Abstract
Background
Growth differentiation factor 15 (GDF15) is significantly correlated with glycolipid metabolic disorders. Increased GDF15 levels are associated with obesity, insulin resistance, and diabetes as well as a poorer diabetes progression and prognosis. This is a prospective cohort study investigated the association between circulating GDF15 and diabetic peripheral artery disease.
Methods
A total of 174 diabetic patients aged 20–80 were enrolled. Plasma GDF15 levels were measured using ELISA. Peripheral Artery Disease (PAD) was evaluated with the Ankle brachial index (ABI) and the Cardio-ankle vascular index (CAVI).
Results
We found that diabetic patients with higher serum GDF15 levels (mean: 2521.5 pg/mL) had a higher incidence of peripheral artery disease. Multivariate logistic regression analysis indicated that patients with high serum GDF15 levels were at an increased risk of developing peripheral artery disease. High GDF15 levels were associated with ABI < 0.9 (right and left mean 19.5% p = 0.80, OR:1.13; 95%CI: [0.44–2.90]). Increased age (p = 0.025 OR:1.02; 95% CI [0.13–0.87]), family history (p = 0.001 OR:1.37; 95%CI: [0.37–5.05]), heart failure (p = 0.002 OR:4.96; 95%CI: [1.76–13.97]), sodium-glucose linked transporter 2 (SGLT 2) inhibitor use (p = 0.026), estimated glomerular filtration rate (eGFR) (p = < 0.001), and uric acid (p = < 0.001) was also positively associated with high GDF15 levels. Urine albumin-to-creatinine ratio (UACR) (p = < 0.010) was associated with higher GDF15 levels after one year of follow up.
Conclusions
Elevated GDF15 was significantly associated with worsening metabolic parameters and an increased risk of peripheral artery disease. Thus, it may be a stronger predictor of these outcomes in people with diabetes.
Keywords: Growth differentiation factor 15, Peripheral artery disease, Type 2 DM
Introduction
Type 2 Diabetes mellitus (DM), characterized by hypoglycemia, is one the most common metabolic disorders globally. It is a worldwide health threat affecting millions of people [1–3], with a high prevalence of chronic complications, which are mainly vascular. It has therefore been recognized as a serious public health concern, with considerable impacts on functional capacities and quality of life [4–6], and leading to significant morbidity and premature mortality [7, 8].
Peripheral arterial disease (PAD) is a dysfunction that limits blood flow to the lower limbs [9, 10], and is a common atherosclerotic macrovascular complication in the diabetic population [11]. It is a leading cause of lower-limb amputation and disabling neuropathic pain [12–14]. Amputations in diabetic patients have a devastating effect on quality of life and physical disability and lead to an alarmingly low life expectancy (mostly only 2 years from the amputation) [15].
The objective of this study is to explore the potential association between GDF15 levels and the severity of Peripheral Artery Disease (PAD) in diabetes patients. Specifically, we hypothesize that higher levels of GDF15 are correlated with increased PAD severity, and that GDF15 may serve as a biomarker for disease progression and prognosis. This hypothesis is based on preliminary data suggesting that GDF15 plays a role in inflammation and vascular remodeling, both of which are central to the pathophysiology of PAD.
Growth differentiation factor 15 (GDF15) is a member of the transforming growth factor β (TGF-β) superfamily, highly expressed in states of inflammatory stress [16, 17], and is significantly correlated with glycolipid metabolic disorders [18]. It is considered a cytokine, with anti-inflammatory effects, and increases insulin sensitivity, reduces body weight, and improves clinical outcomes in patients with diabetes [19]. Its normal range is 200–1200 pg/mL; serum levels increase with age.
In addition to GDF15, several other biomarkers have been implicated in the pathophysiology of PAD, particularly in the context of diabetes, where both inflammation and endothelial dysfunction are prominent features. For example, C-reactive protein (CRP) is widely recognized as a marker of systemic inflammation, and elevated CRP levels have been linked to an increased risk of PAD in diabetic patients. While CRP is well-established, it is a nonspecific marker of inflammation and lacks the sensitivity to detect early vascular changes associated with PAD [20].
Another key marker is interleukin-6 (IL-6), an inflammatory cytokine that has been shown to be elevated in patients with PAD and diabetes. IL-6 plays a critical role in the chronic inflammatory response and has been associated with endothelial dysfunction. However, similar to CRP, IL-6 is a general inflammatory marker and may not fully capture the complex interplay of vascular stress and metabolic dysregulation seen in PAD [21].
Soluble vascular cell adhesion molecule-1 (sVCAM-1) is another biomarker frequently studied in the context of PAD. Elevated levels of sVCAM-1 reflect endothelial activation and the recruitment of leukocytes to sites of vascular injury, which is a critical process in the development of atherosclerosis and PAD. While promising, sVCAM-1 is often seen in association with other inflammatory biomarkers, and its role in the specific context of diabetic PAD remains less well-defined [22].
In comparison to these markers, GDF15 offers a unique advantage. As a stress-induced cytokine, GDF15 is not only a marker of inflammation but also of cellular stress, particularly in endothelial cells and smooth muscle cells. Elevated GDF15 levels have been linked to adverse cardiovascular outcomes and may better capture the stress and damage to the vascular endothelium seen in PAD, particularly in the diabetic population, besides have been positively correlated with an increased risk of Type 2 DM complications, such as cardiovascular events, diabetic nephropathy [23], and retinopathy [24], as demonstrated in previous studies [25–28] These findings align with studies highlighting GDF15’s role as a biomarker of metabolic stress and its contribution to inflammatory pathways in diabetes. Recent studies suggest that GDF15 is a more specific marker of vascular injury and a predictor of future cardiovascular events in PAD, making it a promising biomarker for early detection and monitoring disease progression.
By positioning GDF15 alongside traditional inflammatory markers like CRP, IL-6, and sVCAM-1, this study provides a broader view of the molecular landscape underlying PAD in diabetes. Such a comparison could reveal novel insights into the potential utility of GDF15 as a biomarker that reflects both the inflammatory and stress-related processes driving PAD in diabetic patients.
Methods
Ethics Statement
All procedures adhered to the Declaration of Helsinki. This is a prospective cohort study approved by the Ethics Committee of Kaohsiung Veteran General Hospital Institutional Review Board (IRB) (approval no. VGHKS15-EM10-02). Patient identities in the National Health Insurance Research Database (NHIRD) were concealed, and thus a waiver of informed consent was obtained from the IRB.
Participant population
A total of 174 patients aged 20-80years with Type 2 Diabetes Mellitus were recruited from the Kaohsiung Veterans General Hospital, Taiwan for this study. We selected a total of 174 patients (87 patients per group) and performed the power calculation based on an effect size of 0.5. We set the significance level (α) at 0.05 and aimed for a target power of 0.80, which is the standard power level commonly used in social science research.
The power calculation indicated that with 87 patients per group, the achieved power was 0.906, meaning that our study has a 90.6% chance of correctly detecting a true effect, if one exists. This level of power suggests that the sample size is sufficient to detect a medium-sized effect and provides adequate statistical strength to support the reliability of the results.
Diabetes was defined according to the diagnostic criteria of the American Diabetic Association, namely, fasting plasma glucose (FPG) ≥ 126 mg/dL or glycated hemoglobin A1c (HbA1c) ≥ 6.5%. HbA1c levels ranged from 6.5 to 12%.
Collection of demographic, medical, and laboratory data
Demographic and anthropometric data were collected, including body mass index (ranging from 19.8 to 30 kg/m2), waist circumstance, height, weight, body fat, and sex. The patients’ clinical data, including systolic and diastolic blood pressure, history of hypertension, hyperlipidemia, renal disease, heart failure, types of antidiabetic medication used, and clinical laboratory parameters were obtained at baseline. Clinical laboratory parameters included glycated HbA1c, serum creatinine, blood urea nitrogen, estimated glomerular filtration rate (mL·min/1.73 m2), lipid profiles, fasting glucose, and uric acid. Dyslipidemia was defined as a total cholesterol level of more than 200 mg/dL and/or triglyceride level of more than 150 mg/dL, or treatment with lipid-lowering agents. Albuminuria was assessed by measuring the urinary albumin-to-creatinine ratio (UACR) in spot urine collected from the first voiding of urine in the morning. Hypertension was defined as a systolic blood pressure of more than 140 mmHg, a diastolic blood pressure of more than 90 mmHg, or having a prescription for anti-hypertensive medicine.
Methods for determination of GDF15 (ELISA)
GDF15 levels were measured using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) technique, performed with a commercially available kit from R&D Systems, Inc. (Minneapolis, MN, USA). The assay was conducted according to the manufacturer’s instructions. The sensitivity of the assay is the minimum detectable dose of Rhesus Macaque GDF-15 determined to be 2.2 pg./ml. Minimum detectable dose is defined as the analytic concentration resulting in an absorbance that is 2 standard deviations higher than that of the blank (diluent buffer). This ELISA antibody pair detects Rhesus Macaque GDF-15 and Human GDF-15. Intra-assay variability was assessed by performing the assay on multiple replicates of the same sample, yielding a coefficient of variation (CV) of [CV%: <10%], which reflects the precision of measurements within the same run. Blood samples were centrifuged within 1 hour of collection and frozen at − 20°C until analysis. These parameters confirm the reliability and consistency of GDF15 measurements in this study.”
Assessment of ankle-brachial index and cardio-ankle vascular index
Ankle-brachial index (ABI) and cardio-ankle vascular index (CAVI) were measured using the VaSera VS-1500 N device (Fukuda Denshi Co. Ltd., Tokyo, Japan). The CAVI method estimates arterial stiffness across the aorta and major arteries by analyzing the diastolic-to-systolic ‘stiffening’ of the arteries. This provides a comprehensive assessment of arterial stiffness, taking into account the entire systolic phase. To measure both ABI and CAVI, four blood pressure cuffs were wrapped on the extremities, with electrocardiography electrodes attached to both arms and a microphone placed on the sternum at the second intercostal space. Electrocardiography and phonocardiography were monitored after the patient had been in the supine position for 10 min to stabilize.
The higher of the CAVI measurements from the right or left side was used for analysis. CAVI values were categorized as normal (CAVI ≤ 8.0), borderline (8.0 < CAVI ≤ 9.0), or abnormal (CAVI > 9.0). ABI scores were classified as: (a) hardened vessels (> 1.3), (b) normal arterial flow (0.9–1.3), and (c) arterial occlusion (< 0.9).
Both ABI and CAVI were utilized to provide a more comprehensive assessment of vascular health. ABI is a well-established method for detecting PAD by assessing the ratio of blood pressure at the ankle to the arm, while CAVI provides additional insight into arterial stiffness, which is a key factor in vascular health and a predictor of cardiovascular events. CAVI measures arterial stiffness throughout the systolic phase, which allows it to detect changes in arterial properties that ABI may miss, particularly in patients with borderline ABI values. Therefore, combining both measures offers a more complete picture of vascular function and PAD severity.
Although non-blinded assessors were involved in collecting the data, efforts were made to minimize potential bias through standardized training and calibration of the equipment. However, we acknowledge that the lack of blinding in the assessment process could introduce observer bias. Future studies may benefit from blinding the assessors to the clinical details of the participants to further reduce the risk of bias and improve the reliability of the measurements.
Statistical analysis
Continuous data are reported as mean ± standard deviation, while categorical variables are presented as percentages. Analysis of variance was employed to compare the 50th centiles of GDF15 values among the groups. A p-value less than 0.05 was considered statistically significant, as determined using the chi-square test or t-test. All statistical analyses were conducted using Stata version 17.0 (Stata Corp, College Station, TX, USA).
Results
Comparisons between different levels of GDF15
A total of 174 patients were divided into high (n = 87) and low (n = 87) GDF15 groups according to the median GDF15 level (50th percentile: 2521.5 pg/ml). The group with higher GDF15 levels was older (68 ± 7.7) (p = 0.025) and had a higher prevalence of family history of diabetes (65.5%) (p = 0.001). There were no significant differences in height, weight, BMI, or body fat between the groups. The high GDF15 group also exhibited a significantly higher prevalence of heart failure (26.4%) (p = 0.022) and renal disease (5.8%) (p = 0.023). Additionally, the high GDF15 group used SGLT2 inhibitors less frequently (23.6%) (p = 0.026). (Table 1).
Table 1.
Comparisons of baseline clinical and anthropometric parameters between high and low GDF15 level groups
| Variable | GDF15 < 50th percentile (≤ 2521.5 pg/ml) | GDF15 ≥ 50th percentile (> 2521.5 pg/ml) | p-value |
|---|---|---|---|
| n = 87 | n = 87 | ||
| Age | 64.5 ± 8.1 | 68 ± 7.7 | 0.025* |
| Sex, male | 58(66.7) | 53(60.9) | 0.430 |
| Height | 163.3 ± 8.9 | 161.2 ± 8.2 | 0.115 |
| Weight | 72.7 ± 10.4 | 69.6 ± 11.9 | 0.068 |
| BMI | 27.4 ± 4 | 26.7 ± 4.2 | 0.319 |
| Body fat | 31.4 ± 6.4 | 31.3 ± 5.9 | 0.922 |
| Waist | 92.6 ± 8.2 | 91.7 ± 10.1 | 0.535 |
| Family History of Diabetes mellitus | 35(40.2) | 57(65.5) | 0.001* |
| Hypertension | 54(62.1) | 60(69) | 0.339 |
| Hyperlipidemia | 76(87.4) | 77(88.5) | 0.816 |
| Heart failure | 11(12.6) | 23(26.4) | 0.022* |
| Stroke | 2(2.3) | 2(2.3) | 1.000 |
| Renal disease | 0 | 5(5.8) | 0.023 |
| Systolic BP mmHg | 135.3 ± 15.1 | 138.7 ± 19.3 | 0.195 |
| Diastolic BP mmHg | 77.4 ± 11.6 | 75.6 ± 10.3 | 0.283 |
| Heart rate | 80.1 ± 13.2 | 83.3 ± 13 | 0.109 |
| Thiazolidinedione | 25(28.7) | 33(37.9) | 0.198 |
| SGLT2 inhibitor | 37(42.5) | 23(26.4) | 0.026* |
| Insulin | 27(31) | 23(26.4) | 0.503 |
| AGI | 4(4.6) | 6(6.9) | 0.515 |
| GLP-1 agonist | 6(6.9) | 6(6.9) | 1.000 |
GDF15: Growth differentiation factor 15, BMI: Body mass index, DPP4-inhibitor: Inhibitors of dipeptidyl peptidase 4, SGLT 2-inhibitor: Sodium-glucose linked transporter 2 inhibitor, AGI: Alpha glucosidase inhibitor, GLP-1 agonist: Glucagon-like peptide-1 receptor agonist
The high GDF15 group had a significantly higher BUN (24.9 ± 10.7) (p < 0.001), creatinine (1.29 ± 0.5) (p < 0.001), uric acid (6.4 ± 1.8) (p < 0.001), lower eGFR (60.8 ± 27.8) (p < 0.001) (Table 2).
Table 2.
Comparison of baseline laboratory characteristics between high and low GDF15 level groups
| Variable | GDF15 < 50th | GDF15 > = 50th | |
|---|---|---|---|
| n = 87 | n = 87 | p | |
| Urine | |||
| UACR(mg/g) | 268.6 ± 773.8 | 443.2 ± 1115.2 | 0.232 |
| Biochemistry | |||
| BUN | 18.9 ± 6.5 | 24.9 ± 10.7 | < 0.001* |
| Creatinine | 0.95 ± 0.3 | 1.29 ± 0.5 | < 0.001* |
| Uric Acid | 5.4 ± 1.6 | 6.4 ± 1.8 | < 0.001* |
| eGFR | 79.6 ± 20.9 | 60.8 ± 27.8 | < 0.001* |
| TG | 139.7 ± 116.7 | 141 ± 75.6 | 0.932 |
| CHOL | 160.7 ± 34.5 | 157 ± 30.6 | 0.459 |
| HDL-C | 48.4 ± 12.7 | 46.7 ± 15.6 | 0.423 |
| LDL-C | 88.8 ± 25.5 | 85.5 ± 23.9 | 0.379 |
| FPG | 144.4 ± 44.2 | 144.8 ± 44.6 | 0.961 |
| HbA1c | 7.5 ± 1.1 | 7.7 ± 1.2 | 0.236 |
| HsCRP | 0.24 ± 0.38 | 0.21 ± 0.32 | 0.514 |
BP: Blood pressure, UACR: Urine albumin-to-creatinine ratio, BUN: Blood urea nitrogen, eGFR: Estimated glomerular filtration rate (mL·min/1.73 m2), TG: Triglycerides, CHOL: Cholesterol, HDL-C: High Density Lipoprotein Cholesterol, LDL-C: Low Density Lipoprotein Cholesterol, FPG: Fasting plasma glucose, HbA1c: Glycated hemoglobin A1c, hsCRP: High-sensitivity C-reactive protein
After one year of follow-up, the high GDF15 group continued to show significant differences in various kidney-related biomarkers compared to the low GDF15 group. The high GDF15 group exhibited poorer kidney function and higher kidney-related markers: high BUN (26.8 ± 13), creatinine (1.4 ± 0.7), uric acid (6.3 ± 2), urine albumin-to-creatinine ratio UACR (563 ± 1504.2) and low eGFR (58.9 ± 30.6), (p < 0.001). (Table 3).
Table 3.
Comparison of laboratory characteristics between high and low GDF15 level groups after one year of follow up
| Variable | GDF15 < 50th | GDF15 > = 50th | |
|---|---|---|---|
| n = 87 | n = 87 | ||
| Urine | |||
| UACR | 126.7 ± 298.6 | 563 ± 1504.2 | 0.010* |
| Biochemistry | |||
| BUN | 19.8 ± 7.5 | 26.8 ± 13 | < 0.001* |
| Creatinine | 0.98 ± 0.3 | 1.4 ± 0.7 | < 0.001* |
| Uric acid | 5.3 ± 1.5 | 6.3 ± 2 | 0.001* |
| eGFR | 76.7 ± 21.2 | 58.9 ± 30.6 | < 0.001* |
| TG | 133.3 ± 81.3 | 140.8 ± 78.8 | 0.545 |
| CHOL | 157.3 ± 30.2 | 155.1 ± 37.3 | 0.672 |
| HDL-C | 49 ± 11.5 | 47.9 ± 16.1 | 0.600 |
| LDL-C | 90.2 ± 27.9 | 86.2 ± 25.7 | 0.335 |
| FPG | 138.8 ± 41.1 | 134.7 ± 40.9 | 0.519 |
| HbA1c | 7.6 ± 1.2 | 7.5 ± 1.2 | 0.792 |
| HsCRP | 0.24 ± 0.72 | 0.14 ± 0.16 | 0.222 |
BUN: Blood urea nitrogen, CHOL: Cholesterol, eGFR: Estimated glomerular filtration rate (mL·min/1.73 m2), FPG: Fasting plasma glucose, HbA1c: Glycated hemoglobin A1c, HDL-C: High Density Lipoprotein Cholesterol, LDL-C: Low Density Lipoprotein Cholesterol, UACR: TG: Triglycerides, Urine albumin-to-creatinine ratio, hsCRP: High-sensitivity C-reactive protein
After adjusting for potential confounders, the multivariate logistic regression results presented in Table 3 show that GDF15 levels (≥ 50th percentile) were not significantly associated with abnormal ABI (Ankle-Brachial Index), as indicated by an odds ratio (OR) of 1.13 (95% CI: 0.44–2.90, p = 0.801). This suggests that after controlling for other variables, higher GDF15 levels alone do not significantly increase the risk of abnormal ABI in this study population. However Age was independently associated with an increased risk of abnormal ABI (OR = 1.02 (95% CI: 0.13–0.87, p = 0.025)and Heart failure was strongly associated with an increased risk of abnormal ABI, with a nearly 5-fold higher likelihood of abnormal ABI(OR = 4.96 (95% CI: 1.76–13.97, p = 0.002).Table 4.
Table 4.
Multi variable logistic regression for ABI abnormal
| OR | 95% CI | p | |
|---|---|---|---|
| GDF15 > = 50th | 1.13 | 0.44–2.90 | 0.801 |
| Age | 1.02 | 0.13–0.87 | 0.025* |
| Sex, male | 0.34 | 0.96–1.09 | 0.511 |
| BMI | 1.02 | 0.91–1.14 | 0.739 |
| Hypertension | 1.48 | 0.54–4.05 | 0.445 |
| Hyperlipidemia | 0.85 | 0.22–3.33 | 0.811 |
| Heart failure | 4.96 | 1.76–13.97 | 0.002* |
| Stroke | 4.48 | 0.43–46.80 | 0.210 |
| Renal disease | 1.40 | 0.14–13.58 | 0.771 |
GDF15 levels did not show a statistically significant association with abnormal ABI after adjusting for confounders. However, age and heart failure were independently associated with abnormal ABI, highlighting that heart failure in particular may be a more influential factor for ABI abnormalities in this cohort
Association between GDF15 and ABI and CAVI test
Higher concentrations of GDF15 at baseline were associated with an increased risk of peripheral arterial disease (ABI value < 0.9, right 13.8% and left 12.6%, p = 0.135). There was no significant difference between both groups in the CAVI test (right 9.1 ± 1.3 and 9.4 ± 1.2 p = 0.224; left 9.3 ± 1.4 and 9.5 ± 1.2 p = 0.196), but both groups showed abnormal CAVI test results (Table 5).
Table 5.
Comparison of baseline ABI and CAVI between high and low GDF15 level groups
| Variable | GDF15 < 50th | GDF15 > = 50th | p |
|---|---|---|---|
| n = 87 | n = 87 | ||
| ABI | |||
| Right | 1.07 ± 0.11 | 1.03 ± 0.14 | 0.012* |
| Right < 0.9 | 6(6.9) | 12(13.8) | 0.135 |
| Left | 1.07 ± 0.12 | 1.03 ± 0.13 | 0.055 |
| Left < 0.9 | 7(8) | 11(12.6) | 0.319 |
| CAVI | |||
| Right | 9.1 ± 1.3 | 9.4 ± 1.2 | 0.224 |
| Left | 9.3 ± 1.4 | 9.5 ± 1.2 | 0.196 |
ABI: Ankle-brachial index, CAVI: Cardio-ankle vascular index
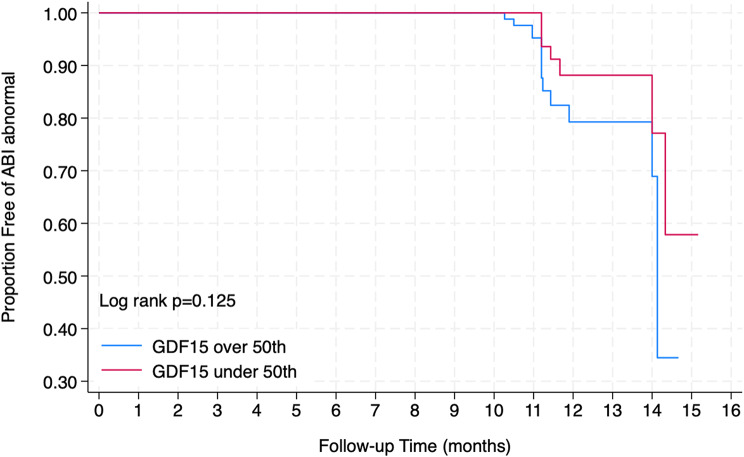
After one year of follow up, high GDF15 levels were still associated with a high risk of PAD (ABI value, R + L < 0.9: 19.5%)(Fig. 1), whereas low GDF15 levels were not (R + L < 0.9: 12.6% p = 0.216); abnormal CAVI test, right 9.2 ± 1.3 and 9.4 ± 1.2 p = 0.308); left 9.2 ± 1.4 and 9.5 ± 1.3 p = 0.191) still present in both groups (Table 6).
Fig. 1.
Kaplan Meier between GDF15 and ABI
Table 6.
Comparison of ABI and CAVI between high and low levels of GDF15 after one year of follow up
| Variable | GDF15 < 50th | GDF15 > = 50th | |
|---|---|---|---|
| n = 87 | n = 87 | p | |
| ABI | |||
| Right | 1.06 ± 0.13 | 1.02 ± 0.16 | 0.068 |
| Right < 0.9 | 10(11.5) | 15(17.2) | 0.280 |
| Left | 1.06 ± 0.13 | 1.03 ± 0.15 | 0.130 |
| Left < 0.9 | 5(5.7) | 12(13.8) | 0.319 |
| R + L < 0.9 | 11(12.6) | 17(19.5) | 0.216 |
| CAVI | |||
| Right | 9.2 ± 1.3 | 9.4 ± 1.2 | 0.308 |
| Left | 9.2 ± 1.4 | 9.5 ± 1.3 | 0.191 |
ABI: Ankle-brachial index, CAVI: Cardio-ankle vascular index
Discussion
While our findings suggest an association between elevated GDF15 levels and an increased likelihood of peripheral arterial disease (PAD), it is important to emphasize the cross-sectional nature of this study. Cross-sectional analyses can identify correlations but cannot establish causality. The observed relationship between high GDF15 levels and PAD may reflect a shared underlying pathophysiological process, or GDF15 could be a marker of existing vascular damage rather than a direct cause of PAD.
Given that we cannot infer temporal causality from this design, further longitudinal studies are needed to explore whether elevated GDF15 levels contribute to the development of PAD or whether they are a consequence of the disease process. While the association observed is clinically relevant, conclusions regarding causality should be made cautiously and in the context of future prospective studies.
These results align with some previous studies that have highlighted GDF15 as a potential biomarker for vascular health, particularly in the context of diabetes and cardiovascular disease. However, it is important to note that other studies have found no significant association between GDF15 levels and PAD. M Barma et al.(2017) [29] and P. Wagne et al., (2023) [30] reported no significant correlation between GDF15 concentrations and PAD, suggesting that the relationship might be context-dependent or influenced by factors such as sample size, population characteristics, or measurement techniques. These discrepancies highlight the complexity of GDF15 as a biomarker and the need for further investigation into its role in vascular diseases like PAD.
Although this study proposes that elevated GDF15 levels may contribute to PAD through mechanisms such as inflammation, these hypotheses remain speculative without direct empirical evidence from the current data. Previous experimental studies have suggested a potential role for GDF15 in modulating inflammatory pathways. For instance, research by Adela, R et al. (2015) [31]demonstrated that GDF15 acts as a regulator of inflammatory cytokines, which could contribute to endothelial dysfunction and atherosclerosis, key features of PAD. Furthermore, a study by A. Schwarz et al. (2023) [32] found that GDF15 expression was upregulated in response to oxidative stress and inflammatory stimuli, suggesting a role in vascular inflammation. These findings are consistent with the hypothesis that elevated GDF15 levels may be a response to vascular injury, potentially exacerbating the pathophysiology of PAD. However, the precise mechanisms through which GDF15 influences PAD remain unclear and warrant further investigation. Experimental models examining the effects of GDF15 inhibition or overexpression in PAD-specific settings would provide more clarity on its functional role in this disease.
The present study observed a modest association between GDF15 levels and PAD outcomes, particularly with respect to ABI measurements. These results are in line with some studies that have highlighted GDF15 as a potential biomarker for vascular health in diabetes.
GDF15, also named macrophage inhibitory cytokine-1, is a homeostatic cytokine that regulates neural and cardio-metabolic functions and is released in response to stress, tissue injury, inflammation, and cancer [33], and thus has been widely explored as a biomarker for disease progression and prognosis [17].
In agreement with previous studies [34, 35] we found that the high serum GDF15 levels are associated with age, family history of DM, heart failure, and renal disease. We also found a lower use of SGLT2 inhibitors in the high GDF15 level group, implying that SGLT2 inhibitors may attenuate GDF15 [36]. Several studies have demonstrated the role of serum GDF15 in diabetic nephropathy and diabetic retinopathy, suggesting that GDF15 is a predictor for diabetic kidney disease [37, 38] and diabetic retinopathy [24, 27]. While we did not specifically investigate diabetic retinopathy, we found high serum GDF15 levels in patients with diabetic nephropathy, which is also positively correlated with urine ACR and eGFR.
We identified a positive correlation between the circulating GDF15 level and PAD. GDF15 predisposes patients not only to the manifestations of the atherosclerotic process but also to its progression. Among the atherosclerotic manifestations, acute coronary syndrome (ACS), ischemic stroke, and peripheral artery disease (PAD) are outcomes of particular importance. ABI and CAVI have been widely used to evaluate arterial stiffening and arterial stenosis/obstruction and are useful diagnostic tools in daily clinical care units for macrovascular complications [39, 40]. Both indices are considered useful for preventing diabetic macroangiopathy. The present study demonstrated that an ABI value < 0.9 was associated with the high GDF15 level group, despite there being no significant difference between both groups. On the other hand, an abnormal CAVI test (> 9.0) was present in both groups.
This study has several important limitations. Firstly, the ethnic homogeneity of the sample, which primarily consisted of Asian patients, limits the generalizability of our findings to broader populations. The results may not be applicable to patients from other ethnic backgrounds, where GDF15 levels and PAD prevalence may differ. Additionally, the retrospective nature of the study introduces potential selection bias, as only patients who met specific inclusion criteria were included. This design cannot account for confounding variables or bias that may have influenced the results, and causality cannot be definitively established from cross-sectional data.
Furthermore, measurement errors, particularly in the assessment of ABI and CAVI, could influence the results. Variability in these measurements could lead to misclassification or underestimation of PAD severity. Although ABI and CAVI are widely used in clinical practice, they are not perfect markers for diagnosing PAD, and their accuracy may vary depending on factors such as operator skill, device calibration, and patient characteristics. There is a possibility that Mönckeberg type arteriosclerosis exists, especially in the lower limb arteries of diabetic patients whose ABI is apparently normal or high. Lastly, the lack of long-term follow-up data beyond one year limits our ability to draw conclusions about the long-term impact of elevated GDF15 on PAD progression or its therapeutic implications. Future prospective studies with larger, more diverse populations and longer follow-up periods are needed to confirm these findings and explore potential causal relationships between GDF15 and PAD in individuals with type 2 diabetes.
In conclusion, our study found that increased plasma GDF15 concentrations were independently and positively associated with metabolic parameters and peripheral artery disease in individuals with type 2 DM. In addition, our findings suggest that GDF15 may be a valuable biomarker for discriminating peripheral artery disease in patients with type 2 DM. Further investigations regarding the underlying mechanisms between GDF15 and PAD in individuals with type 2 DM are warranted and may help in the development of novel therapies for PAD in diabetic patients.
Acknowledgements
The research database was provided by the National Health Insurance Bureau of the National Health Insurance Research Database, managed by the National Health Research Institutes (registration number 101115). Interpretations and conclusions expressed in this article do not represent those of the National Health Insurance Bureau, the Department of Health or the National Health Research Institutes. The authors thank personnel at the Health Examination Center and Department of Medical Education and Research of Kaohsiung Veteran General Hospital for providing information in response to inquiries and assistance in data processing; also would like to thank Convergence CT for assistance with English editing during development of the manuscript.
Author contributions
All authors read and approved the manuscript for publication. CWC was project administrator and contributed to the literature review, funding acquisition, validation, methodology, formal analysis, software, resources, and data curation of the study. CHC contributed to the conceptualization, supervision, and literature review of the study. CSY contributed to software, and resources of the study. MCW, ILH and CML contributed to formal analyses, funding acquisition and data curation of the study. All authors agree to be accountable for all aspects of this work, ensuring integrity and accuracy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethic Committee of Institutional Review Board of Veteran General Hospital of Kaohsiung (IRB No. VGHKS 15-EM10-02) and meets the Helsinki Declaration based ethical principles for medical research involving human subjects. The requirement for informed consent was waived by the Ethic Committee of Institutional Review Board of Veteran General Hospital of Kaohsiung because the study was retrospective.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cardiovascular Disease and Risk Management. Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103–23. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88(11):1254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, Davies J, Vollmer S. Global Economic Burden of Diabetes in adults: projections from 2015 to 2030. Diabetes Care. 2018;41(5):963–70. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakar PK. Pathophysiology of secondary complications of diabetes mellitus. Pathophysiology. 2016;9(1):32–6. [Google Scholar]
- 5.Tafere GG, Wondafrash DZ, Zewdie KA, Assefa BT, Ayza MA. Plasma Adipsin as a Biomarker and its implication in type 2 diabetes Mellitus. Diabetes Metab Syndr Obes. 2020;13:1855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Lawati JA. Diabetes Mellitus: a local and global Public Health Emergency! Oman Med J. 2017;32(3):177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramtahal R, Khan C, Maharaj-Khan K, Nallamothu S, Hinds A, Dhanoo A, Yeh HC, Hill-Briggs F, Lazo M. Prevalence of self-reported sleep duration and sleep habits in type 2 diabetes patients in South Trinidad. J Epidemiol Glob Health. 2015;5(4 Suppl 1):S35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng LN, Tseng YH, Jiang YD, Chang CH, Chung CH, Lin BJ, Chuang LM, Tai TY, Sheu WH. Prevalence of hypertension and dyslipidemia and their associations with micro- and macrovascular diseases in patients with diabetes in Taiwan: an analysis of nationwide data for 2000–2009. J Formos Med Assoc. 2012;111(11):625–36. [DOI] [PubMed] [Google Scholar]
- 9.Mohammedi K, Woodward M, Hirakawa Y, Zoungas S, Williams B, Lisheng L, Rodgers A, Mancia G, Neal B, Harrap S, et al. Microvascular and macrovascular disease and risk for major peripheral arterial disease in patients with type 2 diabetes. Diabetes Care. 2016;39(10):1796–803. [DOI] [PubMed] [Google Scholar]
- 10.Yeboah K, Puplampu P, Ainuson J, Akpalu J, Gyan B, Amoah AG. Peripheral artery disease and exertional leg symptoms in diabetes patients in Ghana. BMC Cardiovasc Disord. 2016;16:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society Consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–67. [DOI] [PubMed] [Google Scholar]
- 12.Tesfaye S, Sloan G. Diabetic polyneuropathy - advances in diagnosis and intervention strategies. Eur Endocrinol. 2020;16(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters EJ, Armstrong DG, Lavery LA. Risk factors for recurrent diabetic foot ulcers: site matters. Diabetes Care. 2007;30(8):2077–9. [DOI] [PubMed] [Google Scholar]
- 14.Artac I, Karakayali M, Omar T, Ilis D, Arslan A, Sahin MH, Kina S, Karabag Y, Rencuzogullari I. Predictive value of the Naples Prognostic score on long-term outcomes in patients with peripheral artery Disease Revascularized via Percutaneous intervention. Ann Vasc Surg. 2024;102:121–32. [DOI] [PubMed] [Google Scholar]
- 15.Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, Tesfaye S. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7(12):938–48. [DOI] [PubMed] [Google Scholar]
- 16.Tan M, Wang Y, Guan K, Sun Y. PTGF-beta, a type beta transforming growth factor (TGF-beta) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-beta signaling pathway. Proc Natl Acad Sci U S A. 2000;97(1):109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wischhusen J, Melero I, Fridman WH. Growth/Differentiation Factor-15 (GDF-15): from biomarker to Novel Targetable Immune Checkpoint. Front Immunol. 2020;11:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adela R, Banerjee SK. GDF-15 as a Target and Biomarker for Diabetes and Cardiovascular Diseases: A Translational Prospective. J Diabetes Res 2015, 2015:490842. [DOI] [PMC free article] [PubMed]
- 19.Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Papadakis M, Nadwa EH, Albogami SM, Alorabi M, Saad HM, Batiha GE. Metformin and growth differentiation factor 15 (GDF15) in type 2 diabetes mellitus: a hidden treasure. J Diabetes. 2022;14(12):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong H, Baek S-Y, Kim SW, Park E-J, Lee J, Kim H, Jeon CH. C reactive protein level as a marker for dyslipidaemia, diabetes and metabolic syndrome: results from the Korea National Health and Nutrition Examination Survey. BMJ open. 2019;9(8):e029861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danielsson P, Truedsson L, Eriksson K-F, Norgren L. Inflammatory markers and IL-6 polymorphism in peripheral arterial disease with and without diabetes mellitus. Vascular Med. 2005;10(3):191–8. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan AD, Rifai N, Ridker PM. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation. 2002;106(7):820–5. [DOI] [PubMed] [Google Scholar]
- 23.Carlsson AC, Nowak C, Lind L, Östgren CJ, Nyström FH, Sundström J, Carrero JJ, Riserus U, Ingelsson E, Fall T. Growth differentiation factor 15 (GDF-15) is a potential biomarker of both diabetic kidney disease and future cardiovascular events in cohorts of individuals with type 2 diabetes: a proteomics approach. Ups J Med Sci. 2020;125(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung JO, Park S-Y, Cho DH, Chung DJ, Chung MY. Relationship between plasma growth differentiation factor-15 levels and diabetic retinopathy in individuals with type 2 diabetes. Sci Rep. 2020;10(1):20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May BM, Pimentel M, Zimerman LI, Rohde LE. GDF-15 as a Biomarker in Cardiovascular Disease. Arq Bras Cardiol. 2021;116(3):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lajer M, Jorsal A, Tarnow L, Parving HH, Rossing P. Plasma growth differentiation factor-15 independently predicts all-cause and cardiovascular mortality as well as deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetes Care. 2010;33(7):1567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu Y, Zhang W, Shi J, Liu Y, Zhang H, Lin N, Li X, Qin L, Yang Z, Su Q. The relationship between circulating growth differentiation factor 15 levels and Diabetic Retinopathy in patients with type 2 diabetes. Front Endocrinol (Lausanne). 2021;12:627395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu X, Zhang Y, Liang F, Yin J, Jiang L, Cai W, Lu J, Zhang C, Xiao Y, Teng H, et al. Relationship between plasma growth differentiation factor 15 levels and complications of type 2 diabetes Mellitus: a cross-sectional study. Can J Diabetes. 2023;47(2):117–e123117. [DOI] [PubMed] [Google Scholar]
- 29.Barma M, Khan F, Price RJ, Donnan PT, Messow CM, Ford I, McConnachie A, Struthers AD, McMurdo ME, Witham MD. Association between GDF-15 levels and changes in vascular and physical function in older patients with hypertension. Aging Clin Exp Res. 2017;29:1055–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skau E, Wagner P, Leppert J, Ärnlöv J, Hedberg P. Determinants of growth differentiation factor 15 plasma levels in outpatients with peripheral arterial disease. Ups J Med Sci 2024, 129. [DOI] [PMC free article] [PubMed]
- 31.Adela R, Banerjee SK. GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J Diabetes Res 2015, 2015(1):490842. [DOI] [PMC free article] [PubMed]
- 32.Schwarz A, Kinscherf R, Bonaterra GA. Role of the stress-and inflammation-induced cytokine GDF-15 in cardiovascular diseases: from basic research to clinical relevance. Rev Cardiovasc Med. 2023;24(3):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie S, Li Q, Luk AOY, Lan HY, Chan PKS, Bayés-Genís A, Chan FKL, Fung E. Major adverse Cardiovascular events and mortality prediction by circulating GDF-15 in patients with type 2 diabetes: a systematic review and Meta-analysis. Biomolecules 2022, 12(7). [DOI] [PMC free article] [PubMed]
- 34.Castiglione V, Aimo A, Vergaro G, Saccaro L, Passino C, Emdin M. Biomarkers for the diagnosis and management of heart failure. Heart Fail Rev. 2022;27(2):625–43. [DOI] [PMC free article] [PubMed]
- 35.Merchant RA, Chan YH, Duque G. GDF-15 Is Associated with Poor Physical Function in Prefrail Older Adults with Diabetes. J Diabetes Res 2023, 2023:2519128. [DOI] [PMC free article] [PubMed]
- 36.Sen T, Li J, Neuen BL, Arnott C, Neal B, Perkovic V, Mahaffey KW, Shaw W, Canovatchel W, Hansen MK, et al. Association between circulating GDF-15 and Cardio-Renal outcomes and Effect of Canagliflozin: results from the CANVAS Trial. J Am Heart Assoc. 2021;10(23):e021661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bansal N, Zelnick L, Shlipak MG, Anderson A, Christenson R, Deo R, deFilippi C, Feldman H, Lash J, He J, et al. Cardiac and stress biomarkers and chronic kidney Disease Progression: the CRIC Study. Clin Chem. 2019;65(11):1448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair V, Robinson-Cohen C, Smith MR, Bellovich KA, Bhat ZY, Bobadilla M, Brosius F, de Boer IH, Essioux L, Formentini I, et al. Growth differentiation Factor-15 and risk of CKD progression. J Am Soc Nephrol. 2017;28(7):2233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishizawa Y, Shoji T, Maekawa K, Nagasue K, Okuno S, Kim M, Emoto M, Ishimura E, Nakatani T, Miki T, et al. Intima-media thickness of carotid artery predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2003;41(3 Suppl 1):S76–79. [DOI] [PubMed] [Google Scholar]
- 40.Gedela S, Appa Rao A, Medicherla NR. Identification of biomarkers for type 2 diabetes and its complications: a bioinformatic approach. Int J Biomed Sci. 2007;3(4):229–36. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.