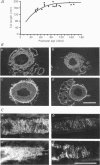
Abstract
1. Intracellular recordings from the smooth muscle of isolated segments of the main caudal artery of rats at various ages between 45 and 150 days postnatal were made in order to relate the spontaneous depolarizations and responses to perivascular stimulation at different levels along the artery to the differences in vessel structure and innervation density during growth of the animals. 2. In the outermost smooth muscle cells close to the neuromuscular junctions, spontaneous depolarizations with fast time courses (spontaneous excitatory junction potentials or SEJPs) were recorded. In cells lying deeper in the media, spontaneous depolarizations had a wide range of time courses and amplitudes, but only a few of those could be attributed to electrotonic attenuation of SEJPs. 3. In arterial segments taken from animals of all ages, stimuli which evoked maximal amplitude excitatory junction potentials (EJPs) 1-2 mm caudal to a suction electrode also evoked neurogenic alpha-depolarizations (NADs) with time to peak of 15 s and duration nearly 1 min. Both responses decreased progressively in amplitude along the length of the artery. NADs were blocked by phentolamine (10(-6) M) or idazoxan (10(-7) M) which were without effects on EJPs. 4. During short trains of stimuli (5 at 1 or 10 Hz), EJPs facilitated but to a lesser extent with distance along the tail. Such trains evoked NADs of greater amplitude than those following a single stimulus; these were often preceded by contractions of the artery which were restricted to the region close to the stimulating electrode. 5. Increasing stimulus voltage led to progressive prolongation of the decay phase of the EJP. After the addition of tetrodotoxin (10(-7) M), or in the presence of reduced Ca2+ and raised Mg2+ concentration, slow depolarizing potentials (SDPs) (with time to peak of 150-300 ms and decay lasting > 2 s) were recorded which were graded in amplitude with stimulus voltage. SDPs were attenuated by increasing Ca2+ concentration to 5 mM. These responses often added to the EJP at supramaximal stimulus voltages. 6. The mean amplitudes of the EJP and NAD declined significantly with age, the former to a greater degree than the latter. These changes may be explained by changes in the electrical properties of the media related to hypertrophy of smooth muscle cells as the animals grew, and emphasize the need to allow for such growth effects in studies of young rats.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF












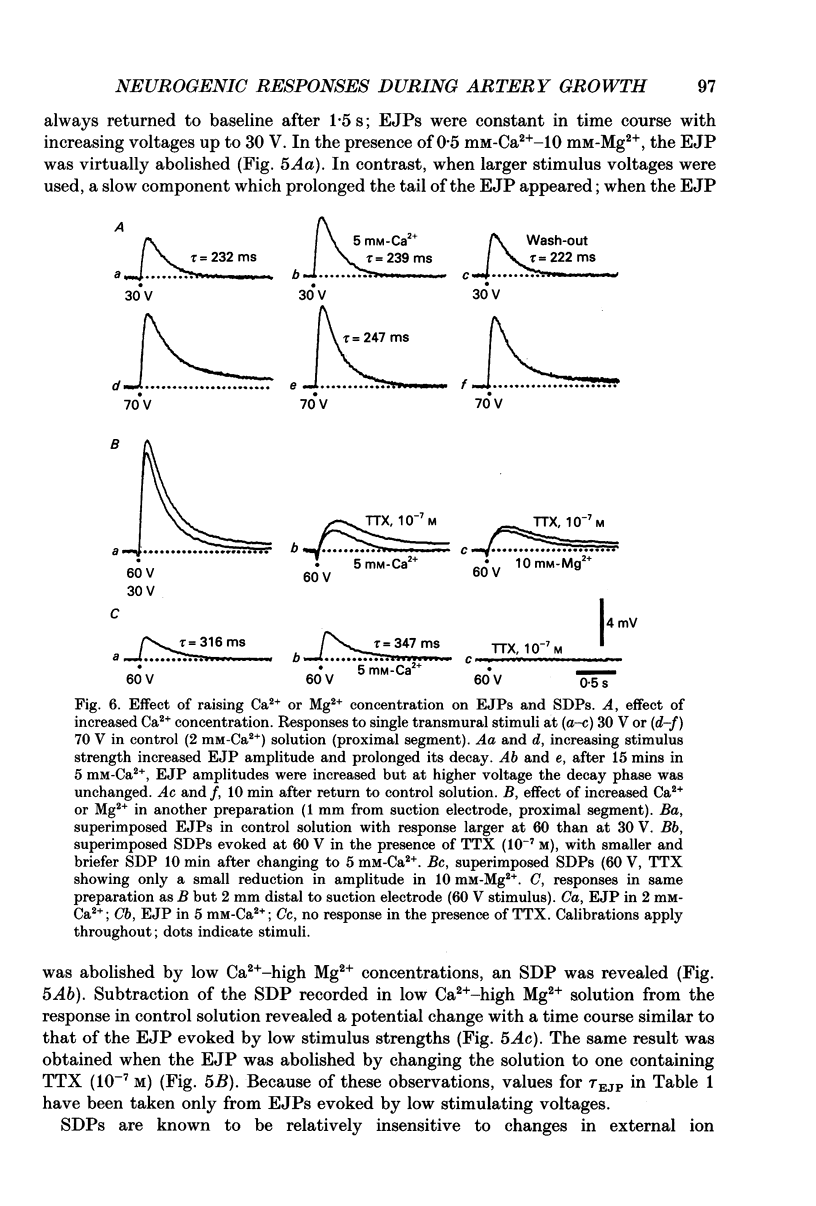








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., McLachlan E. M. The time course of the development of the sympathetic innervation of the vasculature of the rat tail. J Auton Nerv Syst. 1991 Aug;35(2):117–132. doi: 10.1016/0165-1838(91)90055-8. [DOI] [PubMed] [Google Scholar]
- Bao J. X., Eriksson I. E., Stjärne L. Age-related variations in the relative importance of noradrenaline and ATP as mediators of the contractile response of rat tail artery to sympathetic nerve stimulation. Acta Physiol Scand. 1989 Jun;136(2):287–288. doi: 10.1111/j.1748-1716.1989.tb08663.x. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Large W. A. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Q J Exp Physiol. 1986 Jan;71(1):1–28. doi: 10.1113/expphysiol.1986.sp002960. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. A dual function for adenosine 5'-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986 Mar;58(3):319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. The passive membrane properties and excitatory junction potentials of the guinea pig deferens. J Physiol. 1980 Mar;300:303–316. doi: 10.1113/jphysiol.1980.sp013163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell J. F., McLachlan E. M., Sittiracha T. The effect of temperature on neuromuscular transmission in the main caudal artery of the rat. J Physiol. 1988 Mar;397:31–49. doi: 10.1113/jphysiol.1988.sp016986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W. Neural regulation of electrical and mechanical activities in the rat tail artery. Pflugers Arch. 1984 Mar;400(3):335–337. doi: 10.1007/BF00581570. [DOI] [PubMed] [Google Scholar]
- Cheung D. W. Two components in the cellular response of rat tail arteries to nerve stimulation. J Physiol. 1982 Jul;328:461–468. doi: 10.1113/jphysiol.1982.sp014277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Torre J. C. An improved approach to histofluorescence using the SPG method for tissue monoamines. J Neurosci Methods. 1980 Oct;3(1):1–5. doi: 10.1016/0165-0270(80)90029-1. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Hirst G. D., Van Helden D. F. Some properties of excitatory junction currents recorded from submucosal arterioles of guinea-pig ileum. J Physiol. 1984 Jun;351:87–98. doi: 10.1113/jphysiol.1984.sp015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerová M., Gero J., Dolezel S., Konecný M. Postnatal development of sympathetic control in canine femoral artery. Physiol Bohemoslov. 1974;23(4):289–295. [PubMed] [Google Scholar]
- Hill C. E., Hirst G. D., Ngu M. C., van Helden D. F. Sympathetic postganglionic reinnervation of mesenteric arteries and enteric neurones of the ileum of the rat. J Auton Nerv Syst. 1985 Dec;14(4):317–334. doi: 10.1016/0165-1838(85)90079-7. [DOI] [PubMed] [Google Scholar]
- Hill C. E., Hirst G. D., van Helden D. F. Development of sympathetic innervation to proximal and distal arteries of the rat mesentery. J Physiol. 1983 May;338:129–147. doi: 10.1113/jphysiol.1983.sp014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kitamura K., Kuriyama H. Roles of extrajunctional receptors in the response of guinea-pig mesenteric and rat tail arteries to adrenergic nerves. J Physiol. 1983 Dec;345:409–422. doi: 10.1113/jphysiol.1983.sp014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling P., McLachlan E. M., Jänig W., Anderson C. R. Electrophysiological responses in the rat tail artery during reinnervation following lesions of the sympathetic supply. J Physiol. 1992 Aug;454:107–128. doi: 10.1113/jphysiol.1992.sp019256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef K. D., Kreulen D. L. Electrical responses of guinea pig coronary artery to transmural stimulation. Circ Res. 1988 Mar;62(3):585–595. doi: 10.1161/01.res.62.3.585. [DOI] [PubMed] [Google Scholar]
- Luff S. E., McLachlan E. M. Frequency of neuromuscular junctions on arteries of different dimensions in the rabbit, guinea pig and rat. Blood Vessels. 1989;26(2):95–106. doi: 10.1159/000158758. [DOI] [PubMed] [Google Scholar]
- Sittiracha T., McLachlan E. M., Bell C. The innervation of the caudal artery of the rat. Neuroscience. 1987 May;21(2):647–659. doi: 10.1016/0306-4522(87)90150-3. [DOI] [PubMed] [Google Scholar]
- Surprenant A., Neild T. O., Holman M. E. Membrane properties of rabbit basilar arteries and their responses to transmural stimulation. Pflugers Arch. 1987 Sep;410(1-2):92–101. doi: 10.1007/BF00581901. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Effects of endogenous and exogenous noradrenaline on the smooth muscle of guinea-pig mesenteric vein. J Physiol. 1981 Dec;321:495–512. doi: 10.1113/jphysiol.1981.sp013999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helden D. F. Spontaneous and noradrenaline-induced transient depolarizations in the smooth muscle of guinea-pig mesenteric vein. J Physiol. 1991 Jun;437:511–541. doi: 10.1113/jphysiol.1991.sp018609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Hotta K. Electrical responses of the smooth muscle of the guinea-pig cerebral artery to brief electrical stimulation. Jpn J Physiol. 1986;36(1):77–90. doi: 10.2170/jjphysiol.36.77. [DOI] [PubMed] [Google Scholar]