Abstract
Neuro-Behçet’s disease (NBD) is a more severe but rare symptom of Behçet’s disease, which is mainly divided into parenchymal NBD (p-NBD) involving brain stem, spinal cord, and cerebral cortex. Non-p-NBD manifests as intracranial aneurysm, cerebral venous thrombosis, peripheral nervous system injuries, and mixed parenchymal and non-parenchymal disease. p-NBD is pathologically characterized by perivasculitis presenting with cerebrospinal fluid (CSF) pleocytosis, elevated total protein, and central nervous system (CNS) infiltration of macrophages and neutrophils, which are subdivided into acute and chronic progressive stages according to relapsing–remitting courses and responses to steroids. The diagnosis of NBD depends heavily on clinical features and magnetic resonance imaging (MRI) findings. The lack of laboratory biomarkers has hindered standard diagnostics. CSF interleukin (IL)-6 is the most investigated dimension of NBD and correlates with NBD activity, therapeutic responses, and prognosis. Further investigations have focused on inflammatory biomarkers that reflect the activation of innate and adaptive immune responses. Higher levels of CSF migration inhibitory factor and immunosuppressive acidic protein indicated the activation of macrophages in the CNS; increased IL-17, IL-10, T-bet/GATA-3, and retinoic acid related orphan receptor (ROR)-γt/Foxp3 ratios, marking the disrupted scale of the Th1/Th2 and Th17/Treg axis; and elevated B-cell activating factor of the TNF family (BAFF) and IgA/IgM intrathecal synthesis, suggesting that B cells play a dominant role in NBD. CNS destruction and degeneration as a consequence of neuroinflammatory cascades were confirmed by elevated CSF levels of NFL, β2MG, and MBP. Autoantibodies, including anti-STIP-1, anti-Mtch1, anti-B-Crystallin, and anti-m-Hsp65, provide substantial evidence for autoimmune essence and underlying microbiological infections in NBD immunopathogenesis. We summarized opinions on the clinical diagnosis, biomarkers, and pathological findings of NBD.
Keywords: neuro-Behçet’s disease, biomarkers, cerebrospinal fluid, diagnosis, blood–brain barrier, immunopathogenesis
Neuro-Behçet’s disease (NBD) is a more severe but rare symptom of Behçet’s disease (BD), which is mainly divided into parenchymal NBD (p-NBD) involving brain stem, spinal cord, and cerebral cortex. Non-p-NBD manifests as intracranial aneurysm, cerebral venous thrombosis, peripheral nervous system injuries, and mixed parenchymal and non-parenchymal disease. We summarized opinions on the clinical diagnosis, biomarkers, and pathological findings of NBD.
Graphical Abstract
Graphical Abstract.
Introduction
Behçet’s disease (BD), characterized by oral aphthous ulcers, genital ulcers, and ocular lesions, is a variable vasculitis with different disease episodes influencing multiple systems, including arteries, veins, joints, the gastrointestinal tract, and nerves. BD with neurological complications, referred to as neuro-Behçet’s disease (NBD), is the most fatal phenotype, but with a rare prevalence ranging from 3 to 30% [1].
As a clinical subset [2], NBD can be further divided into parenchymal NBD (p-NBD) with magnetic resonance imaging (MRI) abnormalities involving the brain stem, spinal cord, and cerebral hemispheres. Non-p-NBD is characterized by intracranial aneurysm and cerebral venous thrombosis, which is often regarded as an extension of cerebrovascular involvement in BD. Based on the disease courses [3, 4], NBD patients are also classified into acute and chronic progressive disease courses. The identification of NBD is largely dependent on clinical manifestations and MRI findings. To date, there have been no validated clinical guidelines for the diagnosis of NBD. The widely accepted diagnostic criteria for NBD are the 2014 International Consensus Recommendations (ICR) for NBD [5], which emphasize the significance of combining clinical symptoms and ancillary investigations, including MRI screenings and laboratory cerebrospinal fluid (CSF) findings.
MRI evidence is rather objective but can be easily confused with other neurological diseases such as multiple sclerosis (MS) in clinical practice. CSF interleukin(IL)-6 currently serves as a biomarker supporting the diagnosis of NBD [6–10] and has already been included in the 2014 ICR criteria [5]. However, CSF IL-6 is nonspecific for NBD, limiting its diagnostic value. Although NBD is an emerging area of research, there is still no specific biomarker that facilitates its diagnosis.
Moreover, the immunopathogenesis of NBD is not fully understood. The CSF signal of pleocytosis and elevated total protein as well as IL-6 levels [11], previous autopsy examinations identifying multifocal neutrophilic infiltrations around vessels [12], and signs of demyelination and necrosis [13] hint at an immune-mediated pathogenesis of NBD. Future studies analyzing the pathophysiological mechanisms of NBD are necessary to improve our understanding of the disease and to facilitate the detection of novel biomarkers for diagnosis. The present review summarizes existing evidence related to three different aspects: clinical diagnosis, potential biomarkers, and mechanisms underlying the pathogenesis of NBD.
Clinical diagnosis
Clinical subsets of NBD patients
Patients with NBD can be divided into two main groups according to the site of involvement of the neurological system: parenchymal and non-parenchymal. Herein, we compared the clinical and imaging features between parenchymal neuro-Behçet’s disease (p-NBD) and non-p-NBD in Table 1.
Table 1:
comparison of clinical and biological differences between parenchymal and non-parenchymal NBD
| p-NBD | Non-p-NBD | Reference | ||
|---|---|---|---|---|
| Common clinical features |
|
|
[2, 5, 14] | |
| Imaging features | MRI: lesions on the upper brainstem extending into the thalamus and basal ganglia on one side | Venography: evidence of CVST; rare of occluded dural sinus and venous infarcts | [15] | |
| Therapy |
|
|
[5, 16–18] | |
| Biomarkers | CSF OCB |
|
|
[19] |
| CSF pleocytosis | Usually occurs p-NBD | Generally normal | [1, 20] | |
| CSF protein | Usually raises in p-NBD | Generally normal | ||
| CSF pressure | Generally normal | Usually raises in non-p-NBD | ||
| CSF NFL, HoxB3 and YKL-40 | Higher in p-NBD than non-p-NBD | [21] | ||
| CSF IgG against αB-crystallin | Higher in p-NBD than non-p-NBD | [22] | ||
| CSF IL-6 | Higher in p-NBD (both acute and chronic progressive ones) than non-p-NBD | [7] | ||
p-NBD, an inflammatory meningoencephalitic condition, is prevalent in ~75–80% of patients [5, 23]. Those with p-NBD usually manifest with brainstem, cerebral hemispheric, spinal cord, and meningoencephalitis syndromes [1]. The major MRI characteristic of p-NBD is in the upper brainstem extending into the ipsilateral thalamus and basal ganglia [15, 24]. While ophthalmospasms, cranial neuropathy, and cerebellar/pyramidal dysfunctions are commonly recognized in brainstem features, encephalopathy, hemiparesis, hemisensory loss, seizures, acute confusion, and psychiatric manifestations are easily found in hemispheric involvement [2], spinal presentation is always described as transverse myelitis [25]. About 5.2–27.1% of headaches (primarily migraine and tension type) were attributed to NBD [26–28], thus it could be the first hint of central nervous system (CNS) involvement supplemented by abnormalities in neuroimaging or CSF [29] and a sign of increased disease activity among BD patients [27]. However, caution should be exercised if a headache is accompanied by periorbital pain due to uveitis or intracranial infections [30].
Non-p-NBD, usually referred to as neurovascular BD, involves neurovascular structures and can be divided into cerebral venous sinus thrombosis (CVST), large arterial lesions including arterial occlusion, and aneurysms. CVST occurs in 15–20% of NBD patients [2, 31]. Most patients with CVST endure subacute or chronic clinical onset, usually confined to intracranial hypertension without obvious upregulation of CSF inflammatory markers, and have a good prognosis. BD vasculitis is considered an insidious pathological caution for cerebral arterial occlusion and aneurysms because inflammatory cells, including neutrophils, lymphocytes, and plasma cells, infiltrate the media and adventitia in autopsy series [32, 33]. However, several reports have shown no histopathological features of vasculitis among patients with cerebral aneurysms [34, 35], obscuring the causative association between vasculitis and NBD.
Peripheral nervous system (PNS) involvement in NBD is rarely reported, the clinical spectrum of which is composed of neuropathy, myopathy, and neuromuscular junction disorders [5]. Based on a previous report by Al-Araji and Kidd in 2009 [2], PNS involvement in NBD is extremely rare, occurring at 0.8%. The prevalence of peripheral neuropathy in patients with BD ranged from 0.609% (1/164) to 16% (8/50) [36]. This relatively large difference might be explained by the unnoticeable symptoms caused by PNS involvement; electrophysiological examinations are not routinely used in subclinical NBD patients with clinical symptoms of PNS, and some clinicians might consider sensory and motor symptoms to be secondary to CNS involvement in NBD patients rather than peripheral neuropathy.
International diagnostic criteria and differential diagnosis
To date, there are no validated criteria for diagnosing NBD. The current study follows the 2014 ICR for NBD, as proposed by Kalra et al. [5]. The criteria are divided into four subtypes: parenchymal lesions (multifocal, brainstem, spinal cord, cerebral, asymptomatic, and optic neuropathy), non-parenchymal involvement (CVST, intracranial aneurysm, cervical extracranial aneurysm/dissection, and acute meningeal syndrome), PNS injuries (peripheral neuropathy and mononeuritis multiplex, myopathy, and myositis), and mixed parenchymal and non-parenchymal diseases. When diagnosing NBD, patients should first satisfy the International Study Group criteria 1990 [37] or any other generally accepted criteria for BD. Second, patients should have objective neurological abnormalities caused by BD, proven by both neuroimaging and laboratory CSF tests. Third, there is no better explanation for the neurological findings. Clinicians can make a definite diagnosis only when patients meet the above-mentioned criteria. Otherwise, patients with definite NBD syndrome but not satisfying the BD criteria or patients with non-NBD characteristic neurological syndrome should be defined as having probable NBD.
p-NBD, comprising the majority of NBD patients, is commonly mentioned in the differential diagnosis of inflammatory or demyelinating CNS disease and is characterized by the development of immune-mediated meningoencephalitis that predominantly involves the upper brainstem lesion that extends into the thalamus and basal ganglia [15, 24], where it infiltrates with intense inflammatory polymorphs, eosinophils, lymphocytes, macrophages, as well as areas of necrosis and apoptotic neuronal loss [2, 38, 39]. According to Kidd et al., the incidence of brainstem involvement in NBD was as high as 52% [40], and NBD patients developed subacute episodes of neurological dysfunction predominantly situated in the brainstem. There is also an urgent need to discriminate p-NBD from patients with brainstem encephalitis presenting with typical brainstem and diencephalon lesions that mimic NBD on MRI. Therefore, for the differential diagnosis, p-NBD is a masquerader of MS and myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) [31, 41]. However, these can be distinguished in several ways. From the laboratory view, intrathecal oligoclonal IgG bands are found in 95% of MS cases, but only in 6.6–17.5% of NBD [19, 31]. The presence of anti-MOG IgG is fundamental to the diagnosis of MOGAD [42]. Notably, a more inflammatory view in CSF, including higher white blood cells, polymorphonuclear (PMN) dominant pleocytosis, IL-6, and IL-10, together with the absence of anti-MOG IgG and anti-AQP4 IgG, is more suggestive of NBD probability [11, 43]. In terms of clinical and neuroimaging characteristics, patients with NBD usually have less optical nerve involvement than those with MS. Moreover, periventricular, juxtacortical, and corpus callosum lesions are relatively rare in NBD but more common in MS [31]. Therefore, brainstem atrophy in patients with NBD can be used as a powerful discriminator. The clinical and MRI features of NBD, MS, and MOGAD are summarized in Table 2.
Table 2:
differential diagnosis of NBD from MS and MOGAD with brain and brainstem involvement
| Categories | MOGAD with brain and brainstem involvement | NBD | Multiple sclerosis |
|---|---|---|---|
| Pediatric onset | Frequent | Extremely rare | Rare (3–10%) |
| Gender distribution | Female = Male | Female < Male | Female>Male |
| Most common neurological involvements | White matter, deep grey matter, middle cerebellar peduncle, large brainstem, and confluent cortical |
|
|
| Clinical presentations | Encephalopathy, epilepsy, focal deficits, and cerebral cortical encephalitis can occur | Headache, Horner syndrome, pseudobulbar palsy, epilepsy, aseptic meningitis, papilledema, hemiplegia, aphasia, paraplegia, sensory disorders, mental abnormalities |
|
| MRI features |
|
|
|
| Laboratory features |
|
|
|
| Recommended diagnostic criteria |
|
|
|
Considerations of variable clinical courses among p-NBD patients
The clinical presentation of NBD is insidious and comprises recurrent episodes of remission and exacerbation of a wide range of symptoms. p-NBD can generally be classified into acute and chronic progressive NBD. More specifically, patients with acute NBD (earlier stages) were those with acute meningoencephalitis presenting high-intensity areas on T2-weighted or fluid-attenuated inversion recovery images on MRI, attenuated by conventional steroid therapy, and were usually self-limiting [16, 45, 46]. Notably, acute NBD was also included in patients who had endured accumulating recurrency of neurological attacks (headache and fever) and suffered residual permanent damage or disability without disease progression. Another chronic progressive type of NBD represents later stages of disease process; those who were intractable to empirical immunotherapy (corticosteroid, cyclophosphamide, or azathioprine) also manifested with slowly progressive neurological symptoms (dementia, ataxia, and dysarthria), leading to severe disability and a poor prognosis [8, 46]. The major discrepancy of clinical and MRI characteristics among p-NBD at acute and chronic progressive phases were presented in Table 3.
Table 3:
comparisons of clinical and biological differences between acute and chronic progressive NBD
| Category | Acute NBD | Chronic progressive NBD | Reference | |
|---|---|---|---|---|
| Common clinical features |
|
|
[47, 48] | |
| MRI features |
|
|
[1, 47, 49–52] | |
| Therapy |
|
|
[53, 54] | |
| Biomarkers | CSF cell counts |
Sensitivity: 97.4% Specificity: 97.0%, (cutoff 6.2/mm3) for the diagnosis of acute NBD and non-NBD |
[4, 48, 49, 55] | |
| CSF IL-6 |
|
[4, 47, 56] | ||
| CSF BAFF |
|
[57] | ||
| Infiltration pattern in brain tissue from NBD |
|
[58] | ||
It is also noteworthy that those with NBD with chronic progressive symptoms currently have a chance of experiencing preceding attacks of acute NBD; likewise, some of those with acute NBD might also develop chronic progressive symptoms after long-term follow-up. Therefore, the dynamic criteria of the NBD classification are more inclined to delineate different clinical stages that vary over time. According to the theory put forward by Pallis and Fudge in 1956 and Wadia and Williams in 1957, NBD was categorized into three major types: those with brainstem disturbance associated with systemic symptoms; those with meningomyelitis comprising of various neurological signs on the spinal cord and hemisphere; and patients with a confusional syndrome arising from meningoencephalitis without focal neurological signs, which lead to chronic progressive development of dementia, Parkinsonism, pseudobulbar palsy and quadriparesis [59, 60].
Another classification system for NBD was proposed by Akman-Demir et al. [3]. They manually divided NBD into four subgroups: attack and remission course, primary progressive course, secondary progressive course, and silent neurological involvement. Generally, the subgroups of acute and remission from their definition equal to acute NBD in the general classification reference; simultaneously, primary and secondary progression could be considered a chronic progressive subtype. Similarly, as reported by Kidd et al. [40], patients with NBD with one or repeated neurological attacks are referred to as acute NBD, and those with progressive disability are analogous to chronic progressive NBD. Therefore, investigations into clinical phenotypes, including acute and chronic progressive NBD, have already considered the relapsing–remitting form of NBD. It is possible that, at the time of sampling, some patients with NBD were classified as having acute NBD; however, they might have developed chronic progressive NBD when followed-up for a longer period of time. Likewise, patients with chronic progressive NBD might have experienced attacks of acute NBD preceding chronic progressive manifestations. All these circumstances could be classified into ‘secondary progression course’ according to Akman-Demir et al. [3]. We encourage the classification of NBD based strictly on sampling time, considering both primary and secondary progression as chronic progressive NBD. Comparisons of the different classification criteria based on the clinical course of NBD are presented in Table 4.
Table 4:
comparison of different classifications of NBD according to clinical courses
| Publication | Evidence | Category of NBD | Clinical description | Take home message |
|---|---|---|---|---|
| [59] | Common sites involved |
|
|
There is a prototypical conception of chronic progressive classification of NBD |
| [4, 61, 62] | Clinical stages of NBD Responses to steroids |
|
|
There is a chance for acute NBD (early stage) to develop into chronic progressive ones (later stages) |
| [40] | Neurological attacks of NBD |
|
|
— |
| [3] | Disease onset, course, and neurological involvement |
|
|
‘Attack’ equals to ‘acute phases of NBD’ in general classification reference, while ‘primary and secondary progression’ could be considered as chronic progressive subtype. |
It is necessary to differentiate chronic progressive NBD from the recovery phase of acute NBD [20, 55, 63], considering the different therapeutic responses and subsequent clinical outcomes. Moreover, discriminating acute NBD with persistent inflammation, demyelination, and neurological deterioration leading to chronic progressive NBD from those that could completely improve from acute NBD without further progression is an indispensable aspect for future mining of diagnostic markers.
Laboratory biomarkers
Currently, NBD diagnosis is mainly based on clinical features and MRI results. CSF and serum biomarkers of NBD are under consideration and not currently used in the diagnosis of NBD. p-NBD is often characterized by an inflammatory pattern with CSF pleocytosis and elevated CSF protein levels, whereas non-p-NBD normally presents with opening pressure [20]. The CSF total cell count is routinely tested in clinical laboratories and is found to be higher in acute p-NBD than in chronic progressive NBD [4]. A retrospective study consisting of 144 NBD cohorts explored the diagnostic utility of CSF cell count and IL-6 [4], revealing superior sensitivity (97.4%) and specificity (97.0%) of CSF cell counts in terms of distinguishing acute NBD and non-NBD, as well as a preferable diagnostic value of IL-6 in differentiating those with chronic progressive NBD from those in the recovery phase of acute NBD. Moreover, CSF IL-6 is commonly detected in clinical practice, as it is the classical biomarker for NBD and is included in the NBD diagnostic consensus. Increased levels and activities of CSF IL-6 have mostly been reported in NBD patients [6–10]. In most cases, CSF levels of IL-6 are consistently correlated with disease activity [49], in parallel with therapeutic responses and favorable outcomes [7, 16, 53]. However, these biomarkers are nonspecific for subtyping NBD or for differential diagnosis from other neuroinflammatory diseases. Harnessing specific biomarkers originating from NBD pathogenesis is essential for the diagnosis of NBD.
Laboratory parameters detected for NBD in Peking Union Medical College Hospital, 2012–2023
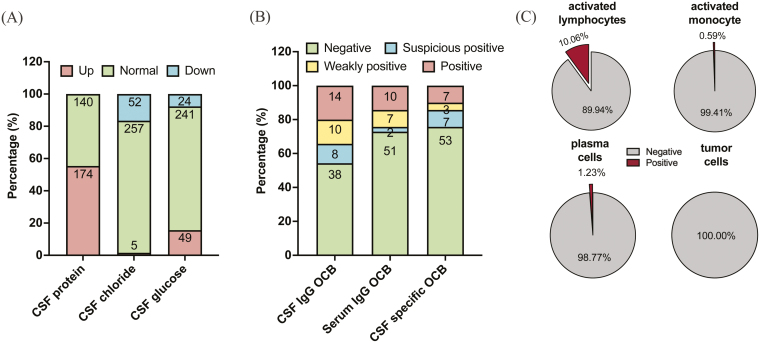
Herein, we share the status of laboratory metrics for NBD tested in Peking Union Medical College Hospital from 2012 to 2023. As shown in Fig. 1, the routine tests for clinical laboratory tests were biochemical dimensions, including CSF protein, CSF chloride, CSF glucose, and oligoclonal bands (OCBs), together with cytological examination of CSF. Of the 314 samples, 174 (55.41%), 5(1.59%), and 49 (15.61%) were found to be upregulated in CSF protein, CSF chloride, and CSF glucose, respectively (Fig. 1A). Among 70 tests of CSF-serum paired NBD samples, 14 (20.00%) were confirmed positive for CSF IgG OCB but were lower in serum IgG OCB (10 tests, 14.29%) and CSF-specific OCB (7 tests, 10.00%) positive rates (Fig. 1B). Mentioning the characteristics of NBD CSF cytology (Fig. 1C), we observed that a higher proportion of CSF samples possessed activated lymphocytes (10.06%) despite being deficient in activated monocytes, plasma cells, and tumor cells. It is noteworthy that 18/43 (41.86%) samples showed elevated CSF myelin basic protein (MBP) levels. Neurological autoantibodies have rarely been tested in patients with NBD. During the 11 years, all detections were negative for antibodies such as anti-GQ1b-IgM, anti-GM1-IgG, anti-GD1b-IgM, anti-GD1b-IgG, anti-Hu, anti-Yo, anti-Ri, anti-CV2/CRMP5, and anti-PNMA2(Ma2/Ta), except for the extremely rare positivity of anti-GQ1b-IgG (1/22) and anti-GM1-IgM (1/18).
Figure 1:
CSF laboratory parameters for NBD patients during routine test of hospitalization in PUMCH. (A) Distribution of upregulated, normal, and downregulated CSF protein (Reference interval: 0.15–0.45 g/l), CSF chloride (Reference interval: 120–132 mmol/l), and CSF glucose (Reference interval: 2.5–4.5 mmol/l) among NBD patients (N = 314). (B) Positivity of OCBs using 70 CSF-serum paired samples of NBD. (C) CSF cytologic features among NBD patients based on morphology and May-Grunwald Giemsa (MGG) staining patterns discerned by two independent experienced lab scientists in PUMCH (N = 169)
Exploring biomarkers relevant to NBD pathogenesis
p-NBD is compactly associated with inflammatory cascades in both peripheral blood and the CNS, characterized by increased CSF PMN leukocytes, elevated protein, and CNS infiltration of macrophages together with neutrophils [12, 64], which is hypothesized to be caused by T helper cells (Th). Investigating cytokines, chemokines, and immune-mediated inflammation is vital to facilitating NBD diagnosis and understanding its pathogenesis.
Biomarkers from innate and adaptive immune responses
The innate immune system is involved in the etiology of NBD. Macrophage migration inhibitory factor (MIF), released by T cells, upregulated the proinflammatory activity of T lymphocytes and macrophages [65]; moreover, MIF was significantly elevated in CSF compared with MS and non-inflammatory neurological disease (NIND) and correlated with CSF cell counts [66]. This suggests a potential role for macrophages in the development of NBDs. Coincidentally, produced mainly by macrophages, CSF immunosuppressive acidic protein (IAP) was considerably higher in those with active NBD than in those in inactive stages [67], which correlated well with disease activity [68]. The close association between CSF IAP and CSF protein [67] indicated that the increased levels of CSF IAP were not only due to blood–brain barrier (BBB) leakage from the serum but also from local production in the CNS. Determining IAP during disease duration seems to reflect the activity of neuroinflammation as well as the extent of immunopathogenesis. Increased proteolytic enzyme activities in mononuclear cells and granulocytes of patients with active NBD compared to normal controls [69] corroborate the existence of a more acute inflammatory process. Previous CSF cytological examination of NBD patients also confirmed these phenomena, observing in them a relatively higher percentage of activated monocytes and lymphocytes than in healthy subjects [70]. In contrast, to prevent and suppress the overactivation of innate inflammation, CSF mRNA expression and protein levels of IL-37 have spontaneously increased, especially in NBD patients after treatment and at remission stages, compared to non-inflammatory NIND, and are inversely correlated with proinflammatory factors such as IL-6, IL-17, IL-21, and thymic stromal lymphopoietin (TSLP) [71]. However, the role of IL-37 in NBD pathogenesis requires further validation due to the small scope of this study.
As the primary source of cytokines, the vital role of T cell responses from the adaptive immune system in neuroinflammatory events needs to be elucidated. The elevated expression of T cell cytokines (IL-17) and transcription factors composing T-bet/GATA-3 and retinoic acid related orphan receptor (ROR)-γt/Foxp3 ratios in CSF emphasizes the disruption of the Th1/Th2 and Th17/Treg axis [6, 72]. In peripheral blood mononuclear cells (PBMCs) from NBD patients, higher-than-NIND levels of T-bet and IFN-γ mRNA expression also suggest a strong response of Th1 cells in NBD pathogenesis [73].
Moreover, the potential game underlying between anti-inflammatory and proinflammatory function of B lymphocytes in NBD pathogenesis should not be ignored, which is facilitated by both the Breg and effector B cells. To begin with, B-cell Activating factor of the TNF family (BAFF), responsible for B cell activation and long-term survival, is shown to rise in CSF samples of NBD [74, 75] and indicates disease status due to its incremental tendency in NBD patients following a more slowly progressive course than in those following an acute course [57]. On one hand, BAFF may lead to the expansion of the IL-10-producing B subpopulation [76]. From the follow-up experience of 19 patients with a first episode of CNS inflammation who subsequently developed NBD [73], a particular increase in IL-10 mRNA in CSF but not in PBMC, together with the lack of correlation between IL-10 and Foxp3 expression, verified the intrathecal production of IL-10 from Breg cells rather than T cells, which characterized anti-inflammatory response in CSF compartment. Additionally, the current study conducted by Maghrebi et al. [77] first came up with the conclusion that, compared with MS, NBD patients possessed upregulation of IL-10-producing cells, which were mainly comprised by B cells rather than CD4 + T cells in the CSF, pointing out the regulatory function of B cells (Breg) in ameliorating inflammatory status and preventing CNS destructions during NBD pathology. However, they didn’t provide data supporting the upregulation of IL-6 release from B cells in CSF. Despite an earlier response (within 1 h) of both IL-6 and IL-10 expression upon stimulation by CpG from PBMC of NBD patients than relapsing–remitting MS (RRMS) and NIND [77], specific cytokines still couldn’t take on their effects without corresponding receptors. So as for the CSF compartment, they’ve shown an elevated mRNA expression level of IL-10Rβ in both the NBD and RRMS samples when compared with NIND. No discrepancies in IL-6 receptors were found between NBD and NIND in CSF. And no difference was provided on the scale of IL-6 or IL-10 receptors in PBMC among NBD and NIND. That’s to say that IL-10 could function well in the CNS with the help of upregulating IL-10Rβ. However, the limitation of this study is that they didn’t prove the upregulation of IL-10Rβ exactly on B cells both in PBMC and CSF, thus hindering us to conclude whether B cells play a regulatory function or not in NBD pathogenesis. Future studies should focus on either elucidating the expression of IL-10/IL-6 receptor expression on B cells in the aspect of mRNA expression and population alteration on cells, or on testing the downstream pathways in response to proinflammatory (IL-6) or anti-inflammatory (IL-10) cytokines as well as their effects on neuronal cells in vitro. There is substantial evidence [78] that the mRNA of CD39 preferentially expresses on regulatory B cells in CSF samples from NBD patients rather than that mainly expressed on Tregs in RRMS CSF, showing a differential inflammatory profile. In return, higher IL-10 and IL-6 expression in the CNS of NBD than in RRMS could promote these Breg cells [9, 79]. When interpreting results generated from cytokines, we should be cautious and aware that cytokines can have pleiotropic effects and different origins from immunocytes.
On the other hand, despite being rarely found in NBD, the considerable involvement of effector B cells, determined by the 10% positivity of OCBs in matched serum and CSF samples of NBD, supports the participation of plasma B cells in the etiology [19]. Intrathecal production of immunoglobulins (Ig), especially IgA and IgM indexes [80], was helpful in monitoring NBD activity but was not helpful in differentiating NBD from MS due to overlapping Ig alterations. A Japanese cohort of patients with NBD has also demonstrated a mere elevation in the IgM index during active NBD courses. Furthermore, the IgM index also shows a remarkable tendency to decrease when neurologic symptoms almost vanish after successful clinical medication [81]. Recently, using 28 Chinese NBD samples, we have validated the pivotal role of the IgA index, which possesses a diagnostic accuracy exceeding 0.80, hinting at the intrathecal involvement of humoral immunity characterized by B cells in NBD [82].
Intriguingly, there is an interplay between innate and adaptive immunological disruptions in NBD pathogenesis. The emergence of enhanced complement 3 (C3) and C4 levels in the CSF of NBD [83], as well as higher C3 conversion products in NBD CSF, has marked complement activation and consumption [83], promoting neuroinflammation. More specifically, from a case study of one NBD patient with acute encephalitis [84], the authors claimed a simultaneous intrathecal production of IgM (IgM index), C3 (C3 index), and CSF immune complexes, as well as increased CSF CD8 + T cell percentage. Considering the previous appearance of IgM serum antibodies to the glycolipids GM and GA1 in NBD, it is reasonable to believe that CSF immune complexes composed of CNS-specific antigens and IgM autoantibodies have the potential to enhance antigen-specific cellular responses, such as CSF CD8 + T cells [85], downstream of Breg activity, thus promoting intrathecal Ig synthesis. The coexistence of humoral and cellular abnormalities warrants further exploration.
Biomarkers of neuro-immunological impairment and degeneration
Perivascular immunocyte infiltration in necrotic injuries constitutes NBD pathology. Matrix metalloproteinase-9 (MMP-9), produced by infiltrating leukocytes, plays a pivotal role in inducing astrocyte chemotactic activity, increasing the invasion of leukocytes by degrading the basal lamina of the CNS border, and contributing to neuroinflammation [86, 87]. A previous report [88] suggested that increased MMP-9 in serum but decreased in CSF, synthesized by the natural killer CD56DIM subset among NBD patients, could be used to distinguish from MS. Similarly, an increased ratio of MMP-9/TIMP-1 in NBD CSF and MMP-9’s correlation with CSF PMN cells [89] demonstrated its underlying origin. Hamzaoui et al. [90] revealed that both the mRNA and protein levels of IL-33 were elevated in CSF samples of NBD compared with those from NIND and BD with a headache. Given that IL-33 is dominantly released from damaged tissues or necrotic cells [91], this result has dropped a hint on the promising utility of IL-33 in identifying the occurrence of neurological damage. Similarly, the pathogenic role of Fas-mediated cell apoptosis has been investigated, since CSF levels of sFas-L were upregulated in patients [92], indicating inflammation-mediated CNS damage in NBD pathogenesis. Tumor necrosis factor-alpha (TNF-α), which induces apoptotic cell death, not only exerts pleiotropic functions in regulating neurogenesis, myelination, BBB permeability, and synaptic plasticity but also contributes to excitotoxicity and inflammation in the CNS [93]. In addition, studies have proven that 80% of patients with NBD experience significant beneficial effects with anti-TNF-α therapy using infliximab and adalimumab [94, 95]. Notably, CSF tumor necrotic factor alpha (TNF-α) concentration responded well to NBD therapy and was markedly downregulated after infliximab [75].
Neuroinflammatory reactions lead to neurological and local dysfunctions. Kuroda et al. reported that the CSF expression of gamma-aminobutyric acid in NBD [96], an inhibitory neurotransmitter, was quite low, identifying the prominent role of CNS dysfunction in NBD. Neurofilament light chain (NFL), a neuronal cytoplasmic protein, increases proportionally with the degree of axonal damage [97]. A recent study [21] showed that p-NBD presented incremental CSF levels of NFL compared with non-p-NBD, which was closely correlated with the modified Rankin score and appeared to predict somatic and cognitive dysfunction, serving as a prognostic biomarker during follow-up.
β2-microglobulin (β2MG) is expressed in neurons and glial cells [98]. A previous study found a dramatically increased intrathecal synthesis (β2MG index) in NBD compared with NIND, and the CSF and quotient were in parallel with beneficial therapeutic responses [45]. They further presumed that the elevation of β2MG was due to transudation of serum and intrathecal synthesis by infiltrating lymphocytes or damaged neurocytes. In parallel, MBP, the most specific protein in nerve tissue, has been identified as a major target of peripheral T cells in MS [99]. Few studies have focused on MBP profiles in the CSF and serum samples of patients with NBD. In 1980, Ohta et al. [100] first measured MBP levels in the CSF of Japanese patients with NBD and pointed out the dramatic increase in MBP, while most patients with other neurological diseases remained at normal levels. Using a 26-patient Chinese p-NBD cohort [101], our recent validation study uncovered the superior value of both CSF and serum MBP for discriminating between NBD, NIND, and CNS idiopathic inflammatory demyelination diseases, as well as the subgrouping of acute and chronic progressive NBD. Long-term supervision of serum MBP levels during the course of NBD sheds light on disease recurrence and therapeutic responses. It is noteworthy that the compact correlation between the IgG index and MBP index further implies demyelination-induced humoral responses in NBD pathogenesis.
Autoantibodies
Several studies have attempted to identify disease/tissue-specific autoantibodies in patients with NBDs. Among the markers developed in other autoimmune diseases, IgM anti-cardiolipin antibodies were found to be higher in NBD patients and reduced along with subsided disease activity [102], but they could appear in other rheumatic diseases with neurological involvement [103], hindering their application in differential diagnosis. Anti-asialo GM1 antibodies were positive in 6/10 NBD serum [104]. The appearance of such products with high molecular weights may permeate through the damaged BBB, giving rise to subsequential neuron destruction.
New antigen targets of NBD were validated using tissue localization and high-throughput technology. After conducting immunohistochemical studies, Vural and colleagues [105] identified tissue-specific antibodies targeting hippocampal and cerebellar-specific antigens in serum and CSF samples of patients with NBD rather than MS. Screening with a protein array and ELISA revealed that higher titers of stress-induced-phosphoprotein-1 (STIP-1) were detected as an intracellular antigenic target of this neural antibody, suggesting that it could be a potential diagnostic marker for NBD but lacks validation in different cohorts. Meanwhile, serum autoantibodies against mitochondrial carrier homolog 1 (Mtch1), an apoptosis-related protein, presented higher specificity (97.6%) in discriminating BD with and without neurological involvement, which is closely associated with NBD severity, such as attacks and disability levels [106]. Elevated serum and CSF IgG antibodies to alpha B-crystallin, which increase with demyelination, have also been reported by Celet et al. [22]. Given that human B cells infected with EBV are able to express alpha B-crystallin and trigger specific Th1 responses [107], we speculate that the cross-reactivity of uncertain virus and tissue antigens initiates autoimmune responses in subjects with NBD. The positivity rates of IgG, IgM, and IgA anti-mycobacterial heat shock protein 65 (m-Hsp65) were as high as 48% in CSF samples of p-NBD [108], local oligodendrocytes (expressing Hsp65), and diffuse BBB damage caused by Hsp65-recognition of γδ T cells, providing novel insights on pathogen involvement during NBD onset [109, 110].
Whether the occurrence of autoantibodies in patients with NBD is a product of autoimmune responses or is generated as an epiphenomenon towards random epitopes of enhanced inflammation from the involved tissue still needs to be further explored.
Microorganism
An unknown infectious pattern is deduced to mediate immune responses in NBD etiology, which was first demonstrated by elevated CSF IL-15, IL-8, and CXCL10 levels in NBD patients [111, 112], then incremental levels of CCL2, CXCL10, and IL-8 were found in both NBD and viral/bacterial meningitis [47, 113]. The upregulated PBMC expression of DEFA1B derived from microbicidal granules of neutrophils and NLRP3 in response to bacteria during an active attack in NBD patients [114] also provided supportive evidence for this hypothesis.
Pathogens have repeatedly been reported to be involved in BD pathogenesis [115]. It is noteworthy that the latest microbiota research [116] found that Prevotella was decreased in both NBD and MS fecal specimens, despite patients with MS experiencing larger microbiota community shifts. In the Prevotella and Bacteroides stratified analysis, opposite tendencies in NBD and MS were observed on the scale of the genera Butyricicoccus and Escherichia/Shigella. For disease-specific microbiological communities, increased levels of Clostridiales were evident in Prevotella-stratified NBD. Also, Prevotella histicola could suppress mice experimental autoimmune encephalomyelitis via downregulating Th1/Th17 cells and upregulating Treg cells [117]. The pathological role of imbalanced internal microbiota niches together with external pathogen invasion in NBD should be emphasized in different cohorts and CSF samples.
Exploring biomarkers relevant to NBD differential diagnosis and disease course
Biomarkers for distinguishing NBD and MS
p-NBD leads to misdiagnosis with MS in clinical practice. Except for clinical symptoms and MRI findings, NBD could be also differentiated from MS in the aspect of laboratory markers. The comparisons of differential biomarkers between NBD and MS are presented in Table 5. As for routine tests, a study pointed out the upregulation of CSF lymphocytes and neutrophils in NBD samples, but a higher positivity of CSF OCB among MS patients [118], claiming a more pronounced neuroinflammatory status in NBD pathology than MS.
Table 5:
comparisons of differential biomarkers between NBD and MS
| Biomarkers | Specimen | Sample size | Alterations | Clinical meanings | Reference |
|---|---|---|---|---|---|
| Lymphocytes (/mm3) | CSF | BD comorbid with MS: n = 18 NBD: n = 172 MS: n = 1574 |
NBD ↑ | More pronounced neuroinflammatory changes in CSF among NBD than MS | [118] |
| Neutrophils (/mm3) | CSF | BD comorbid with MS: n = 18 NBD: n = 172 MS: n = 1574 |
NBD ↑ | ||
| OCB positive/negative | CSF | BD comorbid with MS: n = 18 NBD: n = 172 MS: n = 1574 |
BD comorbid with MS: ↑ MS: ↑ |
||
| IL-22 mRNA IL-22 |
CSF cells CSF |
NBD: n = 27 MS: n = 23 |
NBD ↑ | IL-22 from CNS cells contributes to the infiltration of Th1 and Th17 through the BBB, which collaborate with the IL-6, to exacerbate the inflammation and damage within the CNS during NBD pathogenesis | [113] |
| IL-10 | CSF cells | NBD: n = 27 RRMS: n = 28 |
NBD ↑ |
|
[77] |
| CD19+/IL-10 + B cells % | CSF | NBD: n = 27 RRMS: n = 28 |
NBD ↑ | ||
| CD39 mRNA | PBMC CSF cells |
NBD: n = 20 RRMS: n = 28 |
NBD ↑ | Higher CD39 expression on Treg cells of MS but on B cells of NBD delineate a distinct inflammatory status between MS and NBD | [78] |
| CD73 mRNA | PBMC | NBD: n = 20 RRMS: n = 28 |
NBD ↑ | ||
| IL-6, IL-10, TNF-α mRNA | CSF cells | NBD: n = 20 RRMS: n = 28 |
NBD: IL-6 & IL-10↑ RRMS: TNF-α ↑ |
||
| IL-10 (both protein and mRNA) | CSF | NBD: n = 19 RRMS: n = 21 |
NBD ↑ but dependent from the one in PBMC |
|
[73] |
| T-bet mRNA IL-17 mRNA |
PBMC | NBD ↑ | Emphasize the importance of Th1 and Th17 cells in NBD pathogenesis | ||
| GABA | Stool | NBD: n = 12 RRMS: n = 13 |
NBD ↑ MS ↓ |
Reduced GABA could enhance Th17-type immunity and facilitate inflammation | [119] |
| MMP-9 | CSF | NBD: n = 18 RRMS: n = 15 |
NBD↓ MS ↑ but both are higher than NIND |
MMP-9 could degrade components of basal lamina and disrupt the integrity of BBB and neuroinflammation | [89] |
| IL-6 | CSF | NBD: n = 69 RRMS: n = 9 |
NBD ↑ | Il-6 might potentially involve in CNS tissue destruction and generation of attacks, which could be used to monitor disease activity among NBD | [7] |
| CXCL10 | CSF | NBD: n = 33 MS: n = 25 |
NBD ↑ |
|
[111] |
| CXCL8 | Serum | ||||
| CCL2 | CSF Serum |
||||
| IgG against αB-crystallin | CSF | NBD: n = 27 RRMS: n = 33 |
NBD ↑ | IgG responses to αB-crystallin in NBD confirm local humoral responses in its pathology | [22] |
| IAP (immunosuppressive acidic protein) | Serum | NBD: n = 14 MS: n = 43 |
NBD ↑ | Elevation of IAP is indicative of inflammatory process of CNS among NBD patients | [68] |
Several studies focused on levels of cytokines or chemokines attributed to potential immunocyte infiltration in CNS and peripheral blood. Belghith et al. found an increased expression level of IL-22 in CSF from NBD than MS [113], which affirmed an ongoing Th1/Th17 activation. Upregulated mRNA levels of T-bet and IL-17 in PBMC of NBD [73] than MS also emphasized the specific participation of Th1 and Th17 cells in NBD pathogenesis. Besides, the highly expressive CD39 mRNA of B cells originated from PBMC and CSF among NBD patients, instead of which increased in Tregs from MS [78], delineated a dominant role of B cells participating in NBD etiology than MS. Both the CSF IL-10-producing cells, especially IL-10 + B cells, were observed to be elevated in NBD than MS [77]. Higher CSF expression levels (mRNA and protein) of IL-6 [7, 78] and IL-10 [73, 78] were found in NBD than MS. Featured increment of CXCL10 and CCL2 in CSF samples [111] stood for recruitment of neutrophils and monocytes/macrophages in NBD rather than MS. Thus, these inflammatory factors could not only serve to differentiate NBD and MS patients but also identify a distinct inflammatory profile existing in their pathogenesis.
Biomarkers for discerning p-NBD and non-p-NBD
The most distinct feature between p-NBD and non-p-NBD is the occurrence of CSF pleocytosis in p-NBD rather than non-p-NBD [1, 20]. Besides, the elevation of CSF protein in p-NBD, in contrast to the dramatic increment of CSF pressure in non-p-NBD, consolidates that parenchymal and non-parenchymal involvement in NBD underscores the diverse pathology between them.
Mentioning particular markers for discerning p-NBD and non-p-NBD, Karaaslan et al. [21] suggested a higher CSF NFL, HoxB3, and YKL-40 in p-NBD when compared to non-p-NBD. IgG response to αB-crystallin was relatively higher in p-NBD than in intracranial hypertension type of NBD (considered as non-p-NBD) [22], which showed a potential autoimmune response underlying in p-NBD immunopathogenesis. By recruiting 40 p-NBD, 10 BD with dual sinus thrombosis and 5 with arterial stroke, a previous research has indicated significantly higher CSF IL-6 in p-NBD than both other non-p-NBD groups [7]. Since IL-6 is of importance in CNS destruction due to its neuroinflammatory substance, this result has also hinted that the disease mechanism of p-NBD, different from that of non-p-NBD, might contribute to more CNS dysfunctions and poor prognosis in clinical practice that deserves more attention.
Biomarkers for discerning acute and chronic progressive NBD
Exploring clinical and laboratory features relevant to disease courses is vital for NBD prognosis and therapeutic strategy optimization. However, few studies in this field have profoundly unveiled the discrepancy of acute and chronic progressive NBD from a comprehensive biomarker view.
The most studied CSF IL-6 levels were found to increase in both the acute and chronic progressive NBD but no difference existed [47]. A Japanese cohort including 76 acute and 35 chronic progressive NBD identified an 86.7% sensitivity and 94.7% specificity of CSF IL-6 in differentiating acute and chronic progressive NBD at the recovery phase (cut off: 16.55 pg/ml) [4]. A recent study reported that a dramatic decrement of CSF IL-6 and IL-6 index (stands for IL-6 intracranial synthesis) in chronic progressive NBD after infliximab treatment [56], the higher level of which, additionally, was correlated with long-term disease ( ≥3 years) outcome [7].
Discrepant cellular involvement in CSF and pathology characteristics was also uncovered between acute and chronic progressive NBD. To begin with, raising levels of PMN cells in acute NBD than chronic progressive ones [4] not merely elucidated a remarkable accumulation of neutrophils in acute NBD onset, but also gave rise to differential diagnosis between them with the sensitivity and specificity of 97.4% and 97.0 respectively under the threshold of 6.2/mm3. More specifically, Hirohata [58] found the most prominent feature of chronic progressive NBD was the pathologically aggregating of CD68 + monocytes/macrophages in the whole section while only a small number of which were infiltrated in samples from long-term remission after acute NBD. Apart from that, Sumita et al. [57] also presented higher CSF BAFF in chronic progressive NBD than acute ones, which is significantly downregulated after receiving immunosuppressive therapies and correlated with progressive dementia and psychosis, unveiling a considerable participation of B cell-mediated immune responses in NBD immunopathology, especially chronic progressive ones.
Further insights into NBD pathogenesis
NBS is an inflammatory-vasculitic disorder affecting combined perivascular/capillarity sites with pathological changes such as lymphocytic infiltration, hemorrhage, demyelination, concomitant axonal destruction, and fibrosis. The pathogenesis of NBD is directly informed by clinical pathology.
Based on the autopsy findings of Yamamori et al. [39], diffuse demyelination and gliosis in the front matter have been proven. In a histopathological report of a 38-year-old female with NBD symptoms [38], demyelinating sites were situated in the white matter and basal ganglia, with deleted nerve fibers in a severely demyelinated area. Foci of myelin loss in the damaged regions tended to emerge around blood vessels and fuse into large loci. Moreover, perivascular infiltration, mostly lymphocytes, was observed in the white matter. Microscopic examination of a Japanese NBD patient [12] identified remarkable perivascular lymphocyte and mononuclear cell (foamy macrophage) infiltration in mid-small vessels at the site of necrotic injuries in the cerebrum, whereas neutrophils infiltrated the subarachnoid space. In perivascular and demyelination injuries of the brainstem, acute inflammatory reactions with neutrophilic accumulation were simultaneously accompanied by other sites of chronic inflammation with foamy macrophages and reactive astrocytes. Another postmortem histopathological investigation demonstrated that [120] pan-arteritis cases surrounded by leptomeningitis and demyelinated encephalitis could have coexisted concomitantly in NBD, suggesting a close pathogenic association between NBD and vasculitis. Notably, p-NBS lesions are relatively inclined to gather in areas lacking radial and longitudinal anastomotic channels, a ‘venous watershed concept’ that has been suggested before [15, 121].
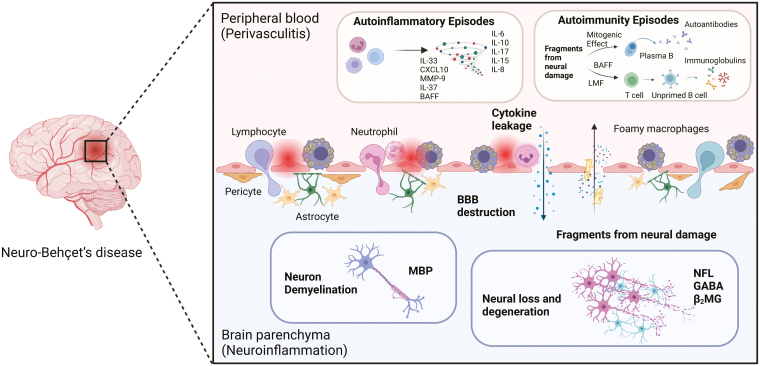
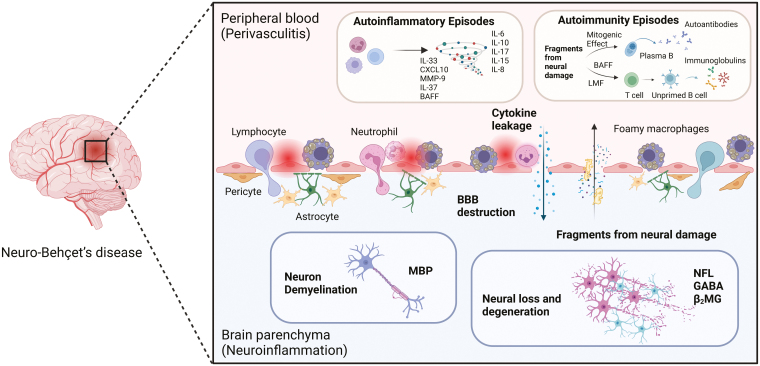
Taken together, NBD mostly manifests as non-bacterial and non-fungal perivasculitis caused by acute inflammation and lymphocytic and neutrophilic infiltration, which subsequently induces demyelination, destroys axons, and leads to necrotized lesions, resulting in progressive neurological dysfunction. The subsequent damage to endothelial cells, pericytes, microglia, and astrocytes leads to disruption of the BBB, which enhances the influx of inflammatory factors (immune cells, cytokines, chemokines, etc.) from the peripheral blood into the CNS and perpetuates inflammatory cascades, as well as more brain damage. The hypothesis of NBD pathology is shown in Fig. 2.
Figure 2:
the hypothetic diagram of NBD immunopathogenesis. NBD pathogenesis first initiates from perivasculitis, with immunocytes (lymphocytes, neutrophils, foamy macrophages, etc.) and cytokines infiltrating in blood vessels, especially influencing CNS branches. The chronic autoinflammatory responses would further lead to the destruction of BBB, allowing more influx of immunopathogenic factors into brain parenchymal tissue, which gives rise to neuron demyelination or degradation events. In turn, the debris and fragments from neuronal damage caused by autoinflammation possess mitogenic and proliferative effects on B cells as well as T cells, promoting hyperfunction of humoral responses and autoimmunity episodes in terminal pathology among NBD patients (Created with BioRender.com)
Conclusions and future directions
NBD is one of the most severe manifestations of BD and is currently considered a consequence of perivasculitis. From the short-term stance, NBD pathology was first initiated by acute perivascular immunocyte infiltration as well as various proinflammatory cytokines. Then the chronic inflammation would enhance the potential release of fragments such as myelin, nerve fibers from severely damaged tissues and might also cause the disruption of BBB perpetuating inflammatory cascades. Promisingly, the long-term interaction of neuroinflammatory factors and abundant neuronal debris might further exacerbate CNS destruction by abnormal excitation of humoral responses.
As for autoimmune responses, identical to MS pathogenesis [122], interminable degradation of myelin sheath processed by acid proteases might cause the formation of motifs with mitogenic and proliferative activity on B lymphoid cells, resulting in excessive immunoglobulin synthesis, leading to autoantibodies targeting myelin debris. Simultaneously, MBP-derived mitogenic fragments also acted as lymphokine to stimulate T cells in inflammatory lesions. After activation, T cells could secret lymphocyte mitogenic factor (LMF) to induce unprimed B cell excitation, thus producing Ig without antigenic reactivity [123]. Our hypothesis could be partially confirmed by the appearance of autoantibodies targeting neuronal antigens like STIP-1 [105] and alpha B-crystallin [22], as well as apoptosis-related proteins such as Mtch1 [106] in NBD serum or CSF, unveiling that CNS destructions caused by continuous neuroinflammation could give rise to autoimmune responses ultimately. Future efforts should be contributed to providing both basic and clinical evidence on proving the existence of autoimmune responses in NBD.
What’s more, the diagnosis of NBD primarily depends on clinical features, MRI findings, and routine nonspecific CSF tests (cell count, proteins, and IL-6). To date, there are no specific diagnostic biomarkers for NBD differential diagnosis, let alone laboratory dimensions related to disease course (acute and chronic progressive, relapsing, and remitting) that correlate with therapeutic response and prognosis. Therefore, research focusing on the identification of disease-specific biomarkers in serum and/or CSF using high-throughput proteomic platforms is urgently needed. In addition, the discovery of novel biomarkers will improve the pathophysiological understanding of NBD and may facilitate targeted treatment approaches in the future.
Acknowledgements
Not applicable.
Contributor Information
Haoting Zhan, Department of Clinical Laboratory, State key Laboratory of Complex, Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Linlin Cheng, Department of Clinical Laboratory, State key Laboratory of Complex, Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Yongzhe Li, Department of Clinical Laboratory, State key Laboratory of Complex, Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Ethical Approval
This study was approved by the Medical Ethics Committee of Peking Union Medical College Hospital (JS-2156).
Conflict of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Funding
This research was supported by grants from the National High Level Hospital Clinical Research Funding (2022-PUMCH-B-124), the Beijing Municipal Natural Science Foundation Project (7234383), and the China Postdoctoral Science Foundation (2023T160060).
Data Availability
Data will be made available on request.
Author Contributions
Haoting Zhan (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Linlin Cheng (Funding acquisition, Investigation, Project administration), and Yongzhe Li (Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision)
References
- 1.Borhani-Haghighi A, Kardeh B, Banerjee S, Yadollahikhales G, Safari A, Sahraian MA, et al. Neuro-Behcet’s disease: An update on diagnosis, differential diagnoses, and treatment. Mult Scler Relat Disord 2020, 39, 101906. doi: https://doi.org/ 10.1016/j.msard.2019.101906 [DOI] [PubMed] [Google Scholar]
- 2.Al-Araji A, Kidd DP.. Neuro-Behçet’s disease: epidemiology, clinical characteristics, and management. Lancet Neurol 2009, 8, 192–204. doi: https://doi.org/ 10.1016/S1474-4422(09)70015-8 [DOI] [PubMed] [Google Scholar]
- 3.Akman-Demir G, Serdaroglu P, Tasçi B.. Clinical patterns of neurological involvement in Behçet’s disease: evaluation of 200 patients. The Neuro-Behçet Study Group . Brain 1999, 122 (Pt 11), 2171–82. doi: https://doi.org/ 10.1093/brain/122.11.2171 [DOI] [PubMed] [Google Scholar]
- 4.Hirohata S, Kikuchi H, Sawada T, Nagafuchi H, Kuwana M, Takeno M, et al. Clinical characteristics of neuro-Behcet’s disease in Japan: a multicenter retrospective analysis. Mod Rheumatol 2012, 22, 405–13. doi: https://doi.org/ 10.1007/s10165-011-0533-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra S, Silman A, Akman-Demir G, Bohlega S, Borhani-Haghighi A, Constantinescu CS, et al. Diagnosis and management of neuro-Behçet’s disease: international consensus recommendations. J Neurol 2014, 261, 1662–76. doi: https://doi.org/ 10.1007/s00415-013-7209-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belghith M, Maghrebi O, Cherif A, Bahrini K, Saied Z, Belal S, et al. Increased T-bet/GATA-3 and ROR-γt /Foxp3 ratios in cerebrospinal fluid as potential criteria for definite neuro-Behçet’s disease. J Clin Med 2022, 11, 4415. doi: https://doi.org/ 10.3390/jcm11154415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akman-Demir G, Tüzün E, Içöz S, Yeşilot N, Yentür SP, Kürtüncü M, et al. Interleukin-6 in neuro-Behçet’s disease: association with disease subsets and long-term outcome. Cytokine 2008, 44, 373–6. doi: https://doi.org/ 10.1016/j.cyto.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 8.Hirohata S, Isshi K, Oguchi H, Ohse T, Haraoka H, Takeuchi A, et al. Cerebrospinal fluid interleukin-6 in progressive neuro-Behçet’s syndrome. Clin Immunol Immunopathol 1997, 82, 12–7. doi: https://doi.org/ 10.1006/clin.1996.4268 [DOI] [PubMed] [Google Scholar]
- 9.Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat Med 2014, 20, 1334–9. doi: https://doi.org/ 10.1038/nm.3680 [DOI] [PubMed] [Google Scholar]
- 10.Borhani Haghighi A, Ittehadi H, Nikseresht AR, Rahmati J, Poorjahromi SG, Pourabbas B, et al. CSF levels of cytokines in neuro-Behçet’s disease. Clin Neurol Neurosurg 2009, 111, 507–10. doi: https://doi.org/ 10.1016/j.clineuro.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 11.Siva A, Saip S.. The spectrum of nervous system involvement in Behçet’s syndrome and its differential diagnosis. J Neurol 2009, 256, 513–29. doi: https://doi.org/ 10.1007/s00415-009-0145-6 [DOI] [PubMed] [Google Scholar]
- 12.Arai Y, Kohno S, Takahashi Y, Miyajima Y, Tsutusi Y.. Autopsy case of neuro-Behçet’s disease with multifocal neutrophilic perivascular inflammation. Neuropathology 2006, 26, 579–85. doi: https://doi.org/ 10.1111/j.1440-1789.2006.00734.x [DOI] [PubMed] [Google Scholar]
- 13.Totsuka S, Midorikawa T.. Some clinical and pathological problems in neuro-Behçet’s syndrome. Folia Psychiatr Neurol Jpn 1972, 26, 275–84. doi: https://doi.org/ 10.1111/j.1440-1819.1972.tb01133.x [DOI] [PubMed] [Google Scholar]
- 14.Diri E, Espinoza LR.. Neuro-Behçet’s syndrome: differential diagnosis and management. Curr Rheumatol Rep 2006, 8, 317–22. doi: https://doi.org/ 10.1007/s11926-006-0016-4 [DOI] [PubMed] [Google Scholar]
- 15.Koçer N, Islak C, Siva A, Saip S, Akman C, Kantarci O, et al. CNS involvement in neuro-Behçet syndrome: an MR study. AJNR Am J Neuroradiol 1999, 20, 1015–24. [PMC free article] [PubMed] [Google Scholar]
- 16.Hirohata S, Suda H, Hashimoto T.. Low-dose weekly methotrexate for progressive neuropsychiatric manifestations in Behcet’s disease. J Neurol Sci 1998, 159, 181–5. doi: https://doi.org/ 10.1016/s0022-510x(98)00165-8 [DOI] [PubMed] [Google Scholar]
- 17.Shugaiv E, Tüzün E, Mutlu M, Kiyat-Atamer A, Kurtuncu M, Akman-Demir G.. Mycophenolate mofetil as a novel immunosuppressant in the treatment of neuro-Behçet’s disease with parenchymal involvement: presentation of four cases. Clin Exp Rheumatol 2011, 29, S64–7. [PubMed] [Google Scholar]
- 18.Ait Ben Haddou EH, Imounan F, Regragui W, Mouti O, Benchakroune N, Abouqal R, et al. Neurological manifestations of Behçet’s disease: evaluation of 40 patients treated by cyclophosphamide. Rev Neurol (Paris) 2012, 168, 344–9. doi: https://doi.org/ 10.1016/j.neurol.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 19.Saruhan-Direskeneli G, Yentür SP, Mutlu M, Shugaiv E, Yesilot N, Kürtüncü M, et al. Intrathecal oligoclonal IgG bands are infrequently found in neuro-Behçet’s disease. Clin Exp Rheumatol 2013, 31, 25–7. [PubMed] [Google Scholar]
- 20.Banerjee S, Hamzoui K, Safari A, Borhani-Haghighi A.. The cerebrospinal fluid presentations of neuro-Bechet disease, a way to know the etiopathogenesis and improve armamentarium. Iran J Immunol 2021, 18, 170–8. doi: https://doi.org/ 10.22034/iji.2021.88326.1864 [DOI] [PubMed] [Google Scholar]
- 21.Karaaslan Z, Şanlı E, Timirci-Kahraman O, Yılmaz V, Akbayır E, Koral G, et al. Cerebrospinal fluid level of neurofilament light chain is associated with increased disease activity in neuro-Behçet’s disease. Turk J Med Sci 2022, 52, 1266–73. doi: https://doi.org/ 10.55730/1300-0144.5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celet B, Akman-Demir G, Serdaroğlu P, Yentür SP, Taşci B, van Noort JM, et al. Anti-alpha B-crystallin immunoreactivity in inflammatory nervous system diseases. J Neurol 2000, 247, 935–9. doi: https://doi.org/ 10.1007/s004150070049 [DOI] [PubMed] [Google Scholar]
- 23.Siva A, Kantarci OH, Saip S, Altintas A, Hamuryudan V, Islak C, et al. Behçet’s disease: diagnostic and prognostic aspects of neurological involvement. J Neurol 2001, 248, 95–103. doi: https://doi.org/ 10.1007/s004150170242 [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Yoon PH, Park SJ, Kim DI.. MRI findings in neuro-Behçet’s disease. Clin Radiol 2001, 56, 485–94. doi: https://doi.org/ 10.1053/crad.2000.0675 [DOI] [PubMed] [Google Scholar]
- 25.Lee HS, Kim do Y, Shin HY, Choi YC, Kim SM.. Spinal cord involvement in Behçet’s disease. Mult Scler 2016, 22, 960–3. doi: https://doi.org/ 10.1177/1352458515613642 [DOI] [PubMed] [Google Scholar]
- 26.Borhani Haghighi A, Aflaki E, Ketabchi L.. The prevalence and characteristics of different types of headache in patients with Behçet’s disease, a case-control study. Headache 2008, 48, 424–9. doi: https://doi.org/ 10.1111/j.1526-4610.2007.01041.x [DOI] [PubMed] [Google Scholar]
- 27.Vinokur M, Burkett JG.. Headache in Behçet’s disease. Curr Pain Headache Rep 2020, 24, 50. doi: https://doi.org/ 10.1007/s11916-020-00882-8 [DOI] [PubMed] [Google Scholar]
- 28.Saip S, Siva A, Altintas A, Kiyat A, Seyahi E, Hamuryudan V, et al. Headache in Behçet’s syndrome. Headache 2005, 45, 911–9. doi: https://doi.org/ 10.1111/j.1526-4610.2005.05160.x [DOI] [PubMed] [Google Scholar]
- 29.Kidd D. The prevalence of headache in Behçet’s syndrome. Rheumatology (Oxford) 2006, 45, 621–3. doi: https://doi.org/ 10.1093/rheumatology/kei255 [DOI] [PubMed] [Google Scholar]
- 30.Yoon DL, Kim YJ, Koo BS, Kim YG, Lee CK, Yoo B.. Neuro-Behçet’s disease in South Korea: clinical characteristics and treatment response. Int J Rheum Dis 2014, 17, 453–8. doi: https://doi.org/ 10.1111/1756-185X.12265 [DOI] [PubMed] [Google Scholar]
- 31.Uygunoglu U, Siva A.. An uncommon disease included commonly in the differential diagnosis of neurological diseases: neuro-Behçet’s syndrome. J Neurol Sci 2021, 426, 117436. doi: https://doi.org/ 10.1016/j.jns.2021.117436 [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto T, Uekusa T, Fukuda Y.. Vasculo-Behçet’s disease: a pathologic study of eight cases. Hum Pathol 1991, 22, 45–51. doi: https://doi.org/ 10.1016/0046-8177(91)90060-3 [DOI] [PubMed] [Google Scholar]
- 33.Pannone A, Lucchetti G, Stazi G, Corvi F, Ferguson TL, Massucci M, et al. Internal carotid artery dissection in a patient with Behçet’s syndrome. Ann Vasc Surg 1998, 12, 463–7. doi: https://doi.org/ 10.1007/s100169900185 [DOI] [PubMed] [Google Scholar]
- 34.Kizilkilic O, Albayram S, Adaletli I, Ak H, Islak C, Kocer N.. Endovascular treatment of Behçet’s disease-associated intracranial aneurysms: report of two cases and review of the literature. Neuroradiology 2003, 45, 328–34. doi: https://doi.org/ 10.1007/s00234-003-0952-x [DOI] [PubMed] [Google Scholar]
- 35.Nakasu S, Kaneko M, Matsuda M.. Cerebral aneurysms associated with Behçet’s disease: a case report. J Neurol Neurosurg Psychiatry 2001, 70, 682–4. doi: https://doi.org/ 10.1136/jnnp.70.5.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atasoy HT, Tunc TO, Unal AE, Emre U, Koca R, Esturk E, et al. Peripheral nervous system involvement in patients with Behçet disease. Neurologist 2007, 13, 225–30. doi: https://doi.org/ 10.1097/NRL.0b013e31805778d1 [DOI] [PubMed] [Google Scholar]
- 37.Criteria for diagnosis of Behçet’s disease. International study group for Behçet’s disease. Lancet 1990, 335, 1078–80. [PubMed] [Google Scholar]
- 38.Miyakawa T, Murayama E, Deshimaru M, Shikai I, Kozuma S.. Neuro-Behcet’s disease showing severe atrophy of the cerebrum. Acta Neuropathol 1976, 34, 95–103. doi: https://doi.org/ 10.1007/BF00684660 [DOI] [PubMed] [Google Scholar]
- 39.Yamamori C, Ishino H, Inagaki T, Seno H, Iijima M, Torii I, et al. Neuro-Behçet disease with demyelination and gliosis of the frontal white matter. Clin Neuropathol 1994, 13, 208–15. [PubMed] [Google Scholar]
- 40.Kidd D, Steuer A, Denman AM, Rudge P.. Neurological complications in Behçet’s syndrome. Brain 1999, 122 ( Pt 11), 2183–94. doi: https://doi.org/ 10.1093/brain/122.11.2183 [DOI] [PubMed] [Google Scholar]
- 41.Ashjazadeh N, Borhani Haghighi A, Samangooie S, Moosavi H.. Neuro-Behcet’s disease: a masquerader of multiple sclerosis. A prospective study of neurologic manifestations of Behcet’s disease in 96 Iranian patients. Exp Mol Pathol 2003, 74, 17–22. doi: https://doi.org/ 10.1016/s0014-4800(03)80004-7 [DOI] [PubMed] [Google Scholar]
- 42.Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, Flanagan EP, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol 2023, 22, 268–82. doi: https://doi.org/ 10.1016/S1474-4422(22)00431-8 [DOI] [PubMed] [Google Scholar]
- 43.Tattevin P, Tchamgoué S, Belem A, Bénézit F, Pronier C, Revest M.. Aseptic meningitis. Rev Neurol (Paris) 2019, 175, 475–80. doi: https://doi.org/ 10.1016/j.neurol.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 44.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018, 17, 162–73. doi: https://doi.org/ 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 45.Kawai M, Hirohata S.. Cerebrospinal fluid beta(2)-microglobulin in neuro-Behçet’s syndrome. J Neurol Sci 2000, 179, 132–9. doi: https://doi.org/ 10.1016/s0022-510x(00)00403-2 [DOI] [PubMed] [Google Scholar]
- 46.Kotake S, Higashi K, Yoshikawa K, Sasamoto Y, Okamoto T, Matsuda H.. Central nervous system symptoms in patients with Behçet disease receiving cyclosporine therapy. Ophthalmology 1999, 106, 586–9. doi: https://doi.org/ 10.1016/S0161-6420(99)90120-3 [DOI] [PubMed] [Google Scholar]
- 47.Ishido M, Horita N, Takeuchi M, Shibuya E, Yamane T, Kawagoe T, et al. Distinct clinical features between acute and chronic progressive parenchymal neuro-Behçet disease: meta-analysis. Sci Rep 2017, 7, 10196. doi: https://doi.org/ 10.1038/s41598-017-09938-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ideguchi H, Suda A, Takeno M, Kirino Y, Ihata A, Ueda A, et al. Neurological manifestations of Behçet’s disease in Japan: a study of 54 patients. J Neurol 2010, 257, 1012–20. doi: https://doi.org/ 10.1007/s00415-010-5454-2 [DOI] [PubMed] [Google Scholar]
- 49.Takahashi S, Sanjo N, Miyamoto S, Hattori T, Oyama J, Tateishi U, et al. Width of the third ventricle as a highly-sensitive biomarker in chronic progressive neuro-Behçet’s disease. J Neurol Sci 2021, 421, 117284. doi: https://doi.org/ 10.1016/j.jns.2020.117284 [DOI] [PubMed] [Google Scholar]
- 50.Banna M, el-Ramahl K.. Neurologic involvement in Behçet disease: imaging findings in 16 patients. AJNR Am J Neuroradiol 1991, 12, 791–6. [PMC free article] [PubMed] [Google Scholar]
- 51.Kürtüncü M, Tüzün E, Akman-Demir G.. Behçet’s disease and nervous system involvement. Curr Treat Options Neurol 2016, 18, 19. doi: https://doi.org/ 10.1007/s11940-016-0405-6 [DOI] [PubMed] [Google Scholar]
- 52.Farahangiz S, Sarhadi S, Safari A, Borhani-Haghighi A.. Magnetic resonance imaging findings and outcome of neuro-Behçet’s disease: the predictive factors. Int J Rheum Dis. 2012, 15, e142–9. doi: https://doi.org/ 10.1111/1756-185X.12013 [DOI] [PubMed] [Google Scholar]
- 53.Hirohata S, Kikuchi H, Sawada T, Nagafuchi H, Kuwana M, Takeno M, et al. Retrospective analysis of long-term outcome of chronic progressive neurological manifestations in Behcet’s disease. J Neurol Sci 2015, 349, 143–8. doi: https://doi.org/ 10.1016/j.jns.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 54.Sota J, Capuano A, Emmi G, Iannone F, Cantarini L, Hatemi G, et al. Therapeutic approach to central nervous system involvement of Behçet’s disease. Semin Arthritis Rheum 2023, 61, 152206. doi: https://doi.org/ 10.1016/j.semarthrit.2023.152206 [DOI] [PubMed] [Google Scholar]
- 55.Hirohata S, Kikuchi H.. Changes in biomarkers focused on differences in disease course or treatment in patients with neuro-Behçet’s disease. Intern Med 2012, 51, 3359–65. doi: https://doi.org/ 10.2169/internalmedicine.51.8583 [DOI] [PubMed] [Google Scholar]
- 56.Hirohata S, Kikuchi H.. Role of intrathecal production of IL-6 in the pathogenesis of chronic progressive neuro-Behçet’s disease. J Neurol Sci 2024, 463, 123145. doi: https://doi.org/ 10.1016/j.jns.2024.123145 [DOI] [PubMed] [Google Scholar]
- 57.Sumita Y, Murakawa Y, Sugiura T, Wada Y, Nagai A, Yamaguchi S.. Elevated BAFF levels in the cerebrospinal fluid of patients with neuro-Behçet’s disease: BAFF is correlated with progressive dementia and psychosis. Scand J Immunol 2012, 75, 633–40. doi: https://doi.org/ 10.1111/j.1365-3083.2012.02694.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirohata S. Histopathological characteristics of central nervous system in chronic progressive neuro-Behçet’s disease. J Neurol Sci 2022, 434, 120127. doi: https://doi.org/ 10.1016/j.jns.2021.120127 [DOI] [PubMed] [Google Scholar]
- 59.Pallis CA, Fudge BJ.. The neurological complications of Behcet’s syndrome. AMA Arch Neurol Psychiatry 1956, 75, 1–14. doi: https://doi.org/ 10.1001/archneurpsyc.1956.02330190009001 [DOI] [PubMed] [Google Scholar]
- 60.Wadia N, Williams E.. Behcet’s syndrome with neurological complications. Brain 1957, 80, 59–71. doi: https://doi.org/ 10.1093/brain/80.1.59 [DOI] [PubMed] [Google Scholar]
- 61.Serdaroğlu P. Behçet’s disease and the nervous system. J Neurol 1998, 245, 197–205. doi: https://doi.org/ 10.1007/s004150050205e2 [DOI] [PubMed] [Google Scholar]
- 62.Uygunoğlu U, Siva A.. Behçet’s syndrome and nervous system involvement. Curr Neurol Neurosci Rep 2018, 18, 35. doi: https://doi.org/ 10.1007/s11910-018-0843-5 [DOI] [PubMed] [Google Scholar]
- 63.Hirohata S, Kikuchi H.. Behçet’s disease. Arthritis Res Ther 2003, 5, 139–46. doi: https://doi.org/ 10.1186/ar757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Totsuka S, Hattori T, Yazaki M, Nagao K, Mizushima S.. Clinicopathologic studies on neuro-Behçet’s disease. Folia Psychiatr Neurol Jpn 1985, 39, 155–66. doi: https://doi.org/ 10.1111/j.1440-1819.1985.tb02899.x [DOI] [PubMed] [Google Scholar]
- 65.David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A 1966, 56, 72–7. doi: https://doi.org/ 10.1073/pnas.56.1.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niino M, Ogata A, Kikuchi S, Tashiro K, Nishihira J.. Macrophage migration inhibitory factor in the cerebrospinal fluid of patients with conventional and optic-spinal forms of multiple sclerosis and neuro-Behçet’s disease. J Neurol Sci 2000, 179, 127–31. doi: https://doi.org/ 10.1016/s0022-510x(00)00397-x [DOI] [PubMed] [Google Scholar]
- 67.Seki H, Tsukamoto T, Aso H, Tamura K.. Intrathecal synthesis of immunosuppressive acidic protein (IAP) in patients with multiple sclerosis and other inflammatory neurological diseases. J Neurol Sci 1988, 85, 259–66. doi: https://doi.org/ 10.1016/0022-510x(88)90185-2 [DOI] [PubMed] [Google Scholar]
- 68.Tsukamoto T, Seki H, Takase S, Sekizawa T, Nakamura S.. Significant increase in immunosuppressive acidic protein (IAP) in serum of patients with multiple sclerosis and other inflammatory neurological disorders. J Neurol Sci 1986, 75, 353–61. doi: https://doi.org/ 10.1016/0022-510x(86)90082-1 [DOI] [PubMed] [Google Scholar]
- 69.Goto I, Shinno N, Kuroiwa Y.. Proteolytic enzyme activities in mononuclear cells and granulocytes of patients with various neurological disorders. J Neurol Sci 1983, 59, 323–9. doi: https://doi.org/ 10.1016/0022-510x(83)90017-5 [DOI] [PubMed] [Google Scholar]
- 70.Nakamura S, Takase S, Itahara K.. Cytological examination of cerebrospinal fluid in eight patients with neuro-Behçet’s disease. Tohoku J Exp Med 1980, 132, 421–30. doi: https://doi.org/ 10.1620/tjem.132.421 [DOI] [PubMed] [Google Scholar]
- 71.Ben Dhifallah I, Borhani-Haghighi A, Hamzaoui A, Hamzaoui K.. Decreased Level of IL-37 correlates negatively with inflammatory cytokines in cerebrospinal fluid of patients with neuro-Behcet’s disease. Iran J Immunol 2019, 16, 299–310. doi: https://doi.org/ 10.22034/IJI.2019.80281 [DOI] [PubMed] [Google Scholar]
- 72.Hamzaoui K, Borhani Haghighi A, Ghorbel IB, Houman H.. RORC and Foxp3 axis in cerebrospinal fluid of patients with neuro-Behçet’s disease. J Neuroimmunol 2011, 233, 249–53. doi: https://doi.org/ 10.1016/j.jneuroim.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 73.Belghith M, Bahrini K, Kchaou M, Maghrebi O, Belal S, Barbouche MR.. Cerebrospinal fluid IL-10 as an early stage discriminative marker between multiple sclerosis and neuro-Behçet disease. Cytokine 2018, 108, 160–7. doi: https://doi.org/ 10.1016/j.cyto.2018.03.039 [DOI] [PubMed] [Google Scholar]
- 74.Hamzaoui K, Houman H, Hentati F, Hamzaoui A.. BAFF is up-regulated in central nervous system of neuro-Behçet’s disease. J Neuroimmunol 2008, 200, 111–4. doi: https://doi.org/ 10.1016/j.jneuroim.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 75.Fujikawa K, Aratake K, Kawakami A, Aramaki T, Iwanaga N, Izumi Y, et al. Successful treatment of refractory neuro-Behcet’s disease with infliximab: a case report to show its efficacy by magnetic resonance imaging, transcranial magnetic stimulation and cytokine profile. Ann Rheum Dis 2007, 66, 136–7. doi: https://doi.org/ 10.1136/ard.2006.056804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, et al. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol 2010, 184, 3321–5. doi: https://doi.org/ 10.4049/jimmunol.0902551 [DOI] [PubMed] [Google Scholar]
- 77.Maghrebi O, Belghith M, Jeridi C, Rachdi A, Fatnassi FN, Saied Z, et al. B cells specific CpG induces high IL-10 and IL-6 expression in vitro in neuro-Behçet’s disease. Cells 2022, 11, 1306. doi: https://doi.org/ 10.3390/cells11081306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bahrini K, Belghith M, Maghrebi O, Bekir J, Kchaou M, Jeridi C, et al. Discriminative expression of CD39 and CD73 in cerebrospinal fluid of patients with multiple sclerosis and neuro-Behçet’s disease. Cytokine 2020, 130, 155054. doi: https://doi.org/ 10.1016/j.cyto.2020.155054 [DOI] [PubMed] [Google Scholar]
- 79.Figueiró F, Muller L, Funk S, Jackson EK, Battastini AM, Whiteside TL.. Phenotypic and functional characteristics of CD39(high) human regulatory B cells (Breg). Oncoimmunology 2016, 5, e1082703. doi: https://doi.org/ 10.1080/2162402X.2015.1082703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharief MK, Hentges R, Thomas E.. Significance of CSF immunoglobulins in monitoring neurologic disease activity in Behçet’s disease. Neurology 1991, 41, 1398–401. doi: https://doi.org/ 10.1212/wnl.41.9.1398 [DOI] [PubMed] [Google Scholar]
- 81.Hirohata S, Takeuchi A, Miyamoto T.. Association of cerebrospinal fluid IgM index with central nervous system involvement in Behçet’s disease. Arthritis Rheum 1986, 29, 793–6. doi: https://doi.org/ 10.1002/art.1780290614 [DOI] [PubMed] [Google Scholar]
- 82.Zhan H, Cheng L, Liu Y, Xu H, Feng X, Liu Y, et al. Significance of immunoglobulins synthesis with central nervous system involvement in neuro-Behçet’s disease. Clin Chim Acta 2024, 559, 119681. doi: https://doi.org/ 10.1016/j.cca.2024.119681 [DOI] [PubMed] [Google Scholar]
- 83.Aoyama J, Inaba G, Shimizu T.. Third complement component in cerebrospinal fluid in neuro-Behçet’s syndrome. Conversion patterns by crossed immunoelectrophoresis. J Neurol Sci 1979, 41, 183–90. doi: https://doi.org/ 10.1016/0022-510x(79)90037-6 [DOI] [PubMed] [Google Scholar]
- 84.Jongen PJ, Daelmans HE, Bruneel B, den Hartog MR.. Humoral and cellular immunologic study of cerebrospinal fluid in a patient with Behçet encephalitis. Arch Neurol 1992, 49, 1075–8. doi: https://doi.org/ 10.1001/archneur.1992.00530340101024 [DOI] [PubMed] [Google Scholar]
- 85.Marusić-Galesić S, Pavelić K, Pokrić B.. Cellular immune response to the antigen administered as an immune complex. Immunology 1991, 72, 526–31. [PMC free article] [PubMed] [Google Scholar]
- 86.Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y, et al. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep 2015, 10, 1040–54. doi: https://doi.org/ 10.1016/j.celrep.2015.01.037 [DOI] [PubMed] [Google Scholar]
- 87.Tian W, Kyriakides TR.. Matrix metalloproteinase-9 deficiency leads to prolonged foreign body response in the brain associated with increased IL-1beta levels and leakage of the blood-brain barrier. Matrix Biol 2009, 28, 148–59. doi: https://doi.org/ 10.1016/j.matbio.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aldinucci A, Bonechi E, Biagioli T, Repice AM, D’Elios MM, Emmi L, et al. CSF/serum matrix metallopeptidase-9 ratio discriminates neuro Behçet from multiple sclerosis. Ann Clin Transl Neurol 2018, 5, 493–8. doi: https://doi.org/ 10.1002/acn3.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamzaoui K, Maître B, Hamzaoui A.. Elevated levels of MMP-9 and TIMP-1 in the cerebrospinal fluid of neuro-Behçet’s disease. Clin Exp Rheumatol 2009, 27, S52–7. [PubMed] [Google Scholar]
- 90.Hamzaoui K, Borhani-Haghighi A, Kaabachi W, Hamzaoui A.. Increased interleukin 33 in patients with neuro-Behcet’s disease: correlation with MCP-1 and IP-10 chemokines. Cell Mol Immunol 2014, 11, 613–6. doi: https://doi.org/ 10.1038/cmi.2014.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liew FY, Pitman NI, McInnes IB.. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 2010, 10, 103–10. doi: https://doi.org/ 10.1038/nri2692 [DOI] [PubMed] [Google Scholar]
- 92.Aoyama-Hayashi E, Matsuda T, Ohya N, Tanaka C, Kongozi M, Kamo C, et al. Soluble Fas ligand levels in cerebrospinal fluid in neuro-Behçet’s disease. Adv Exp Med Biol 2003, 528, 389–91. doi: https://doi.org/ 10.1007/0-306-48382-3_80 [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez Caldito N. Role of tumor necrosis factor-alpha in the central nervous system: a focus on autoimmune disorders. Front Immunol 2023, 14, 1213448. doi: https://doi.org/ 10.3389/fimmu.2023.1213448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alpsoy E, Leccese P, Emmi G, Ohno S.. Treatment of Behçet’s disease: an algorithmic multidisciplinary approach. Front Med (Lausanne) 2021, 8, 624795. doi: https://doi.org/ 10.3389/fmed.2021.624795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Desbois AC, Addimanda O, Bertrand A, Deroux A, Pérard L, Depaz R, et al. Efficacy of Anti-TNFα in severe and refractory neuro-behcet disease: an observational study. Medicine (Baltim) 2016, 95, e3550. doi: https://doi.org/ 10.1097/MD.0000000000003550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuroda H, Ogawa N, Yamawaki Y, Nukina I, Ofuji T, Yamamoto M, et al. Cerebrospinal fluid GABA levels in various neurological and psychiatric diseases. J Neurol Neurosurg Psychiatry 1982, 45, 257–60. doi: https://doi.org/ 10.1136/jnnp.45.3.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H.. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 2019, 90, 870–81. doi: https://doi.org/ 10.1136/jnnp-2018-320106 [DOI] [PubMed] [Google Scholar]
- 98.Dorval G, Welsh KI, Nilsson K, Wigzell H.. Quantitation of beta2-microglobulin and HLA on the surface of human cells. I. T and B lymphocytes and lymphoblasts. Scand J Immunol 1977, 6, 255–63. doi: https://doi.org/ 10.1111/j.1365-3083.1977.tb00392.x [DOI] [PubMed] [Google Scholar]
- 99.van Noort JM, van Sechel A, Boon J, Boersma WJ, Polman CH, Lucas CJ.. Minor myelin proteins can be major targets for peripheral blood T cells from both multiple sclerosis patients and healthy subjects. J Neuroimmunol 1993, 46, 67–72. doi: https://doi.org/ 10.1016/0165-5728(93)90234-p [DOI] [PubMed] [Google Scholar]
- 100.Ohta M, Nishitani H, Matsubara F, Inaba G.. Myelin basic protein in spinal fluid from patients with neuro-Behcet’s disease. N Engl J Med 1980, 302, 1093. doi: https://doi.org/ 10.1056/NEJM198005083021922 [DOI] [PubMed] [Google Scholar]
- 101.Zhan H, Cheng L, Wang X, Jin H, Liu Y, Li H, et al. Myelin basic protein and index for neuro-Behçet’s disease. Clin Immunol 2023, 250, 109286. doi: https://doi.org/ 10.1016/j.clim.2023.109286 [DOI] [PubMed] [Google Scholar]
- 102.Wang CR, Chuang CY, Chen CY.. Anticardiolipin antibodies and interleukin-6 in cerebrospinal fluid and blood of Chinese patients with neuro-Behçet’s syndrome. Clin Exp Rheumatol 1992, 10, 599–602. [PubMed] [Google Scholar]
- 103.Yeh TS, Wang CR, Jeng GW, Lee GL, Chen MY, Wang GR, et al. The study of anticardiolipin antibodies and interleukin-6 in cerebrospinal fluid and blood of Chinese patients with systemic lupus erythematosus and central nervous system involvement. Autoimmunity 1994, 18, 169–75. doi: https://doi.org/ 10.3109/08916939409007993 [DOI] [PubMed] [Google Scholar]
- 104.Hirano T, Miyajima H, Taniguchi O, Ueda A, Takai S, Hashimoto H, et al. Anti asialo GM1 antibody detected in the patients’ sera from systemic lupus erythematosus and Behçet’s diseases with neurological manifestations. Jpn J Med 1988, 27, 167–71. doi: https://doi.org/ 10.2169/internalmedicine1962.27.167 [DOI] [PubMed] [Google Scholar]
- 105.Vural B, Uğurel E, Tüzün E, Kürtüncü M, Zuliani L, Cavuş F, et al. Anti-neuronal and stress-induced-phosphoprotein 1 antibodies in neuro-Behçet’s disease. J Neuroimmunol 2011, 239, 91–7. doi: https://doi.org/ 10.1016/j.jneuroim.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 106.Vural B, Sehitoğlu E, Cavuş F, Yalçınkaya N, Haytural H, Küçükerden M, et al. Mitochondrial carrier homolog 1 (Mtch1) antibodies in neuro-Behçet’s disease. J Neuroimmunol 2013, 263, 139–44. doi: https://doi.org/ 10.1016/j.jneuroim.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 107.Young DB. Heat-shock proteins: immunity and autoimmunity. Curr Opin Immunol 1992, 4, 396–400. doi: https://doi.org/ 10.1016/s0952-7915(06)80029-4 [DOI] [PubMed] [Google Scholar]
- 108.Taşçi B, Direskeneli H, Serdaroglu P, Akman-Demir G, Eraksoy M, Saruhan-Direskeneli G.. Humoral immune response to mycobacterial heat shock protein (hsp)65 in the cerebrospinal fluid of neuro-Behçet patients. Clin Exp Immunol 1998, 113, 100–4. doi: https://doi.org/ 10.1046/j.1365-2249.1998.00620.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hasan A, Fortune F, Wilson A, Warr K, Shinnick T, Mizushima Y, et al. Role of gamma delta T cells in pathogenesis and diagnosis of Behcet’s disease. Lancet 1996, 347, 789–94. doi: https://doi.org/ 10.1016/s0140-6736(96)90868-5 [DOI] [PubMed] [Google Scholar]
- 110.Selmaj K, Brosnan CF, Raine CS.. Expression of heat shock protein-65 by oligodendrocytes in vivo and in vitro: implications for multiple sclerosis. Neurology 1992, 42, 795–800. doi: https://doi.org/ 10.1212/wnl.42.4.795 [DOI] [PubMed] [Google Scholar]
- 111.Saruhan-Direskeneli G, Yentür SP, Akman-Demir G, Işik N, Serdaroğlu P.. Cytokines and chemokines in neuro-Behçet’s disease compared to multiple sclerosis and other neurological diseases. J Neuroimmunol 2003, 145, 127–34. doi: https://doi.org/ 10.1016/j.jneuroim.2003.08.040 [DOI] [PubMed] [Google Scholar]
- 112.Hamzaoui K, Hamzaoui A, Ghorbel I, Khanfir M, Houman H.. Levels of IL-15 in serum and cerebrospinal fluid of patients with Behçet’s disease. Scand J Immunol 2006, 64, 655–60. doi: https://doi.org/ 10.1111/j.1365-3083.2006.01844.x [DOI] [PubMed] [Google Scholar]
- 113.Belghith M, Maghrebi O, Ben Laamari R, Hanachi M, Hrir S, Saied Z, et al. Increased IL-22 in cerebrospinal fluid of neuro-Behçet’s disease patients. Cytokine 2024, 179, 156617. doi: https://doi.org/ 10.1016/j.cyto.2024.156617 [DOI] [PubMed] [Google Scholar]
- 114.Ugurel E, Erdag E, Kucukali CI, Olcay A, Sanli E, Akbayir E, et al. Enhanced NLRP3 and DEFA1B expression during the active stage of parenchymal neuro-Behçet’s disease. In Vivo 2019, 33, 1493–7. doi: https://doi.org/ 10.21873/invivo.11629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng L, Zhan H, Liu Y, Chen H, Zhang F, Zheng W, et al. Infectious agents and pathogenesis of Behçet’s disease: An extensive review. Clin Immunol 2023, 251, 109631. doi: https://doi.org/ 10.1016/j.clim.2023.109631 [DOI] [PubMed] [Google Scholar]
- 116.Oezguen N, Yalcinkaya N, Kücükali CI, Dahdouli M, Hollister EB, Luna RA, et al. Microbiota stratification identifies disease-specific alterations in neuro-Behçet’s disease and multiple sclerosis. Clin Exp Rheumatol 2019, 37, 58–66. [PubMed] [Google Scholar]
- 117.Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N, et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep 2017, 20, 1269–77. doi: https://doi.org/ 10.1016/j.celrep.2017.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.İsmayılov R, Talibov T, Gündüz T, Kürtüncü M.. Parenchymal Neuro-Behçet’s disease or Comorbid Behçet’s disease with multiple sclerosis: A discriminative analysis of a complex clinical entity. Mult Scler Relat Disord 2024, 87, 105684. doi: https://doi.org/ 10.1016/j.msard.2024.105684 [DOI] [PubMed] [Google Scholar]
- 119.Yalçınkaya N, Akcan U, Örçen A, Küçükali C, Türkoğlu R, Kürtüncü M, et al. Reduced fecal GABA levels in multiple sclerosis patients. Mult Scler Relat Disord 2016, 9, 60–1. doi: https://doi.org/ 10.1016/j.msard.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 120.Nishimura M, Satoh K, Suga M, Oda M.. Cerebral angio- and neuro-Behçet’s syndrome: neuroradiological and pathological study of one case. J Neurol Sci 1991, 106, 19–24. doi: https://doi.org/ 10.1016/0022-510x(91)90188-d [DOI] [PubMed] [Google Scholar]
- 121.Uygunoglu U, Zeydan B, Ozguler Y, Ugurlu S, Seyahi E, Kocer N, et al. Myelopathy in Behçet’s disease: The Bagel Sign. Ann Neurol 2017, 82, 288–98. doi: https://doi.org/ 10.1002/ana.25004 [DOI] [PubMed] [Google Scholar]
- 122.Paterson PY, Whitacre CC.. The enigma of oligoclonal immunoglobulin G in cerebrospinal fluid from multiple sclerosis patients. Immunol Today 1981, 2, 111–7. doi: https://doi.org/ 10.1016/0167-5699(81)90044-X [DOI] [PubMed] [Google Scholar]
- 123.Geha RS, Mudawwar F, Schneeberger E.. The specificity of T-cell helper factor in man. J Exp Med 1977, 145, 1436–48. doi: https://doi.org/ 10.1084/jem.145.6.1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.