubs/water-eau/sum_guide-res_recom/sum_guide-res_recom-eng.pdf). [Google Scholar]
- 137.U.S. Environmental Protection Agency, National Primary Drinking Water Regulations, https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations). [Google Scholar]
- 138.European Parliament and Council, Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption, Official Journal of the European Union, 1998, vol. L330, 32–54. [Google Scholar]
- 139.European Parliament and Council, Directive 2000/60/EC of the of 23 October 2000 establishing a framework for community action in the field of water policy, Official Journal of the European Union, 2000, L327. [Google Scholar]
- 140.European Parliament and Council, Directive 2006/118/EC of 12 December 2006 on the protection of groundwater against pollution and deterioration, Official Journal of the European Union L, 2006, 372, 19–31. [Google Scholar]
- 141.European Parliament and Council, Directive 2013/39/EU of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy, Official Journal of the European Union, 2013, L226. [Google Scholar]
- 142.European Parliament and Council, European Union Directive 2008/105/EC of the European Parliament and of the Council on environmental quality standards in the field of water policy, Official Journal of European Union L, 2008, 348, 84–97. [Google Scholar]
- 143.Nowell LH, Moran PW, Schmidt TS, Norman JE, Nakagaki N, Shoda ME, Mahler BJ, Van Metre PC, Stone WW and Sandstrom MW, Complex mixtures of dissolved pesticides show potential aquatic toxicity in a synoptic study of Midwestern US streams, Sci. Total Environ, 2018, 613, 1469–1488. [DOI] [PubMed] [Google Scholar]
- 144.U.S. Food and Drug Administration,, Education and Welfare Food Memorandum of Understanding Between The Environmental Protection Agency and The United States Department of Healthand Drug Administration, https://www.fda.gov/about-fda/domestic-mous/mou-225-73-8010). [Google Scholar]
- 145.Craddock HA, Huang D, Turner PC, Quirós-Alcalá L. and Payne-Sturges DC, Trends in neonicotinoid pesticide residues in food and water in the United States, 1999–2015, Environ. Health, 2019, 18, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Klarich Wong KL, Webb DT, Nagorzanski MR, Kolpin DW, Hladik ML, Cwiertny DM and LeFevre GH, Chlorinated byproducts of neonicotinoids and their metabolites: An unrecognized human exposure potential?, Environmental Science and Technology Letters, 2019, 6, 98–105. [Google Scholar]
- 147.Sultana T, Murray C, Kleywegt S. and Metcalfe CD, Neonicotinoid pesticides in drinking water in agricultural regions of southern Ontario, Canada, Chemosphere, 2018, 202, 506–513. [DOI] [PubMed] [Google Scholar]
- 148.Montiel-León JM, Duy SV, Munoz G, Amyot M. and Sauvé S, Evaluation of on-line concentration coupled to liquid chromatography tandem mass spectrometry for the quantification of neonicotinoids and fipronil in surface water and tap water, Anal. Bioanal. Chem, 2018, 410, 2765–2779. [DOI] [PubMed] [Google Scholar]
- 149.World Health Organization, Drinking-water, https://www.who.int/news-room/fact-sheets/detail/drinking-water). [Google Scholar]
- 150.Dieter CA, Maupin MA, Caldwell RR, Harris MA, Ivahnenko T, Lovelace JK, Barber NL and Linsey KS, Water Availability and Use Science Program: Estimated Use of Water in the United States In 2015, U.S. Geological Survey Circular 1441, 2018. [Google Scholar]
- 151.U.S. Environmental Protection Agency, Potential Well Water Contaminants and Their Impacts, https://www.epa.gov/privatewells/potential-well-water-contaminants-and-their-impacts, (accessed August 30, 2019).
- 152.Schmoll O, Howard G, Chilton J. and Chorus I, Protecting groundwater for health: managing the quality of drinking-water sources, World Health Organization, 2006. [Google Scholar]
- 153.DeSimone LA and Hamilton PA, Quality of water from domestic wells in principal aquifers of the United States, 1991–2004, U.S. Geological Survey Circular 1332, 2009. [Google Scholar]
- 154.Gatto NM, Cockburn M, Bronstein J, Manthripragada AD and Ritz B, Well-water consumption and Parkinson’s disease in rural California, Environ. Health Perspect, 2009, 117, 1912–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yoshida T, Yamauchi H. and Sun GF, Chronic health effects in people exposed to arsenic via the drinking water: dose–response relationships in review, Toxicol. Appl. Pharmacol, 2004, 198, 243–252. [DOI] [PubMed] [Google Scholar]
- 156.Ayotte JD, Baris D, Cantor KP, Colt J, Robinson GR, Lubin JH, Karagas M, Hoover RN, Fraumeni JF and Silverman DT, Bladder cancer mortality and private well use in New England: an ecological study, J. Epidemiol. Community Health, 2006, 60, 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Inoue-Choi M, Jones RR, Anderson KE, Cantor KP, Cerhan JR, Krasner S, Robien K, Weyer PJ and Ward MH, Nitrate and nitrite ingestion and risk of ovarian cancer among postmenopausal women in Iowa, Int. J. Cancer, 2015, 137, 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sanders AP, Desrosiers TA, Warren JL, Herring AH, Enright D, Olshan AF, Meyer RE and Fry RC, Association between arsenic, cadmium, manganese, and lead levels in private wells and birth defects prevalence in North Carolina: a semi-ecologic study, BMC Public Health, 2014, 14, 955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Magalhaes LC, Hunt TE and Siegfried BD, Efficacy of neonicotinoid seed treatments to reduce soybean aphid populations under field and controlled conditions in Nebraska, J. Econ. Entomol, 2009, 102, 187–195. [DOI] [PubMed] [Google Scholar]
- 160.Stoner KA and Eitzer BD, Movement of soil-applied imidacloprid and thiamethoxam into nectar and pollen of squash (Cucurbita pepo), PLoS One, 2012, 7, e39114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bonmatin J, Moineau I, Charvet R, Fleche C, Colin M. and Bengsch E, A LC/APCI-MS/MS method for analysis of imidacloprid in soils, in plants, and in pollens, Anal. Chem, 2003, 75, 2027–2033. [DOI] [PubMed] [Google Scholar]
- 162.Chauzat M-P, Faucon J-P, Martel A-C, Lachaize J, Cougoule N. and Aubert M, A survey of pesticide residues in pollen loads collected by honey bees in France, J. Econ. Entomol, 2006, 99, 253–262. [DOI] [PubMed] [Google Scholar]
- 163.Girolami V, Mazzon L, Squartini A, Mori N, Marzaro M, Di Bernardo A, Greatti M, Giorio C. and Tapparo A, Translocation of neonicotinoid insecticides from coated seeds to seedling guttation drops: a novel way of intoxication for bees, J. Econ. Entomol, 2009, 102, 1808–1815. [DOI] [PubMed] [Google Scholar]
- 164.Wise JC, Vanderpoppen R. and Vandervoort C, Curative activity of insecticides on Rhagoletis pomonella (Diptera: Tephritidae) in apples, J. Econ. Entomol, 2009, 102, 1884–1890. [DOI] [PubMed] [Google Scholar]
- 165.Mota-Sanchez D, Cregg B, Hoffmann E, Flore J. and Wise JC, Penetrative and dislodgeable residue characteristics of 14C-insecticides in apple fruit, J. Agric. Food Chem, 2012, 60, 2958–2966. [DOI] [PubMed] [Google Scholar]
- 166.Hoffmann EJ, Vandervoort C. and Wise JC, Plum curculio (Coleoptera: Curculionidae) adult mortality and associated fruit injury after exposure to field-aged insecticides on tart cherry branches, J. Econ. Entomol, 2010, 103, 1196–1205. [DOI] [PubMed] [Google Scholar]
- 167.Pramanik SK, Bhattacharyya J, Dutta S, Dey PK and Bhattacharyya A, Persistence of Acetamiprid in/on Mustard (Brassica juncea L.), Bull. Environ. Contam. Toxicol, 2006, 76. [DOI] [PubMed] [Google Scholar]
- 168.Sanyal D, Chakma D. and Alam S, Persistence of a neonicotinoid insecticide, acetamiprid on chili (Capsicum annum L.), Bull. Environ. Contam. Toxicol, 2008, 81, 365–368. [DOI] [PubMed] [Google Scholar]
- 169.Venkateswarlu P, Mohan KR, Kumar CR and Seshaiah KR, Monitoring of multi-class pesticide residues in fresh grape samples using liquid chromatography with electrospray tandem mass spectrometry, Food Chem, 2007, 105, 1760–1766. [Google Scholar]
- 170.Yu Y.-l., Wu J.-l., Stahler M. and Pestemer W, Residual dynamics of thiacloprid in medical herbs marjoram, thyme, and camomile in soil, Journal of environmental sciences, 2007, 19, 205–209. [DOI] [PubMed] [Google Scholar]
- 171.Romeh AA, Mekky TM, Ramadan RA and Hendawi MY, Dissipation of profenofos, imidacloprid and penconazole in tomato fruits and products, Bull. Environ. Contam. Toxicol, 2009, 83, 812. [DOI] [PubMed] [Google Scholar]
- 172.Wang P, Yang X, Wang J, Cui J, Dong A, Zhao H, Zhang L, Wang Z, Xu R. and Li W, Multi-residue method for determination of seven neonicotinoid insecticides in grains using dispersive solid-phase extraction and dispersive liquid–liquid micro-extraction by high performance liquid chromatography, Food Chem, 2012, 134, 1691–1698. [DOI] [PubMed] [Google Scholar]
- 173.Liu S, Zheng Z, Wei F, Ren Y, Gui W, Wu H. and Zhu G, Simultaneous determination of seven neonicotinoid pesticide residues in food by ultraperformance liquid chromatography tandem mass spectrometry, J. Agric. Food Chem, 2010, 58, 3271–3278. [DOI] [PubMed] [Google Scholar]
- 174.Kapoor U, Srivastava M, Srivastava AK, Patel D, Garg V. and Srivastava L, Analysis of imidacloprid residues in fruits, vegetables, cereals, fruit juices, and baby foods, and daily intake estimation in and around Lucknow, India, Environ. Toxicol. Chem, 2013, 32, 723–727. [DOI] [PubMed] [Google Scholar]
- 175.Abd-Alrahman SH, Residue and dissipation kinetics of thiamethoxam in a vegetable-field ecosystem using QuEChERS methodology combined with HPLC–DAD, Food Chem, 2014, 159, 1–4. [DOI] [PubMed] [Google Scholar]
- 176.Bakırcı GT, Acay DBY, Bakırcı F. and Ötleş S, Pesticide residues in fruits and vegetables from the Aegean region, Turkey, Food Chem, 2014, 160, 379–392. [DOI] [PubMed] [Google Scholar]
- 177.Andrade GC, Monteiro SH, Francisco JG, Figueiredo LA, Rocha AA and Tornisielo VL, Effects of types of washing and peeling in relation to pesticide residues in tomatoes, J. Braz. Chem. Soc, 2015, 26, 1994–2002. [Google Scholar]
- 178.Huang JX, Liu CY, Lu DH, Chen JJ, Deng YC and Wang FH, Residue behavior and risk assessment of mixed formulation of imidacloprid and chlorfenapyr in chieh-qua under field conditions, Environ. Monit. Assess, 2015, 187, 650. [DOI] [PubMed] [Google Scholar]
- 179.Teló GM, Marchesan E, Zanella R, Limberger de Oliveira M, Coelho LL and Martins ML, Residues of fungicides and insecticides in rice field, Agron. J, 2015, 107, 851–863. [Google Scholar]
- 180.Bhattacherjee AK and Dikshit A, Dissipation kinetics and risk assessment of thiamethoxam and dimethoate in mango, Environ. Monit. Assess, 2016, 188, 165. [DOI] [PubMed] [Google Scholar]
- 181.Balfour NJ, Carreck NL, Blanchard HE and Ratnieks FLW, Size matters: Significant negative relationship between mature plant mass and residual neonicotinoid levels in seed-treated oilseed rape and maize crops, Agric., Ecosyst. Environ, 2016, 215, 85–88. [Google Scholar]
- 182.Fang Q, Shi Y, Cao H, Tong Z, Xiao J, Liao M, Wu X. and Hua R, Degradation dynamics and dietary risk assessments of two neonicotinoid insecticides during lonicera japonica planting, drying, and tea brewing processes, J. Agric. Food Chem, 2017, 65, 1483–1488. [DOI] [PubMed] [Google Scholar]
- 183.Golge O, Hepsag F. and Kabak B, Health risk assessment of selected pesticide residues in green pepper and cucumber, Food Chem. Toxicol, 2018, 121, 51–64. [DOI] [PubMed] [Google Scholar]
- 184.Lu C, Chang C-H, Palmer C, Zhao M. and Zhang Q, Neonicotinoid residues in fruits and vegetables: An integrated dietary exposure assessment approach, Environ. Sci. Technol, 2018, 52, 3175–3184. [DOI] [PubMed] [Google Scholar]
- 185.Zywitz D, Anastassiades M. and Scherbaum E, Analysis of Neonicotinoid Insecticides in Fruits and Vegetables using LC-MS (MS), http://cvuas.xn--untersuchungsmter-bw-nzb.de/pdf/poster_neonicotinoid_insecticides_eprw.pdf). [Google Scholar]
- 186.U.S. Department of Agriculture, Pesticide Data Program, Annual Summary Calendar Year 2015, https://www.ams.usda.gov/datasets/pdp, (accessed 09/28, 2018).
- 187.Ueyama J, Harada KH, Koizumi A, Sugiura Y, Kondo T, Saito I. and Kamijima M, Temporal Levels of Urinary Neonicotinoid and Dialkylphosphate Concentrations in Japanese Women Between 1994 and 2011, Environ. Sci. Technol, 2015, 49, 14522–14528. [DOI] [PubMed] [Google Scholar]
- 188.Juraske R, Castells F, Vijay A, Muñoz P. and Antón A, Uptake and persistence of pesticides in plants: measurements and model estimates for imidacloprid after foliar and soil application, J. Hazard. Mater, 2009, 165, 683–689. [DOI] [PubMed] [Google Scholar]
- 189.Kamel A, Refined Methodology for the Determination of Neonicotinoid Pesticides and Their Metabolites in Honey Bees and Bee Products by Liquid Chromatography−Tandem Mass Spectrometry (LC-MS/MS), J. Agric. Food Chem, 2010, 58, 5926–5931. [DOI] [PubMed] [Google Scholar]
- 190.Mitchell EAD, Mulhauser B, Mulot M, Mutabazi A, Glauser G. and Aebi A, A worldwide survey of neonicotinoids in honey, Science, 2017, 358, 109–111. [DOI] [PubMed] [Google Scholar]
- 191.Sanchez-Bayo F. and Goka K, Pesticide residues and bees--a risk assessment, PLoS One, 2014, 9, e94482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Blacquiere T, Smagghe G, van Gestel CAM and Mommaerts V, Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment, Ecotoxicology, 2012, 21, 973–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Campillo N, Vinas P, Ferez-Melgarejo G. and Hernandez-Cordoba M, Liquid chromatography with diode array detection and tandem mass spectrometry for the determination of neonicotinoid insecticides in honey samples using dispersive liquid-liquid microextraction, J. Agric. Food Chem, 2013, 61, 4799–4805. [DOI] [PubMed] [Google Scholar]
- 194.Gbylik-Sikorska M, Sniegocki T. and Posyniak A, Determination of neonicotinoid insecticides and their metabolites in honey bee and honey by liquid chromatography tandem mass spectrometry, J. Chromatogr. B, 2015, 990, 132–140. [DOI] [PubMed] [Google Scholar]
- 195.Tanner G. and Czerwenka C, LC-MS/MS Analysis of Neonicotinoid Insecticides in Honey: Methodology and Residue Findings in Austrian Honeys, J. Agric. Food Chem, 2011, 59, 12271–12277. [DOI] [PubMed] [Google Scholar]
- 196.Jones A. and Turnbull G, Neonicotinoid concentrations in UK honey from 2013, Pest Manage. Sci, 2016, 72, 1897–1900. [DOI] [PubMed] [Google Scholar]
- 197.Codling G, Al Naggar Y, Giesy JP and Robertson AJ, Concentrations of neonicotinoid insecticides in honey, pollen and honey bees (Apis mellifera L.) in central Saskatchewan, Canada, Chemosphere, 2016, 144, 2321–2328. [DOI] [PubMed] [Google Scholar]
- 198.Harada KH, Tanaka K, Sakamoto H, Imanaka M, Niisoe T, Hitomi T, Kobayashi H, Okuda H, Inoue S, Kusakawa K, Oshima M, Watanabe K, Yasojima M, Takasuga T. and Koizumi A, Biological Monitoring of Human Exposure to Neonicotinoids Using Urine Samples, and Neonicotinoid Excretion Kinetics, PLoS One, 2016, 11, e0146335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang Q, Lu Z, Chang C-H, Yu C, Wang X. and Lu C, Dietary risk of neonicotinoid insecticides through fruit and vegetable consumption in school-age children, Environ. Int, 2019, 126, 672–681. [DOI] [PubMed] [Google Scholar]
- 200.New Zealand Food Safety, Pesticide maximum residue level legislation around the world, https://www.mpi.govt.nz/growing-and-harvesting/plant-products/pesticide-maximum-residue-levels-mrls-for-plant-based-foods/pesticide-maximum-residue-level-legislation-around-the-world/, (accessed August 22, 2019).
- 201.U.S. Environmental Protection Agency, Pesticide Tolerances: Thiamethoxam, https://www.regulations.gov/document?D=EPA-HQ-OPP-2015-0705-0006, (accessed August 22, 2019).
- 202.U.S. Environmental Protection Agency, Pesticide Tolerances: Thiacloprid, https://www.regulations.gov/document?D=EPA-HQ-OPP-2010-0311–0004, (accessed August 22, 2019).
- 203.U.S. Environmental Protection Agency, Pesticide Tolerances: Dinotefuran, https://www.regulations.gov/document?D=EPA-HQ-OPP-2012-0060-0005, (accessed August 22, 2019).
- 204.U.S. Environmental Protection Agency, Pesticide Tolerances: Acetamiprid, https://www.regulations.gov/document?D=EPA-HQ-OPP-2011-0792-0005, (accessed August 22, 2019).
- 205.U.S. Environmental Protection Agency, Pesticide Tolerances: Clothianidin, https://www.regulations.gov/document?D=EPA-HQ-OPP-2010-0217-0044, (accessed August 22, 2019).
- 206.U.S. Environmental Protection Agency, Pesticide Tolerances: Imidacloprid, https://www.regulations.gov/document?D=EPA-HQ-OPP-2012-0204-0005, (accessed August 22, 2019).
- 207.U.S. Environmental Protection Agency, Acetamiprid. Human Health Assessment Scoping Document in Support of Registration Review, https://www.regulations.gov/document?D=EPA-HQ-OPP-2012-0329–0004). [Google Scholar]
- 208.Health Canada, Maximum Residue Limits for Pesticides, http://pr-rp.hc-sc.gc.ca/mrl-lrm/index-eng.php, (accessed September 9. 2019). [Google Scholar]
- 209.The Japan Food Chemical Research Foundation, Maximum Residue Limits (MRLs) List of Agricultural Chemicals in Foods, http://db.ffcr.or.jp/front/, (accessed September 9, 2019).
- 210.World Health Organization, Inventory of evaluations performed by the Joint Meeting on Pesticide Residues (JMPR), http://apps.who.int/pesticide-residues-jmpr-database, (accessed August 22, 2019).
- 211.Casida JE, Neonicotinoid metabolism: compounds, substituents, pathways, enzymes, organisms, and relevance, J. Agric. Food Chem, 2011, 59, 2923–2931. [DOI] [PubMed] [Google Scholar]
- 212.Tomizawa M, Lee DL and Casida JE, Neonicotinoid Insecticides: Molecular Features Conferring Selectivity for Insect versus Mammalian Nicotinic Receptors, J. Agric. Food Chem, 2000, 48, 6016–6024. [DOI] [PubMed] [Google Scholar]
- 213.Dai Y-J, Ji W-W, Chen T, Zhang W-J, Liu Z-H, Ge F. and Yuan S, Metabolism of the Neonicotinoid insecticides acetamiprid and thiacloprid by the yeast Rhodotorula mucilaginosa strain IM-2, J. Agric. Food Chem, 2010, 58, 2419–2425. [DOI] [PubMed] [Google Scholar]
- 214.Zhang H-J, Zhou Q-W, Zhou G-C, Cao Y-M, Dai Y-J, Ji W-W, Shang G-D and Yuan S, Biotransformation of the neonicotinoid insecticide thiacloprid by the bacterium Variovorax boronicumulans strain J1 and mediation of the major metabolic pathway by nitrile hydratase, J. Agric. Food Chem, 2012, 60, 153–159. [DOI] [PubMed] [Google Scholar]
- 215.Dai Y-J, Yuan S, Ge F, Chen T, S.-c. Xu and J.-p. Ni, Microbial hydroxylation of imidacloprid for the synthesis of highly insecticidal olefin imidacloprid, Appl. Microbiol. Biotechnol, 2006, 71, 927–934. [DOI] [PubMed] [Google Scholar]
- 216.Wang J, Hirai H. and Kawagishi H, Biotransformation of acetamiprid by the white-rot fungus Phanerochaete sordida YK-624, Appl. Microbiol. Biotechnol, 2012, 93, 831–835. [DOI] [PubMed] [Google Scholar]
- 217.Lu Z, Challis JK and Wong CS, Quantum Yields for Direct Photolysis of Neonicotinoid Insecticides in Water: Implications for Exposure to Nontarget Aquatic Organisms, Environmental Science and Technology Letters, 2015, 2, 188–192. [Google Scholar]
- 218.Todey SA, Fallon AM and Arnold WA, Neonicotinoid insecticide hydrolysis and photolysis: Rates and residual toxicity, Environ. Toxicol. Chem, 2018, 37, 2797–2809. [DOI] [PubMed] [Google Scholar]
- 219.Aregahegn KZ, Shemesh D, Gerber RB and Finlayson-Pitts BJ, Photochemistry of Thin Solid Films of the Neonicotinoid Imidacloprid on Surfaces, Environ. Sci. Technol, 2017, 51, 2660–2668. [DOI] [PubMed] [Google Scholar]
- 220.Moza P, Hustert K, Feicht EA and Kettrup A, Photolysis of imidacloprid in aqueous solution, Chemosphere, 1998, 36, 497–502. [Google Scholar]
- 221.Zabar R, Dolenc D, Jerman T, Franko M. and Trebse P, Photolytic and photocatalytic degradation of 6-chloronicotinic acid, Chemosphere, 2011, 85, 861–868. [DOI] [PubMed] [Google Scholar]
- 222.Gong Y, Chen J, Wang H. and Li J, Separation and Identification of Photolysis Products of Clothianidin by Ultra-Performance Liquid Tandem Mass Spectrometry, Anal. Lett, 2012, 45, 2483–2492. [Google Scholar]
- 223.Dell'arciprete ML, Santos-Juanes L, Sanz AA, Vicente R, Amat AM, Furlong JP, Martire DO and Gonzalez MC, Reactivity of hydroxyl radicals with neonicotinoid insecticides: mechanism and changes in toxicity, Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 2009, 8, 1016–1023. [DOI] [PubMed] [Google Scholar]
- 224.Karmakar R, Singh SB and Kulshrestha G, Persistence and transformation of thiamethoxam, a neonicotinoid insecticide, in soil of different agroclimatic zones of India, Bull. Environ. Contam. Toxicol, 2006, 76, 400–406. [DOI] [PubMed] [Google Scholar]
- 225.Karmakar R, Singh SB and Kulshrestha G, Kinetics and mechanism of the hydrolysis of thiamethoxam, J. Environ. Sci. Health, Pt. B: Pestic., Food Contam., Agric. Wastes, 2009, 44, 435–441. [DOI] [PubMed] [Google Scholar]
- 226.Liqing Z, Guoguang L, Dezhi S. and Kun Y, Hydrolysis of Thiamethoxam, Bull. Environ. Contam. Toxicol, 2006, 76, 942–949. [DOI] [PubMed] [Google Scholar]
- 227.Leiva JA, Nkedi-Kizza P, Morgan KT and Qureshi JA, Imidacloprid Sorption Kinetics, Equilibria, and Degradation in Sandy Soils of Florida, J. Agric. Food Chem, 2015, 63, 4915–4921. [DOI] [PubMed] [Google Scholar]
- 228.Pandey G, Dorrian SJ, Russell RJ and Oakeshott JG, Biotransformation of the neonicotinoid insecticides imidacloprid and thiamethoxam by Pseudomonas sp. 1G, Biochem. Biophys. Res. Commun, 2009, 380, 710–714. [DOI] [PubMed] [Google Scholar]
- 229.Zhou G-C, Wang Y, Zhai S, Ge F, Liu Z-H, Dai Y-J, Yuan S. and J.-y. Hou, Biodegradation of the neonicotinoid insecticide thiamethoxam by the nitrogen-fixing and plant-growth-promoting rhizobacterium Ensifer adhaerens strain TMX-23, Appl. Microbiol. Biotechnol, 2013, 97, 4065–4074. [DOI] [PubMed] [Google Scholar]
- 230.Yang H, Wang X, Zheng J, Wang G, Hong Q, Li S, Li R. and Jiang J, Biodegradation of acetamiprid by Pigmentiphaga sp. D-2 and the degradation pathway, Int. Biodeterior. Biodegradation, 2013, 85, 95–102. [Google Scholar]
- 231.Wang G, Yue W, Liu Y, Li F, Xiong M. and Zhang H, Biodegradation of the neonicotinoid insecticide Acetamiprid by bacterium Pigmentiphaga sp. strain AAP-1 isolated from soil, Bioresour. Technol, 2013, 138, 359–368. [DOI] [PubMed] [Google Scholar]
- 232.Tang H, Li J, Hu H. and Xu P, A newly isolated strain of Stenotrophomonas sp. hydrolyzes acetamiprid, a synthetic insecticide, Process Biochem, 2012, 47, 1820–1825. [Google Scholar]
- 233.Chen T, Dai Y-J, Ding J-F, Yuan S. and Ni J-P, N-demethylation of neonicotinoid insecticide acetamiprid by bacterium Stenotrophomonas maltophilia CGMCC 1.1788, Biodegradation, 2008, 19, 651–658. [DOI] [PubMed] [Google Scholar]
- 234.Ge F, Zhou L-Y, Wang Y, Ma Y, Zhai S, Liu Z-H, Dai Y-J and Yuan S, Hydrolysis of the neonicotinoid insecticide thiacloprid by the N2-fixing bacterium Ensifer meliloti CGMCC 7333, Int. Biodeterior. Biodegrad, 2014, 93, 10–17. [Google Scholar]
- 235.Zhou LY, Zhang LJ, Sun SL, Ge F, Mao SY, Ma Y, Liu ZH, Dai YJ and Yuan S, Degradation of the neonicotinoid insecticide acetamiprid via the N-carbamoylimine derivate (IM-1–2) mediated by the nitrile hydratase of the nitrogen-fixing bacterium Ensifer meliloti CGMCC 7333, J. Agric. Food Chem, 2014, 62, 9957–9964. [DOI] [PubMed] [Google Scholar]
- 236.Cycon M, Markowicz A, Borymski S, Wojcik M. and Piotrowska-Seget Z, Imidacloprid induces changes in the structure, genetic diversity and catabolic activity of soil microbial communities, J. Environ. Manage, 2013, 131, 55–65. [DOI] [PubMed] [Google Scholar]
- 237.Cycon M. and Piotrowska-Seget Z, Biochemical and microbial soil functioning after application of the insecticide imidacloprid, Journal of environmental sciences, 2015, 27, 147–158. [DOI] [PubMed] [Google Scholar]
- 238.Mahapatra B, Adak T, Patil NKB, Pandi G GP, Gowda GB, Jambhulkar NN, Yadav MK, Panneerselvam P, Kumar U, Munda S. and Jena M, Imidacloprid application changes microbial dynamics and enzymes in rice soil, Ecotoxicol. Environ. Saf, 2017, 144, 123–130. [DOI] [PubMed] [Google Scholar]
- 239.Chagnon M, Kreutzweiser D, Mitchell EA, Morrissey CA, Noome DA and Van der Sluijs JP, Risks of large-scale use of systemic insecticides to ecosystem functioning and services, Environ. Sci. Pollut. Res. Int, 2015, 22, 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mori T, Wang J, Tanaka Y, Nagai K, Kawagishi H. and Hirai H, Bioremediation of the neonicotinoid insecticide clothianidin by the white-rot fungus Phanerochaete sordida, J. Hazard. Mater, 2017, 321, 586–590. [DOI] [PubMed] [Google Scholar]
- 241.Brunet JL, Badiou A. and Belzunces LP, In vivo metabolic fate of [14C]-acetamiprid in six biological compartments of the honeybee, Apis mellifera L, Pest Manage. Sci, 2005, 61, 742–748. [DOI] [PubMed] [Google Scholar]
- 242.Ford KA and Casida JE, Chloropyridinyl neonicotinoid insecticides: diverse molecular substituents contribute to facile metabolism in mice., Chem. Res. Toxicol, 2006, 19, 944–951. [DOI] [PubMed] [Google Scholar]
- 243.Ford KA and Casida JE, Comparative Metabolism and Pharmacokinetics of Seven Neonicotinoid Insecticides in Spinach, J. Agric. Food Chem, 2008, 56, 10168–10175. [DOI] [PubMed] [Google Scholar]
- 244.Tokieda M, Ozawa M, Kobayashi S. and Gomyo T, Methods of determination of acetamiprid and its degradation products in soil by gas chromatography, J. Pestic. Sci, 1999, 24, 181–185. [Google Scholar]
- 245.Elbert A, Haas M, Springer B, Thielert W. and Nauen R, Applied aspects of neonicotinoid uses in crop protection, Pest Manage. Sci, 2008, 64: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 246.Ford KA, Metabolism, pharmacokinetics and toxicology of the neonicotinoid insecticides in mice, University of California, Berkeley, 2008. [Google Scholar]
- 247.Ford KA, Casida JE, Chandran D, Gulevich AG, Okrent RA, Durkin KA, Sarpong R, Bunnelle EM and Wildermuth MC, Neonicotinoid insecticides induce salicylate-associated plant defense responses, Proc. Natl. Acad. Sci. U. S. A, 2010, 107, 17527–17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Muerdter CP and LeFevre GH, Synergistic Lemna Duckweed and Microbial Transformation of Imidacloprid and Thiacloprid Neonicotinoids, Environmental Science & Technology Letters, 2019. [Google Scholar]
- 249.Shi X, Dick RA, Ford KA and Casida JE, Enzymes and inhibitors in neonicotinoid insecticide metabolism, J. Agric. Food Chem, 2009, 57, 4861–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Marfo JT, Fujioka K, Ikenaka Y, Nakayama SMM, Mizukawa H, Aoyama Y, Ishizuka M. and Taira K, Relationship between Urinary N-Desmethyl-Acetamiprid and Typical Symptoms including Neurological Findings: A Prevalence Case-Control Study, PLoS One, 2015, 10, e0142172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Renaud HJ, Cui JY, Khan M. and Klaassen CD, Tissue distribution and gender-divergent expression of 78 cytochrome P450 mRNAs in mice, Toxicol. Sci, 2011, 124, 261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Vila R, Kurosaki M, Barzago MM, Kolek M, Bastone A, Colombo L, Salmona M, Terao M. and Garattini E, Regulation and Biochemistry of Mouse Molybdo-flavoenzymes The DBA/2 Mouse is selectively deficient in the expression of aldehyde oxidase homologues 1 and 2 and represents a unique source for purification and characterization of aldehyde oxidase, J. Biol. Chem, 2004, 279, 8668–8683. [DOI] [PubMed] [Google Scholar]
- 253.Kasiotis KM and Marchera K, Neonicotinoids and their Metabolites in Human Biomonitoring: A Review, Hellenic Plant Protection Journal, 2015, 8, 33–45. [Google Scholar]
- 254.Taira K, Human neonicotinoids exposure in Japan, Japanese Journal of Clinical Ecology, 2014, 23, 14–24. [Google Scholar]
- 255.Taira K, Aoyama Y, Kawakami T, Kamata M. and Aoi T, Detection of chrolopyridinyl neonicotinoid insecticide metabolite 6-chloronicotinic acid in the urine: Six cases with subacute nicotinic symptoms, Japan Journal of Clinical Toxicology, 2011, 24, 222–230. [PubMed] [Google Scholar]
- 256.Taira K, Fujioka K. and Aoyama Y, Qualitative profiling and quantification of neonicotinoid metabolites in human urine by liquid chromatography coupled with mass spectrometry, PLoS One, 2013, 8, e80332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Uroz FJ, Arrebola FJ, Egea-Gonzalez FJ and Martinez-Vidal JL, Monitoring of 6-chloronicotinic acid in human urine by gas chromatography-tandem mass spectrometry as indicator of exposure to the pesticide imidacloprid, Analyst, 2001, 126, 1355–1358. [DOI] [PubMed] [Google Scholar]
- 258.Nomura H, Ueyama J, Kondo T, Saito I, Murata K, Iwata T, Wakusawa S. and Kamijima M, Quantitation of neonicotinoid metabolites in human urine using GC-MS, J. Chromatogr. B, 2013, 941, 109–115. [DOI] [PubMed] [Google Scholar]
- 259.Schulz-Jander DA and Casida JE, Imidacloprid insecticide metabolism: human cytochrome P450 isozymes differ in selectivity for imidazolidine oxidation versus nitroimine reduction, Toxicol. Lett, 2002, 132, 65–70. [DOI] [PubMed] [Google Scholar]
- 260.Schulz-Jander DA, Leimkuehler WM and Casida JE, Neonicotinoid insecticides: reduction and cleavage of imidacloprid nitroimine substituent by liver microsomal and cytosolic enzymes, Chem. Res. Toxicol, 2002, 15, 1158–1165. [DOI] [PubMed] [Google Scholar]
- 261.Brunet J-L, Maresca M, Fantini J. and Belzunces LP, Human intestinal absorption of imidacloprid with Caco-2 cells as enterocyte model, Toxicol. Appl. Pharmacol, 2004, 194, 1–9. [DOI] [PubMed] [Google Scholar]
- 262.Gibbons D, Morrissey C. and Mineau P, A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife, Environmental Science and Pollution Research, 2015, 22, 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Han W, Tian Y. and Shen X, Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview, Chemosphere, 2018, 192, 59–65. [DOI] [PubMed] [Google Scholar]
- 264.Roberts JR and Reigart JR, Recognition and management of pesticide poisonings, U.S. Environmental Protection Agency,, 1999. [Google Scholar]
- 265.Freed V, Haque R, Schmedding D. and Kohnert R, Physicochemical properties of some organophosphates in relation to their chronic toxicity, Environ. Health Perspect, 1976, 13, 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.U.S. Environmental Protection Agency, Chapter 5: Organophosphate insecticides, https://www.epa.gov/pesticide-worker-safety/recognition-and-management-pesticide-poisonings). [Google Scholar]
- 267.Annabi A, Dhouib IB, Lamine AJ, Golli NE, Gharbi N, Fazâa SE and Lasram MM, Recovery by N-acetylcysteine from subchronic exposure to Imidacloprid-induced hypothalamic–pituitary–adrenal (HPA) axis tissues injury in male rats, Toxicol. Mech. Methods, 2015, 25, 524–531. [DOI] [PubMed] [Google Scholar]
- 268.Kapoor U, Srivastava MK, Bhardwaj S. and Srivastava LP, Effect of imidacloprid on antioxidant enzymes and lipid peroxidation in female rats to derive its No Observed Effect Level (NOEL), The Journal of toxicological sciences, 2010, 35, 577–581. [DOI] [PubMed] [Google Scholar]
- 269.Lonare M, Kumar M, Raut S, Badgujar P, Doltade S. and Telang A, Evaluation of imidacloprid-induced neurotoxicity in male rats: a protective effect of curcumin, Neurochem. Int, 2014, 78, 122–129. [DOI] [PubMed] [Google Scholar]
- 270.Duzguner V. and Erdogan S, Chronic exposure to imidacloprid induces inflammation and oxidative stress in the liver & central nervous system of rats, Pestic. Biochem. Physiol, 2012, 104, 58–64. [Google Scholar]
- 271.Aydin B, Effects of thiacloprid, deltamethrin and their combination on oxidative stress in lymphoid organs, polymorphonuclear leukocytes and plasma of rats, Pestic. Biochem. Physiol, 2011, 100, 165–171. [Google Scholar]
- 272.El-Gendy KS, Aly NM, Mahmoud FH, Kenawy A. and El-Sebae AK, The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid, Food Chem. Toxicol, 2010, 48, 215–221. [DOI] [PubMed] [Google Scholar]
- 273.Tanaka T, Reproductive and neurobehavioral effects of clothianidin administered to mice in the diet, Birth Defects Research. Part B, Developmental and Reproductive Toxicology, 2012, 95, 151–159. [DOI] [PubMed] [Google Scholar]
- 274.Wang X, Anadón A, Wu Q, Qiao F, Ares I, Martínez-Larrañaga M-R, Yuan Z. and Martínez M-A, Mechanism of neonicotinoid toxicity: Impact on oxidative stress and metabolism, Annu. Rev. Pharmacol. Toxicol, 2018, 58, 471–507. [DOI] [PubMed] [Google Scholar]
- 275.Gu Y.-h., Li Y, Huang X.-f., Zheng J.-f., Yang J, Diao H, Yuan Y, Xu Y, Liu M, Shi H-J and Xu W.-p., Reproductive effects of two neonicotinoid insecticides on mouse sperm function and early embryonic development in vitro, PLoS One, 2013, 8, e70112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Berheim EH, Jenks JA, Lundgren JG, Michel ES, Grove D. and Jensen WF, Effects of Neonicotinoid Insecticides on Physiology and Reproductive Characteristics of Captive Female and Fawn White-tailed Deer, Sci. Rep, 2019, 9, 4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bal R, Turk G, Yilmaz O, Etem E, Kuloglu T, Baydas G. and Naziroglu M, Effects of clothianidin exposure on sperm quality, testicular apoptosis and fatty acid composition in developing male rats, Cell Biol. Toxicol, 2012, 28, 187–200. [DOI] [PubMed] [Google Scholar]
- 278.Najafi G, Razi M, Hoshyar A, Shahmohamadloo S. and Feyzi S, The effect of chronic exposure with imidacloprid insecticide on fertility in mature male rats, International Journal of Fertility and Sterility, 2010, 4, 9–16. [Google Scholar]
- 279.Bal R, Türk G, Tuzcu M, Yilmaz O, Kuloglu T, Gundogdu R, Gür S, Agca A, Ulas M. and Çambay Z, Assessment of imidacloprid toxicity on reproductive organ system of adult male rats, J. Environ. Sci. Health, Pt. B: Pestic., Food Contam., Agric. Wastes, 2012, 47, 434–444. [DOI] [PubMed] [Google Scholar]
- 280.Bal R, Türk G, Tuzcu M, Yılmaz Ö, Kuloğlu T, Baydaş G, Naziroğlu M, Yener Z, Etem E. and Tuzcu Z, Effects of the neonicotinoid insecticide, clothianidin, on the reproductive organ system in adult male rats, Drug Chem. Toxicol, 2013, 36, 421–429. [DOI] [PubMed] [Google Scholar]
- 281.Terayama H, Qu N, Endo H, Ito M, Tsukamoto H, Umemoto K, Kawakami S, Fujino Y, Tatemichi M. and Sakabe K, Effect of acetamiprid on the immature murine testes, Int. J. Environ. Health Res, 2018, 28, 683–696. [DOI] [PubMed] [Google Scholar]
- 282.Mohany M, Badr G, Refaat I. and El-Feki M, Immunological and histological effects of exposure to imidacloprid insecticide in male albino rats, African Journal of Pharmacy and Pharmacology 2011, 5, 2106–2114. [Google Scholar]
- 283.Toor HK, Sangha GK and Khera KS, Imidacloprid induced histological and biochemical alterations in liver of female albino rats, Pestic. Biochem. Physiol, 2013, 105, 1–4. [DOI] [PubMed] [Google Scholar]
- 284.Badgujar PC, Jain SK, Singh A, Punia JS, Gupta RP and Chandratre GA, Immunotoxic effects of imidacloprid following 28 days of oral exposure in BALB/c mice, Environ Toxicology and Pharmacology, 2013, 35, 408–418. [DOI] [PubMed] [Google Scholar]
- 285.Green T, Toghill A, Lee R, Waechter F, Weber E. and Noakes J, Thiamethoxam induced mouse liver tumors and their relevance to humans. Part 1: mode of action studies in the mouse, Toxicol. Sci, 2005, 86, 36–47. [DOI] [PubMed] [Google Scholar]
- 286.Bianchi J, Cabral-de-Mello DC and Marin-Morales MA, Toxicogenetic effects of low concentrations of the pesticides imidacloprid and sulfentrazone individually and in combination in in vitro tests with HepG2 cells and Salmonella typhimurium, Ecotoxicol. Environ. Saf, 2015, 120, 174–183. [DOI] [PubMed] [Google Scholar]
- 287.Karabay NU and Oguz MG, Cytogenetic and genotoxic effects of the insecticides, imidacloprid and methamidophos, Genet. Mol. Res, 2005, 4, 653–662. [PubMed] [Google Scholar]
- 288.Calderón-Segura ME, Gómez-Arroyo S, Villalobos-Pietrini R, Martínez-Valenzuela C, Carbajal-López Y, del Calderón-Ezquerro M, Cortés-Eslava J, García-Martínez R, Flores-Ramírez D, Rodríguez-Romero M, Méndez-Pérez P. and Bañuelos-Ruíz E, Evaluation of genotoxic and cytotoxic effects in human peripheral blood lymphocytes exposed in vitro to neonicotinoid insecticides news, J. Toxicol, 2012, 2012, 612647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Costa C, Silvari V, Melchini AA, Catania S, Heffron JJA, Trovato A. and De Pasquale RD, Genotoxicity of imidacloprid in relation to metabolic activation and composition of the commercial product, Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2009, 672, 40–44. [DOI] [PubMed] [Google Scholar]
- 290.Feng S, Kong Z, Wang X, Peng P. and Zeng EY, Assessing the genotoxicity of imidacloprid and RH-5849 in human peripheral blood lymphocytes in vitro with comet assay and cytogenetic tests, Ecotoxicol. Environ. Saf, 2005, 61, 239–246. [DOI] [PubMed] [Google Scholar]
- 291.Demsia G, Vlastos D, Goumenou M. and Matthopoulos DP, Assessment of the genotoxicity of imidacloprid and metalaxyl in cultured human lymphocytes and rat bone-marrow, Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 2007, 634, 32–39. [DOI] [PubMed] [Google Scholar]
- 292.Bagri P, Kumar V. and Sikka AK, Assessment of imidacloprid-induced mutagenic effects in somatic cells of Swiss albino male mice, Drug Chem. Toxicol, 2016, 39, 412–417. [DOI] [PubMed] [Google Scholar]
- 293.Kocaman AY and Topaktas M, In vitro evaluation of the genotoxicity of acetamiprid in human peripheral blood lymphocytes, Environ. Mol. Mutag, 2007, 48, 483–490. [DOI] [PubMed] [Google Scholar]
- 294.Zang Y, Zhong Y, Luo Y. and Kong Z, Genotoxicity of two novel pesticides for the earthworm, Eisenia fetida, Environ. Pollut, 2000, 108, 271–278. [DOI] [PubMed] [Google Scholar]
- 295.Kocaman AY, Rencuzogullari E. and Topaktas M, In vitro investigation of the genotoxic and cytotoxic effects of thiacloprid in cultured human peripheral blood lymphocytes, Environ. Toxicol, 2014, 29, 631–641. [DOI] [PubMed] [Google Scholar]
- 296.Sekeroglu V, Sekeroglu ZA and Kefelioglu H, Cytogenetic effects of commercial formulations of deltamethrin and/or thiacloprid on Wistar rat bone marrow cells, Environ. Toxicol, 2013, 28, 524–531. [DOI] [PubMed] [Google Scholar]
- 297.Pastoor T, Rose P, Lloyd S, Peffer R. and Green T, Case study: weight of evidence evaluation of the human health relevance of thiamethoxam-related mouse liver tumors, Toxicol. Sci, 2005, 86, 56–60. [DOI] [PubMed] [Google Scholar]
- 298.Green T, Toghill A, Lee R, Waechter F, Weber E, Peffer R, Noakes J. and Robinson M, Thiamethoxam induced mouse liver tumors and their relevance to humans. Part 2: species differences in response, Toxicol. Sci, 2005, 86, 48–55. [DOI] [PubMed] [Google Scholar]
- 299.Sheets LP, in Handbook of Neurotoxicology: Volume I, ed. Massaro EJ, Humana Press, Totowa NJ, 2002, DOI: 10.1007/978-1-59259-132-9_6, pp. 79–87. [DOI] [Google Scholar]
- 300.Bhardwaj S, Srivastava MK, Kapoor U. and Srivastava LP, A 90 days oral toxicity of imidacloprid in female rats: morphological, biochemical and histopathological evaluations, Food Chem. Toxicol, 2010, 48, 1185–1190. [DOI] [PubMed] [Google Scholar]
- 301.Hirano T, Yanai S, Omotehara T, Hashimoto R, Umemura Y, Kubota N, Minami K, Nagahara D, Matsuo E, Aihara Y, Shinohara R, Furuyashiki T, Mantani Y, Yokoyama T, Kitagawa H. and Hoshi N, The combined effect of clothianidin and environmental stress on the behavioral and reproductive function in male mice, The Journal of Veterinary Medical Science, 2015, 77, 1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Sheets LP, Li AA, Minnema DJ, Collier RH, Creek MR and Peffer RC, A critical review of neonicotinoid insecticides for developmental neurotoxicity, Crit. Rev. Toxicol, 2016, 46, 153–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Özdemir HH, Kara M, Yumrutas O, Uckardes F, Eraslan E, Demir CF and Bal R, Determination of the effects on learning and memory performance and related gene expressions of clothianidin in rat models, Cogn. Neurodyn, 2014, 8, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kimura-Kuroda J, Komuta Y, Kuroda Y, Hayashi M. and Kawano H, Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats, PLoS One, 2012, 7, e32432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.de Oliveria IM, Nunes BV, Barbosa DR, Pallares AM and Faro LRF, Effects of the neonicotinoids thiametoxam and clothianidin on in vivo dopamine release in rat striatum, Toxicol. Lett, 2010, 192, 294–297. [DOI] [PubMed] [Google Scholar]
- 306.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U. and Silberberg SD, A call for transparent reporting to optimize the predictive value of preclinical research, Nature, 2012, 490, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Caron-Beaudoin E, Viau R, Hudon-Thibeault A-A, Vaillancourt C. and Sanderson JT, The use of a unique co-culture model of fetoplacental steroidogenesis as a screening tool for endocrine disruptors: The effects of neonicotinoids on aromatase activity and hormone production, Toxicol. Appl. Pharmacol, 2017, 332, 15–24. [DOI] [PubMed] [Google Scholar]
- 308.Caron-Beaudoin E, Viau R. and Sanderson JT, Effects of Neonicotinoid Pesticides on Promoter-Specific Aromatase (CYP19) Expression in Hs578t Breast Cancer Cells and the Role of the VEGF Pathway, Environ. Health Perspect, 2018, 126, 047014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Caron-Beaudoin É, Denison MS and Sanderson JT, Effects of neonicotinoids on promoter-specific expression and activity of aromatase (CYP19) in human adrenocortical carcinoma (H295R) and primary umbilical vein endothelial (HUVEC) cells, Toxicol. Sci, 2015, 149, 134–144. [DOI] [PubMed] [Google Scholar]
- 310.Schmidt S, Promotional Consideration: A Potential Mechanistic Link between Neonicotinoid Insecticides and Hormone-Dependent Breast Cancer, Environ. Health Perspect, 2018, 126, 114001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Agarwal VR, Bulun SE, Leitch M, Rohrich R. and Simpson ER, Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients, The Journal of Clinical Endocrinology and Metabolism, 1996, 81, 3843–3849. [DOI] [PubMed] [Google Scholar]
- 312.Irahara N, Miyoshi Y, Taguchi T, Tamaki Y. and Noguchi S, Quantitative analysis of aromatase mRNA expression derived from various promoters (I. 4, I. 3, PII and I. 7) and its association with expression of TNF-α, IL-6 and COX-2 mRNAs in human breast cancer, Int. J. Cancer, 2006, 118, 1915–1921. [DOI] [PubMed] [Google Scholar]
- 313.Nauen R, Ebbinghaus-Kintscher U. and Schmuck R, Toxicity and nicotinic acetylcholine receptor interaction of imidacloprid and its metabolites in Apis mellifera (Hymenoptera: Apidae). Pest Manage. Sci, 2001, 57, 577–586. [DOI] [PubMed] [Google Scholar]
- 314.la Farre M, Perez S, Kantiani L. and Barcelo D, Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment, TrAC, Trends Anal. Chem, 2008, 27, 991–1007. [Google Scholar]
- 315.Nakayama H, Numakawa T, Ikeuchi T. and Hatanaka H, Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells, J. Neurochem, 2001, 79, 489–498. [DOI] [PubMed] [Google Scholar]
- 316.LaLone CA, Villeneuve DL, Wu-Smart J, Milsk RY, Sappington K, Garber KV, Housenger J. and Ankley GT, Weight of evidence evaluation of a network of adverse outcome pathways linking activation of the nicotinic acetylcholine receptor in honey bees to colony death, Sci. Total Environ, 2017, 584, 751–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zhang Q, Li Z, Chang C, Lou J, Zhao M. and Lu C, Potential human exposures to neonicotinoid insecticides: A review, Environ. Pollut, 2018, 236, 71–81. [DOI] [PubMed] [Google Scholar]
- 318.Osaka A, Ueyama J, Kondo T, Nomura H, Sugiura Y, Saito I, Nakane K, Takaishi A, Ogi H. and Wakusawa S, Exposure characterization of three major insecticide lines in urine of young children in Japan—neonicotinoids, organophosphates, and pyrethroids, Environ. Res, 2016, 147, 89–96. [DOI] [PubMed] [Google Scholar]
- 319.Ueyama J, Nomura H, Kondo T, Saito I, Ito Y, Osaka A. and Kamijima M, Biological monitoring method for urinary neonicotinoid insecticides using LC-MS/MS and its application to Japanese adults, J. Occup. Health, 2014, 56, 461–468. [DOI] [PubMed] [Google Scholar]
- 320.Uroz FJ, Arrebola FJ, Egea-González FJ and Martinez-Vidal JL, Monitoring of 6-chloronicotinic acid in human urine by gas chromatography-tandem mass spectrometry as indicator of exposure to the pesticide imidacloprid, Analyst, 2001, 126, 1355–1358. [DOI] [PubMed] [Google Scholar]
- 321.Taira K, Aoyama Y, Kawakami T, Kamata M. and Aoi T, [Detection of chloropyridinyl neonicotinoid insecticide metabolite 6-chloronicotinic acid in the urine: six cases with subacute nicotinic symptoms], Japanese Journal of Toxicology, 2011, 24, 222–230. [PubMed] [Google Scholar]
- 322.Taira K, Health effects of neonicotinoid insecticides-Part1: Physicochemical characteristics and case reports, Jap. J. Ecol, 2012, 21, 24–34. [Google Scholar]
- 323.Kavvalakis MP, Tzatzarakis MN, Theodoropoulou EP, Barbounis EG, Tsakalof AK and Tsatsakis AM, Development and application of LC–APCI–MS method for biomonitoring of animal and human exposure to imidacloprid, Chemosphere, 2013, 93, 2612–2620. [DOI] [PubMed] [Google Scholar]
- 324.Wang L, Liu T, Liu F, Zhang J, Wu Y. and Sun H, Occurrence and profile characteristics of the pesticide imidacloprid, preservative parabens, and their metabolites in human urine from rural and urban China, Environ. Sci. Technol, 2015, 49, 14633–14640. [DOI] [PubMed] [Google Scholar]
- 325.Kabata R, Nanayakkara S, STMLD S, Harada KH, Chandrajith R, Hitomi T, Abeysekera T, Takasuga T. and Koizumi A, Neonicotinoid concentrations in urine from chronic kidney disease patients in the North Central Region of Sri Lanka, J. Occup. Health, 2015, 15–0140-BR. [DOI] [PubMed] [Google Scholar]
- 326.López-García M, Romero-González R, Lacasaña M. and Frenich AG, Semiautomated determination of neonicotinoids and characteristic metabolite in urine samples using TurboFlow™ coupled to ultra high performance liquid chromatography coupled to Orbitrap analyzer, J. Pharm. Biomed. Anal, 2017, 146, 378–386. [DOI] [PubMed] [Google Scholar]
- 327.Zhang Q, Wang X, Li Z, Jin H, Lu Z, Yu C, Y.-f. Huang and M. Zhao, Simultaneous determination of nine neonicotinoids in human urine using isotope-dilution ultra-performance liquid chromatography–tandem mass spectrometry, Environ. Pollut, 2018, 240, 647–652. [DOI] [PubMed] [Google Scholar]
- 328.Baker SE, Serafim AB, Morales-Agudelo P, Vidal M, Calafat AM and Ospina M, Quantification of DEET and neonicotinoid pesticide biomarkers in human urine by online solid-phase extraction high-performance liquid chromatography-tandem mass spectrometry, Anal. Bioanal. Chem, 2019, 411, 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Honda M, Robinson M. and Kannan K, A simple method for the analysis of neonicotinoids and their metabolites in human urine, Environmental Chemistry, 2019, 16, 171–178. [Google Scholar]
- 330.Ikenaka Y, Miyabara Y, Ichise T, Nakayama S, Nimako C, Ishizuka M. and Tohyama C, Exposures of children to neonicotinoids in pine wilt disease control areas, Environ. Toxicol. Chem, 2019, 38, 71–79. [DOI] [PubMed] [Google Scholar]
- 331.Ospina M, Wong L-Y, Baker S, Serafim AB, Morales-Agudelo P. and Calafat AM, Exposure to neonicotinoid insecticides in the US general population: Data from the 2015–2016 national health and nutrition examination survey, Environ. Res, 2019, 108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Tao Y, Phung D, Dong F, Xu J, Liu X, Wu X, Liu Q, He M, Pan X. and Li R, Urinary monitoring of neonicotinoid imidacloprid exposure to pesticide applicators, Sci. Total Environ, 2019, 669, 721–728. [DOI] [PubMed] [Google Scholar]
- 333.Zhang T, Song S, Bai X, He Y, Zhang B, Gui M, Kannan K, Lu S, Huang Y. and Sun H, A nationwide survey of urinary concentrations of neonicotinoid insecticides in China, Environ. Int, 2019, 132, 105114. [DOI] [PubMed] [Google Scholar]
- 334.Hernández AF, Casado I, Pena G, Gil F, Villanueva E. and Pla A, Low level of exposure to pesticides leads to lung dysfunction in occupationally exposed subjects, Inhal. Toxicol, 2008, 20, 839–849. [DOI] [PubMed] [Google Scholar]
- 335.Lin P-C, Lin H-J, Liao Y-Y, Guo H-R and Chen K-T, Acute Poisoning with Neonicotinoid Insecticides: A Case Report and literature Review, Basic and Clinical Pharmacology and Toxicology, 2013, 112, 282–286. [DOI] [PubMed] [Google Scholar]
- 336.Wu I-W, Lin J-L and Cheng E-T, Acute poisoning with the neonicotinoid insecticide imidacloprid in N-methyl pyrrolidone, J. Toxicol. Clin. Toxicol, 2001, 39, 617–621. [DOI] [PubMed] [Google Scholar]
- 337.Phua DH, Lin CC, Wu M-L, Deng J-F and Yang C-C, Neonicotinoid insecticides: an emerging cause of acute pesticide poisoning, Clin. Toxicol, 2009, 47, 336–341. [DOI] [PubMed] [Google Scholar]
- 338.Mohamed F, Gawarammana I, Robertson TA, Roberts MS, Palangasinghe C, Zawahir S, Jayamanne S, Kandasamy J, Eddleston M. and Buckley NA, Acute human self-poisoning with imidacloprid compound: a neonicotinoid insecticide, PLoS One, 2009, 4, e5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Huang N-C, Lin S-L, Chou C-H, Hung Y-M, Chung H-M and Huang S-T, Fatal ventricular fibrillation in a patient with acute imidacloprid poisoning, The American Journal of Emergency Medicine, 2006, 24, 883–885. [DOI] [PubMed] [Google Scholar]
- 340.Agarwal R. and Srinivas R, Severe neuropsychiatric manifestations and rhabdomyolysis in a patient with imidacloprid poisoning, The American Journal of Emergency Medicine, 2007, 25, 844–845. [DOI] [PubMed] [Google Scholar]
- 341.Shadnia S. and Moghaddam HH, Fatal intoxication with imidacloprid insecticide, The American Journal of Emergency Medicine, 2008, 26, 634. e631–634. e634. [DOI] [PubMed] [Google Scholar]
- 342.Todani M, Kaneko T, Hayashida H, Kaneda K, Tsuruta R, Kasaoka S. and Maekawa T, Acute poisoning with neonicotinoid insecticide acetamiprid, Japanese Journal of Toxicology, 2008, 21, 387–390. [PubMed] [Google Scholar]
- 343.Panigrahi AK, Subrahmanyam D. and Mukku KK, Imidacloprid poisoning: a case report, The American Journal of Emergency Medicine, 2009, 27, 256. e255–256. e256. [DOI] [PubMed] [Google Scholar]
- 344.Karatas AD, Severe central nervous system depression in a patient with acute imidacloprid poisoning, The American Journal of Emergency Medicine, 2009, 27, 1171. e1175–1171. e1177. [DOI] [PubMed] [Google Scholar]
- 345.Imamura T, Yanagawa Y, Nishikawa K, Matsumoto N. and Sakamoto T, Two cases of acute poisoning with acetamiprid in humans, Clin. Toxicol, 2010, 48, 851–853. [DOI] [PubMed] [Google Scholar]
- 346.Iyyadurai R, George IA and Peter JV, Imidacloprid poisoning—newer insecticide and fatal toxicity, J. Med. Toxicol, 2010, 6, 77–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Yeh I-J, Lin T-J and Hwang D-Y, Acute multiple organ failure with imidacloprid and alcohol ingestion, The American Journal of Emergency Medicine, 2010, 28, 255. e251–255. e253. [DOI] [PubMed] [Google Scholar]
- 348.Chwaluk P, Acute inhalation imidacloprid poisoning--a case report, Przegl. Lek, 2010, 67, 619–620. [PubMed] [Google Scholar]
- 349.Lin PC, Lin HJ, Liao YY, Guo HR and Chen KT, Acute poisoning with neonicotinoid insecticides: a case report and literature review, Basic and Clinical Pharmacology and Toxicology, 2013, 112, 282–286. [DOI] [PubMed] [Google Scholar]
- 350.David D, George IA and Peter JV, Toxicology of the newer neonicotinoid insecticides: imidacloprid poisoning in a human, Clin. Toxicol, 2007, 45, 485–486. [DOI] [PubMed] [Google Scholar]
- 351.Proença P, Teixeira H, Castanheira F, Pinheiro J, Monsanto PV, Marques EP and Vieira DN, Two fatal intoxication cases with imidacloprid: LC/MS analysis, Forensic Sci. Int, 2005, 153, 75–80. [DOI] [PubMed] [Google Scholar]
- 352.Fuke C, Nagai T, Ninomiya K, Fukasawa M, Ihama Y. and Miyazaki T, Detection of imidacloprid in biological fluids in a case of fatal insecticide intoxication, Leg. Med, 2014, 16, 40–43. [DOI] [PubMed] [Google Scholar]
- 353.Vinod KV, Srikant S, Thiruvikramaprakash G. and Dutta TK, A fatal case of thiacloprid poisoning, The American Journal of Emergency Medicine, 2015, 33, 310. e315–310. e316. [DOI] [PubMed] [Google Scholar]
- 354.Nistor N, Frăsinariu OE and Ştreangă V, Acute Poisoning with Neonicotinoid Insecticide, Poisoning: From Specific Toxic Agents to Novel Rapid and Simplified Techniques for Analysis, 2017, 107. [Google Scholar]
- 355.Kim JC, So BH, Kim HJ, Kim HM, Park JH, Choi SM, Park KN and Choi KH, Clinical characteristics of patients with neonicotinoid insecticide poisoning, Journal of the Korean Society of Clinical Toxicology, 2010, 8, 24. [Google Scholar]
- 356.Forrester M, Neonicotinoid insecticide exposures reported to six poison centers in Texas, Hum. Exp. Toxicol, 2014, 33, 568–573. [DOI] [PubMed] [Google Scholar]
- 357.Koureas M, Tsezou A, Tsakalof A, Orfanidou T. and Hadjichristodoulou C, Increased levels of oxidative DNA damage in pesticide sprayers in Thessaly Region (Greece). Implications of pesticide exposure, Sci. Total Environ, 2014, 496, 358–364. [DOI] [PubMed] [Google Scholar]
- 358.Keil AP, Daniels JL and Hertz-Piccioto I, Autism Spectrum disorder, flea and tick medication, and adjustments for exposure misclassification: the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study, Environ. Health, 2014, 13, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Carmichael SL, Yang W, Roberts E, Kegley SE, Padula AM, English PB, Lammer EJ and Shaw GM, Residential agricultural pesticide exposures and risk of selected congenital heart defects among offspring in the San Joaquin Valley of California, Environ. Res, 2014, 135, 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Yang W, Carmichael SL, Roberts EM, Kegley SE, Padula AM, English PB and Shaw GM, Residential Agricultural Pesticide Exposures and Risk of Neural Tube Defects and Orofacial Clefts among Offspring in the San Joaquin Valley of California, Am. J. Epidemiol, 2014, 179, 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Ichikawa G, Kuribayashi R, Ikenaka Y, Ichise T, Nakayama SM, Ishizuka M, Taira K, Fujioka K, Sairenchi T. and Kobashi G, LC-ESI/MS/MS analysis of neonicotinoids in urine of very low birth weight infants at birth, PLoS One, 2019, 14, e0219208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Schmuck R, Stadler T. and Schmidt HW, Field relevance of a synergistic effect observed in the laboratory between an EBI fungicide and a chloronicotinyl insecticide in the honeybee (Apis mellifera L, Hymenoptera), Pest Manage. Sci, 2003, 59, 279–286. [DOI] [PubMed] [Google Scholar]
- 363.Chang C-H, MacIntosh D, Lemos B, Zhang Q. and Lu C, Characterization of daily dietary intake and the health risk of neonicotinoid insecticides for the US population, J. Agric. Food Chem, 2018, 66, 10097–10105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.