Abstract
Despite their high degree of effectiveness in the management of psychiatric conditions, exposure to anti-psychotic drugs, including olanzapine and risperidone, is frequently associated with substantial weight gain and the development of diabetes. Even prior to weight gain, a rapid rise in circulating leptin concentrations can be observed in most patients taking anti-psychotic drugs. To date, the contribution of this hyperleptinemia to weight gain and metabolic deterioration has not been defined. Here, with an established mouse model that recapitulates anti-psychotic drug-induced obesity and insulin resistance, we not only confirm that hyperleptinemia occurs before weight gain, but also demonstrate that hyperleptinemia contributes directly to the development of obesity and associated metabolic disorders. By suppressing the rise in leptin through the use of a monoclonal leptin-neutralizing antibody, we effectively prevented weight gain, restored glucose tolerance, and preserved adipose tissue and liver function in the antipsychotic drug-treated mice. Mechanistically, suppressing excess leptin resolved local tissue and systemic inflammation typically associated with anti-psychotic drug treatment. We conclude that hyperleptinemia is a key contributor to anti-psychotic drug-associated weight gain and metabolic deterioration. Leptin suppression is a highly effective approach to reduce the undesirable side-effects of anti-psychotic drugs.
One sentence summary:
Anti-psychotic drug-induced hyperleptinemia contributes to weight gain and its associated metabolic disorders.
Introduction
Psychotic disorders, including schizophrenia, schizoaffective disorders, brief psychotic disorders, and delusional disorders, are major mental health issues that severely burden affected patients (1). Therapeutically, several second-generation atypical anti-psychotic drugs, including clozapine, ziprasidone, paliperidone, olanzapine, and risperidone, are being used clinically. Although these drugs are highly effective in treating mental health disorders, they are also associated with common side effects, including notable weight gain and hyperglycemia (2).
Several different mechanisms, including multiple neuron engagement and inflammatory processes, have been proposed to contribute to the detrimental effects of anti-psychotic drugs. Previously, treatment with a serotonin receptor 2C (HTR2C)-specific agonist has been shown to reverse olanzapine-induced weight gain and hyperglycemia (3). We recently demonstrated that hypothalamic melanocortin 4 receptor (MC4R) participates in risperidone-induced weight gain, as acute risperidone treatment inhibits MC4R activity by increasing post-synaptic potassium conductance (4). Additionally, the involvement of critical hypothalamic appetite regulatory neurons in antipsychotic-induced hyperphagia has been well-documented (5). Olanzapine treatment decreases proopiomelanocortin (Pomc) mRNA expression, POMC neuron numbers, and POMC projections prior to the onset of obesity (6). Moreover, an elevation in the expression of orexygenic neuropeptides Y (Npy) and agouti-related protein (Agrp) has been described (5, 7, 8). Aside from various neuronal circuits being affected, inflammation has been reported to participate in anti-psychotic drug-induced metabolic disorders. Chronic treatment with olanzapine induces inflammatory responses in peripheral tissues as well as the central nervous system, primarily by promoting macrophage infiltration into adipose tissue. It also increases circulating pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor α (TNFα) (9, 10). Furthermore, there is considerable variation in response, with some individuals being highly prone to develop obesity whereas others gain substantially less weight. More specifically, gene expression profiles in circulating blood cells in both humans and mice with differential weight gain reveal that transcripts elevated in obese-prone individuals relative to obese-resistant participants are enriched for numerous inflammatory and immunomodulatory signaling nodes at baseline, prior to drug exposure (11). Moreover, the second-generation tetracycline antibiotic minocycline, which displays robust anti-inflammatory effects, mitigates weight gain and hyperglycemia when added to olanzapine treatment (12, 13).
Beyond its role in the regulation of body fat mass, leptin has received much attention over recent years for its pro-inflammatory actions (14). In several clinical studies, circulating leptin has been shown to rapidly increase prior to massive weight gain in patients undergoing anti-psychotic therapy (15, 16). This raises the question as to whether leptin may be causally involved in weight gain and metabolic deterioration (17). Using in vitro-differentiated human adipocytes, the anti-psychotic drug clozapine was shown to directly regulate leptin gene expression (18). Of note, we recently demonstrated that hyperleptinemia is not only a phenomenon associated with weight gain, but rather a vastly underestimated driving force for diet-induced obesity. Increasing leptin concentrations, either in a murine transgenic setting or with recombinant leptin administration, accelerates diet-induced obesity and deteriorates glucose intolerance and insulin resistance (18–20). Based on these observations, we hypothesized that anti-psychotic drug-induced hyperleptinemia may be a key contributor to therapy-associated weight gain and metabolic dysfunction. By utilizing a well-established mouse model that recapitulates the spectrum of detrimental effects of antipsychotic drug treatment, we show that hyperleptinemia occurs before any increases in body weight. Furthermore, preventing the leptin surge through the use of a monoclonal leptin-neutralizing antibody at least partially mitigates risperidone-induced obesity and prevents metabolic dysfunction.
Results
Olanzapine-induced weight gain is indicated by an increase in leptin expression
Olanzapine can induce substantial weight gain in female mice. However, in response to olanzapine treatment, some female mice are more prone to develop obesity, whereas others are more resistant to weight gain (Fig. S1A). It is not clear what differentiates individual mice to the various degrees of weight gain. To answer this question, we analyzed mice that ended up gaining weight (“Prone”) and mice resistant to weight gain (“Resistant”) upon exposure to olanzapine. We performed gene analysis in gonadal fat isolated from Prone and Resistant mice. Compared to Resistant mice, the mice Prone to weight gain displayed a substantial increase in leptin and a dramatic reduction in adiponectin (Fig. S1B-C). Other genes, including Acc1, Fasn, and Cpt1a, were comparable between the Prone and Resistant mice (Fig. S1D-F). This observation highlights the importance of leptin expression as an indicator of olanzapine-induced weight gain.
Hyperleptinemia occurs prior to body weight gain in risperidone and olanzapine-treated mice
To delineate the underlying mechanisms that mediate anti-psychotic drug-induced weight gain, we utilized an established mouse model in which young female mice are fed a high-fat diet (HFD) (45% energy from fat) supplemented with either olanzapine or risperidone (4). This mouse model replicates the phenotype of anti-psychotic drug-induced weight gain and hyperglycemia. In this model, we initially examined the circulating leptin concentrations in response to acute olanzapine and risperidone treatment. Clinical observations in patients taking antipsychotic drugs indicate a rapid rise in leptin (21). Mice administered either olanzapine or risperidone exhibited no acute changes in body weight or food intake (Fig. 1A, B) but a rapid increase in circulating leptin (Fig. 1C). This increase occurred within 3 days of drug exposure (Fig. 1C). Concomitantly, leptin (Lep) gene expression was increased both in subcutaneous adipose tissue (SAT) and gonadal adipose tissue (GAT) depots (Fig. 1D, E). We further observed a reduction in adiponectin gene expression, albeit only in SAT, indicating fat depot-specific regulation (Fig. 1F, G). In addition, a marked increase in total adipose tissue macrophages, as assessed by gene expression of the macrophage marker F4/80 (Adgre1), was evident in both fat depots (Fig. 1H, I), with risperidone exerting a more pronounced proinflammatory effect than olanzapine. Based on these data, we selected risperidone to further explore the contributions of leptin to the undesired side effects of antipsychotic drug treatment on body weight and systemic metabolism.
Figure 1. Effect of acute olanzapine and risperidone treatment on body weight, food intake, and circulating leptin.
Female mice (n = 5 per group) were placed on a high-fat diet alone (Ctrl) or a high-fat diet supplemented with olanzapine or risperidone for 2 weeks. Body weight, food intake, and circulating leptin concentrations were measured at the indicated time points. At the end of the experiment, subcutaneous adipose tissue (SAT) and gonadal adipose tissue (GAT) were collected for gene expression analysis. (A) Body weight; (B) food intake; (C) circulating leptin; (D) leptin gene expression in SAT; (E) adiponectin gene expression in SAT; (F) F4/80 expression in SAT; (G) leptin expression in GAT; (H) adiponectin gene expression in GAT; (I) F4/80 expression in GAT. Data are mean ± SEM. Student’s t test or one-way ANOVA: *p < 0.05, **p < 0.01, ***p < 0.001 for olanzapine vs Ctrl or risperidone vs Ctrl.
Leptin neutralization reduces risperidone-induced weight gain and improves glucose tolerance
If hyperleptinemia contributes to anti-psychotic drug-induced weight gain and glucose intolerance, we would indeed expect that neutralizing excess leptin can prevent these undesired side effects. We examined the efficacy of our home-made leptin neutralizing antibody towards reducing the activity of leptin signaling. With HEK293 cells stably transfected with the long form of the leptin receptor, we stimulated the cells in the absence and presence of leptin and different doses of leptin neutralizing antibody and measured the abundance of phospho-STAT3 (p-STAT3). The results indicate that our customized leptin neutralizing antibody effectively blocked leptin signaling in a dose-dependent manner (Fig. S2A). Based on these in vitro studies, we selected an optimal concentration for in vivo studies. We also tested whether the acute treatment with leptin neutralizing antibody affected insulin sensitivity in obese mice. We performed hyperinsulinemic euglycemic clamps (HIEC) before and after acute leptin neutralizing antibody treatment in HFD-fed mice. Before treatment, all mice were clamped at similar glycemia and displayed a similar glucose infusion rate (GIR) (Fig. S2B). However, after acute antibody treatment (16h), the mice receiving leptin neutralizing antibody displayed a significantly increased glucose infusion rate (GIR) to maintain similar glycemia (Fig. S2B), indicating an increase in insulin sensitivity. These results indicate our leptin neutralizing antibody can effectively bind circulating leptin and modulate leptin signaling in a highly acute setting.
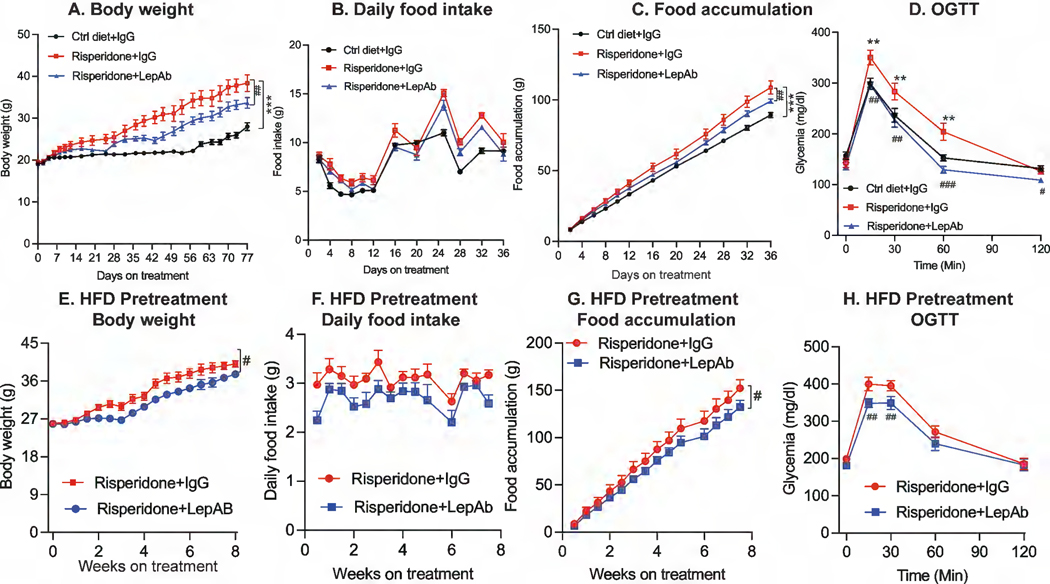
We applied this approach to female mice that were fed a HFD supplemented with risperidone. As expected, female mice on risperidone treatment gained far more weight than mice fed a HFD alone (Fig. 2A). The rapid weight gain induced by risperidone was likely caused by a substantial increase in daily and cumulative food consumption (Fig. 2B, C). In this setting, leptin antibody (LepAb) administration to mice on a risperidone-supplemented diet reduced body weight gain, in part by reducing food intake (Fig. 2A-C). Risperidone treatment has previously been shown to lead to an impairment in glucose tolerance in mice (22, 23). In line with these previous observations, mice on a risperidone-supplemented diet exhibited glucose intolerance, a phenomenon that was fully reversed by LepAb treatment (Fig 2D). Collectively, these observations indicate that risperidone-induced hyperleptinemia directly promotes weight gain as well as glucose intolerance.
Figure 2. Effect of risperidone and risperidone + LepAb on body weight, food intake, and glucose tolerance.
Female mice (n = 9) were placed on a high-fat diet alone (Ctrl) or a high-fat diet supplemented with risperidone. Mice were treated with either control IgG or LepAb twice weekly. Body weight and food intake were measured. Glucose tolerance was measured by a glucose tolerance test (GTT) at the end of the experiment. (A) Body weight; (B) daily food intake; (C) cumulative food intake; (D) glucose tolerance. A second cohort of female mice (n = 8 per group) were placed on HFD plus risperidone diet for 4 weeks to achieve significant obesity. The mice were then injected with IgG or LepAb for another 8 weeks. (E) Body weight; (F) Daily food intake; (G) Food accumulation; (H) OGTT. Data are mean ± SEM. Student’s t test or one-way ANOVA: *p < 0.05, **p < 0.01, ***p < 0.001 for risperidone + IgG vs Ctrl diet + IgG; # p < 0.05, ##p < 0.01, ###p < 0.001 for risperidone + LepAb vs risperidone + IgG.
As treatment with the leptin neutralizing antibody led to a partial prevention of risperidone-induced weight gain, we wondered whether leptin neutralization would produce similar effects in mice with pre-existing obesity due to risperidone treatment. We exposed female mice to HFD and risperidone for 4 weeks to allow the mice to develop obesity. Only at that point, we initiated exposure to the leptin neutralizing antibody (or control IgG) in these obese mice. Our results indicate that leptin neutralization showed similar effects in slowing down further weight gain (Fig. 2E), reducing daily food intake and food accumulation over the remaining risperidone treatment period (Fig. 2F-G). Moreover, compared to IgG-treated mice, the obese mice exposed to leptin neutralizing antibody greatly increased glucose tolerance (Fig. 2H). These observations support that reducing leptin can counteract the side effects induced anti-psychotic drugs at various stages of risperidone treatment.
As acute exposure to risperidone elicited a pronounced increase in systemic leptin concentrations, we also measured leptin, adiponectin, and insulin dynamics after chronic drug treatment. Our analyses revealed that leptin (Lep) gene expression was increased in both SAT and GWAT (Fig. 3 A, B) and that circulating leptin was also substantially elevated after chronic risperidone exposure (Fig. 3C). After LepAb treatment, this surge in leptin gene expression and circulating concentrations was largely blunted (Fig. 3A-C). Risperidone treatment also caused a marked reduction of adiponectin (Adipoq) gene expression in SAT, which was successfully restored by LepAb treatment (Fig. 3D). In contrast, adiponectin gene expression and circulating concentrations in GAT were not significantly altered (Fig. 3E-F). With regards to insulin, risperidone induced a notable increase in the circulating concentration of insulin, reflecting prevailing systemic insulin resistance (Fig. 3G). LepAb treatment also potently reversed this hyperinsulinemia, even though it only partially alleviated weight gain. These results suggest the existence of a weight-independent effect of leptin neutralization on glucose tolerance and insulin sensitivity.
Figure 3. Circulating leptin, adiponectin, and insulin concentrations in response to risperidone and risperidone + LepAb treatment.
(A) Leptin expression in SAT; (B) leptin expression in GAT; (C) circulating leptin; (D) adiponectin expression in SAT; (E) adiponectin expression in GAT; (F) circulating adiponectin; and (G) circulating insulin in the fed state. Data are mean ± SEM. Student’s t test: *p < 0.05, **p < 0.01, ***p < 0.001 for risperidone + IgG vs Ctrl diet + IgG; #p < 0.05, ##p < 0.01, ###p < 0.001 for risperidone + LepAb vs risperidone + IgG.
Risperidone-induced hyperleptinemia promotes liver fibrosis and whitening of brown fat
Several studies have shown that leptin plays a crucial role in the development of liver fibrosis in response to chronic liver injury (24, 25). We thus investigated whether the hyperleptinemia caused by risperidone treatment also promoted liver fibrosis and furthermore assessed its involvement in other common forms of obesity-associated tissue dysfunction such as brown fat whitening. We observed severe liver fibrosis in response to chronic risperidone treatment as indicated by a substantial increase in the expression of fibrogenic genes such as collagen type 1 alpha 1 chain (Col1a1), Col4a4, and smooth muscle actin (SMA; Acta2) (Fig. 4A-C). This was substantiated by trichrome staining, with a clearcut darker blue stain in risperidone-treated mice, reflecting exacerbated tissue fibrosis (Fig. 4D). LepAb treatment completely prevented risperidone-driven liver fibrosis (Fig. 4A-D). Risperidone also caused a considerable increase in hepatic steatosis as judged by lipid droplet accumulation, which was partially reversed by LepAb treatment.
Figure 4. Effects of risperidone and risperidone + LepAb on liver and brown adipose tissue function.

(A) Col1a1 expression in liver; (B) Col4a4 expression in liver; (C) Acta2 expression in liver; (D) Trichrome staining of liver; (E) Prdm16 expression in brown fat; (F) Pgc1a expression in brown fat; (G) Ucp1 expression in brown fat; (H) H&E staining of brown fat. Data are mean ± SEM. Student’s t test: *p < 0.05, **p < 0.01, ***p < 0.001 for risperidone + IgG vs Ctrl diet + IgG; #p < 0.05, ##p < 0.01, ###p < 0.001 for risperidone + LepAb vs risperidone + IgG.
Obesity coincides with a whitening of brown fat in mice (26). The role of obesity-associated hyperleptinemia in this process has however remained controversial (27). Mice that were fed a risperidone-supplemented diet exhibited a more pronounced whitening of brown fat as indicated by a decrease in the expression of specific brown fat markers, such as Prdm16, Pgc1α (Ppargc1a), and Ucp1 (Fig. 4E-G), concomitant with increased accumulation of larger lipid droplets (Fig. 4H). Similar to what we observed in the liver, these effects of risperidone treatment on brown fat were mostly prevented by application of the LepAb.
Risperidone-induced hyperleptinemia increases local tissue and systemic inflammation
Inflammation is considered a driving force in the development of antipsychotic drug-induced metabolic disorders (28). As leptin constitutes a key regulator of immune function (29), the hyperleptinemia triggered by anti-psychotic drug treatment may be an underappreciated factor in establishing systemic inflammation and metabolic deregulation. In the context of obesity, GWAT undergoes massive expansion and severe inflammation, which is often considered to contribute immediately to systemic inflammation. Accompanying weight gain, chronic risperidone treatment increased the expression of inflammation markers such as F4/80 (Adgre1), MCP1 (Ccl2), and Ccl4 in GAT (Fig. 5A-C). GWAT from risperidone-treated mice furthermore displayed increased macrophage infiltration, as evident by enhanced Mac2 staining (Fig. 5D). LepAb treatment effectively reduced the expression of these inflammation markers as well as macrophage infiltration in GWAT, highlighting the profound role that hyperleptinemia plays in the development and maintenance of GWAT inflammation.
Figure 5. Adipose tissue and systemic inflammation in response to risperidone and risperidone + LepAb.
(A) Adgre1 expression in GAT; (B) Mcp1 expression in GAT; (C) Ccl4 expression in GAT; (D) Mac2 staining in GAT; (E) circulating MCP1; (F) circulating IL-1β; (G) circulating TNFα. Data are mean ± SEM. Student’s t test: *p < 0.05, **p < 0.01, ***p < 0.001 for risperidone + IgG vs Ctrl diet + IgG; #p < 0.05, ##p < 0.01, ###p < 0.001 for risperidone + LepAb vs risperidone + IgG.
Beyond GWAT, the liver is similarly affected by obesity-associated inflammatory processes. In line with this, mice fed a risperidone-supplemented diet also displayed increased expression of inflammation markers in the liver, which was successfully alleviated by LepAb treatment (Fig. S3A-D). Risperidone-treated mice also displayed enhanced hepatic staining of F4/80, which was reduced by leptin neutralization as well (Fig. S3A-D). Last, reduced hepatic smooth muscle actin (SMA) staining in LepAb-treated mice further confirmed its protective effects against liver fibrosis.
Accompanying increased tissue inflammation, the circulating concentrations of inflammatory cytokines such as MCP1, IL-1β, and TNFα were elevated in risperidone-treated mice (Fig. 5E-G). This treatment-induced elevation of inflammation markers was equally prevented by LepAb treatment (Fig. 5E-G). Collectively, these observations robustly support a model in which risperidone-induced hyperleptinemia directly contributes to local tissue and systemic inflammation, ultimately resulting in diverse metabolic manifestations.
Leptin neutralization dampens hypothalamic inflammation.
It has previously been shown that risperidone treatment is associated with a reduction of POMC expression in neuron populations critically involved in the regulation of energy balance (30). Related to this, we have shown that leptin reduction restores leptin sensitivity in the hypothalamus (20, 31). With these previous findings in mind, we investigated whether LepAB treatment is capable of restoring the reduced expression of hypothalamic Pomc in risperidone-treated mice. Whereas risperidone treatment was associated with a robust reduction of hypothalamic Agrp and Pomc gene expression, leptin neutralization by LepAb application indeed normalized the expression of these factors (Fig. 6A-C). We also observed a marked increase in hypothalamic inflammation genes in risperidone-treated mice, against which LepAb treatment exerted similarly potent protective effects (Fig. 6D-F). Taken together, these observations further support the notion that risperidone-induced hyperleptinemia is a driving force for central and peripheral systemic inflammation as well as central dysregulation of energy balance.
Figure 6. Effects of risperidone and risperidone + LepAB on hypothalamic gene expression.
(A) Agrp expression; (B) Npy expression; (C) Pomc expression; (D) Mcp1 expression; (E) Ccl8 expression; (F) Adgre1 expression. Data are mean ± SEM. Student’s t test: *p < 0.05, **p < 0.01, ***p < 0.001 for risperidone + IgG vs Ctrl diet + IgG; #p < 0.05, ##p < 0.01, ###p < 0.001 for risperidone + LepAB vs risperidone + IgG.
Risperidone treatment is associated with widespread mammary duct development
A salient side effect of risperidone is that it drastically increases the plasma concentrations of prolactin in patients (32). Prolactin however also is a key factor in the formation of the mammary ductal network during pregnancy (33, 34). After chronic risperidone treatment we observed the formation of structures, similar to those observed during pregnancy-induced mammary gland development, in the SWAT of non-pregnant mice (Fig. 7A). Of note, these structures were not present in control mice that were fed HFD alone. Histological staining of cluster of differentiation 31 (CD31) confirmed that these structures also exhibited cellular resemblances to mammary ducts, consistent with widespread features of mammary gland development induced by anti-psychotic drug exposure (Fig. 7B). LepAb treatment partially suppressed the development of these mammary gland-like structures in SWAT (Fig. 7A, B). Previous reports implicated prolactin in this process of inducing mammary gland expansion during pregnancy (33). We therefore wondered whether our observed expansion of these mammary gland structures is associated with an increase in circulating prolactin. To this end, we measured circulating prolactin concentrations in mice with and without risperidone treatment. As expected, we found that risperidone significantly increased circulating prolactin (p < 0.001), and this increase was partially blocked by leptin neutralization (Fig 7C). These observations suggest that leptin neutralization may be beneficial in reducing hyperprolactinemia-induced mammary gland development in response to risperidone treatment.
Figure 7. Risperidone induces mammary gland development.
(A) H&E staining of SAT; (B) CD31/Mac2 staining of mammary duct-like structures in mouse SAT. CD31 stained as “green” and Mac2 stained as “red”; (C). Prolactin concentrations. Data are mean ± SEM. Student’s t test: *p < 0.05, **p < 0.01, ***p < 0.001 for risperidone + IgG vs Ctrl diet + IgG; #p < 0.05, ##p < 0.01, ###p < 0.001 for risperidone + LepAb vs risperidone + IgG.
Reducing circulating leptin acts similarly in olanzapine-treated female mice
As both risperidone and olanzapine show similar effects in inducing circulating leptin, we wondered whether the increased circulating leptin would be a universal mechanism associated with anti-psychotic drug-associated weight gain and glucose intolerance. We therefore administered olanzapine to female mice instead of risperidone. We initiated the IgG and LepAb injections at the time of olanzapine administration. Similar to our observations made with risperidone, the leptin neutralizing antibody effectively prevented olanzapine-induced weight gain (fig. S4A) by reducing daily food intake and food accumulation over the course of the olanzapine treatment period (Fig S4B-C). In addition, leptin neutralizing antibody reversed olanzapine-induced glucose intolerance (Fig S4D). Furthermore, we found that leptin neutralization significantly alleviated liver fibrosis-associated gene expression (Fig S4E) and inflammation-associated gene expression in adipose tissue (Fig S4F) in olanzapine-treated mice, similar to the effects observed in risperidone-treated mice. These observations further support the notion that anti-psychotic drug-induced hyperleptinemia contributes to weight gain and glucose intolerance. Thus, a leptin reduction strategy, induced by a leptin-neutralizing antibody, offers a potential means to treat anti-psychotic drug-associated side effects.
Discussion
Anti-psychotic drugs are highly effective in alleviating symptoms of psychosis. However, they are also generally associated with a series of common side effects including massive weight gain, development and of progression of diabetes, and liver disease (35). The mechanisms underlying these unwanted side effects are largely unknown. As we show herein that anti-psychotic drug exposure is associated with increased circulating leptin concentrations even before weight gain occurs, proposing an involvement of leptin in mediating the drugs’ side effects. However, to date, this proposition has not been examined experimentally. The system we used here reproduces central side effects of anti-psychotic drug exposure. Taking advantage of this preclinical model, we provide evidence that the anti-psychotic drug-induced hyperleptinemia contributes to weight gain, glucose intolerance, and tissue dysfunction. More specifically, we show that suppressing leptin signaling through application of a monoclonal leptin-neutralizing antibody constitutes an effective approach to alleviate anti-psychotic drug-induced weight gain and reverse several other detrimental aspects of these drugs, overall improving tissue and systemic functions.
The overall impact of hyperleptinemia on body weight regulation remains somewhat controversial. Leptin transgenic mice display high circulating leptin as early as the embryonic stage, which results in an extremely lean phenotype with improved glucose tolerance, improved insulin sensitivity, and reduced systemic triglyceride-rich lipoprotein levels (27, 36). These studies, however, are confounded by considerable developmental impact of hyperleptinemia. Recently, we and others demonstrated that hyperleptinemia can in fact promote diet-induced obesity and glucose intolerance (19, 20), which aligns well with a model that implicates hyperleptinemia directly in the development of leptin resistance (37). Based on our observations and those of others in the field, we were encouraged to examine the contribution of anti-psychotic drug-induced hyperleptinemia into weight gain and metabolic deterioration. Our results strongly support the concept that anti-psychotic drug-induced hyperleptinemia is a driving force behind the observed resulting body weight gain. We demonstrate that suppressing leptin signaling systemically is an effective intervention that reverses several side effects of pre-clinical anti-psychotic drug treatment. Our study further supports the importance of leptin neutralization in the context of common obesity, further substantiating our previous studies.
One of the mechanisms by which hyperleptinemia and anti-psychotic drugs promote body weight gain may lie in the induction of systemic inflammation. Given leptin is a potent regulator of immune function, hyperleptinemia promotes a high degree of monocyte proliferation and subsequent maturation of these cells to macrophages, which can effectively infiltrate adipose tissue and the liver, culminating in local tissue and systemic inflammation. Here, we observed enhanced inflammation in several tissues in treated mice, specifically in adipose tissue, the liver, and the hypothalamus. Neutralizing circulating leptin effectively prevents these inflammatory processes, leading to restored tissue homeostasis and improved glucose tolerance.
It is well established that hyperleptinemia can induce robust suppressor of cytokine signaling 3 (SOCS3) expression and phosphorylation in the hypothalamus to impair specific aspects of insulin and leptin signaling (38). However, although risperidone too has previously been shown to up-regulate SOCS3 expression in cells (39), it has remained unknown whether or not hypothalamic SOCS3 up-regulation in response to risperidone treatment critically depends on the hyperleptinemia this drug induces. If so, neutralizing circulating leptin could be a viable approach to restore normal SOCS3 expression. Beyond this, we show herein that mice consuming a risperidone-supplemented diet exhibited a substantial reduction in hypothalamic Pomc and Agrp expression, which was indeed normalized by leptin neutralization. What constitutes possible mechanism(s) associated with the change of Pomc and Agrp? Reduced inflammation in the hypothalamic region may be the major driver. As previously reported, leptin is a master of regulator of innate and adaptive immune responses. Anti-psychotic drug-induced hyperleptinemia over-activates the immune system, leading to peripheral and central inflammation, which in turn affects Pomc and Agrp gene expression. As a result, normalization of leptin concentrations by a leptin-neutralizing antibody effectively reduces inflammatory states in adipose tissues and the CNS and normalizes the expression of Pomc and Agrp. In addition, another interesting observation is that the changes of Pomc and Agrp are in the same direction. Generally speaking, POMC and AGRP act in an opposite fashion: fasting conditions stimulate Agrp expression, whereas fed conditions promote Pomc expression. Generally, one would expect to see changes of Pomc and Agrp expression in opposite directions, which is seen in some, but not all publications. Celastrol induces leptin sensitivity promoting weight loss, but regulates the expression of Pomc and Agrp in the same direction, similar to our current observations (40). Based on these observations, we conclude that anti-psychotic drug-induced obesity and associated metabolic disorders have their roots in hyperleptinemia. As such, our proposed leptin neutralization strategy may be a possible addition to current anti-psychotic therapy in the clinic.
The observation that the mouse model we use here also exhibits enhanced mammary gland development furthermore suggests that it can serve as a useful pre-clinical system to study this side effect of anti-psychotic drug treatment. Furthermore, the widespread mammary gland development observed in non-pregnant young female mice on anti-psychotics is a possible drawback of using this drug in treating younger patients, especially children. Currently, we do not have any data on the use of risperidone and the incidence of breast cancer. Due to the increased mammary gland development upon drug treatment, this issue deserves further evaluation.
There are limitations of our study. Here, we established a mouse model recapturing many of the side effects of anti-psychotic drugs in the clinic. As with all preclinical models, there are limitations to each model. Although we believe that the model presented here reflects many responses to the drugs seen clinically as far as metabolic changes are concerned, we will have to await validation of the key premise (leptin as a driver of weight gain and insulin resistance) in the clinic. To determine the appropriate dose of anti-psychotic compounds, we performed studies with various amounts of risperidone and olanzapine and settled on a minimal dosage that gives us the metabolic changes seen in our study here. However, compared to the doses conventionally used in the clinic, the doses used here in mice was considerably higher. To establish a preclinical model reflecting the clinical readouts, there are no other alternatives at present. Another limitation of our study is that only female, but not male, mice respond to anti-psychotic drug exposure. Male mice on the same drug regimen fail to display a substantial increase in body weight. Future work is warranted to address these limitations.
In conclusion, anti-psychotic drug therapy induces high concentrations of circulating leptin prior to massive weight gain. This leptin surge is a driving force for the development of obesity, tissue dysfunction, and impaired glucose tolerance, which likely occurs primarily through increased systemic inflammation. Leptin neutralization in the context of anti-psychotic drug treatment is greatly beneficial to the management of the weight gain and metabolic dysfunction. In the future, the addition of leptin-neutralizing antibodies to anti-psychotic drug regimens may serve to prevent metabolic side effects in patients treated for psychotic conditions.
Materials and Methods
Study design
The aim of this study is to investigate the contribution of anti-psychotic-drug induced hyperleptinemia in weight gain and its associated metabolic disorders. To explore our hypothesis, we selected a mouse model that mimics the side effects of anti-psychotic drug in humans According to our observations and previous publications, only female mice were used in this study. We administrated two common anti-psychotic drugs (risperidone and olanzapine) to the mice, in the absence or presence of our leptin-neutralizing antibody. In each experiment, age-matched female mice were randomly assigned to experimental groups. All the female mice were fed a diet supplemented either with risperidone or olanzapine, followed by IgG or LepAb neutralizing antibody injection. Food intake and body weight were monitored. Investigators were not blinded to genotyping or treatment group at Touchstone Diabetes Center, Dallas, Texas, USA. Mice were maintained and studies performed according to protocols approved by the Institutional Animal Care and Use Committee of UT Southwestern Medical Center. Standard food or special diet was provided ad libitum throughout the experiments. We replicated experiments at least once to ensure biological reproducibility and adequate statistical analysis for comparisons.
Animals
All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of University of Texas Southwestern Medical Center at Dallas. Mice were housed under standard laboratory conditions (12 h on/off; lights on at 6:00 a.m.) in a temperature-controlled environment with food and water available ad libitum. Female mice (Cat #000664) were obtained from Jax lab at approximately 8 weeks of age.
Antibody preparation
The parental LepAb was isolated from a phage displayed human ScFv antibody library. To avoid immunogenicity against the human antibody during long term and multiple dosing treatment in mice, we mouserized the parental LepAB. Mouserization was accomplished by using a combined KABAT/IMGT CDR-grafting method. The VH and VL DNA sequences of the parental antibody were blasted against the mouse germline gene sequence database with IgBLAST or IMGT/V-QUEST. The most similar mouse germline VH and VL sequences were selected as templates. The CDRs defined by KABAT/IMGT were grafted onto the framework regions of corresponding templates. The CDR-grafted VH and VL were cloned into mouse IgG1 and light chain backbone to express the full-length antibody. Antibody expression and purification were based on protocols described previously. Monoclonal anti-leptin antibody (LepAb) and isotype control (IgG) were transiently expressed in ExpiHEK293 cells in shake flask cultures according to manufacturer’s protocol (Thermo Scientific, Invitrogen). Briefly, variable heavy and light sequences (Patent # 17/124,481; US2021–0188970-A1) were constructed into two separate expression vectors for co-transfection and expression in HEK293 cells using polyethylenimine (PEI, Sigma) to mediate cell transfection. Cell culture supernatants were harvested by centrifugation at 4000g for 10 minutes after 7 days of culturing in a shaker incubator with 8% CO2 and 80% humidity. Monoclonal antibodies secreted in cultures were purified using protein A affinity resin (Repligen) as described previously.
Mouse treatment
After one weeks’ acclimation in the mouse room, female mice were placed either on control HFD diet (D09092903, Research Diet), olanzapine diet (D12040807, Research Diet), or risperidone diet (D09092903, Research Diet) for various periods, as indicated in figures. For experiment related to LepAB injection, there were three groups of mice: Control diet with Control IgG (I-536, Leinco Technologies) injection; Risperidone diet with control IgG injection and risperidone diet with monoclonal leptin neutralizing antibody injection (Lot #20200821 with an endotoxin concentration of 4.66 EU per mg Ig). The control IgG and LepAB were given at a dose of 1mg per kg body weight at a frequency of twice a week (Monday and Thursday).
Food intake and body weight
In order to measure food intake and body weight gain, all female mice were singly housed. Before each experiment, mice were acclimated in the single cage for at least one week to reduce stress. Food intake and body weight were measured before each injection.
Glucose tolerance and insulin tolerance tests
Glucose tolerance tests (GTTs) were performed as previously described (41). For the GTTs, mice were fasted for 4–6 h in the morning and then orally gavaged 2 g of glucose per kg body weight (dissolved in phosphate buffered saline) (Cat. 806552, Sigma-Aldrich). Blood glucose was measured using a Contour glucometer.
Hyperinsulinemic euglycemic clamp studies
Clamp studies were performed as previously described in Tao et al (42).
Blood parameters
Blood was taken from fed animals in the morning, allowed to clot, centrifuged for 5 min at 8000 g to isolate serum for multiple analyses. Leptin and adiponectin were measured using appropriate ELISA kits (Crystal Chem, #90080 and Thermo Fisher Scientific, #EZMADP-60K). For the leptin measurement, we first removed IgG-bounded leptin by precipitating the plasma with anti-mouse IgG beads. After centrifugation, the supernatant was used for leptin measurement. We referred this leptin as “free” leptin.
RT-qPCR
RNA was extracted from fresh or frozen tissues by homogenization in TRIzol reagent (Thermo Fisher Scientific) as previously described (43). cDNA was prepared using the iScript Reverse Transcription kit (Bio-Rad) and analyses were performed using PowerUp SYBR Green Master Mix (Thermo Fisher Scientific) on a QuantStudio 6 system (Thermo Fisher Scientific). Most RT-qPCR primers were from Harvard PrimerBank (https://pga.mgh.harvard.edu/primerbank/). Relative expression were calculated using the comparative threshold cycle method and normalized to the housekeeping gene Rps16.
Histology
Histology was performed as previously described (44). In brief, adipose tissue and liver tissues were collected and fixed overnight in 10% PBS-buffered formalin and thereafter stored in 50% ethanol. Tissues were further processed by the UTSW Molecular Pathology Core.
Cell culture and Western blots
HEK293 cells (ATCC, Cat #CRL-1573) was transfected with lentivirus to allow stable expression of long form leptin receptor (LepRb). This cell line was seeded into 6-well plate and reached 80% confluence. The cells were then treated with DMEM medium without serum for 6 hr, and incubated in the absence and presence of 10ng leptin and different doses of leptin neutralizing antibody (LepAb). Before adding to the wells, leptin and leptin-neutralizing antibodies were mixed together in a 1.5ml Eppendorf tubes with gentle shaking for 1hr. Then the mixture was added into the well for 10 mins. After that, the wells were washed with cold PBS twice and then stored for further analysis. Western blot was performed as previously done. The antibody p-Stat3 was obtained from Cell Signaling (Cat#9145, RRID: AB_2491009) with a dilution 1:1000.
Immunofluorescence
Immunofluorescence was performed as previously described (45). In brief, formalin-fixed, paraffin-embedded sections from adipose tissues or liver were blocked in PBST containing 5% BSA. Primary antibodies used were perilipin (1:500 dilution, Novus, #NB100–60554; RRID: AB_922242), CD31 (1:250 dilution, Abcam, #ab124432; RRID: N/A), and Mac2 (1:500 dilution, BioLegend, #125401; RRID: AB_1134237). Secondary antibodies (1:250 dilution) used were Alexa Fluor 488 or 594 donkey anti-rabbit IgG (HCL) or Alexa Fluor 488 or 594 donkey anti-goat IgG (HCL) (Thermo Fisher Scientific). Slides were counterstained with DAPI. Fluorescent Images were acquired using an AxioObserver Epifluorescence Microscope (Zeiss) or FSX100 microscope (Olympus).
Statistical Analysis
For all animal studies, we designed experiments to address the parameter(s) of interest with utmost efforts to minimize and control for confounding variables such as mouse strain, gender, and age, tissue sampling time-of-day, fed/fasted state, diet composition, and light cycle. Based on our previous experience, we can use 5 animals per group to achieve sufficient statistical power to detect significant differences for measures of RNA, protein, and metabolites. For studies that require mice to undergo surgery (as for clamp studies), 8 animals were used to account for the variability that occurs due to differences in recovery time and experienced stress. All values were expressed as the mean ± SEM. Pairwise comparison of means was accomplished under the assumptions of normality using Student’s t test (two-sided) for comparison of two groups. One way or two-way ANOVA was used for comparisons of more than two groups. P ≤ 0.05 was regarded as statistically significant.
Supplementary Material
Acknowledgments
Funding:
This work was supported by US National Institutes of Health grants R01-DK55758, R01-DK099110, RC2-DK118620, R01-DK127274, R01-DK131537 and P01-AG051459 (to P.E.S), US National Institutes of Health grants R01-DK118725 and R01-DK088423 (to J.K.E), US National Institutes of Health grants R01 DK114036, DK130892 (to C.L) as well as US National Institutes of Health grants R01 DK117872 (to O.O). In addition, this study was also supported by the Cancer Prevention and Research Institute of Texas (CPRIT) Grants RP190561 and Welch Foundation grant no. AU-0042–20030616 (to Z.A). S.Z. was supported by a US National Institutes of Health grant R00-AG068239 and a Voelcker Fund Young Investigator Pilot Grant. Y.Z. was supported by a US National Institutes of Health grant R01-DK136619 and R01-DK136532; L.L was supported by AHA Postdoc Fellowship 23POST1019715; Q.Z. was supported by AHA Career Development Award 855170. Research reported in this publication was also supported by the UTSWNORC grant under NIDDK/NIH award number P30-DK127984.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability:
All data associated with this study can be found in the paper or supplementary materials. All materials and mouse models are available through request to Dr. Philipp Scherer (philipp.scherer@utsouthwestern.edu) without MTA. Raw data are provided in data file S1.
References
- 1.Lieberman JA, First MB, Psychotic Disorders. N Engl J Med 379, 270–280 (2018); published online EpubJul 19 ( 10.1056/NEJMra1801490). [DOI] [PubMed] [Google Scholar]
- 2.Henderson DC, Weight gain with atypical antipsychotics: evidence and insights. J Clin Psychiatry 68 Suppl 12, 18–26 (2007). [PubMed] [Google Scholar]
- 3.Lord CC, Wyler SC, Wan R, Castorena CM, Ahmed N, Mathew D, Lee S, Liu C, Elmquist JK, The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J Clin Invest 127, 3402–3406 (2017); published online EpubSep 1 ( 10.1172/JCI93362). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L, Yoo ES, Li X, Wyler SC, Chen X, Wan R, Arnold AG, Birnbaum SG, Jia L, Sohn JW, Liu C, The atypical antipsychotic risperidone targets hypothalamic melanocortin 4 receptors to cause weight gain. J Exp Med 218, (2021); published online EpubJul 5 ( 10.1084/jem.20202484). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukherjee S, Skrede S, Milbank E, Andriantsitohaina R, Lopez M, Ferno J, Understanding the Effects of Antipsychotics on Appetite Control. Front Nutr 8, 815456 (2021) 10.3389/fnut.2021.815456). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Lee N, Suh SB, Jang S, Kim S, Kim DG, Park JK, Lee KW, Choi SY, Lee CH, Metformin ameliorates olanzapine-induced disturbances in POMC neuron number, axonal projection, and hypothalamic leptin resistance. BMB Rep 55, 293–298 (2022); published online EpubJun ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lian J, De Santis M, He M, Deng C, Risperidone-induced weight gain and reduced locomotor activity in juvenile female rats: The role of histaminergic and NPY pathways. Pharmacol Res 95–96, 20–26 (2015); published online EpubMay-Jun ( 10.1016/j.phrs.2015.03.004). [DOI] [PubMed] [Google Scholar]
- 8.Martins PJ, Haas M, Obici S, Central nervous system delivery of the antipsychotic olanzapine induces hepatic insulin resistance. Diabetes 59, 2418–2425 (2010); published online EpubOct ( 10.2337/db10-0449). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Peng S, Li S, Liu S, Lv Y, Yang N, Yu L, Deng YH, Zhang Z, Fang M, Huo Y, Chen Y, Sun T, Li W, Chronic olanzapine administration causes metabolic syndrome through inflammatory cytokines in rodent models of insulin resistance. Sci Rep 9, 1582 (2019); published online EpubFeb 7 ( 10.1038/s41598-018-36930-y). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Victoriano M, de Beaurepaire R, Naour N, Guerre-Millo M, Quignard-Boulange A, Huneau JF, Mathe V, Tome D, Hermier D, Olanzapine-induced accumulation of adipose tissue is associated with an inflammatory state. Brain Res 1350, 167–175 (2010); published online EpubSep 2 ( 10.1016/j.brainres.2010.05.060). [DOI] [PubMed] [Google Scholar]
- 11.Zapata RC, Chaudry BS, Valencia ML, Zhang D, Ochsner SA, McKenna NJ, Osborn O, Conserved immunomodulatory transcriptional networks underlie antipsychotic-induced weight gain. Transl Psychiatry 11, 405 (2021); published online EpubJul 22 ( 10.1038/s41398-021-01528-y). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Gomez A, Carretero M, Weber N, Peterka V, To A, Titova V, Solis G, Osborn O, Petrascheck M, A phenotypic Caenorhabditis elegans screen identifies a selective suppressor of antipsychotic-induced hyperphagia. Nat Commun 9, 5272 (2018); published online EpubDec 10 ( 10.1038/s41467-018-07684-y). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zapata RC, Rosenthal SB, Fisch K, Dao K, Jain M, Osborn O, Metabolomic profiles associated with a mouse model of antipsychotic-induced food intake and weight gain. Sci Rep 10, 18581 (2020); published online EpubOct 29 ( 10.1038/s41598-020-75624-2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Cava A, Matarese G, The weight of leptin in immunity. Nat Rev Immunol 4, 371–379 (2004); published online EpubMay ( 10.1038/nri1350). [DOI] [PubMed] [Google Scholar]
- 15.Srisawasdi P, Vanwong N, Hongkaew Y, Puangpetch A, Vanavanan S, Intachak B, Ngamsamut N, Limsila P, Sukasem C, Kroll MH, Impact of risperidone on leptin and insulin in children and adolescents with autistic spectrum disorders. Clin Biochem 50, 678–685 (2017); published online EpubAug ( 10.1016/j.clinbiochem.2017.02.003). [DOI] [PubMed] [Google Scholar]
- 16.Hendouei N, Hosseini SH, Panahi A, Khazaeipour Z, Barari F, Sahebnasagh A, Ala S, Negative Correlation between Serum S100B and Leptin Levels in Schizophrenic Patients During Treatment with Clozapine and Risperidone: Preliminary Evidence. Iran J Pharm Res 15, 323–330 (2016); published online EpubWinter ( [PMC free article] [PubMed] [Google Scholar]
- 17.Baptista T, Beaulieu S, Are leptin and cytokines involved in body weight gain during treatment with antipsychotic drugs? Can J Psychiatry 47, 742–749 (2002); published online EpubOct ( 10.1177/070674370204700805). [DOI] [PubMed] [Google Scholar]
- 18.Sarvari AK, Vereb Z, Uray IP, Fesus L, Balajthy Z, Atypical antipsychotics induce both proinflammatory and adipogenic gene expression in human adipocytes in vitro. Biochem Biophys Res Commun 450, 1383–1389 (2014); published online EpubAug 8 ( 10.1016/j.bbrc.2014.07.005). [DOI] [PubMed] [Google Scholar]
- 19.Pretz D, Le Foll C, Rizwan MZ, Lutz TA, Tups A, Hyperleptinemia as a contributing factor for the impairment of glucose intolerance in obesity. FASEB J 35, e21216 (2021); published online EpubFeb ( 10.1096/fj.202001147R). [DOI] [PubMed] [Google Scholar]
- 20.Zhao S, Zhu Y, Schultz RD, Li N, He Z, Zhang Z, Caron A, Zhu Q, Sun K, Xiong W, Deng H, Sun J, Deng Y, Kim M, Lee CE, Gordillo R, Liu T, Odle AK, Childs GV, Zhang N, Kusminski CM, Elmquist JK, Williams KW, An Z, Scherer PE, Partial Leptin Reduction as an Insulin Sensitization and Weight Loss Strategy. Cell Metab 30, 706–719 e706 (2019); published online EpubOct 1 ( 10.1016/j.cmet.2019.08.005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melkersson KI, Hulting AL, Brismar KE, Elevated levels of insulin, leptin, and blood lipids in olanzapine-treated patients with schizophrenia or related psychoses. J Clin Psychiatry 61, 742–749 (2000); published online EpubOct ( 10.4088/jcp.v61n1006). [DOI] [PubMed] [Google Scholar]
- 22.Boyda HN, Pham M, Huang J, Ho AA, Procyshyn RM, Yuen JWY, Honer WG, Barr AM, Antipsychotic Drug-Induced Increases in Peripheral Catecholamines are Associated With Glucose Intolerance. Front Pharmacol 13, 765905 (2022) 10.3389/fphar.2022.765905). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai HP, Hou PH, Mao FC, Chang CC, Yang WC, Wu CF, Liao HJ, Lin TC, Chou LS, Hsiao LW, Chang GR, Risperidone Exacerbates Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Renal Impairment in Obese Mice. Int J Mol Sci 22, (2021); published online EpubJan 2 ( 10.3390/ijms22010409). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leclercq IA, Farrell GC, Schriemer R, Robertson GR, Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol 37, 206–213 (2002); published online EpubAug ( 10.1016/s0168-8278(02)00102-2). [DOI] [PubMed] [Google Scholar]
- 25.Dai K, Qi JY, Tian DY, Leptin administration exacerbates thioacetamide-induced liver fibrosis in mice. World J Gastroenterol 11, 4822–4826 (2005); published online EpubAug 21 ( 10.3748/wjg.v11.i31.4822). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Li N, Huang M, Chen X, An YA, Li J, Zhao S, Funcke JB, Cao J, He Z, Zhu Q, Zhang Z, Wang ZV, Xu L, Williams KW, Li C, Grove K, Scherer PE, Activating Connexin43 gap junctions primes adipose tissue for therapeutic intervention. Acta Pharm Sin B 12, 3063–3072 (2022); published online EpubJul ( 10.1016/j.apsb.2022.02.020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa Y, Masuzaki H, Hosoda K, Aizawa-Abe M, Suga J, Suda M, Ebihara K, Iwai H, Matsuoka N, Satoh N, Odaka H, Kasuga H, Fujisawa Y, Inoue G, Nishimura H, Yoshimasa Y, Nakao K, Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes 48, 1822–1829 (1999); published online EpubSep ( 10.2337/diabetes.48.9.1822). [DOI] [PubMed] [Google Scholar]
- 28.Suvisaari J, Mantere O, Inflammation theories in psychotic disorders: a critical review. Infect Disord Drug Targets 13, 59–70 (2013); published online EpubFeb ( 10.2174/18715265112129990032). [DOI] [PubMed] [Google Scholar]
- 29.Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gomez-Reino JJ, Mera A, Lago F, Gomez R, Gualillo O, Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol 13, 100–109 (2017); published online EpubFeb ( 10.1038/nrrheum.2016.209). [DOI] [PubMed] [Google Scholar]
- 30.Kursungoz C, Ak M, Yanik T, Effects of risperidone treatment on the expression of hypothalamic neuropeptide in appetite regulation in Wistar rats. Brain Res 1596, 146–155 (2015); published online EpubJan 30 ( 10.1016/j.brainres.2014.10.070). [DOI] [PubMed] [Google Scholar]
- 31.Zhao S, Li N, Zhu Y, Straub L, Zhang Z, Wang MY, Zhu Q, Kusminski CM, Elmquist JK, Scherer PE, Partial leptin deficiency confers resistance to diet-induced obesity in mice. Mol Metab 37, 100995 (2020); published online EpubJul ( 10.1016/j.molmet.2020.100995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleinberg DL, Davis JM, de Coster R, Van Baelen B, Brecher M, Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 19, 57–61 (1999); published online EpubFeb ( 10.1097/00004714-199902000-00011). [DOI] [PubMed] [Google Scholar]
- 33.Oakes SR, Rogers RL, Naylor MJ, Ormandy CJ, Prolactin regulation of mammary gland development. J Mammary Gland Biol Neoplasia 13, 13–28 (2008); published online EpubMar ( 10.1007/s10911-008-9069-5). [DOI] [PubMed] [Google Scholar]
- 34.Stojkovic M, Radmanovic B, Jovanovic M, Janjic V, Muric N, Ristic DI, Risperidone Induced Hyperprolactinemia: From Basic to Clinical Studies. Front Psychiatry 13, 874705 (2022) 10.3389/fpsyt.2022.874705). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pouget JG, Shams TA, Tiwari AK, Muller DJ, Pharmacogenetics and outcome with antipsychotic drugs. Dialogues Clin Neurosci 16, 555–566 (2014); published online EpubDec ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuoka N, Ogawa Y, Masuzaki H, Ebihara K, Aizawa-Abe M, Satoh N, Ishikawa E, Fujisawa Y, Kosaki A, Yamada K, Kuzuya H, Nakao K, Decreased triglyceride-rich lipoproteins in transgenic skinny mice overexpressing leptin. Am J Physiol Endocrinol Metab 280, E334–339 (2001); published online EpubFeb ( 10.1152/ajpendo.2001.280.2.E334). [DOI] [PubMed] [Google Scholar]
- 37.Knight ZA, Hannan KS, Greenberg ML, Friedman JM, Hyperleptinemia is required for the development of leptin resistance. PLoS One 5, e11376 (2010); published online EpubJun 29 ( 10.1371/journal.pone.0011376). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS, Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1, 619–625 (1998); published online EpubMar ( 10.1016/s1097-2765(00)80062-3). [DOI] [PubMed] [Google Scholar]
- 39.Piao L, Park J, Li Y, Shin S, Shin S, Kong G, Shrestha R, Tran Q, Hur GM, Kim JL, Park J, SOCS3 and SOCS6 are required for the risperidone-mediated inhibition of insulin and leptin signaling in neuroblastoma cells. Int J Mol Med 33, 1364–1370 (2014); published online EpubMay ( 10.3892/ijmm.2014.1693). [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U, Treatment of obesity with celastrol. Cell 161, 999–1011 (2015); published online EpubMay 21 ( 10.1016/j.cell.2015.05.011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao S, Mugabo Y, Iglesias J, Xie L, Delghingaro-Augusto V, Lussier R, Peyot ML, Joly E, Taib B, Davis MA, Brown JM, Abousalham A, Gaisano H, Madiraju SR, Prentki M, alpha/beta-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab 19, 993–1007 (2014); published online EpubJun 3 ( 10.1016/j.cmet.2014.04.003). [DOI] [PubMed] [Google Scholar]
- 42.Tao C, Holland WL, Wang QA, Shao M, Jia L, Sun K, Lin X, Kuo YC, Johnson JA, Gordillo R, Elmquist JK, Scherer PE, Short-Term Versus Long-Term Effects of Adipocyte Toll-Like Receptor 4 Activation on Insulin Resistance in Male Mice. Endocrinology 158, 1260–1270 (2017); published online EpubMay 1 ( 10.1210/en.2017-00024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Y, Zhao S, Deng Y, Gordillo R, Ghaben AL, Shao M, Zhang F, Xu P, Li Y, Cao H, Zagnitko O, Scott DA, Gupta RK, Xing C, Zhang BB, Lin HV, Scherer PE, Hepatic GALE Regulates Whole-Body Glucose Homeostasis by Modulating Tff3 Expression. Diabetes 66, 2789–2799 (2017); published online EpubNov ( 10.2337/db17-0323). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z, Shao M, Hepler C, Zi Z, Zhao S, An YA, Zhu Y, Ghaben AL, Wang MY, Li N, Onodera T, Joffin N, Crewe C, Zhu Q, Vishvanath L, Kumar A, Xing C, Wang QA, Gautron L, Deng Y, Gordillo R, Kruglikov I, Kusminski CM, Gupta RK, Scherer PE, Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J Clin Invest 129, 5327–5342 (2019); published online EpubDec 2 ( 10.1172/JCI130239). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Y, Gao Y, Tao C, Shao M, Zhao S, Huang W, Yao T, Johnson JA, Liu T, Cypess AM, Gupta O, Holland WL, Gupta RK, Spray DC, Tanowitz HB, Cao L, Lynes MD, Tseng YH, Elmquist JK, Williams KW, Lin HV, Scherer PE, Connexin 43 Mediates White Adipose Tissue Beiging by Facilitating the Propagation of Sympathetic Neuronal Signals. Cell Metab 24, 420–433 (2016); published online EpubSep 13 ( 10.1016/j.cmet.2016.08.005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study can be found in the paper or supplementary materials. All materials and mouse models are available through request to Dr. Philipp Scherer (philipp.scherer@utsouthwestern.edu) without MTA. Raw data are provided in data file S1.