Abstract
A two-microelectrode current-voltage clamp and Cl(-)-selective microelectrodes were used to examine the effects of gamma-aminobutyric acid (GABA) on membrane potential, current and intracellular Cl- activity (aiCl) in the crayfish stretch receptor neurone. All experimental solutions were CO2-HCO3- free. 2. GABA (500 microM) produced a mono- or biphasic depolarization (amplitude < or = 10 mV), often with a prominent initial depolarizing component followed by a transient shift to a more negative level. In some neurones, an additional depolarizing phase was seen upon washout of GABA. Receptor desensitization, being absent, played no role in the multiphasic actions of GABA. 3. The pronounced increase in membrane conductance evoked by GABA (500 microM) was associated with an increase in aiCl which indicates that the depolarizing action was not due to a current carried by Cl- ions. 4. The currents activated by GABA under voltage clamp conditions were inwardly directed when recorded at the level of the resting membrane potential, and they often revealed a biphasic character. The reversal potential of peak currents activated by pulses of 500 microM-GABA (EGABA) was 9-12 mV more positive than the reversal potential of the simultaneously measured net Cl- flux (ECl). ECl was 2-7 mV more negative than the resting membrane potential. 5. EGABA (measured using pulses of 500 microM-GABA) was about 10 mV more positive than the reversal potential of the current activated by 500 microM-muscimol, a GABA agonist that is a poor substrate of the Na(+)-dependent GABA uptake system. 6. In the absence of Na+, the depolarization and inward current caused by 500 microM-GABA were converted to a hyperpolarization and to an outward current. Muscimol produced an immediate outward current both in the presence and absence of Na+. 7. Following block of the inhibitory channels by picrotoxin (100-200 microM), the depolarizing effect of 500 microM-GABA was enhanced and the transient hyperpolarizing shifts were abolished. 8. In the presence of picrotoxin, GABA (> or = 2 microM) produced a concentration-dependent monophasic inward current which had a reversal potential of +30 to +60 mV. This current was inhibited in the absence of Na+ and by the GABA uptake blocker, nipecotic acid. Unlike the channel-mediated current, the picrotoxin-insensitive current was activated without delay also at low (2-10 microM) concentrations of GABA.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



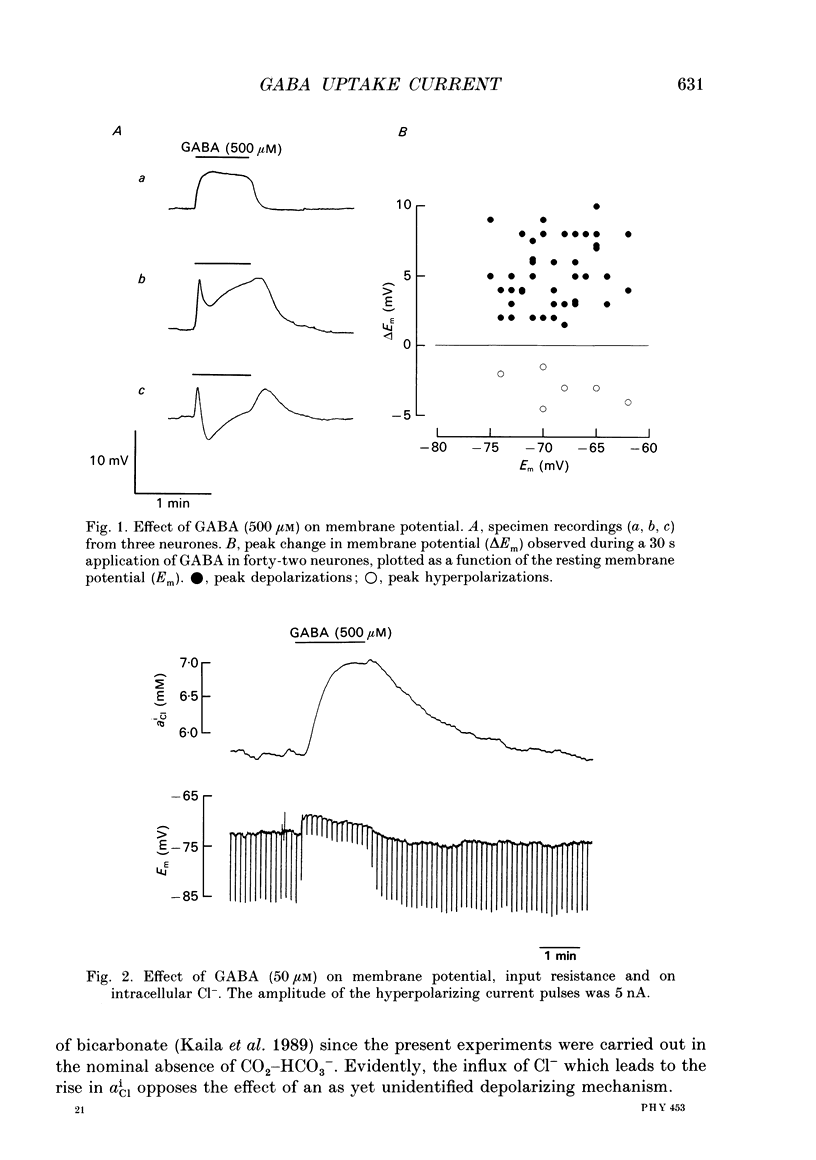
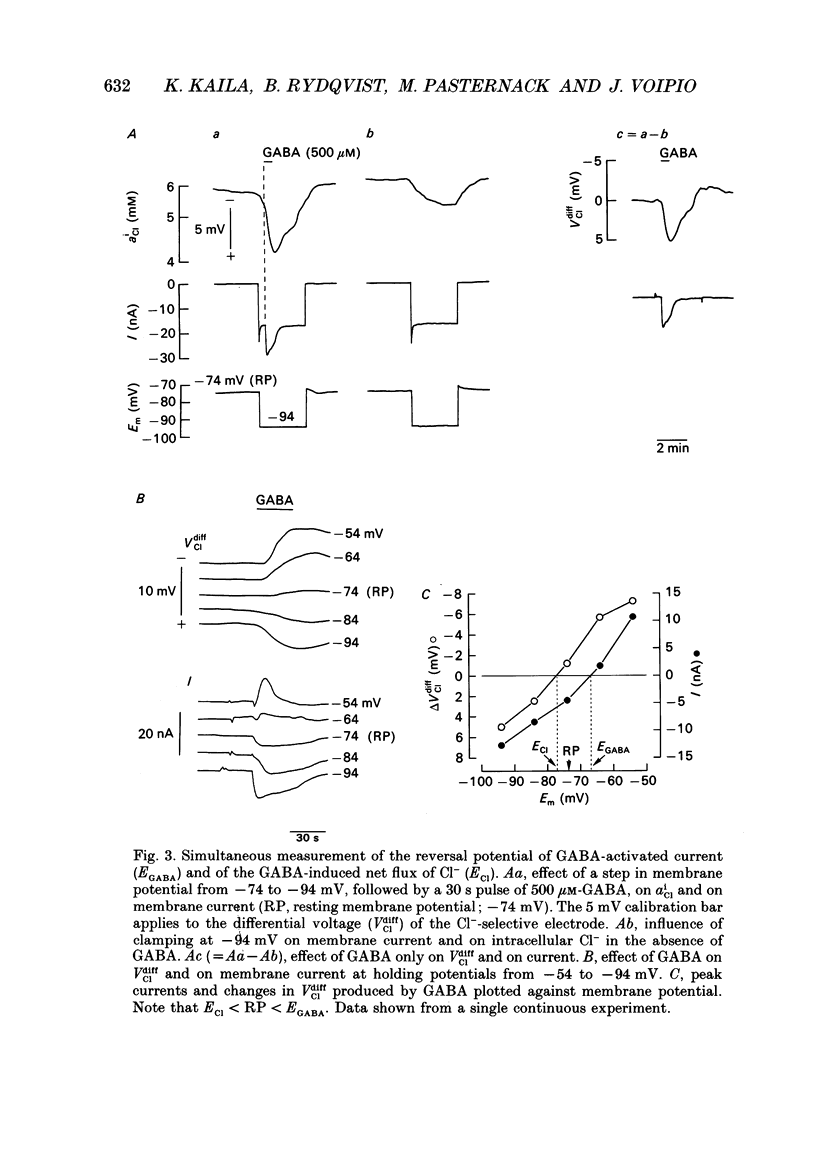
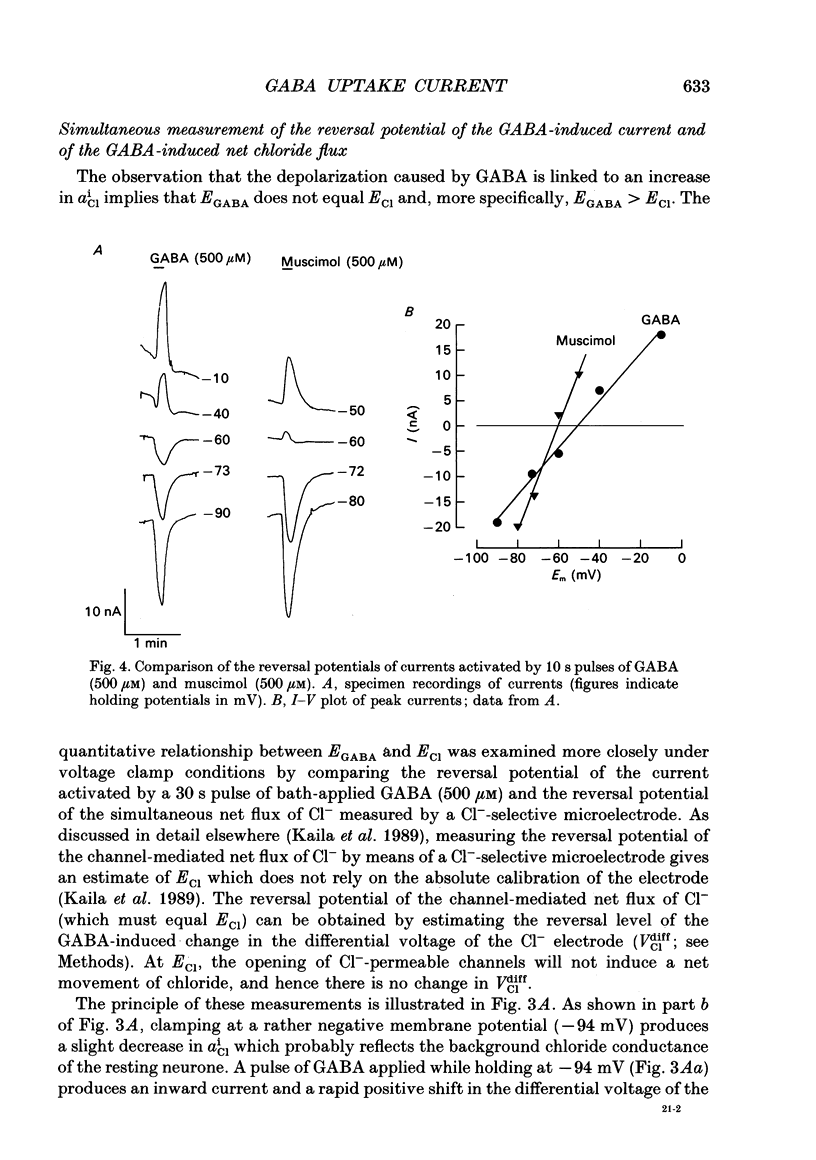









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
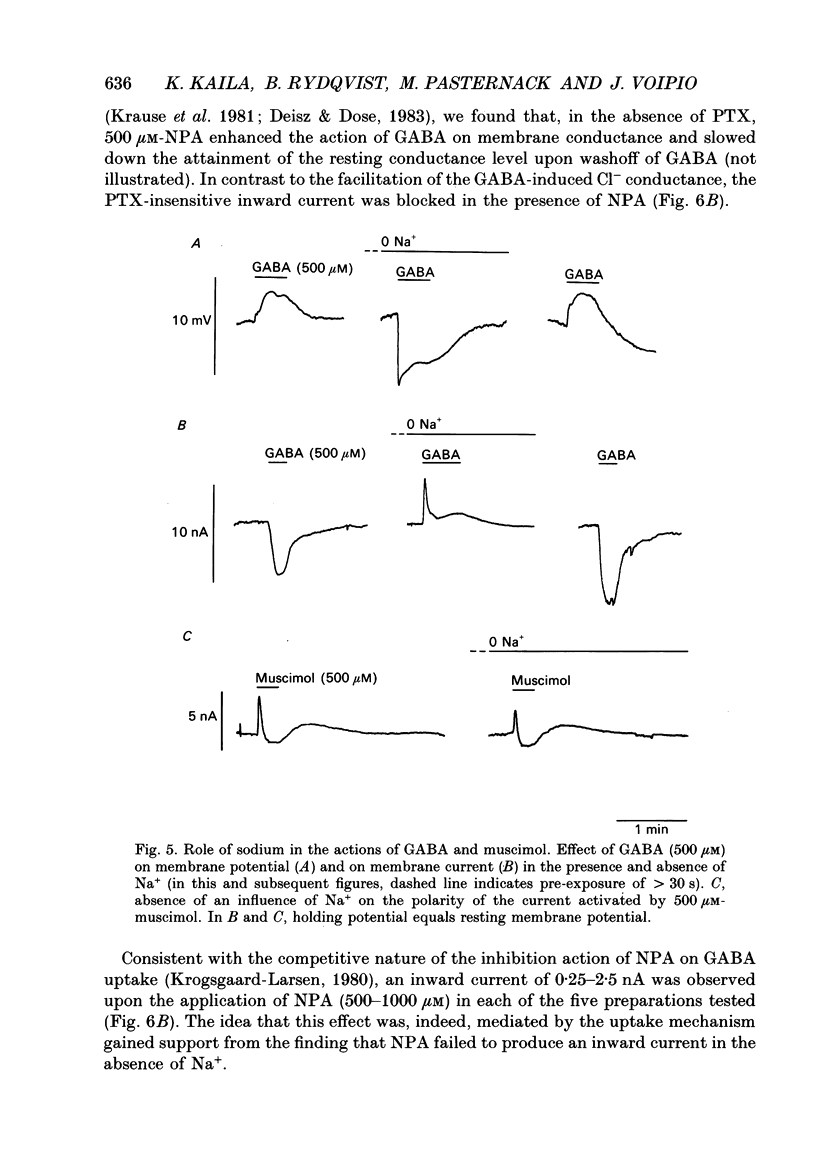
- Adams P. R., Constanti A., Banks F. W. Voltage clamp analysis of inhibitory synaptic action in crayfish stretch receptor neurons. Fed Proc. 1981 Sep;40(11):2637–2641. [PubMed] [Google Scholar]
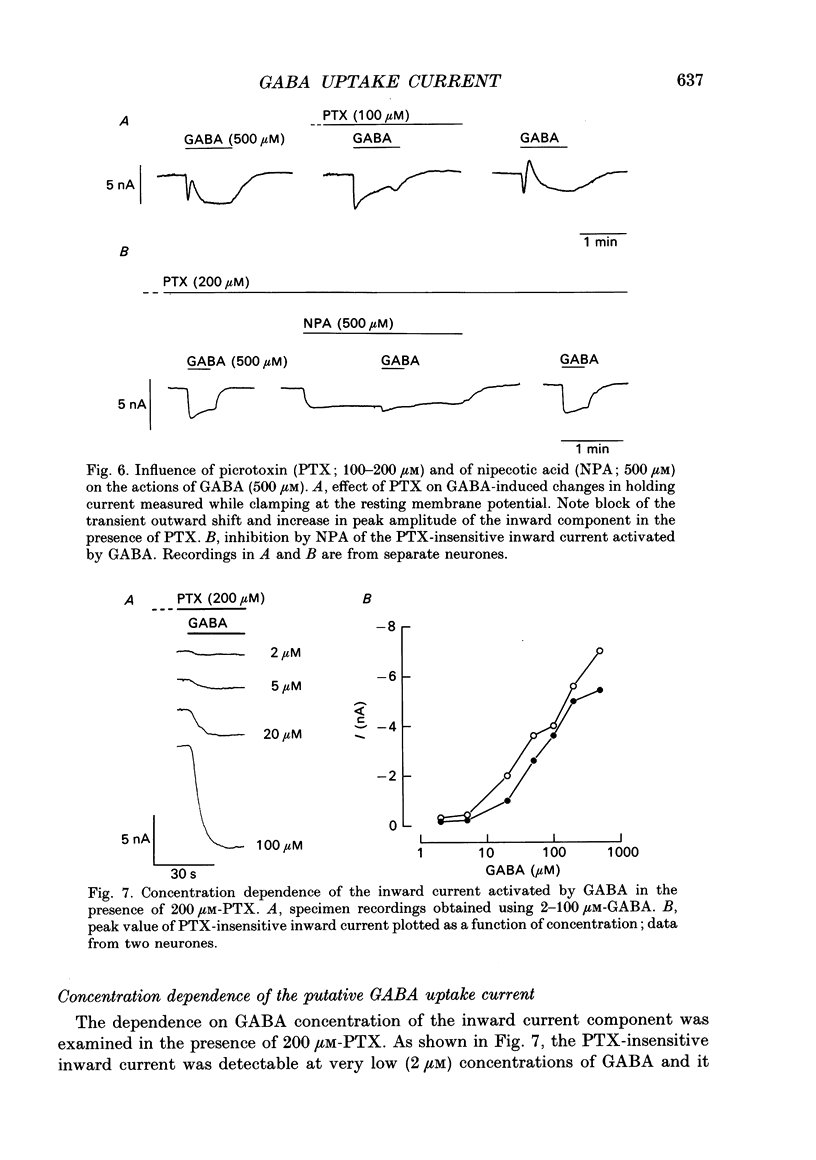
- Aickin C. C., Deisz R. A., Lux H. D. Ammonium action on post-synaptic inhibition in crayfish neurones: implications for the mechanism of chloride extrusion. J Physiol. 1982 Aug;329:319–339. doi: 10.1113/jphysiol.1982.sp014305. [DOI] [PMC free article] [PubMed] [Google Scholar]
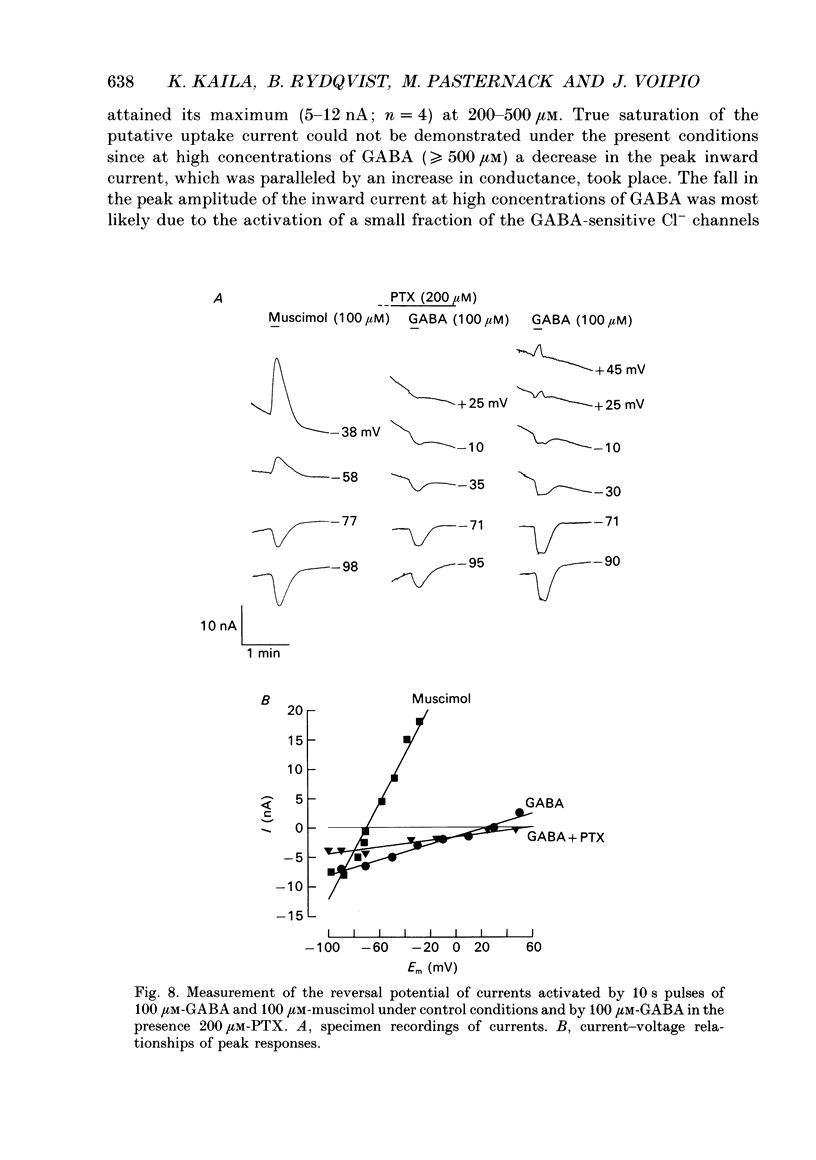
- Aickin C. C., Deisz R. A., Lux H. D. On the action of the anticonvulsant 5,5-diphenylhydantoin and the convulsant picrotoxin in crayfish stretch receptor. J Physiol. 1981 Jun;315:157–173. doi: 10.1113/jphysiol.1981.sp013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Maruyama T., Sikdar S. K., Yasui S. Sodium-dependent suppression of gamma-aminobutyric-acid-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1987 Nov;392:543–562. doi: 10.1113/jphysiol.1987.sp016796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. GABA-mediated biphasic inhibitory responses in hippocampus. Nature. 1979 Sep 27;281(5729):315–317. doi: 10.1038/281315a0. [DOI] [PubMed] [Google Scholar]
- Atwood H. L. Organization and synaptic physiology of crustacean neuromuscular systems. Prog Neurobiol. 1976;7(Pt 4):291–391. doi: 10.1016/0301-0082(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Amino acid pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:331–354. doi: 10.1113/jphysiol.1978.sp012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J., Zaafrani M., Bergman C. Electrophysiological investigation of the amino acid carrier selectivity in epithelial cells from Xenopus embryo. J Membr Biol. 1989 Nov;111(3):241–251. doi: 10.1007/BF01871009. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Inhibition of GABA uptake potentiates the conductance increase produced by GABA-mimetic compounds on single neurones in isolated olfactory cortex slices of the guinea-pig. Br J Pharmacol. 1984 Sep;83(1):195–202. doi: 10.1111/j.1476-5381.1984.tb10135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Ottoson D., Rydqvist B. Crayfish stretch receptor: an investigation with voltage-clamp and ion-sensitive electrodes. J Physiol. 1978 Nov;284:155–179. doi: 10.1113/jphysiol.1978.sp012533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Game C. J., Lodge D. The in vivo inactivation of GABA and other inhibitory amino acids in the cat nervous system. Exp Brain Res. 1976 Jun 30;25(4):413–428. doi: 10.1007/BF00241731. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Dose M. Comparison of GABA analogues at the crayfish stretch receptor neurone. Brain Res Bull. 1983 Sep;11(3):283–288. doi: 10.1016/0361-9230(83)90161-2. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Dose M., Lux H. D. The time course of GABA action on the crayfish stretch receptor: evidence for a saturable GABA uptake. Neurosci Lett. 1984 Jun 29;47(3):245–250. doi: 10.1016/0304-3940(84)90521-4. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Lux H. D. The role of intracellular chloride in hyperpolarizing post-synaptic inhibition of crayfish stretch receptor neurones. J Physiol. 1982 May;326:123–138. doi: 10.1113/jphysiol.1982.sp014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS C., KUFFLER S. W. The blocking effect of gamma-aminobutyric acid (GABA) and the action of related compounds on single nerve cells. J Neurochem. 1959 Apr;4(1):19–30. doi: 10.1111/j.1471-4159.1959.tb13170.x. [DOI] [PubMed] [Google Scholar]
- Elekes K., Florey E. Immunocytochemical evidence for the GABAergic innervation of the stretch receptor neurons in crayfish. Neuroscience. 1987 Sep;22(3):1111–1122. doi: 10.1016/0306-4522(87)92986-1. [DOI] [PubMed] [Google Scholar]
- Erecińska M. The neurotransmitter amino acid transport systems. A fresh outlook on an old problem. Biochem Pharmacol. 1987 Nov 1;36(21):3547–3555. doi: 10.1016/0006-2952(87)90001-3. [DOI] [PubMed] [Google Scholar]
- Finger W., Stettmeier H. Analysis of miniature spontaneous inhibitory postsynaptic currents (sIPSCs) from current noise in crayfish opener muscle. Pflugers Arch. 1981 Dec;392(2):157–162. doi: 10.1007/BF00581265. [DOI] [PubMed] [Google Scholar]
- Horwitz I. S., Orkand R. K. GABA inactivation at the crayfish neuromuscular junction. J Neurobiol. 1980 Sep;11(5):447–458. doi: 10.1002/neu.480110504. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Kravitz E. A. The metabolism of gamma-aminobutyric acid (GABA) in the lobster nervous system--uptake of GABA in the nerve-muscle preparations. J Neurochem. 1968 Jul;15(7):609–620. doi: 10.1111/j.1471-4159.1968.tb08960.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki S., Florey E. Inhibitory miniature potentials in the stretch receptor neurons of crayfish. J Gen Physiol. 1969 May;53(5):666–682. doi: 10.1085/jgp.53.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., EDWARDS C. Mechanism of gamma aminobutyric acid (GABA) action and its relation to synaptic inhibition. J Neurophysiol. 1958 Nov;21(6):589–610. doi: 10.1152/jn.1958.21.6.589. [DOI] [PubMed] [Google Scholar]
- Kaila K., Pasternack M., Saarikoski J., Voipio J. Influence of GABA-gated bicarbonate conductance on potential, current and intracellular chloride in crayfish muscle fibres. J Physiol. 1989 Sep;416:161–181. doi: 10.1113/jphysiol.1989.sp017755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987 Nov 12;330(6144):163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22(1):1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- Kehoe J. S. Electrogenic effects of neutral amino acids on neurons of Aplysia californica. Cold Spring Harb Symp Quant Biol. 1976;40:145–155. doi: 10.1101/sqb.1976.040.01.016. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Dingledine R. Inhibition of GABA uptake in the rat hippocampal slice. Brain Res. 1986 Mar 19;368(2):247–255. doi: 10.1016/0006-8993(86)90568-8. [DOI] [PubMed] [Google Scholar]
- Krause D. N., Ikeda K., Roberts E. Dose-conductance relationships for GABA agonists and the effect of uptake inhibitors in crayfish stretch receptor neurons. Brain Res. 1981 Nov 30;225(2):319–332. doi: 10.1016/0006-8993(81)90839-8. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P. Inhibitors of the GABA uptake systems. Mol Cell Biochem. 1980 Jun 18;31(2):105–121. doi: 10.1007/BF00240816. [DOI] [PubMed] [Google Scholar]
- Lodge D., Johnston G. A., Curtis D. R., Brand S. J. Effects of the Areca nut constituents arecaidine and guvacine on the action of GABA in the cat central nervous system. Brain Res. 1977 Nov 18;136(3):513–522. doi: 10.1016/0006-8993(77)90075-0. [DOI] [PubMed] [Google Scholar]
- Macaskill J. B., Mohan M. S., Bates R. G. Activity coefficients and osmotic coefficients in aqueous solutions of choline chloride at 25 degrees C. Anal Chem. 1977 Feb;49(2):209–212. doi: 10.1021/ac50010a010. [DOI] [PubMed] [Google Scholar]
- Nistri A., Constanti A. Pharmacological characterization of different types of GABA and glutamate receptors in vertebrates and invertebrates. Prog Neurobiol. 1979;13(2):117–235. doi: 10.1016/0301-0082(79)90016-9. [DOI] [PubMed] [Google Scholar]
- Orkand P. M., Kravitz E. A. Localization of the sites of gamma-aminobutyric acid (GABA) uptake in lobster nerve-muscle preparations. J Cell Biol. 1971 Apr;49(1):75–89. doi: 10.1083/jcb.49.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling J. C., Jahnsen H., Mosfeldt Laursen A. The effect of two lipophilic gamma-aminobutyric acid uptake blockers in CA1 of the rat hippocampal slice. Br J Pharmacol. 1990 Jan;99(1):103–106. doi: 10.1111/j.1476-5381.1990.tb14661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira C., Ben-Ari Y., Cherubini E. Somatic and dendritic actions of gamma-aminobutyric acid agonists and uptake blockers in the hippocampus in vivo. Neuroscience. 1984 Jun;12(2):543–555. doi: 10.1016/0306-4522(84)90072-1. [DOI] [PubMed] [Google Scholar]
- Rydqvist B., Zhou J. Y. Potential-dependent potassium currents in the slowly adapting stretch receptor neuron of the crayfish. Acta Physiol Scand. 1989 Nov;137(3):409–419. doi: 10.1111/j.1748-1716.1989.tb08771.x. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Strickholm A. Reduction of response time for potential in salt bridge reference electrodes for electrophysiology. Nature. 1968 Jan 6;217(5123):80–81. doi: 10.1038/217080a0. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J Physiol. 1969 Nov;205(2):377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voipio J., Pasternack M., Rydqvist B., Kaila K. Effect of gamma-aminobutyric acid on intracellular pH in the crayfish stretch-receptor neurone. J Exp Biol. 1991 Mar;156:349–360. doi: 10.1242/jeb.156.1.349. [DOI] [PubMed] [Google Scholar]


