Abstract
Activation of β-adrenoceptors in the basolateral complex of the amygdala (BLA) modulates memory storage processes and long-term potentiation in downstream targets of BLA efferents, including the hippocampus. Here, we show that this activation also increases hippocampal levels of activity-regulated cytoskeletal protein (Arc), an immediate-early gene (also termed Arg 3.1) implicated in hippocampal synaptic plasticity and memory consolidation processes. Infusions of the β-adrenoreceptor agonist, clenbuterol, into the BLA immediately after training on an inhibitory avoidance task enhanced memory tested 48 h later. The same dose of clenbuterol significantly increased Arc protein levels in the dorsal hippocampus. Additionally, posttraining intra-BLA infusions of a memory-impairing dose of lidocaine significantly reduced Arc protein levels in the dorsal hippocampus. Increases in Arc protein levels were not accompanied by increases in Arc mRNA, suggesting that amygdala modulation of Arc protein and synaptic plasticity in efferent brain regions occurs at a posttranscriptional level. Finally, infusions of Arc antisense oligodeoxynucleotides into the dorsal hippocampus impaired performance of an inhibitory avoidance task, indicating that the changes in Arc protein expression are related to the observed changes in memory performance.
Keywords: activity-regulated cytoskeletal-associated gene, emotional memory, immediate-early gene, memory consolidation, memory systems
Emotionally arousing events are typically well remembered. Extensive evidence indicates that the enhancing effect of emotional arousal on memory consolidation is mediated by release of adrenal stress hormones (i.e., epinephrine and glucocorticoids) that, in turn, affects neurotransmitter systems that converge in the basolateral amygdala (BLA) to alter noradrenergic activity (1). Direct infusions of drugs into the BLA that target noradrenergic receptors affect the consolidation of memory for a variety of behavioral tasks. For example, posttraining intra-BLA infusions of the β-adrenoreceptor agonist, clenbuterol, enhance memory of inhibitory avoidance training (2-4), and infusions of norepinephrine enhance retention of contextual fear conditioning (5), conditioned taste aversion (6), and spatial water maze training (7).
Amygdala activation influences the consolidation of memories for such an array of behavioral tasks by modulating neuroplasticity in many other brain regions engaged in memory processing (1, 8). For example, posttraining infusions of d-amphetamine into the amygdala enhance retention of both spatial and cued versions of a water maze task. Inactivating the hippocampus after training with infusion of lidocaine prevents the amygdala-induced enhancement of memory for the spatial version of the task and posttraining infusions of lidocaine into the caudate nucleus prevent the amygdala-induced enhancement of memory for training on the cued version of the task (9). These findings suggest that the amygdala modulates the consolidation of memory for the spatial training by influencing the hippocampus and the consolidation of the cued training by influencing the caudate nucleus. The interaction of the noradrenergic system of the BLA with the dorsal hippocampus appears to be critical for modulation of memory consolidation. Posttraining infusions of β-adrenoreceptor antagonists into the BLA block the memory enhancing effects of a glucocorticoid-receptor agonist administered into the hippocampus after inhibitory avoidance training (10). These behavioral findings are complemented by research indicating a β-noradrenergic influence on modulation of hippocampal long-term potentiation (LTP) by emotional arousal or amygdala stimulation (11-13). However, the cellular mechanisms supporting this BLA-influenced hippocampal synaptic plasticity remain to be identified.
Immediate-early genes (IEGs) are rapidly induced in the brain in response to synaptic activity and can serve as “reporters” for critical molecular processes involved in synaptic plasticity. The IEG Arc (also termed Arg 3.1) is especially relevant because it is targeted specifically to regions of dendrites that receive direct synaptic stimulation (14). The evidence that infusions of Arc antisense oligodeoxynucleotides into the hippocampus block expression of the protein, disrupt the maintenance of LTP and impair memory for a spatial water maze task (15) suggests that Arc plays a role in the synaptic plasticity underlying the consolidation of long-term memory. Changes in Arc expression may thus provide an indicator of such synaptic plasticity. To address the mechanisms involved in BLA-influenced memory consolidation, the present experiments examined the effect of noradrenergic stimulation of the BLA on long-term memory and Arc expression in the hippocampus.
Materials and Methods
Subjects. One hundred and fifty-nine male Sprague-Dawley rats weighing 300-350 g (Charles River Breeding Laboratories) were housed individually in a temperature-controlled room on a 12-h light/12-h dark cycle. Animals were treated in accordance with National Institutes of Health guidelines, and procedures were approved by the University of California, Irvine, Institutional Animal Care and Use Committee.
Surgery. Rats were anesthetized for surgery with sodium pentobarbital (50 mg/kg). Bilateral guide cannulae were implanted dorsal to the BLA (anteriorposterior, -3.0; mediolateral, ±5.0 mm from bregma; and dorsoventral, -6.0 mm from skull) or the dorsal hippocampus (anteriorposterior, -3.6; mediolateral, ±2.2 mm from bregma; and dorsoventral, -2.5 mm from skull). After 2 days of recovery from surgery, rats were handled daily for 5 days (5 min/day).
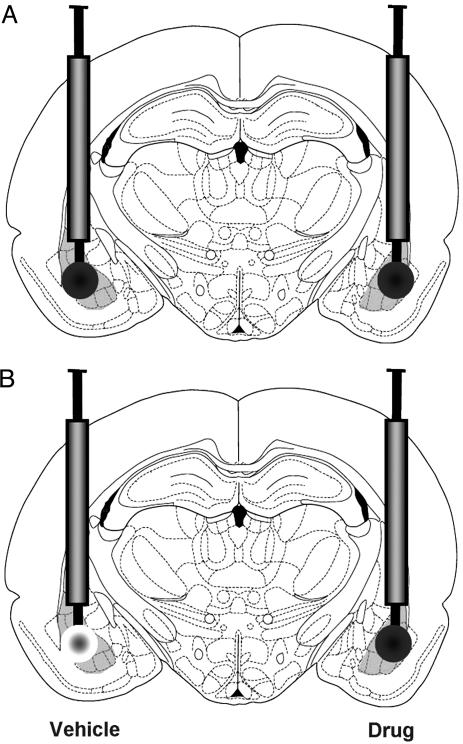
Inhibitory Avoidance (IA). The rat was placed in the illuminated compartment of an IA apparatus (a trough-shaped alley, half illuminated and half dark, divided by a retractable door) and after it crossed from the illuminated to the dark compartment of the alley, the door was closed and a single foot shock was delivered. Rats receiving posttraining treatment with the memory-enhancing drug clenbuterol (Sigma) received a lower intensity foot shock (0.38 mA for 1 sec) than that of rats given the memory-impairing drug, lidocaine (0.48 mA for 1 sec). Ten seconds after the foot shock, rats were removed from the apparatus and immediately received bilateral intra-BLA infusions of the β-adrenoreceptor agonist clenbuterol (4 ng per 0.2 μl), the local anesthetic lidocaine (2% in 0.2 μl), or PBS (vehicle 0.2 μl) through a 30-gauge microinjection needle extending 2 mm beyond the cannulae (Fig. 1A). The duration of all 0.2-μl infusions was 1 min. The dose of clenbuterol was determined in a previous report to be effective in enhancing memory (2). Forty-eight hours after training, rats were again placed in the lighted compartment of the IA alley. Latency to enter the dark compartment was considered a measure of memory retention for the aversive stimulus.
Fig. 1.
Intra-BLA drug infusions. (A) Bilateral, intra-BLA infusions of either vehicle, clenbuterol (4 ng), or lidocaine (2%) were given in 0.2 μl over the course of 1 min immediately after IA training. (B) For protein and mRNA analysis, rats were infused with vehicle into the BLA of one hemisphere and either clenbuterol or lidocaine into the BLA of the other hemisphere immediately after IA training. The side of the vehicle/drug infusion was counter-balanced.
Rats used in the protein and mRNA analysis experiments received unilateral, posttraining intra-BLA infusions of clenbuterol or lidocaine and infusions of the vehicle into the contralateral BLA (Fig. 1B). Thus, each animal served as its own control. The drug-infused hemisphere was counterbalanced. Rats were deeply anesthetized with sodium pentobarbital (300 mg i.p.) 30 or 45 min after the beginning of training for mRNA and protein experiments, respectively. Brains were rapidly removed and flash frozen by submersion (2 min) in a beaker of 2-methylbutane sitting in a dry ice-ethanol bath. Untrained control rats were taken from the vivarium and remained in their home cages in the holding area, along with trained rats, before being infused with drugs and killed.
Immunoblot Analysis. Using a sliding microtome, 1-mm-thick coronal sections were taken from the dorsal hippocampus (-2.3 to -3.3 mm from bregma) and ventral hippocampus (-4.8 to -5.8 mm from bregma). From the 1-mm-thick coronal section containing the dorsal hippocampus and amygdala, several 40-μm sections were taken and mounted on slides and stained with thionin. For experiments examining only dorsal hippocampus (not ventral), frozen brains were cut horizontally just above the level of the rhinal fissure. The ventral part of the brain was placed in 10% formalin for 1 week, then sectioned (40 μm) by using a sliding microtome and mounted on slides and stained with thionin. Brain sections were analyzed under a light microscope to identify the location of cannula placement. Only brains with needle tracks in the BLA were used for analysis. Tissue punches (1 mm in diameter) were taken with a glass pipette. Tissue punches were sonicated in 0.1 M phosphate buffer, pH 7.4 [containing 2% SDS, 10% glycerol, 20% protease inhibitor cocktail (Sigma), and 10% protease inhibitor cocktail II (Sigma)]. Protein concentrations were determined with a microplate reader and Pierce protein assay kit.
Approximately 15 μg of protein from each sample were heated in sample buffer with reducing agent (Bio-Rad), loaded and run on 12% Tris·HCl gels (Bio-Rad). Samples from left and right hippocampus of an individual animal were loaded in adjacent wells. Gels were then electroblotted to nitrocellulose membranes. Membranes were washed in Tris-buffered saline (TBS; 150 mM NaCl/100 mM Tris Base, pH 7.5) and incubated with primary antibody diluted in blocking solution (5% Carnation nonfat dry milk in TBS) overnight at 4°C. Immunoreactivity was measured by chemiluminescence (ECL Western blot kit; Amersham Pharmacia). Blots were first processed to detect Arc immunoreactivity and then directly reprobed with antibody to actin. In some cases, blots were stripped and reprobed with antibody to cFos. Bio-Rad markers were run on all gels to determine the relative mobility of the immunoreactive bands. The following rabbit polyclonal antibodies and dilutions were used: anti-Arc (1:2,000; ref. 16), anti-Actin (1:200; Sigma), and anti-cFos (1:800; Santa Cruz Biotechnology). For quantification of immunoblot results, films were scanned and converted into TIF files for analysis using Scion image software. Arc and cFos immunoreactivity levels were normalized by using the actin immunoreactivity value for each sample.
Fluorescence in Situ Hybridization (FISH). FISH was performed as reported (17). Brain sections (20 μm) were taken by using a cryostat and were mounted on slides so that a brain from each group was on a single slide. Intermittent sections were mounted and stained to determine cannula placement. Only those brains with needle tracks in the BLA were used for analysis. Dioxigenin-labeled full-length Arc antisense riboprobe was hybridized with tissue overnight, detected with anti-dioxigenin-horseradish peroxidase conjugate (Roche Applied Science), and revealed with a cyanine-3 substrate kit (CY3 Direct FISH; PerkinElmer Life Sciences). Nuclei were counterstained with DAPI (Molecular Probes). Twelve-bit gray scale images were acquired on a Nikon TE2000 epifluorescence microscope using a ×4 plan fluor objective. Regions of interest (ROIs) were traced on the DAPI images and transferred to the corresponding CY3 images. Mean gray scale intensities were determined for each ROI. Background ROIs were traced over the corpus callosum for the purposes of background subtraction. Images were analyzed for CA1, CA2, CA3, and dentate gyrus of the dorsal hippocampus (anterior-posterior, approximately -3.2 to -3.6 from bregma) on two to three different slides for each animal. metamorph software (Universal Imaging) was used both for image acquisition and image analyses.
Continuous Multiple Trial IA (CMIA). Either Arc antisense or scrambled control oligodeoxynucleotides (ODNs, Midland Certified Reagent Company) were delivered through guide cannulae directed at the dorsal hippocampus 3 h before CMIA training. ODNs (1.0 mM in 1.0 μl PBS) were infused over 154 sec. The biochemical specificity and efficacy of the antisense and scrambled sequences used in the current studies were validated in a past study (15). During training, rats were retained in the IA apparatus and received a 0.35-mA foot shock upon each entry into the dark compartment and training continued until the rat reached a criterion of remaining in the light compartment for 100 consecutive seconds. Retention was tested 48 h later, using the same procedures as those described for one-trial inhibitory avoidance. After testing, the rats were killed with an overdose of sodium pentobarbital (100 mg/kg i.p.) and perfused. Brains were sectioned at a thickness of 40 μm, mounted, and stained. Only brains with cannulae located in the dorsal hippocampus were used for analysis.
Data Analysis. Because of nonnormal distributions of latencies, comparisons for behavioral procedures used the nonparametric Mann-Whitney U test. Densitometry results from immunoblots were compared across groups by taking the normalized values for each rat to make a ratio of drug-infused vs. vehicle-infused hemisphere. This ratio was compared, using an ANOVA with Fisher's posttest, to results from vehicle-only-infused rats and to results from rats receiving the same treatment but without training. To determine whether there were differences in mRNA levels, the gray scale ratio (gray scale value for a given ROI in the dorsal hippocampus ipsilateral to the drug-infused BLA/gray scale value for the corresponding ROI in the dorsal hippocampus ipsilateral to the vehicle-infused BLA) was compared across groups for each area of the hippocampus independently (CA1, CA2, CA3, and dentate gyrus) and again with ratios taken from the sum of all areas of the hippocampus.
Results
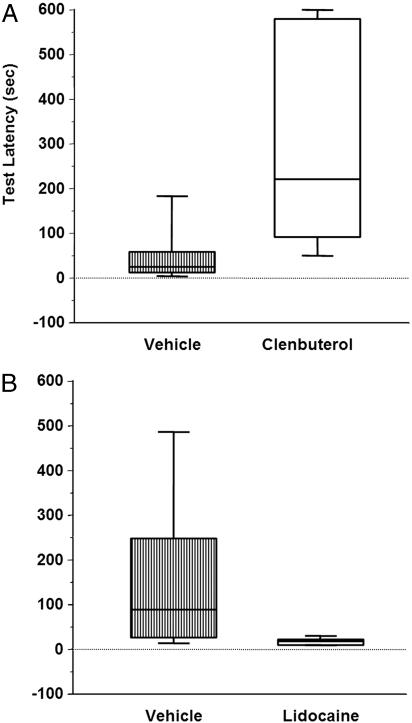
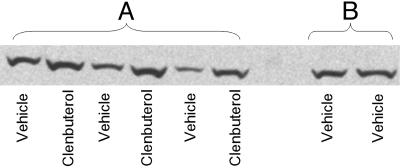
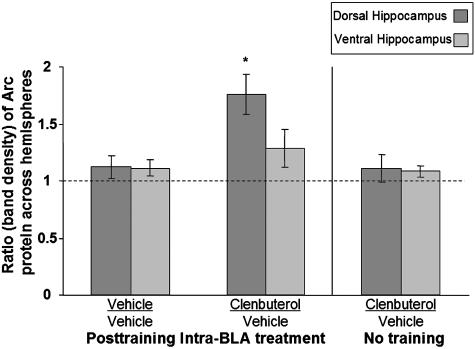
Posttraining intra-BLA infusions of clenbuterol enhanced 48-h inhibitory avoidance retention performance (Fig. 2A; U = 12.5; P < 0.01). Separate groups of rats were trained in IA, given posttraining drug infusions, and killed 45 min later. Protein extracts were prepared from tissue of the dorsal and ventral hippocampus, and levels of Arc protein were quantified by Western blot as described in Materials and Methods. Figs. 3 and 4 illustrate the ratio of Arc protein expression in the dorsal hippocampus across the two hemispheres. An ANOVA revealed a significant difference across groups (Fig. 4; F2,17 = 8.065; P < 0.005). For rats given infusions of clenbuterol into the BLA of one hemisphere and vehicle infusions into the BLA of the other hemisphere, the ratio of Arc protein levels in the dorsal hippocampus between the two hemispheres was significantly greater than that seen in rats receiving bilateral vehicle infusions (P < 0.005, Fisher's posttest). This ratio was also significantly greater than that seen in drug-infused rats that were not trained on the IA task (P < 0.005). There was no significant effect of clenbuterol on expression of cFos protein in the dorsal hippocampus (F2,11 = 0.151; P > 0.05). There were no significant differences between the groups in Arc protein expression in the ventral hippocampus (F2,15 = 1.024; P > 0.05).
Fig. 2.
Posttraining intra-BLA infusions of clenbuterol enhance and lidocaine impair IA retention performance. (A) Box and whisker plot indicates median latency in seconds + interquartile range for rats receiving intra-BLA infusions of vehicle (24 + 22.12) and intra-BLA infusions of clenbuterol (220 + 243.12). Clenbuterol-treated rats spent significantly more time in the light compartment on the retention test than vehicle-treated rats (P < 0.01). (B) Posttraining intra-BLA infusions of lidocaine impair IA retention performance. Box and whisker plot indicates median latency in seconds + interquartile range for rats receiving intra-BLA infusions of vehicle (85 + 109) and intra-BLA infusions of lidocaine (13 + 5.87). Lidocaine-treated rats spent significantly less time in the light compartment on the retention test than did vehicle-treated rats (P < 0.01).
Fig. 3.
Western blot for Arc protein in the dorsal hippocampus from rats infused with clenbuterol into the BLA of one hemisphere and vehicle into the BLA of the other hemisphere (A) and from a rat receiving bilateral vehicle infusions (B).
Fig. 4.
Posttraining intra-BLA clenbuterol enhances Arc protein expression in the dorsal hippocampus. The ratio of Arc protein across hemispheres is significantly greater in the dorsal hippocampus of rats that received posttraining intra-BLA clenbuterol than in vehicle-infused rats (P < 0.005) or in clenbuterol-infused rats that were not trained (P < 0.01).
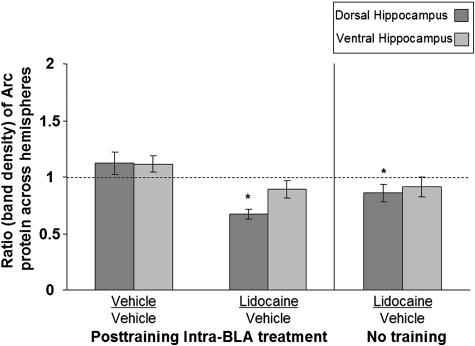
Posttraining intra-BLA infusions of lidocaine impaired IA retention performance tested 48 h later (Fig. 2B; U = 14.0, P < 0.05). Western blot analysis indicated that lidocaine treatment also decreased expression of Arc protein in the dorsal hippocampus ipsilateral to the drug infusion (Fig. 5). An ANOVA revealed that, when the memory-impairing dose of lidocaine was infused into the BLA of one hemisphere and vehicle was infused into the BLA of the other hemisphere, the ratio of Arc expression in the dorsal hippocampus across the two hemispheres was significantly reduced when compared with that seen in rats receiving bilateral vehicle infusions [Fig. 5; overall ANOVA (F2,17 = 8.282), P < 0.005; P < 0.001]. Intra-BLA infusions of lidocaine, in the absence of behavioral training, modestly but significantly decreased Arc expression in the dorsal hippocampus when compared with vehicle-only infused rats (P < 0.05). As with clenbuterol-treatment, the lidocaine treatment did not affect the expression of cFos at this time point (F2,10 = 0.722; P > 0.05). The groups did not differ in Arc protein expression in the ventral hippocampus (F2,13 = 2.670; P > 0.05).
Fig. 5.
Posttraining intra-BLA lidocaine reduces Arc protein expression in the dorsal hippocampus. The ratio of Arc protein across hemispheres is significantly lower in the dorsal hippocampus of rats that received posttraining intra-BLA lidocaine than in vehicle-infused rats (P < 0.001). The ratio of Arc expression across hemispheres is also significantly different in lidocaine-infused rats that were not trained on the IA task versus that seen in vehicle-infused rats (P < 0.05).
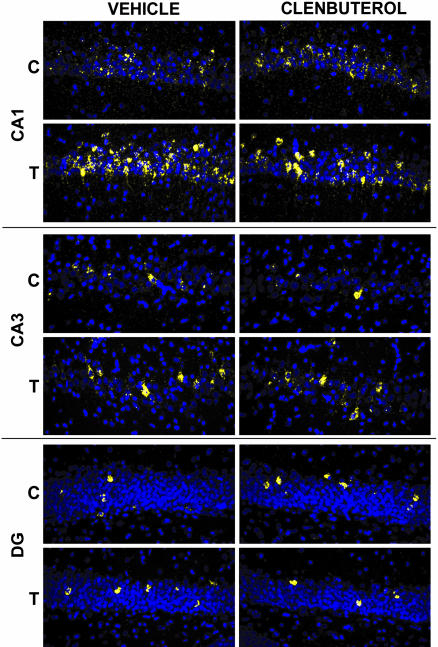
Examination of ratios of Arc mRNA levels in CA1, CA2, CA3, and DG of drug/vehicle-infused hemispheres, revealed no significant differences across treatment groups in any area of the hippocampus. Similarly, no significant differences were observed when considering across-hemisphere ratios of total hippocampal Arc mRNA levels. Because it was predicted that training would induce an increase in hippocampal Arc mRNA, and amygdala stimulation would further enhance this increase, one-tailed t tests were performed to compare gray scale intensities taken from the vehicle-infused hemisphere in trained versus untrained rats. Gray-scale intensities taken from vehicle-infused hemisphere versus drug infused hemisphere were compared for each area of the hippocampus independently, then a separate comparison was made for the sum of the intensity values in all four regions in the vehicle-infused hemisphere versus the drug-infused hemisphere. A significant effect was seen in the comparison of gray scale intensities in vehicle-infused hemispheres of trained versus nontrained rats in areas CA1 [t(14) = 1.75; P < 0.05] and DG [t(14) = 1.92; P < 0.05]. No other comparisons were significant. Fig. 6 illustrates representative sections of hybridized dorsal hippocampus.
Fig. 6.
Memory-enhancing, posttraining intra-BLA clenbuterol infusions do not affect Arc mRNA levels in the dorsal hippocampus. Blue pseudocoloring indicates nuclei, and yellow indicates Arc mRNA in three areas of the dorsal hippocampus. The dorsal hippocampus ipsilateral to the vehicle infusion is compared to the hippocampus ipsilateral to the clenbuterol infusion in an individual rat. Arc mRNA levels were greater in trained (T) rats than in cage controls (C), but were no different across hemispheres in rats receiving posttraining infusions of clenbuterol into the BLA of one hemisphere and vehicle into the BLA of the other hemisphere.
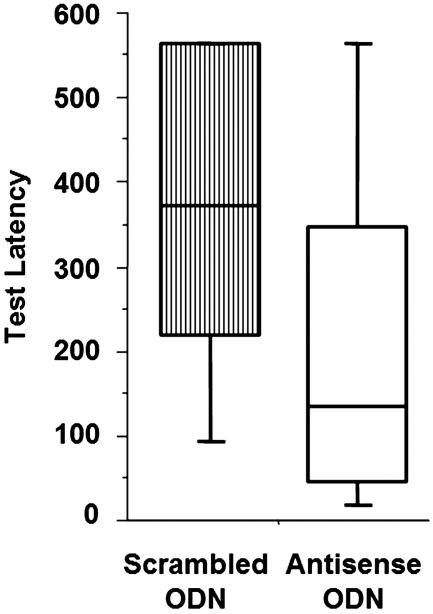
To determine whether Arc protein expression in the dorsal hippocampus is related to consolidation of IA training, rats were given bilateral infusions of either Arc antisense or scrambled control ODNs 3 h before training in CMIA (Fig. 7). There were no significant differences between scrambled- and antisense ODN-infused rats in trials to criterion (U = 134; P = 0.53) or latency to enter the dark compartment (U = 151; P = 0.95) during training. However, intradorsal hippocampus infusions of Arc antisense ODNs significantly impaired 48-h retention performance on the CMIA task (U = 86; P < 0.05).
Fig. 7.
Intradorsal hippocampus infusions of Arc antisense oligodeoxynucleotides impairs retention performance on continuous multiple trial IA. Retention latency was significantly greater in scrambled ODN-infused controls than in antisense-infused rats (P < 0.05).
Discussion
The major finding of the present experiments is that infusions of memory-modulating drugs into the BLA have parallel effects on memory consolidation and Arc protein expression in the dorsal hippocampus. Changes in Arc protein levels were not reflected by changes in mRNA expression, suggesting that amygdala modulation of synaptic plasticity in efferent brain regions is at a posttranscriptional level. The changes in Arc protein levels appear to be related to the observed changes in memory performance, as infusions of Arc antisense oligodeoxynucleotides into the dorsal hippocampus impaired performance of an inhibitory avoidance task.
Extensive findings indicate an ipsilateral interaction between the BLA and hippocampus. Ipsilateral, not contralateral, BLA lesions impair induction of hippocampal LTP (18). Inactivation of the ipsilateral, but not the contralateral, BLA blocked the enhancing effects of a glucocorticoid receptor agonist infused into the hippocampus on memory for IA (10). Lesions of the ipsilateral, but not the contralateral, BLA also blocked the enhancing effect of a cAMP analog infused into the entorhinal cortex on memory for IA (18). Here, we have shown that Arc expression in the dorsal hippocampus ipsilateral to an intra-BLA drug treatment may be compared to the expression of Arc in the dorsal hippocampus ipsilateral to an intra-BLA vehicle infusion in the same animal. Thus, each animal serves as its own control. This paradigm is useful in studies examining IEG expression, as variability across animals may obscure subtle differences.
The dose of clenbuterol that enhanced memory on the IA task when infused into the BLA immediately after training also increased protein levels of the immediate-early gene Arc in the dorsal hippocampus relative to the contralateral (vehicle-infused) hippocampus. Conversely, a memory impairing dose of lidocaine resulted in a decrease in Arc protein expression in the dorsal hippocampus relative to the contralateral (vehicle-infused) side of the brain. The same treatments did not significantly affect levels of Arc protein in the ventral hippocampus, consistent with the view that the dorsal hippocampus plays a unique role in memory consolidation (20-22). Interestingly, similar effects were not seen with another IEG (cFos), suggesting a distinct role for Arc in modulating hippocampal synaptic plasticity. This finding indicates that genes localized to the synapse may be preferentially modulated by input from the amygdala.
Although both clenbuterol and lidocaine significantly altered Arc protein levels in the dorsal hippocampus in trained rats, neither treatment significantly affected mRNA levels as compared to the vehicle treated hemisphere. Therefore, any hemispheric differences in hippocampal Arc protein expression cannot be explained by differences in hemispheric Arc mRNA expression. These findings are not likely due to a difference in sensitivities of the techniques used because the FISH technique, and RNA detection methods in general, tend to be more sensitive for detecting such changes than Western blotting. The FISH protocol used in the current study has previously been shown to be very sensitive to changes in Arc mRNA levels over a broad range of conditions, including behavioral activation under emotionally neutral conditions (17, 23, 24) and under stressful conditions such as water maze learning (25) as well as seizures (23, 26). The negative findings of amygdala lidocaine or clenbuterol infusions on hippocampal Arc mRNA expression determined with FISH also corroborate similar negative results determined by using an RNase protection assay of whole hippocampal punches (e.g., ref. 25 and B.S. and J.F.G., unpublished findings). These findings indicate that noradrenergic activation of the BLA alters functional protein expression in the dorsal hippocampus, and that this effect appears to be posttranscriptional; that is, on translation or turnover. Our findings suggest that the effect is more likely to be due to an influence on translation rather than on turnover or stability of the protein. In the current experiments, protein levels were assessed in animals killed 45 min after training. Previous reports indicate that Arc protein levels continue to increase up to 60 min after training (17, 27). Therefore, breakdown is unlikely to be responsible for the elevated levels of protein, as translation appears to be the dominant process occurring at this time point.
Although previous findings indicate that behavioral stimulation affects Arc mRNA levels in the hippocampus (15, 23), our findings suggest that amygdala activity influences memory through a different mechanism. Other investigators have also found disparities between levels of Arc mRNA and protein. For example, Kelly and Deadwyler (28) reported that mRNA levels remained elevated, whereas protein levels returned to baseline in rats that were overtrained on a lever-press task. Furthermore, in a mouse model of Fragile X syndrome, mutant mice display more Arc protein than do wild-type mice, whereas transcript levels are not different (29), indicating a role for Fragile X mental retardation protein in translational suppression of Arc mRNA. Thus, the translation of Arc mRNA can be negatively or positively regulated.
Previous studies indicate that translational changes underlying synaptic plasticity may occur at stimulated synapses (30). Additionally, application of brain-derived neurotrophic factor (BDNF) to synaptosomal fractions results in increased levels of newly synthesized and total Arc protein, demonstrating dendritically localized translation of Arc as well as an influence of BDNF treatment on the degradation of Arc protein (31). Recent findings indicate that activation of the amygdala can transform transient into long-lasting plasticity in the hippocampus (32). The findings reported here are consistent with earlier studies (17, 23, 33) indicating that the cell firing activity associated with processing of contextual information initiates transcription and then translocation of Arc mRNA to the dendritic arbor in a proportion of CA1, CA3, and DG neurons. Subsequent posttraining stimulation of the BLA might then enhance translation at those recently activated synapses. Alternatively, or in addition, the BLA stimulation could lower the threshold for translation such that it would occur in synapses that were only weakly active during training. In support of this observation, recent findings indicate that pairing of subthreshold electrical stimulation with β-adenoreceptor activation is sufficient to induce long-lasting LTP in a hippocampal slice preparation and that this effect requires local, dendritic protein synthesis but is independent of transcription (34). The net effect of either of these two possibilities would be a strengthened network of synaptically coupled cells encoding relevant information about the IA training.
Arc is colocalized with the actin cytoskeletal matrix (16) and has been found to interact with other cytoskeletal proteins such as MAP2 (35) and CAMKII (36). Furthermore, recent research using electron microscopy confirms the enrichment of Arc protein in postsynaptic densities within stimulated dendritic spines (37). Thus, an increase in Arc activity may facilitate dendritic remodeling underlying long-term memory. Here, to determine whether the observed increase in Arc protein levels may play a role in long-term memory for inhibitory avoidance, Arc antisense ODNs were infused into the dorsal hippocampus before training on the continuous multiple trial IA task. The ODNs were infused before training to assure cellular uptake of the ODN by the time the memory consolidation process occurred. Earlier biochemical characterization of the identical sequence Arc antisense ODNs, at the same dose, were shown to lead to a significant (≈60%) decrease of Arc protein in antisense-treated hemispheres as compared to scrambled-treated hemispheres, but did not affect the expression of another IEG protein, Narp, or a neuronal specific protein, NP1 (15). Arc antisense ODN infusions impaired 48-h retention performance on the IA task, but did not affect acquisition. Intradorsal hippocampus treatment with this sequence of Arc antisense ODNs has also been found to impair memory, but not acquisition, of a spatial water-maze task and maintenance, but not induction, of LTP (17). Therefore, Arc expression in the hippocampus appears to play an important role in the maintenance of the synaptic modifications that underlie long-term memory for an IA task.
The findings presented here support the hypothesis that BLA activity influences memory storage by modulating synaptic plasticity in efferent areas of the brain through a mechanism involving the action(s) of Arc protein. These findings thus provide further evidence consistent with the hypothesis that the basolateral amygdala plays a critical role in enabling significant experiences to be well-remembered.
Acknowledgments
We thank Dr. Paul Worley (Johns Hopkins School of Medicine, Baltimore) for the kind gift of Arc antibody and Levi Maes and Amin Abdinezhad for technical assistance. We appreciate Katherine Reissner, Justin Shobe, and Dr. Shiv Sharma for help with the Western blotting technique. We thank Aryeh Routtenberg for helpful comments on a previous version of this manuscript. This research was supported by National Institutes of Health Grants MH12526 and DC-05592 (to J.L.M.), MH060123 (to J.F.G.), NS12333 (to O.S.), and MH12646 (to C.K.M.).
Abbreviations: BLA, basolateral amygdala; LTP, long-term potentiation; IEG, intermediate-early gene; IA, inhibitory avoidance; ROI, region of interest; CMIA, continuous multiple trial IA; ODN, oligodeoxynucleotide.
References
- 1.McGaugh, J. L. (2004) Annu. Rev. Neurosci. 27, 1-28. [DOI] [PubMed] [Google Scholar]
- 2.Ferry, B. & McGaugh, J. L. (1999) Neurbiol. Learn. Mem. 72, 8-12. [DOI] [PubMed] [Google Scholar]
- 3.Ferry, B., Roozendaal, B. & McGaugh, J. L. (1999) Eur. J. Pharmacol. 1, 9-16. [DOI] [PubMed] [Google Scholar]
- 4.Quirarte, G. L., Roozendaal, B. & McGaugh, J. L. (1997) Proc. Natl. Acad. Sci. USA 94, 14048-14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalumiere, R. T., Buen, T. V. & McGaugh, J. L. (2003) J. Neurosci. 23, 6754-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miranda, M. I., LaLumiere, R. T., Buen, T. V., Bermudez-Rattoni, F. & McGaugh, J. L. (2003) Eur. J. Neurosci. 18, 2605-2610. [DOI] [PubMed] [Google Scholar]
- 7.Hatfield, T. & McGaugh, J. L. (1999) Neurobiol. Learn. Mem. 71, 232-239. [DOI] [PubMed] [Google Scholar]
- 8.Richter-Levin, G. & Akirav, I. (2003) Brain Res. Rev. 43, 247-256. [DOI] [PubMed] [Google Scholar]
- 9.Packard, M. G., Cahill, L. & McGaugh, J. L. (1994) Proc. Natl. Acad. Sci. USA 91, 8477-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roozendaal, B., Nguyen, B. T., Power, A. E. & McGaugh, J. L. (1999) Proc. Natl. Acad. Sci. USA 28, 11642-11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidenbecher, T., Reymann, K. G. & Balschun, D. (1997) Proc. Natl. Acad. Sci. USA 94, 1494-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikegaya, Y., Nakanishi, K., Saito, H. & Abe, K. (1997) NeuroReport 8, 3143-3146. [DOI] [PubMed] [Google Scholar]
- 13.Akirav, I. & Richter-Levin, G. (2002) J. Neurosci. 22, 9912-9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steward, O., Wallace, C. S., Lyford, G. L. & Worley, P. F. (1998) Neuron 21, 741-751. [DOI] [PubMed] [Google Scholar]
- 15.Guzowski, J. F., Lyford, G. L., Stevenson, G. D., Houston, F. P., McGaugh, J. L., Worley, P. F. & Barnes, C. A. (2000) J. Neurosci. 20, 3993-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyford, G. L., Yamagata, K., Kaufman, W. E., Barnes, C. A., Sanders, L. K., Copeland, N. G., Gilbert, D. J., Jenkins, N. A., Lanahan, A. A. & Worley, P. F. (1998) Neuron 14, 433-445. [DOI] [PubMed] [Google Scholar]
- 17.Guzowski, J. F., McNaughton, B. L., Barnes, C. A. & Worley, P. F. (1999) Nat. Neurosci. 2, 1120-1124. [DOI] [PubMed] [Google Scholar]
- 18.Ikegaya, Y., Saito, H. & Abe, K. (1994) Brain Res. 656, 157-164. [DOI] [PubMed] [Google Scholar]
- 19.Roesler, R., Roozendaal, B. & McGaugh, J. L. (2002) Eur. J. Neurosci. 15, 905-910. [DOI] [PubMed] [Google Scholar]
- 20.Bannerman, D. M., Grubb, M., Deacon, R. M. J., Yee, B. K., Feldon, J. & Rawlins, J. N. P. (2003) Behav. Brain Res. 139, 197-213. [DOI] [PubMed] [Google Scholar]
- 21.Zhang, W.-N., Pothuizen, H. H. J., Feldon, J. & Rawlins, J. N. P. (2004) Neuroscience 127, 289-300. [DOI] [PubMed] [Google Scholar]
- 22.Matus-Amat, P., Higgins, E. A., Barrientos, R. M. & Rudy, J. W. (2004) J. Neurosci. 24, 2431-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazdarjanova, A., McNaughton, B. L., Barnes, C. A., Worley, P. F. & Guzowski, J. F. (2002) J. Neurosci. 22, 10067-10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazdarjanova, A. & Guzowski, J. F. (2004) J. Neurosci. 24, 6489-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzowski, J. F., Setlow, B., Wagner, E. K. & McGaugh, J. L. (2001) J. Neurosci. 21, 5089-5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bottai, D., Guzowski, J. F., Schwarz, M. K., Kang, S. H., Xiao, B., Lanahan, A., Worley, P. F. & Seeburg, P. H. (2002) J. Neurosci. 22, 167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez-Amaya, V., Vazdarjanova, A., Mikhael, D., Rosi, S., Worley, P. F. & Barnes, C. A. (2005) J. Neurosci. 25, 1761-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly, M. P. & Deadwyler, S. A. (2003) J. Neurosci. 23, 6443-6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zalfa, F., Giorgi, M., Primerano, B., Moro, A., Di Penta, A., Reis, S., Oostra, B. & Bagni, C. (2003) Cell 112, 317-327. [DOI] [PubMed] [Google Scholar]
- 30.Steward, O. & Shuman, E. M. (2001) Annu. Rev. Neurosci. 24, 299-325. [DOI] [PubMed] [Google Scholar]
- 31.Yin, Y., Edelman, G. M. & Vanderklish, P. W. (2002) Proc. Natl. Acad. Sci. USA 99, 2368-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frey, S., Bergado-Rosado, J., Seidenbecher, T., Pape, H. C. & Frey, J. U. (2001) J. Neurosci. 21, 3697-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steward, O. & Worley, P. (2002) Neurobiol. Learn. Mem. 78, 508-527. [DOI] [PubMed] [Google Scholar]
- 34.Gelinas, J. N. & Nguyen, P. V. (2005) J. Neurosci. 25, 3294-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimoto, T., Tanaka, H., Kumamaru, E., Okamura, K. & Miki, N. (2004) J. Neurosci. Res. 76, 51-63. [DOI] [PubMed] [Google Scholar]
- 36.Donai, H., Sugura, H., Ara, D., Yoshimura, Y., Yamagata, K. & Yamauchi, T. (2003) Neurosci. Res. 47, 399-408. [DOI] [PubMed] [Google Scholar]
- 37.Moga, D. E., Calhoun, M. E., Chowdhury, A., Worley, P., Morrison, J. H. & Shapiro, M. L. (2004) Neuroscience 125, 7-11. [DOI] [PubMed] [Google Scholar]