Abstract
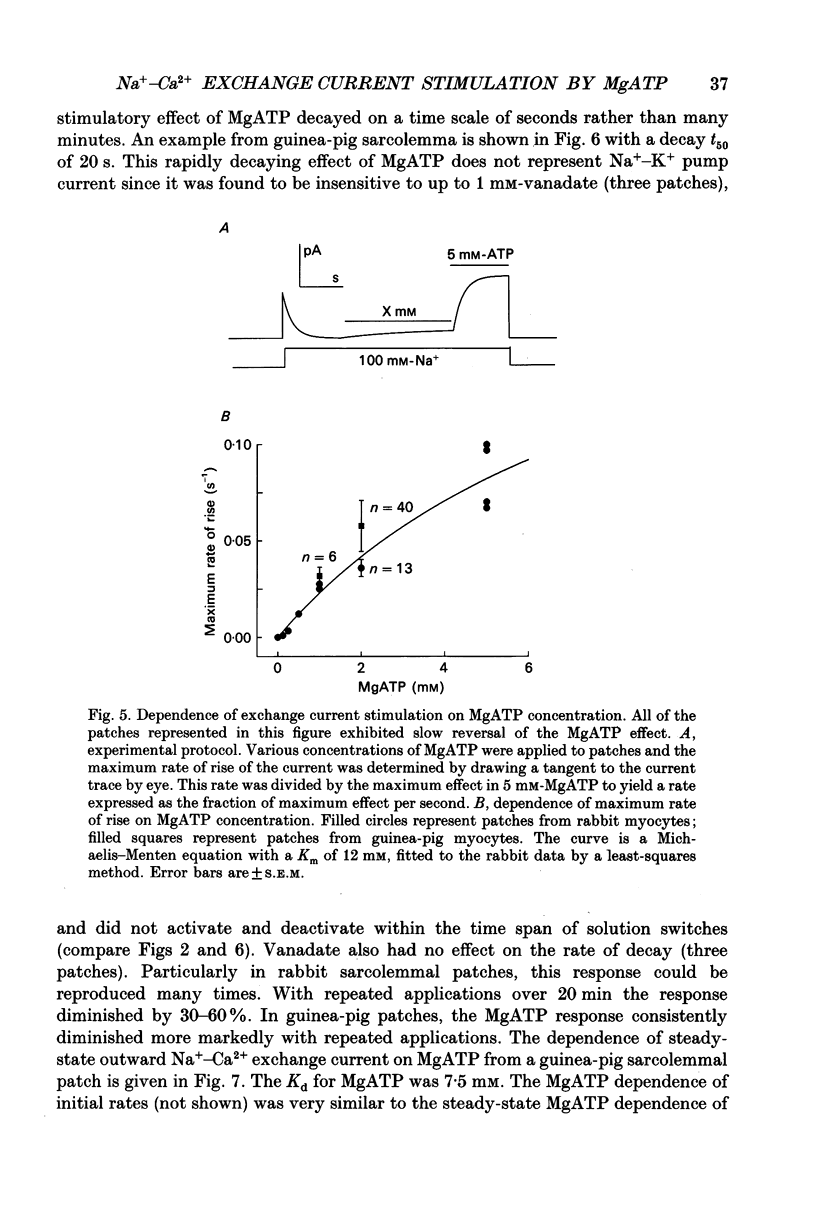
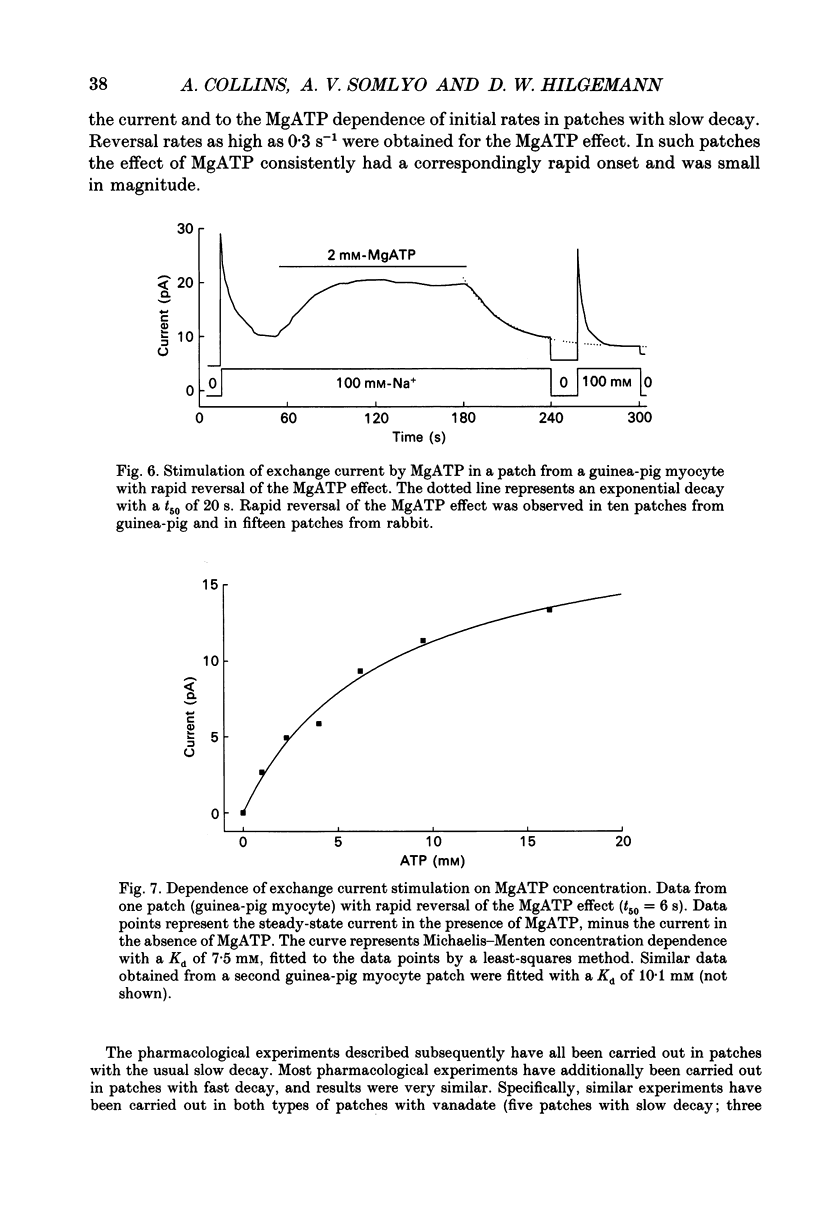
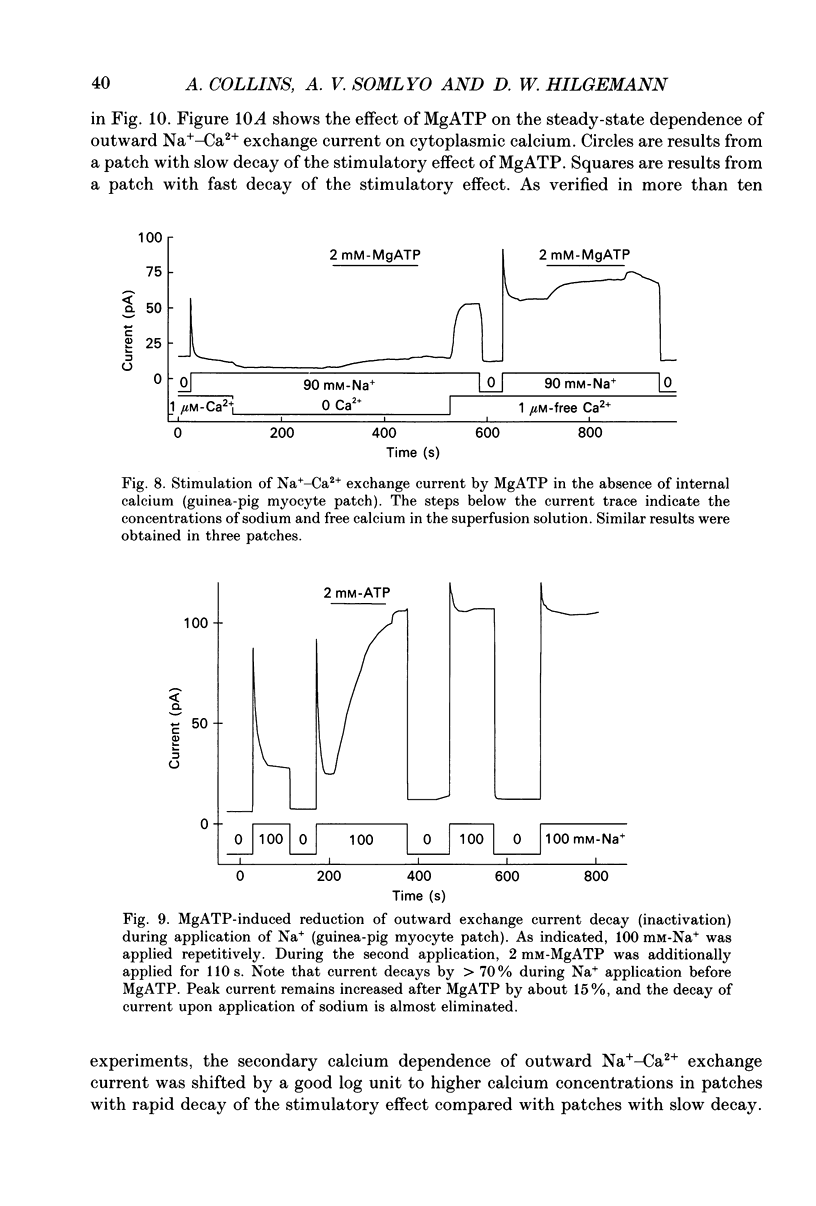
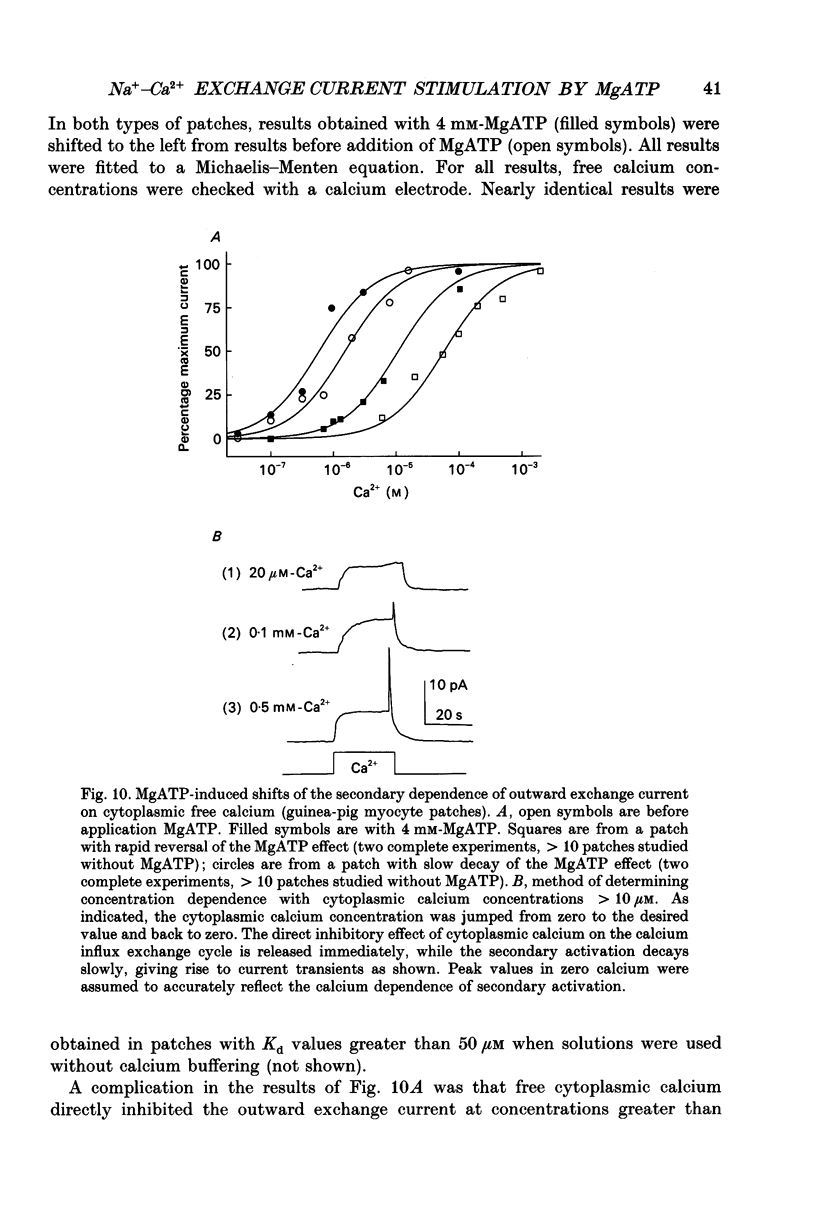
1. A giant patch method was used to study the stimulatory effect of cytoplasmic MgATP on outward Na(+)-Ca2+ exchange current in inside-out cardiac membrane patches (1-10 G omega seals with 14-24 microns pipette tip diameters) excised from guinea-pig, rabbit and mouse myocytes. 2. To establish the validity of the method with respect to structure, bleb formation was examined with electron microscopy and with confocal fluorescence light microscopy. The blebs, which form as the sarcolemma detaches, excluded intracellular organelles and transverse tubules. The blebbed cells contained normal sarcomeres, sarcoplasmic reticulum, triads and diads. 3. To further establish the validity of the method for ion transport studies, measurements of Na(+)-K+ pump currents and charge movements are described briefly which demonstrate (i) free access to the cytoplasmic membrane side, (ii) MgATP dependence comparable to reconstituted pump (Kd, 94 microns), (iii) fast, rigorous concentration control and (iv) Na(+)-K+ pump densities in the range of whole-cell densities. 4. Stimulation of outward Na(+)-Ca2+ exchange current by MgATP attenuated exchange current decay during step increments of cytoplasmic sodium, shifted the secondary activation of outward exchange current by cytoplasmic calcium to lower free calcium concentrations and, particularly in mouse cardiac sarcolemma, induced cytoplasmic calcium-independent current. 5. Upon removal of MgATP the stimulatory effect usually decayed with a t50 (half-time) of about 3 min. However, the reversal took place much more rapidly (t50, 5-20 s) in patches from individual guinea-pig and rabbit myocyte batches. When decay was rapid, secondary activation by cytoplasmic calcium was shifted to higher free cytoplasmic calcium concentrations (Kd, 10-65 microns-free calcium). 6. With repeated applications of MgATP the rate and magnitude of the stimulatory effect progressively decreased. 7. The Kd for MgATP of the initial rate of stimulation of outward exchange current was 3 mM or greater. When decay was rapid, the steady-state dependence of exchange current on MgATP also had a Kd of 3 mM or greater. 8. Stimulation of Na(+)-Ca2+ exchange current by MgATP occurred in the absence of cytoplasmic calcium with 9 mM-EGTA. 9. The stimulatory effect of 2 mM-MgATP was not inhibited by up to 200 microM of the protein kinase inhibitor 1-(5-isoquinoline sulphonyl)-2-methylpiperazine (H7), or by peptide inhibitors of cyclic AMP-dependent protein kinase, protein kinase C and calcium-calmodulin-dependent protein kinase II.(ABSTRACT TRUNCATED AT 400 WORDS)
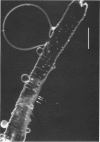
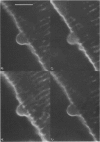
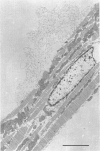
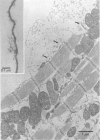
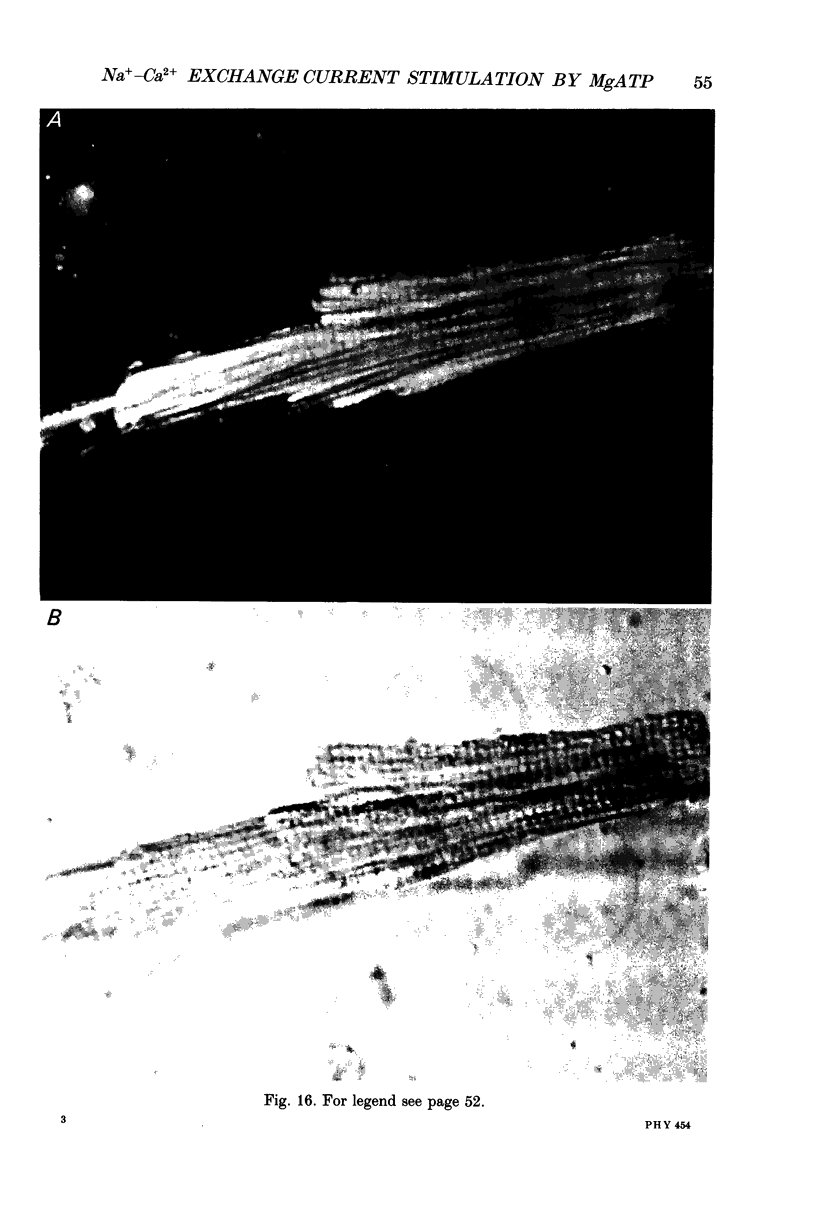
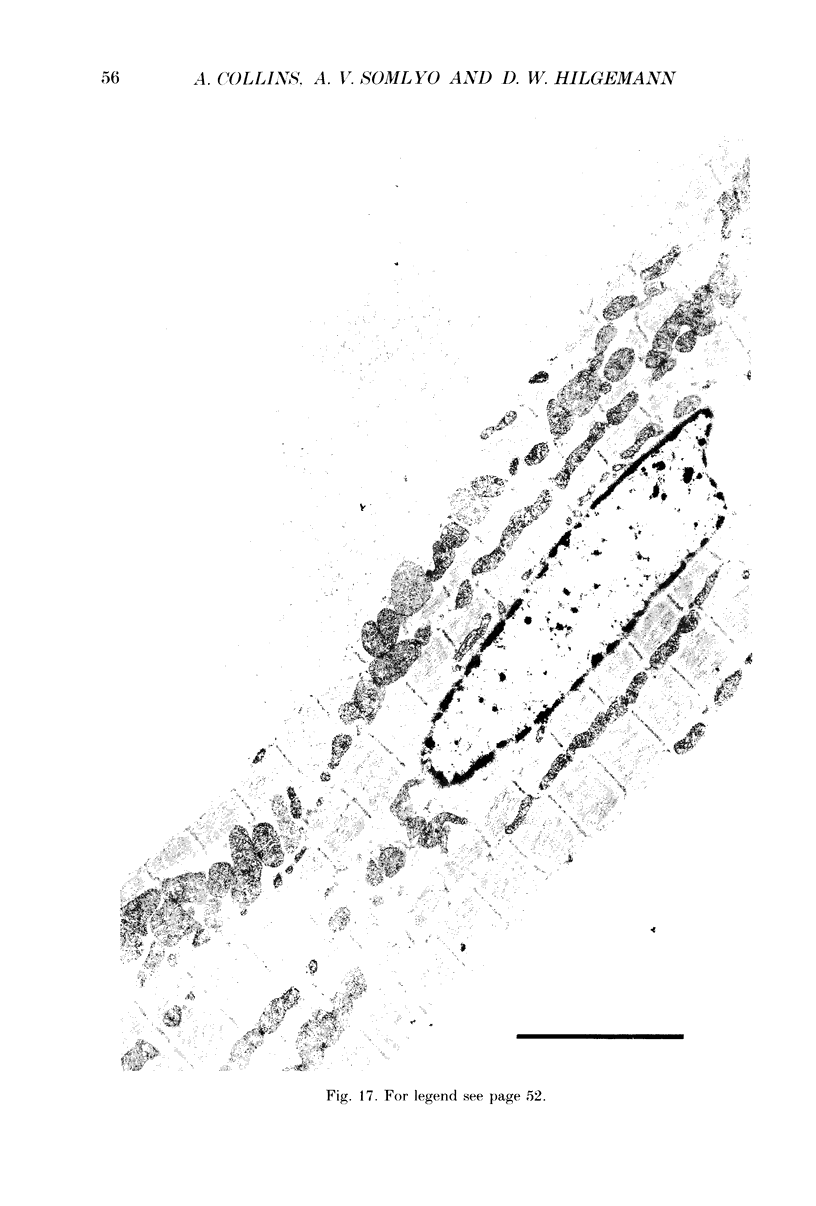
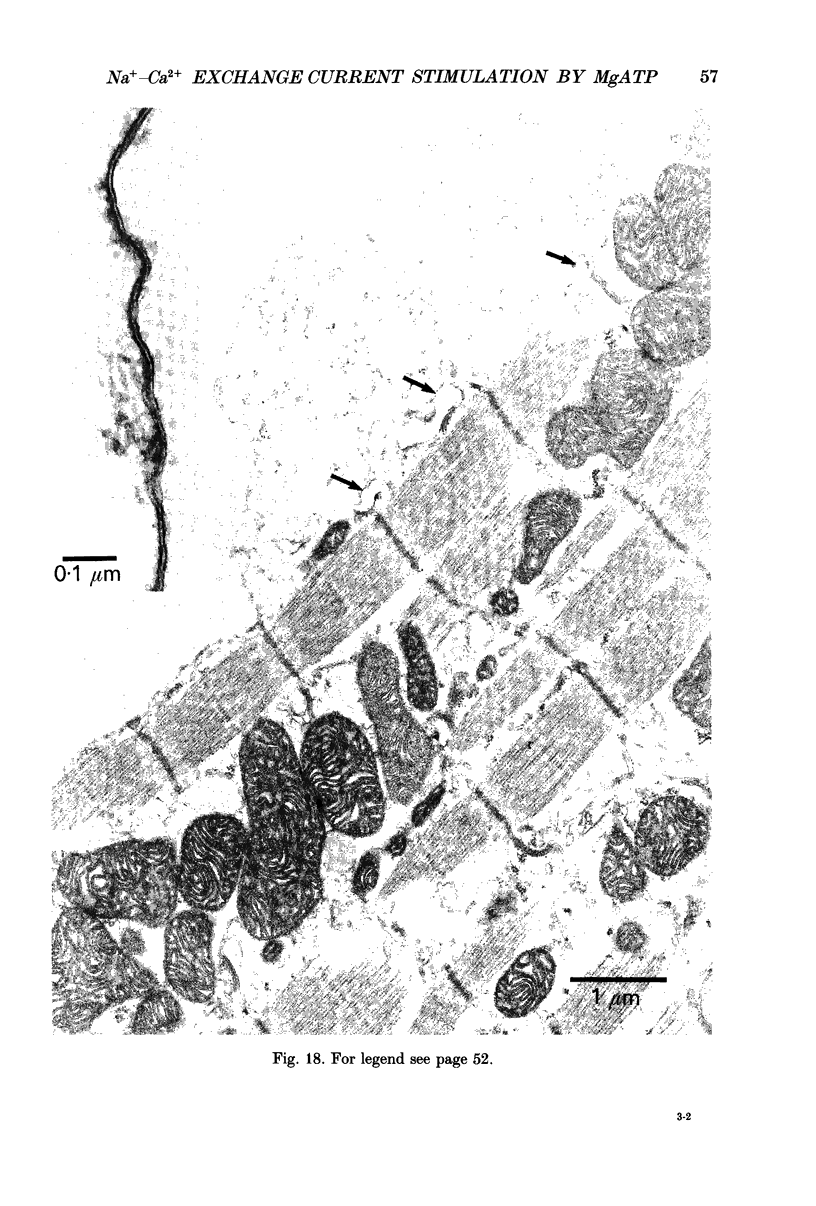
Full text
PDF



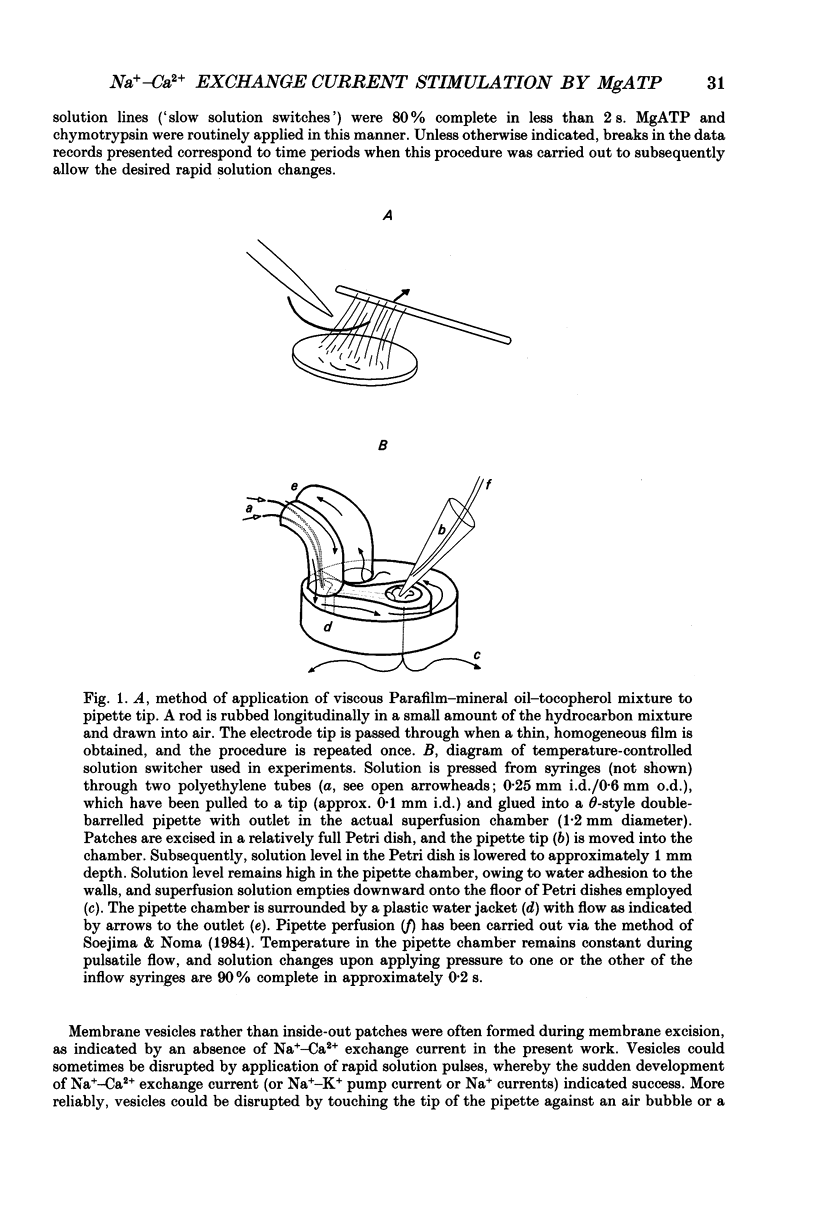

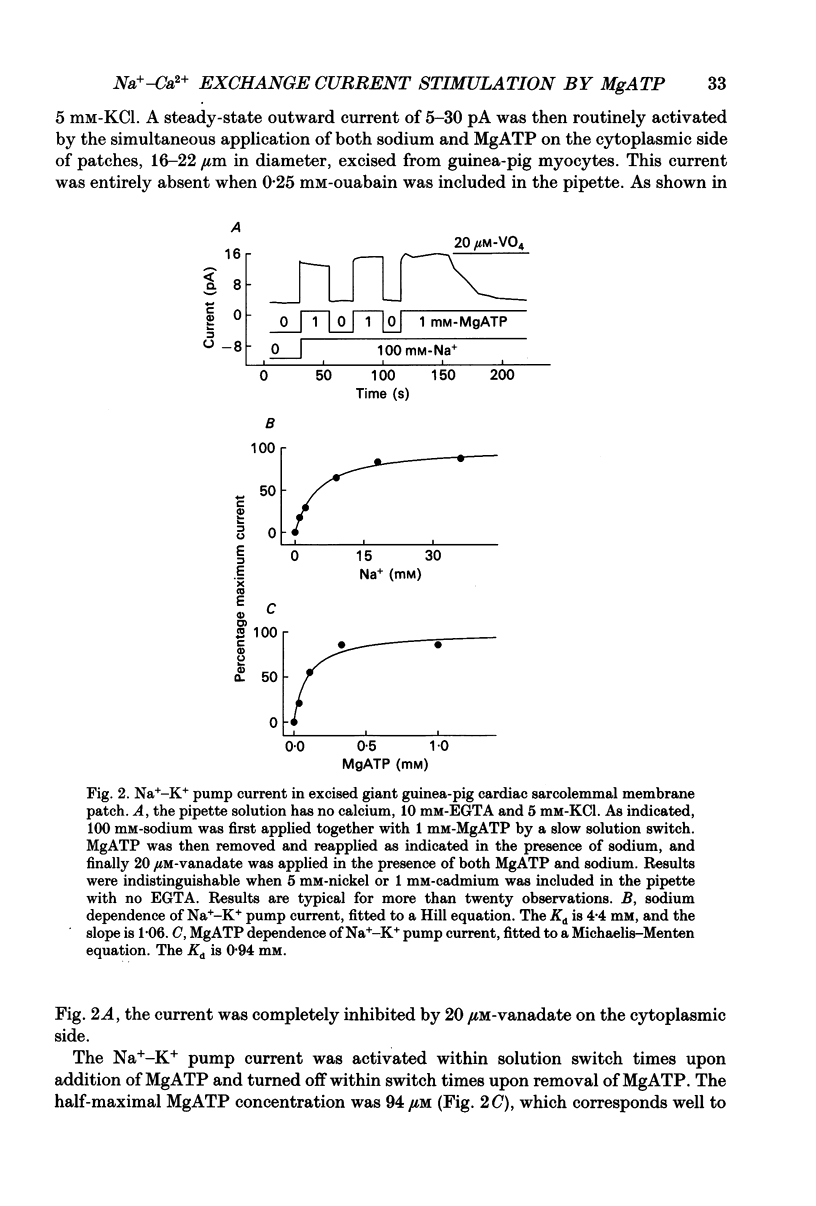
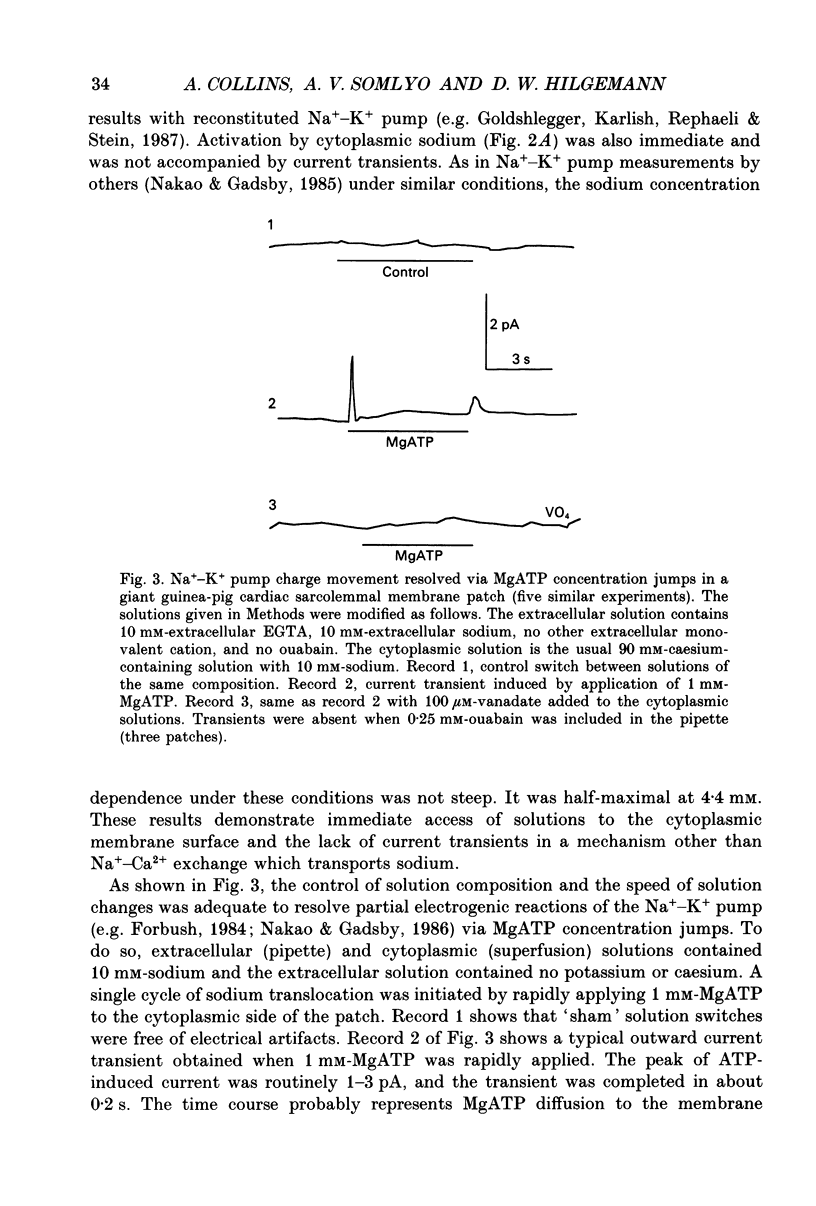

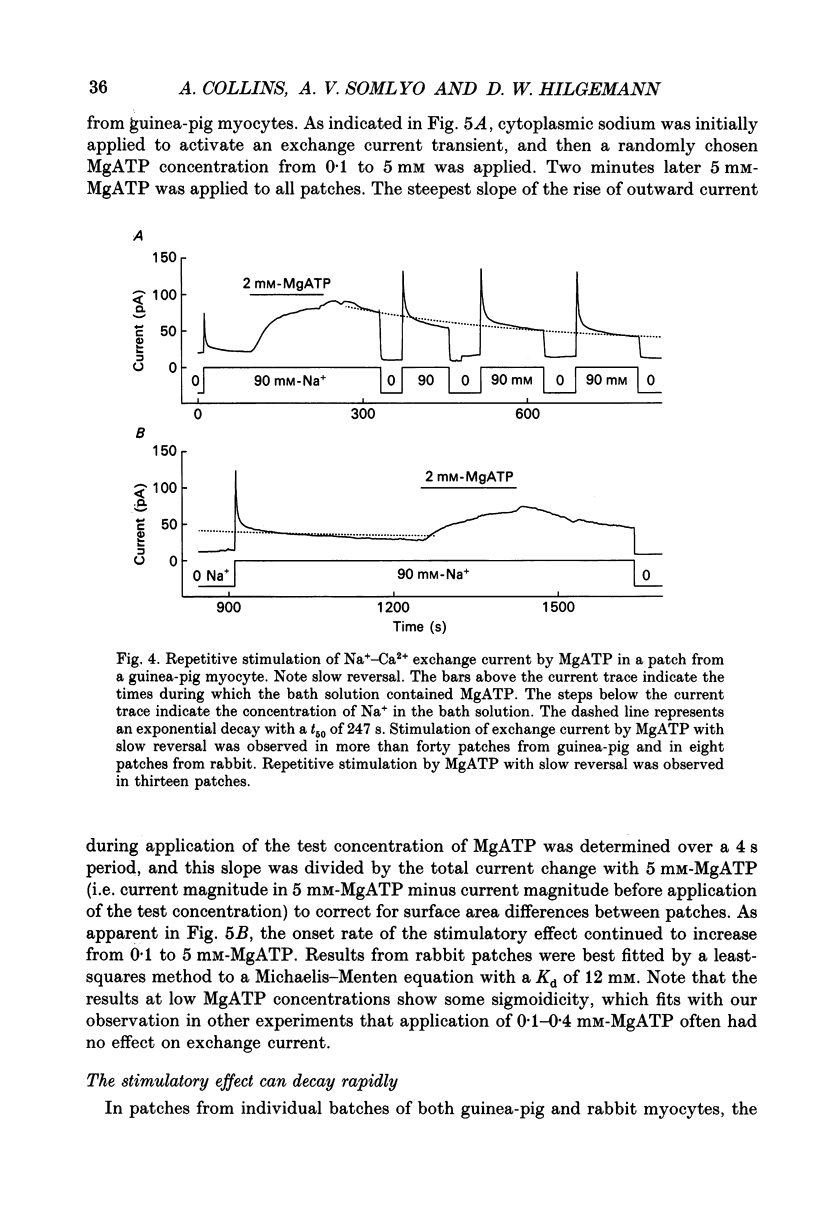

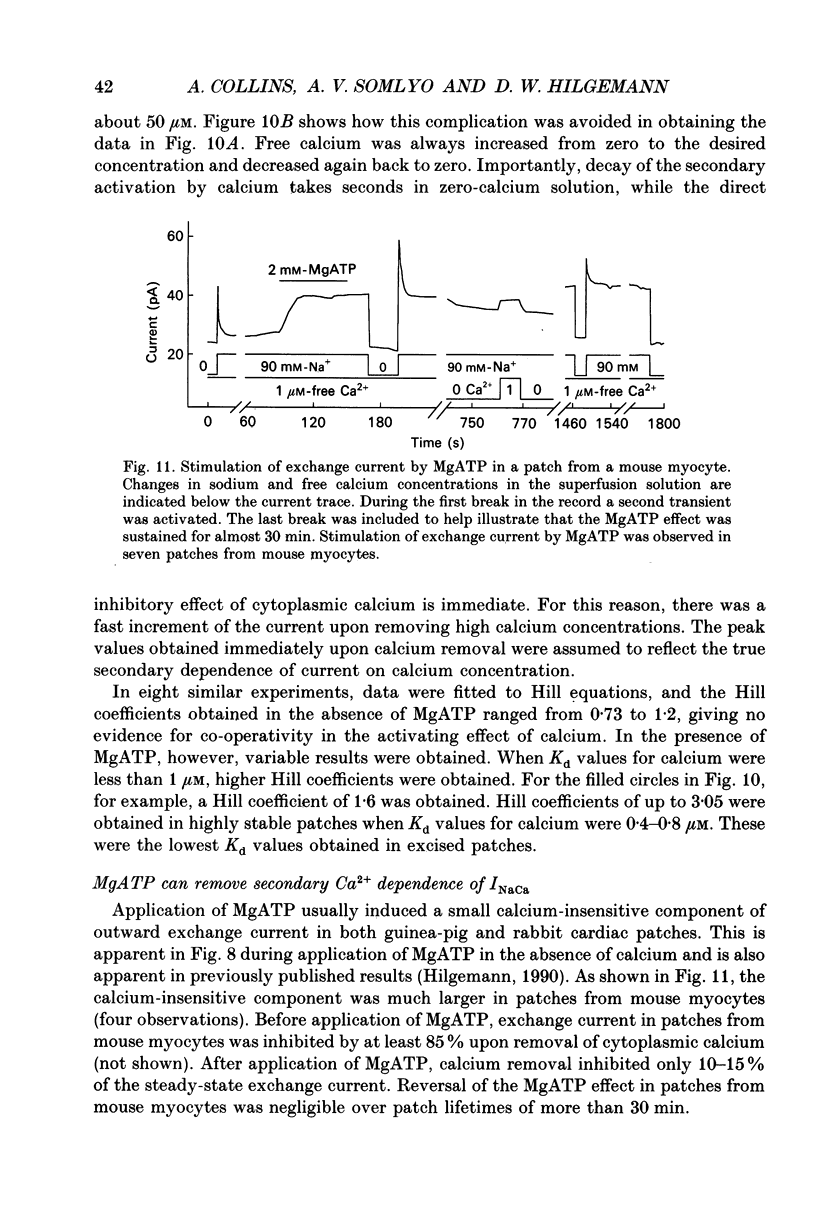









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcenas-Ruiz L., Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in heart: membrane currents and changes in [Ca2+]i. Science. 1987 Dec 18;238(4834):1720–1722. doi: 10.1126/science.3686010. [DOI] [PubMed] [Google Scholar]
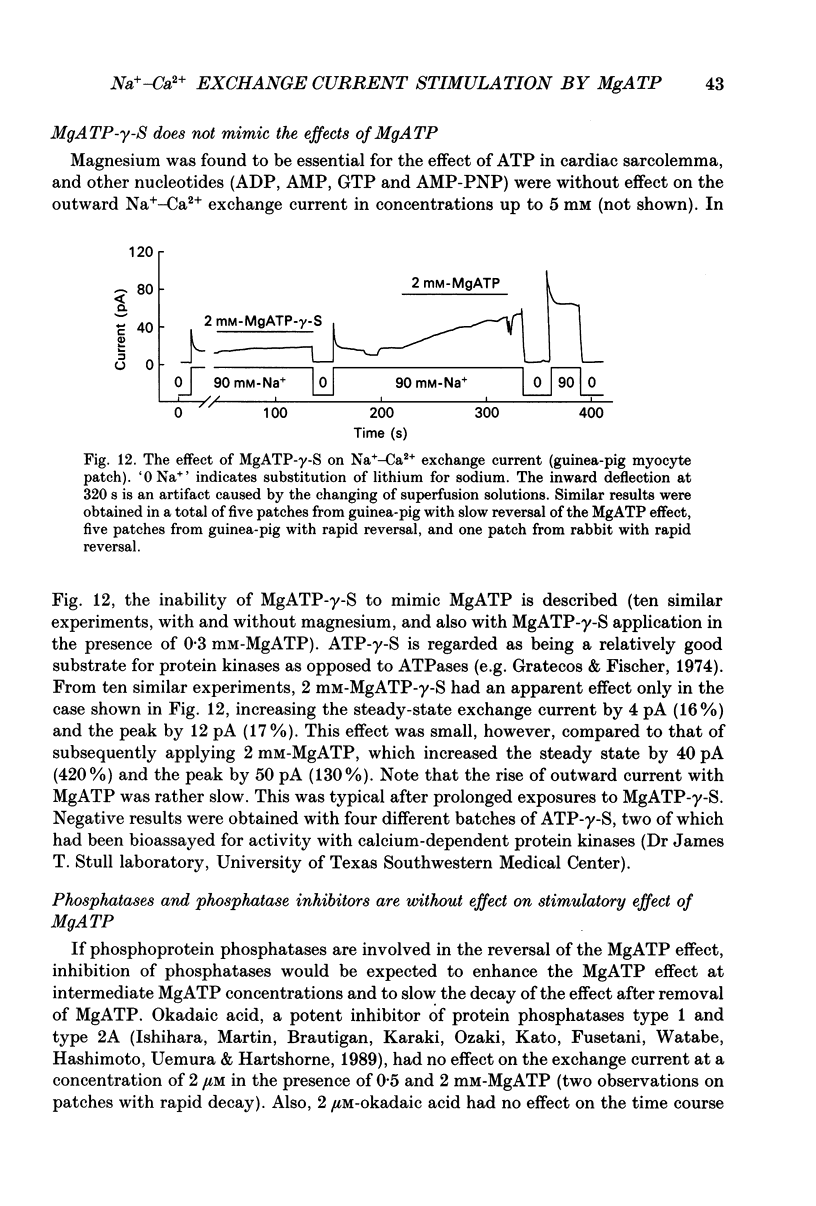
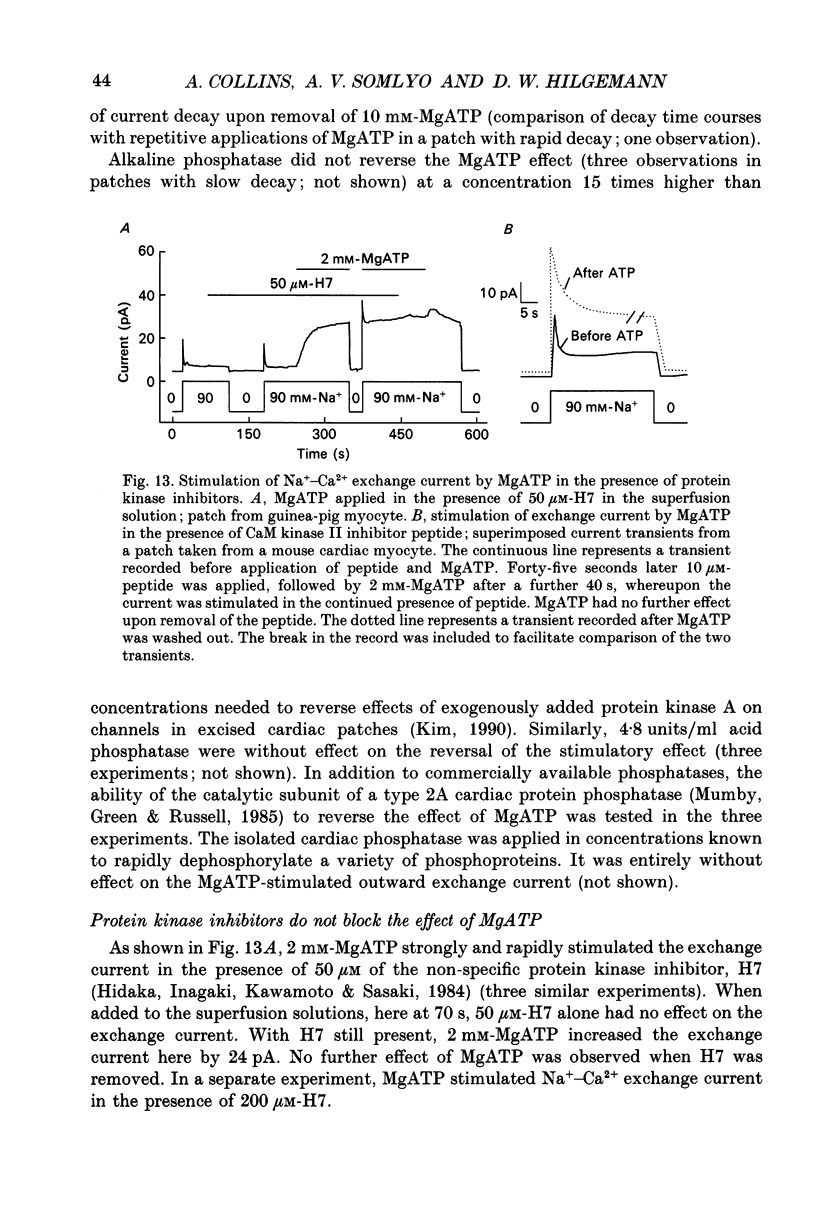
- Baumann O., Kitazawa T., Somlyo A. P. Laser confocal scanning microscopy of the surface membrane/T-tubular system and the sarcoplasmic reticulum in insect striated muscle stained with DilC18(3). J Struct Biol. 1990 Oct-Dec;105(1-3):154–161. doi: 10.1016/1047-8477(90)90109-p. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Santiago E. M. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J. 1977 Oct;20(1):79–111. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal D. K., Takio K., Edelman A. M., Charbonneau H., Titani K., Walsh K. A., Krebs E. G. Identification of the calmodulin-binding domain of skeletal muscle myosin light chain kinase. Proc Natl Acad Sci U S A. 1985 May;82(10):3187–3191. doi: 10.1073/pnas.82.10.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L., Giles W. R., Robinson K., Shibata E. F. Studies of the sodium-calcium exchanger in bull-frog atrial myocytes. J Physiol. 1988 Sep;403:317–340. doi: 10.1113/jphysiol.1988.sp017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The regulation of the Na+ -Ca2+ exchanger of heart sarcolemma. Eur J Biochem. 1983 May 16;132(3):451–460. doi: 10.1111/j.1432-1033.1983.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Coutinho O. P., Carvalho A. P., Carvalho C. A. Effect of monovalent cations on Na+/Ca2+ exchange and ATP-dependent Ca2+ transport in synaptic plasma membranes. J Neurochem. 1983 Sep;41(3):670–676. doi: 10.1111/j.1471-4159.1983.tb04793.x. [DOI] [PubMed] [Google Scholar]
- Crespo L. M., Grantham C. J., Cannell M. B. Kinetics, stoichiometry and role of the Na-Ca exchange mechanism in isolated cardiac myocytes. Nature. 1990 Jun 14;345(6276):618–621. doi: 10.1038/345618a0. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Asymmetrical properties of the Na-Ca exchanger in voltage-clamped, internally dialyzed squid axons under symmetrical ionic conditions. J Gen Physiol. 1990 May;95(5):819–835. doi: 10.1085/jgp.95.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Ca2+ transport in nerve fibers. Biochim Biophys Acta. 1988 Oct 11;947(3):549–569. doi: 10.1016/0304-4157(88)90007-x. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Characterization of the reverse Na/Ca exchange in squid axons and its modulation by Cai and ATP. Cai-dependent Nai/Cao and Nai/Nao exchange modes. J Gen Physiol. 1987 Oct;90(4):505–525. doi: 10.1085/jgp.90.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. In squid axons, ATP modulates Na+-Ca2+ exchange by a Ca2+i-dependent phosphorylation. Biochim Biophys Acta. 1987 Mar 12;897(3):347–354. doi: 10.1016/0005-2736(87)90432-9. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. The effects of vanadate on calcium transport in dialyzed squid axons. Sidedness of vanadate-cation interactions. Biochim Biophys Acta. 1981 Jul 20;645(2):229–236. doi: 10.1016/0005-2736(81)90193-0. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Spindler A. J., Twist V. W. Sodium-calcium exchange during the action potential in guinea-pig ventricular cells. J Physiol. 1989 Apr;411:639–661. doi: 10.1113/jphysiol.1989.sp017596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Noble D., Shimoni Y., Spindler A. J. Inward current related to contraction in guinea-pig ventricular myocytes. J Physiol. 1987 Apr;385:565–589. doi: 10.1113/jphysiol.1987.sp016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Fox A. P., Krasne S. Membrane patches and whole-cell membranes: a comparison of electrical properties in rat clonal pituitary (GH3) cells. J Physiol. 1984 Nov;356:565–585. doi: 10.1113/jphysiol.1984.sp015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshlegger R., Karlish S. J., Rephaeli A., Stein W. D. The effect of membrane potential on the mammalian sodium-potassium pump reconstituted into phospholipid vesicles. J Physiol. 1987 Jun;387:331–355. doi: 10.1113/jphysiol.1987.sp016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratecos D., Fischer E. H. Adenosine 5'-O(3-thiotriphosphate) in the control of phosphorylase activity. Biochem Biophys Res Commun. 1974 Jun 18;58(4):960–967. doi: 10.1016/s0006-291x(74)80237-8. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Goknur A. B., Hunter D. R., Hegge J. O., Berkoff H. A. Inhibition of calcium influx in isolated adult rat heart cells by ATP depletion. Circ Res. 1987 Apr;60(4):586–594. doi: 10.1161/01.res.60.4.586. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W., Collins A. Mechanism of cardiac Na(+)-Ca2+ exchange current stimulation by MgATP: possible involvement of aminophospholipid translocase. J Physiol. 1992 Aug;454:59–82. doi: 10.1113/jphysiol.1992.sp019254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D. W. Extracellular calcium transients and action potential configuration changes related to post-stimulatory potentiation in rabbit atrium. J Gen Physiol. 1986 May;87(5):675–706. doi: 10.1085/jgp.87.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D. W. Giant excised cardiac sarcolemmal membrane patches: sodium and sodium-calcium exchange currents. Pflugers Arch. 1989 Nov;415(2):247–249. doi: 10.1007/BF00370601. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W., Noble D. Excitation-contraction coupling and extracellular calcium transients in rabbit atrium: reconstruction of basic cellular mechanisms. Proc R Soc Lond B Biol Sci. 1987 Mar 23;230(1259):163–205. doi: 10.1098/rspb.1987.0015. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W. Regulation and deregulation of cardiac Na(+)-Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature. 1990 Mar 15;344(6263):242–245. doi: 10.1038/344242a0. [DOI] [PubMed] [Google Scholar]
- Honig M. G., Hume R. I. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989 Sep;12(9):333-5, 340-1. [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Kim D. Beta-adrenergic regulation of the muscarinic-gated K+ channel via cyclic AMP-dependent protein kinase in atrial cells. Circ Res. 1990 Nov;67(5):1292–1298. doi: 10.1161/01.res.67.5.1292. [DOI] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Noma A., Irisawa H. Na-Ca exchange current in mammalian heart cells. Nature. 1986 Feb 13;319(6054):596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- Li Z., Nicoll D. A., Collins A., Hilgemann D. W., Filoteo A. G., Penniston J. T., Weiss J. N., Tomich J. M., Philipson K. D. Identification of a peptide inhibitor of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. J Biol Chem. 1991 Jan 15;266(2):1014–1020. [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Miura Y., Kimura J. Sodium-calcium exchange current. Dependence on internal Ca and Na and competitive binding of external Na and Ca. J Gen Physiol. 1989 Jun;93(6):1129–1145. doi: 10.1085/jgp.93.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby M. C., Green D. D., Russell K. L. Structural characterization of cardiac protein phosphatase with a monoclonal antibody. Evidence that the Mr = 38,000 phosphatase is the catalytic subunit of the native enzyme(s). J Biol Chem. 1985 Nov 5;260(25):13763–13770. [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. Voltage dependence of Na translocation by the Na/K pump. Nature. 1986 Oct 16;323(6089):628–630. doi: 10.1038/323628a0. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Blaustein M. P. Effects of ATP and vanadate on calcium efflux from barnacle muscle fibres. Nature. 1981 Jan 22;289(5795):314–316. doi: 10.1038/289314a0. [DOI] [PubMed] [Google Scholar]
- Requena J. Calcium efflux from squid axons under constant sodium electrochemical gradient. J Gen Physiol. 1978 Oct;72(4):443–470. doi: 10.1085/jgp.72.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z. F., Baumann O., Somlyo A. P. Axial resolution of confocal microscopes with parallel-beam detection. J Microsc. 1991 Oct;164(Pt 1):13–19. doi: 10.1111/j.1365-2818.1991.tb03187.x. [DOI] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R., Ward T. A., Wilson S. W. A new preparation for recording single-channel currents from skeletal muscle. Proc R Soc Lond B Biol Sci. 1984 Jun 22;221(1225):455–464. doi: 10.1098/rspb.1984.0044. [DOI] [PubMed] [Google Scholar]
- Terasaki M., Song J., Wong J. R., Weiss M. J., Chen L. B. Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell. 1984 Aug;38(1):101–108. doi: 10.1016/0092-8674(84)90530-0. [DOI] [PubMed] [Google Scholar]
- Valdiosera R., Clausen C., Eisenberg R. S. Impedance of frog skeletal muscle fibers in various solutions. J Gen Physiol. 1974 Apr;63(4):460–491. doi: 10.1085/jgp.63.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]