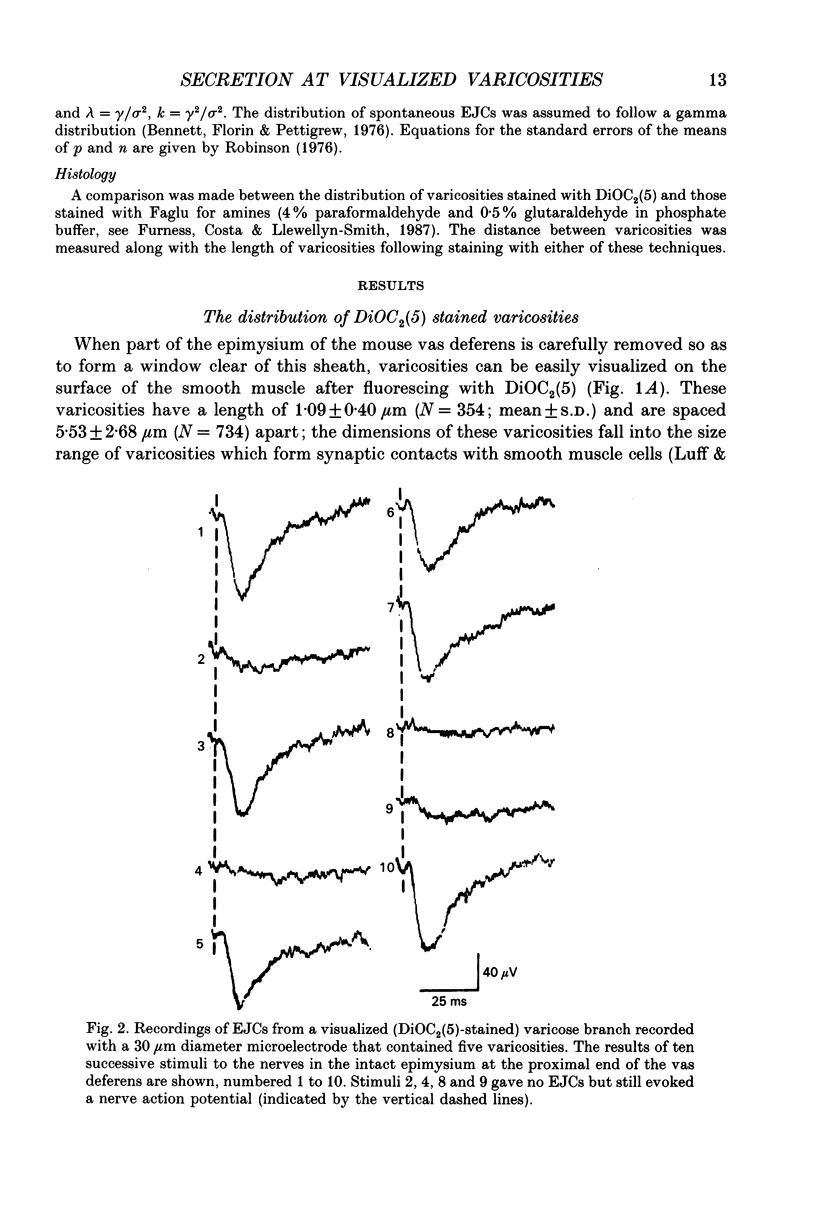
Abstract
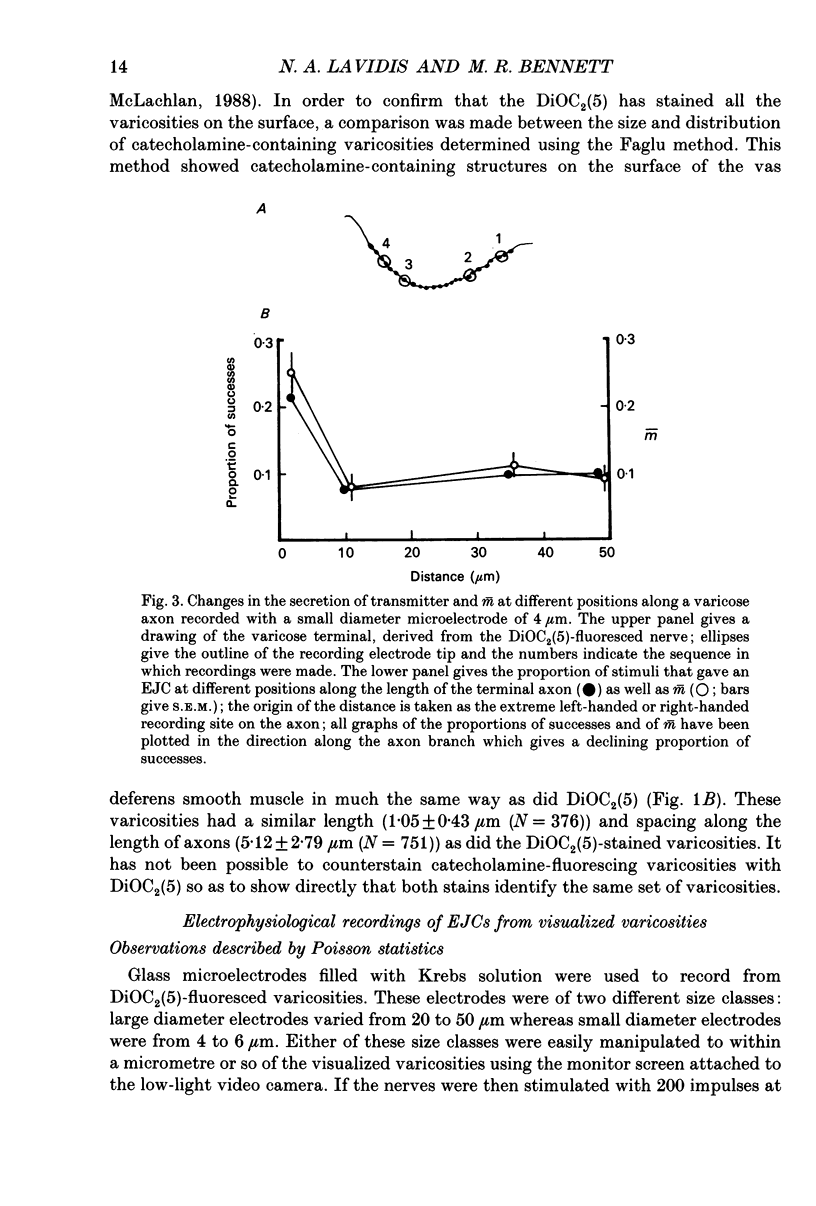
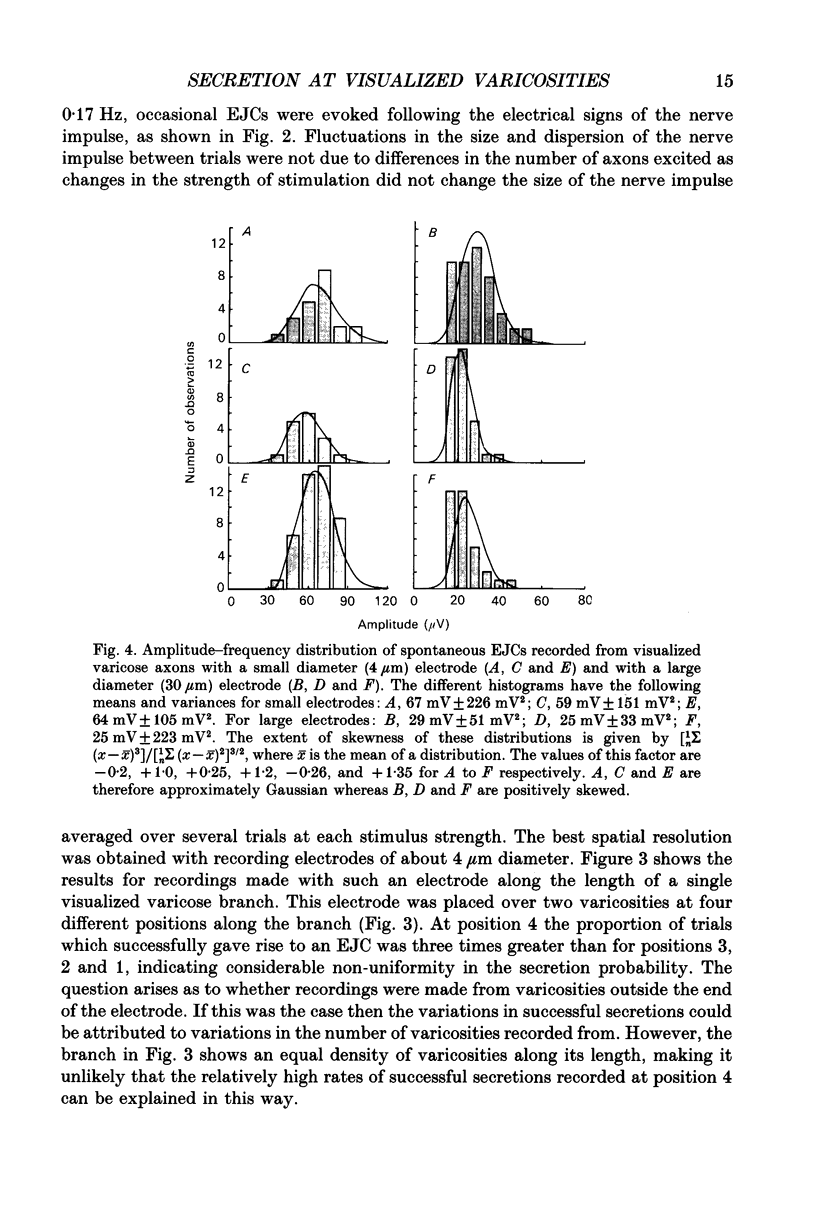
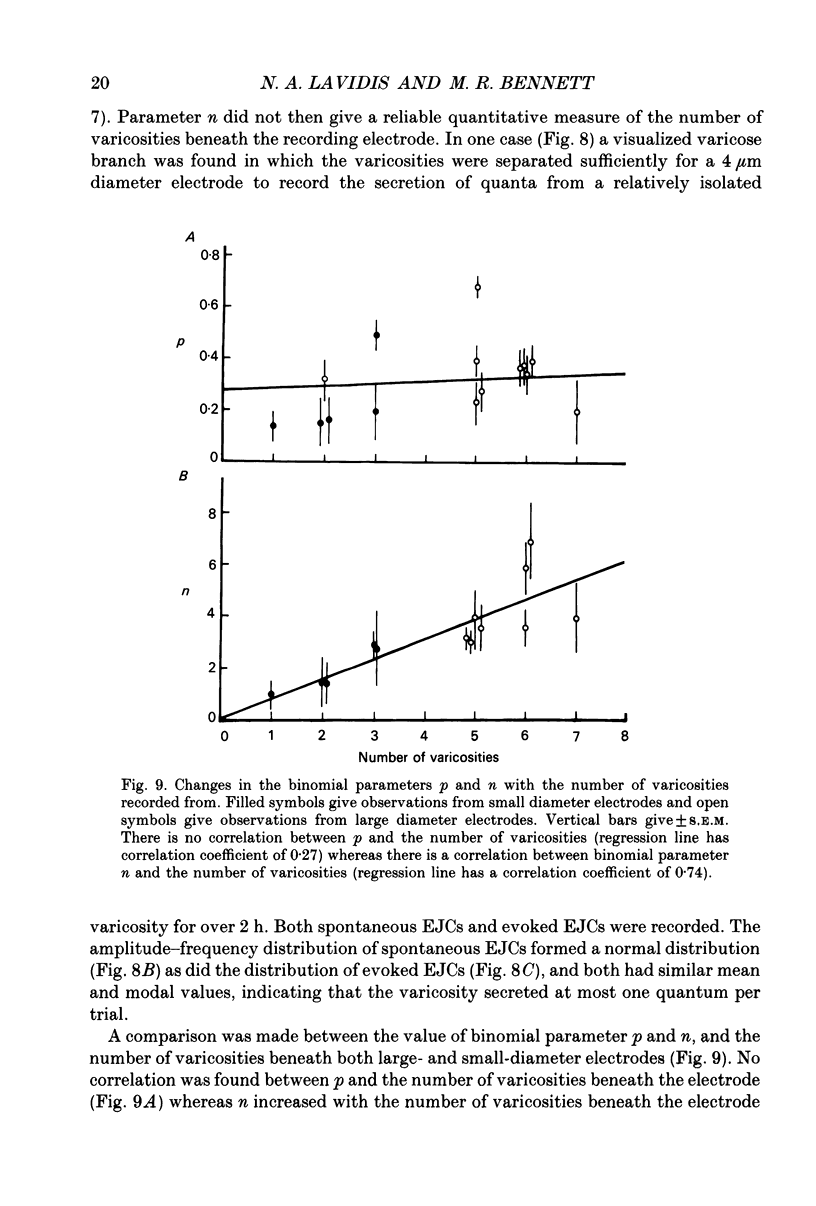
1. Sympathetic varicosities on the surface of smooth muscle cells of the mouse vas deferens were visualized with the fluorescent dye 3-3 Diethyloxardicarbocyanine iodide (DiOC2(5)) and quantal secretion recorded from these with both small diameter (4-6 microns) and large diameter (20-50 microns) microelectrodes. Small diameter electrodes were placed over one to three varicosities and large diameter electrodes over three to seven varicosities. 2. The size and distribution of varicosities along individual terminal branches was about the same when these were fluoresced with DiOC2(5) (length 1.09 +/- 0.40 microns (mean +/- S.D.); intervaricosity distance 5.53 +/- 2.68 microns) as when they were stained for catecholamines using Faglu fluorescence (length 1.05 +/- 0.43 microns; intervaricosity distance 5.12 +/- 2.79 microns) suggesting that DiOC2(5) does allow for identification of the catecholamine-containing varicosities. 3. The spontaneous excitatory junctional currents (EJCs) recorded from visualized varicosities with small diameter electrodes (amplitudes 59-67 microV) were much larger than those recorded with large diameter electrodes (amplitudes 25-29 microV). The frequency of evoked EJCs as well as the amplitude-frequency distribution of these EJCs varied greatly between sets of visualized varicosities recorded along individual branches, either with a small or large diameter electrode. These amplitude-frequency distributions typically followed Poisson statistics, in which the mean quantal content of the EJC (m) varied by over threefold for different sets of varicosities on the same branch (m was 0.07-0.21 for small electrodes whereas m was 1-3 for large electrodes). 4. Although m varied considerably for a constant number of varicosities beneath the electrode at different sites along a single branch, there was an overall correlation between m and the number of varicosities, m increasing on average 0.25 for each additional varicosity in a [Ca2+]o of 4.0 mM. 5. The frequency of evoked EJCs at visualized sets of varicosities along some branches was sufficiently high to allow binomial statistics to predict the amplitude-frequency distributions of evoked EJCs. In these cases m was again shown to vary considerably along single terminal branches, and this was primarily due to variation in the probability of secretion (p) between sets of varicosities and not to variation in binomial parameter n. 6. In one case a relatively isolated varicosity, over 3 microns from adjacent varicosities, was recorded for 30 min with a 4 microns diameter electrode. The mean and variance of the evoked EJC was similar to that of the spontaneous EJCs suggesting that this varicosity secreted at most one quantum on arrival of the nerve impulse.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
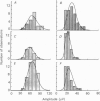
PDF



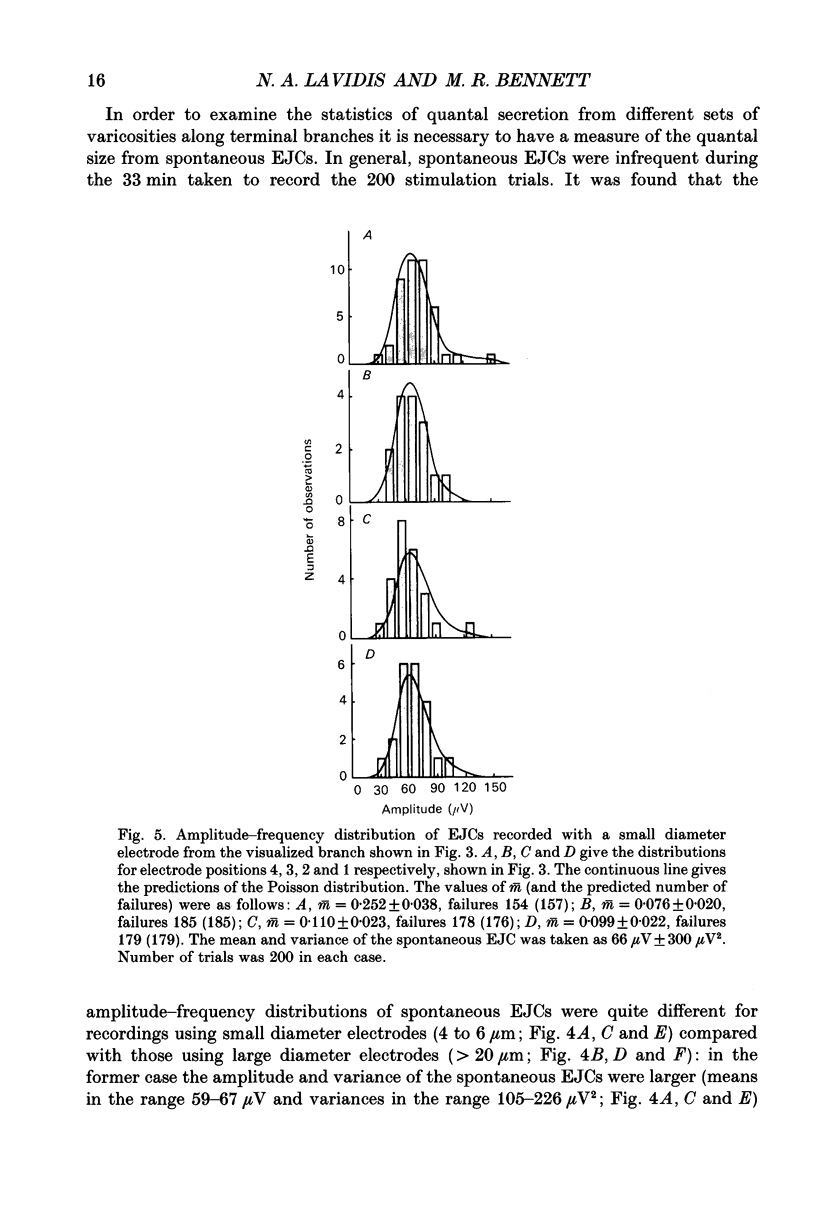
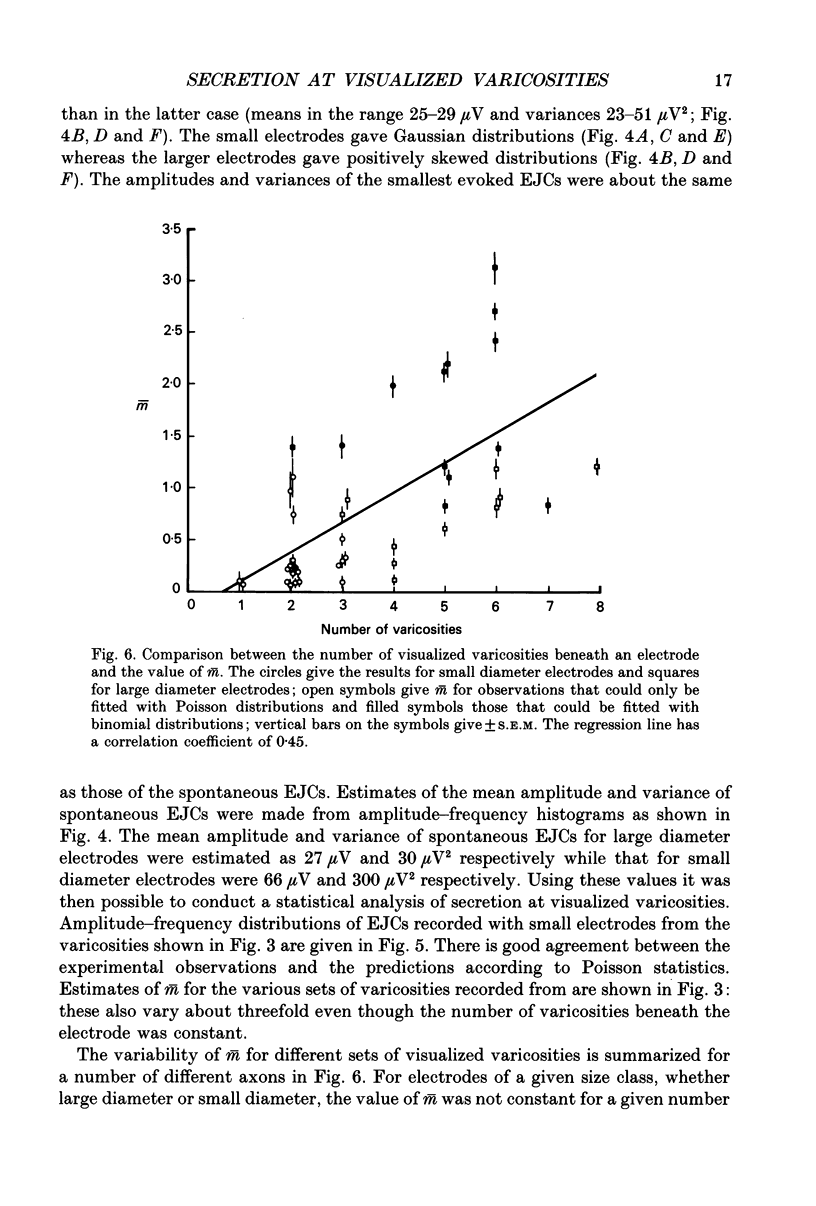
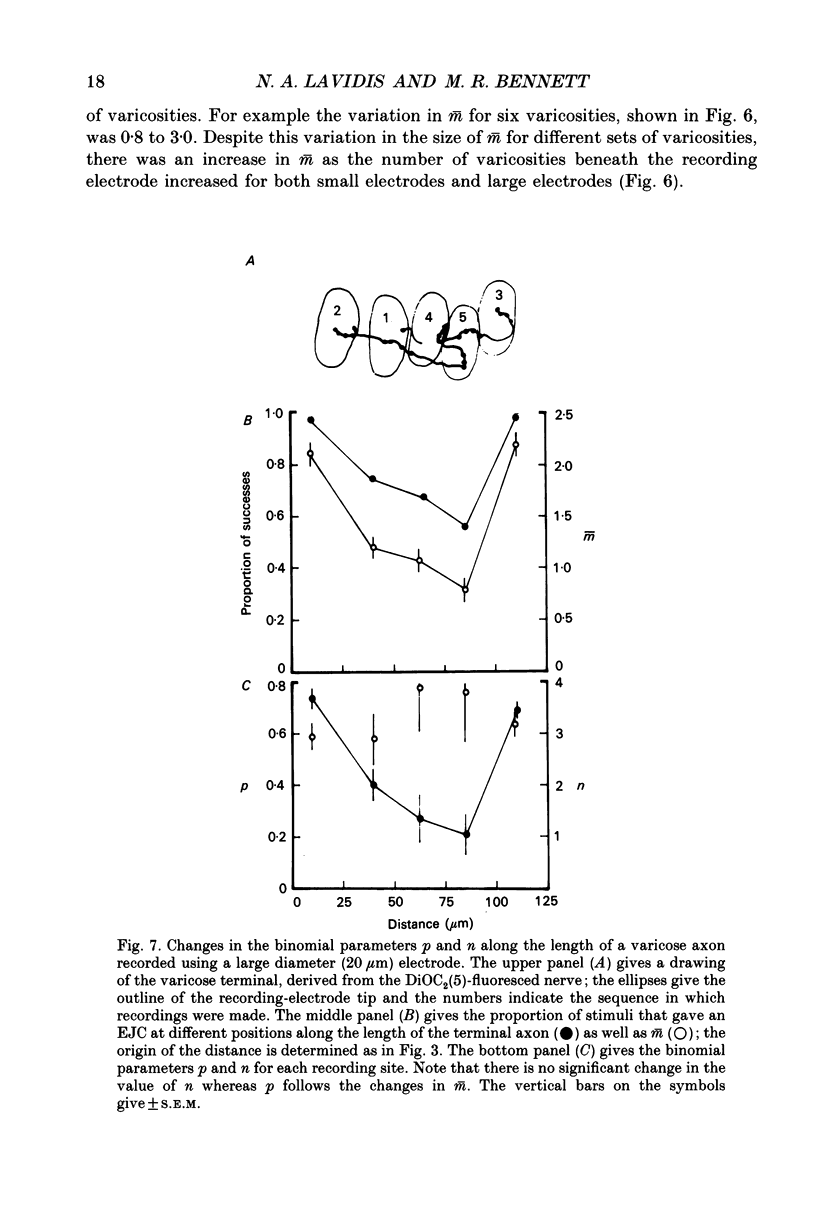
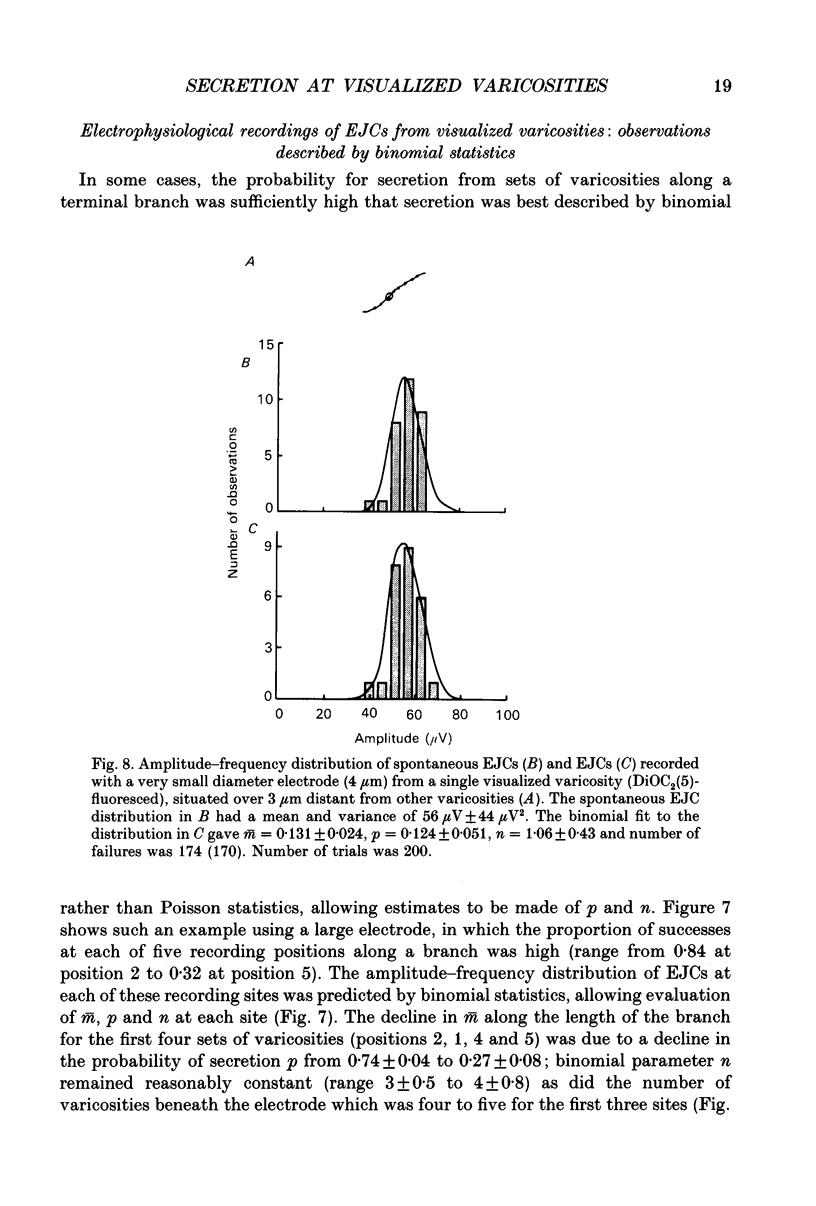






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allcorn R. J., Cunnane T. C., Kirkpatrick K. Actions of alpha, beta-methylene ATP and 6-hydroxydopamine on sympathetic neurotransmission in the vas deferens of the guinea-pig, rat and mouse: support for cotransmission. Br J Pharmacol. 1986 Dec;89(4):647–659. doi: 10.1111/j.1476-5381.1986.tb11169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrand P., Brock J. A., Cunnane T. C. Time course of transmitter action at the sympathetic neuroeffector junction in rodent vascular and non-vascular smooth muscle. J Physiol. 1988 Jul;401:657–670. doi: 10.1113/jphysiol.1988.sp017185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrand P., Stjärne L. On the secretory activity of single varicosities in the sympathetic nerves innervating the rat tail artery. J Physiol. 1989 Feb;409:207–220. doi: 10.1113/jphysiol.1989.sp017493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet M. R., Lavidis N. A. Variation in quantal secretion at different release sites along developing and mature motor terminal branches. Brain Res. 1982 Sep;281(1):1–9. doi: 10.1016/0165-3806(82)90107-9. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Pettigrew A. G. The effect of calcium ions on the binomial statistic parameters that control acetylcholine release at preganglionic nerve terminals. J Physiol. 1976 Jun;257(3):597–620. doi: 10.1113/jphysiol.1976.sp011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Jones P., Lavidis N. A. The probability of quantal secretion along visualized terminal branches at amphibian (Bufo marinus) neuromuscular synapses. J Physiol. 1986 Oct;379:257–274. doi: 10.1113/jphysiol.1986.sp016252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. Quantal secretion at release sites of nerve terminals in toad (Bufo marinus) muscle during formation of topographical maps. J Physiol. 1988 Jul;401:567–579. doi: 10.1113/jphysiol.1988.sp017180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. The effect of calcium ions on the secretion of quanta evoked by an impulse at nerve terminal release sites. J Gen Physiol. 1979 Oct;74(4):429–456. doi: 10.1085/jgp.74.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. The probability of quantal secretion at release sites in different calcium concentrations in toad (Bufo marinus) muscle. J Physiol. 1989 Nov;418:219–233. doi: 10.1113/jphysiol.1989.sp017836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Merrillees N. C. An analysis of the transmission of excitation from autonomic nerves to smooth muscle. J Physiol. 1966 Aug;185(3):520–535. doi: 10.1113/jphysiol.1966.sp008000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Robinson J. Probabilistic secretion of quanta from nerve terminals at synaptic sites on muscle cells: non-uniformity, autoinhibition and the binomial hypothesis. Proc R Soc Lond B Biol Sci. 1990 Apr 23;239(1296):329–358. doi: 10.1098/rspb.1990.0020. [DOI] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Electrical activity at the sympathetic neuroeffector junction in the guinea-pig vas deferens. J Physiol. 1988 May;399:607–632. doi: 10.1113/jphysiol.1988.sp017099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Local application of drugs to sympathetic nerve terminals: an electrophysiological analysis of the role of prejunctional alpha-adrenoceptors in the guinea-pig vas deferens. Br J Pharmacol. 1991 Mar;102(3):595–600. doi: 10.1111/j.1476-5381.1991.tb12218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Relationship between the nerve action potential and transmitter release from sympathetic postganglionic nerve terminals. Nature. 1987 Apr 9;326(6113):605–607. doi: 10.1038/326605a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Sympathetic purinergic transmission in small blood vessels. Trends Pharmacol Sci. 1988 Apr;9(4):116–117. doi: 10.1016/0165-6147(88)90185-x. [DOI] [PubMed] [Google Scholar]
- Couteaux R., Pécot-Dechavassine M. Vésicules synaptiques et poches au niveau des "zones actives" de la jonction neuromusculaire. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2346–2349. [PubMed] [Google Scholar]
- Cunnane T. C., Manchanda R. Electrophysiological analysis of the inactivation of sympathetic transmitter in the guinea-pig vas deferens. J Physiol. 1988 Oct;404:349–364. doi: 10.1113/jphysiol.1988.sp017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alonzo A. J., Grinnell A. D. Profiles of evoked release along the length of frog motor nerve terminals. J Physiol. 1985 Feb;359:235–258. doi: 10.1113/jphysiol.1985.sp015583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. L., Burnstock G. Angiotensin neuromodulation of adrenergic and purinergic co-transmission in the guinea-pig vas deferens. Br J Pharmacol. 1989 Aug;97(4):1157–1164. doi: 10.1111/j.1476-5381.1989.tb12574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried G. Small noradrenergic storage vesicles isolated from rat vas deferens--biochemical and morphological characterization. Acta Physiol Scand Suppl. 1980;493:1–28. [PubMed] [Google Scholar]
- Herrera A. A., Banner L. R. The use and effects of vital fluorescent dyes: observation of motor nerve terminals and satellite cells in living frog muscles. J Neurocytol. 1990 Feb;19(1):67–83. doi: 10.1007/BF01188440. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Some properties of spontaneous excitatory junction potentials recorded from arterioles of guinea-pigs. J Physiol. 1980 Jun;303:43–60. doi: 10.1113/jphysiol.1980.sp013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff S. E., McLachlan E. M., Hirst G. D. An ultrastructural analysis of the sympathetic neuromuscular junctions on arterioles of the submucosa of the guinea pig ileum. J Comp Neurol. 1987 Mar 22;257(4):578–594. doi: 10.1002/cne.902570407. [DOI] [PubMed] [Google Scholar]
- Luff S. E., McLachlan E. M. The form of sympathetic postganglionic axons at clustered neuromuscular junctions near branch points of arterioles in the submucosa of the guinea pig ileum. J Neurocytol. 1988 Aug;17(4):451–463. doi: 10.1007/BF01189802. [DOI] [PubMed] [Google Scholar]
- MacKenzie I., Kirkpatrick K. A., Burnstock G. Comparative study of the actions of AP5A and alpha,beta-methylene ATP on nonadrenergic, noncholinergic neurogenic excitation in the guinea-pig vas deferens. Br J Pharmacol. 1988 Jul;94(3):699–706. doi: 10.1111/j.1476-5381.1988.tb11578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrillees N. C. The nervous environment of individual smooth muscle cells of the guinea pig vas deferens. J Cell Biol. 1968 Jun;37(3):794–817. doi: 10.1083/jcb.37.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. M., Heuser J. E. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J Cell Biol. 1984 Feb;98(2):685–698. doi: 10.1083/jcb.98.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990 Jan;70(1):165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Robinson J. Estimation of parameters for a model of transmitter release at synapses. Biometrics. 1976 Mar;32(1):61–68. [PubMed] [Google Scholar]
- Robitaille R., Tremblay J. P. Non-uniform release at the frog neuromuscular junction: evidence of morphological and physiological plasticity. Brain Res. 1987 Mar;434(1):95–116. doi: 10.1016/0165-0173(87)90019-1. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikami D., Okun L. M. Staining of living presynaptic nerve terminals with selective fluorescent dyes. Nature. 1984 Jul 5;310(5972):53–56. doi: 10.1038/310053a0. [DOI] [PubMed] [Google Scholar]
- Zefirov A. L. Sekretsiia mediatora v proksimal'nykh i distal'nykh uchastkakh nervnogo okonchaniia portniazhnoi myshtsy liagushki. Neirofiziologiia. 1983;15(4):362–369. [PubMed] [Google Scholar]