Abstract
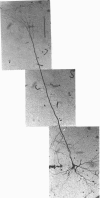
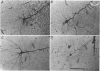
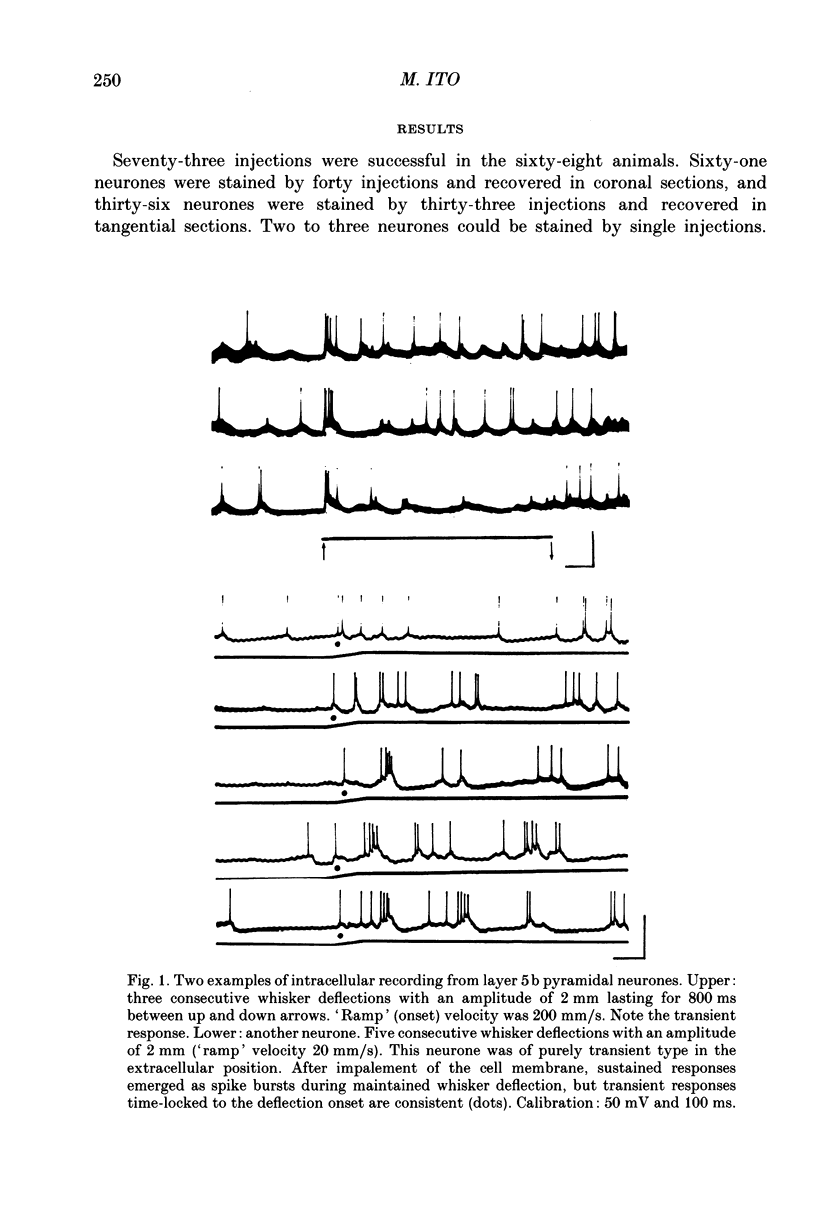
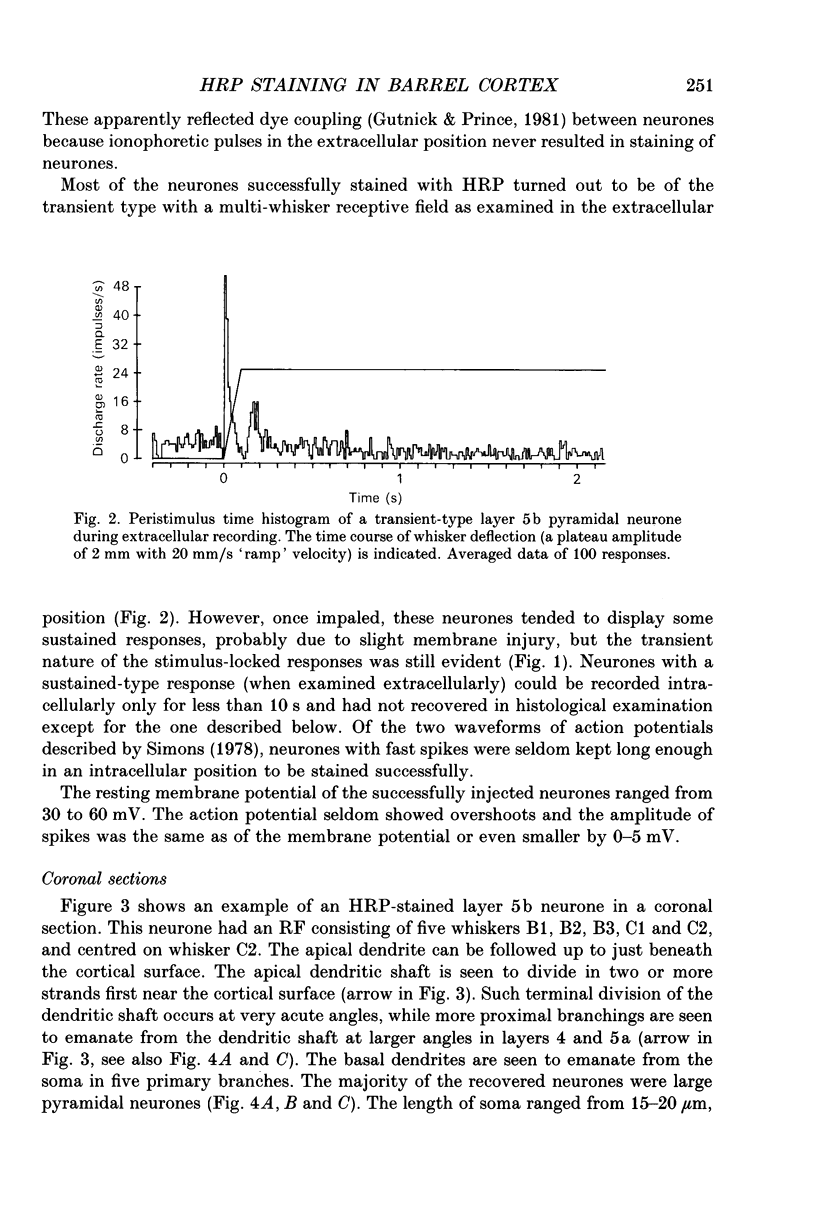
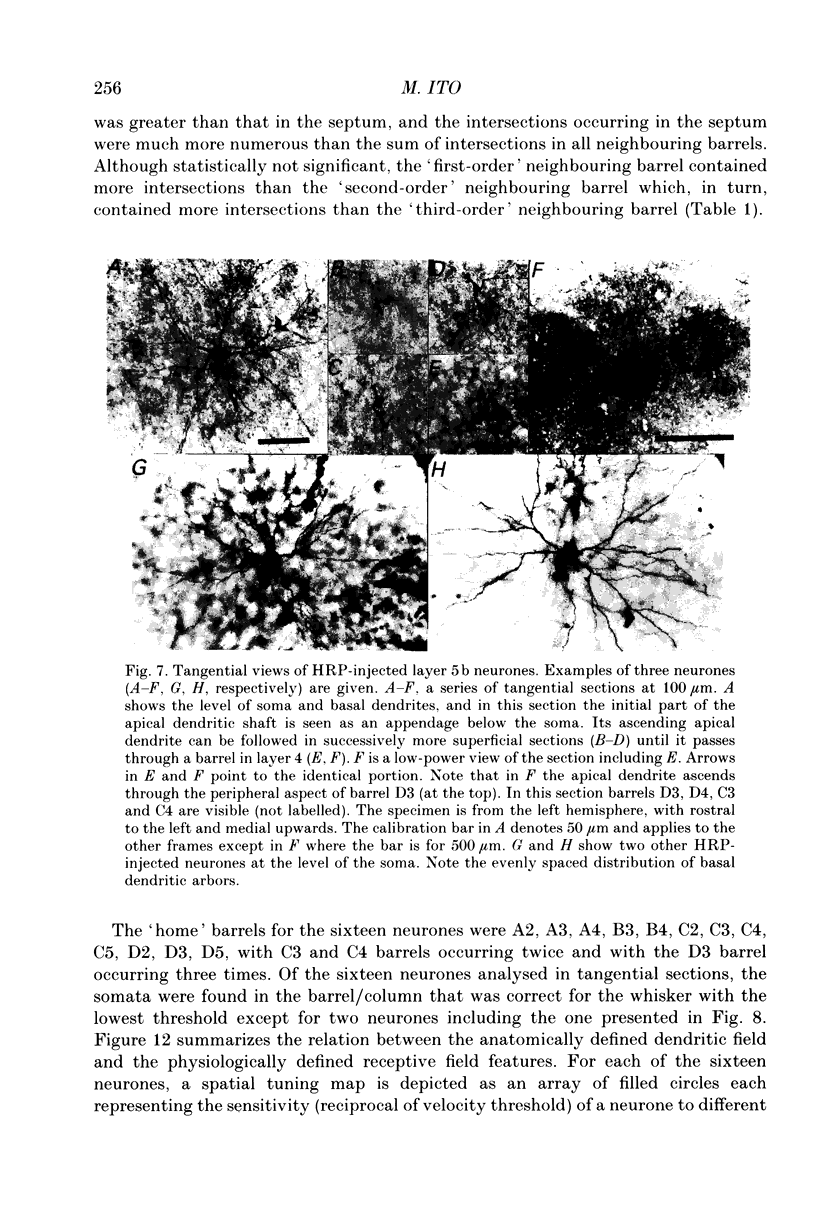
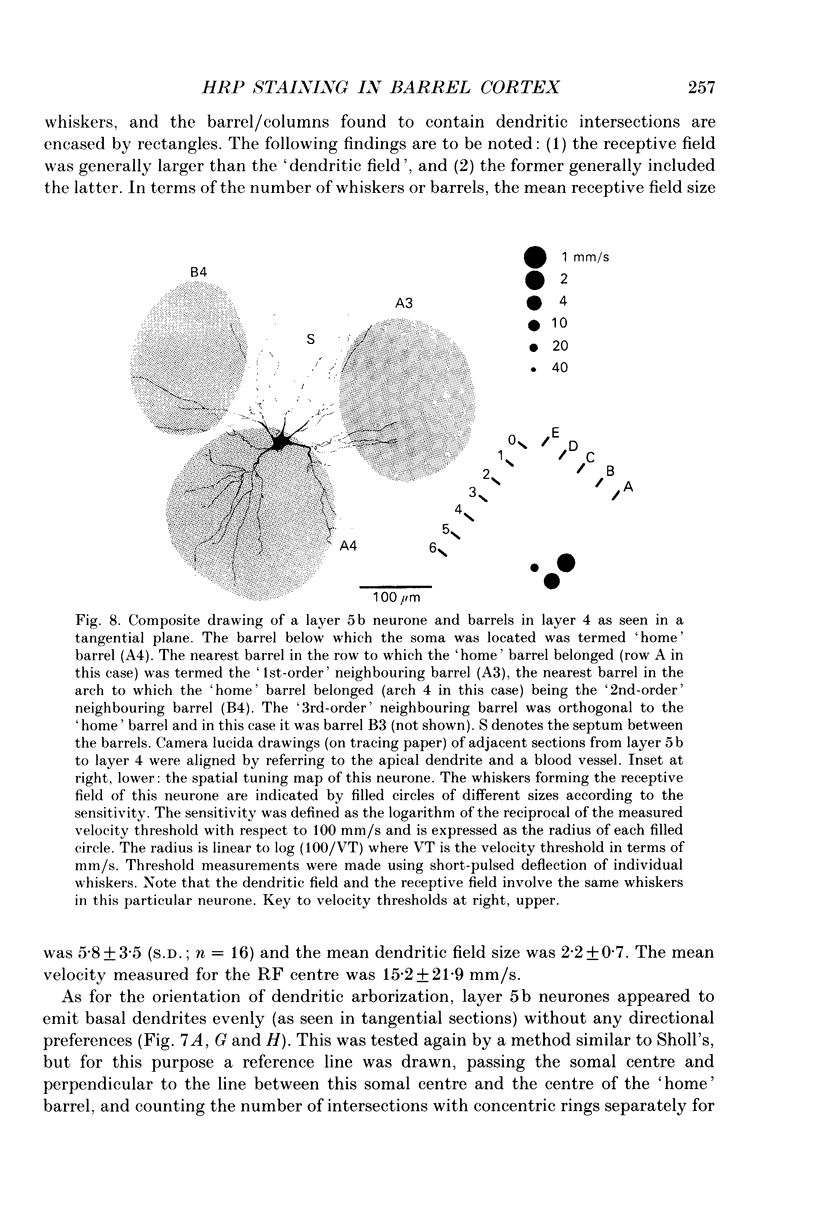
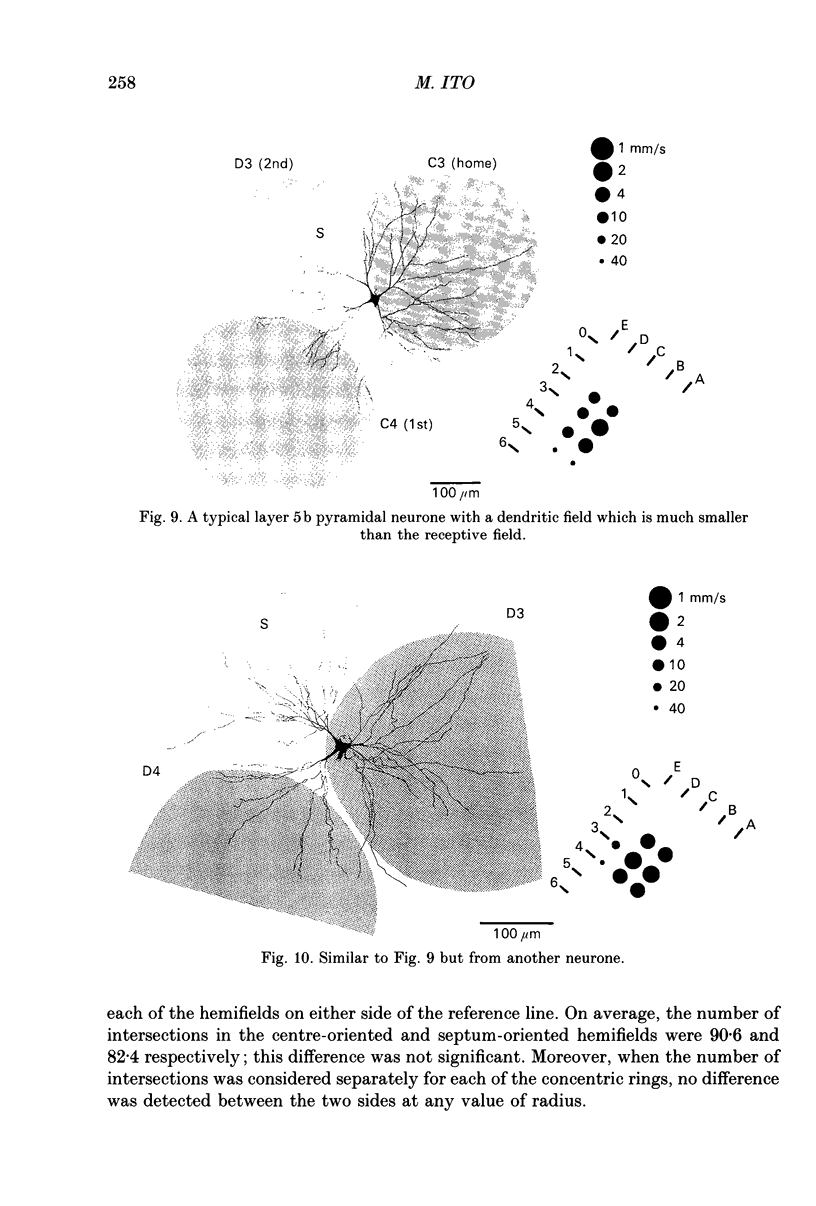
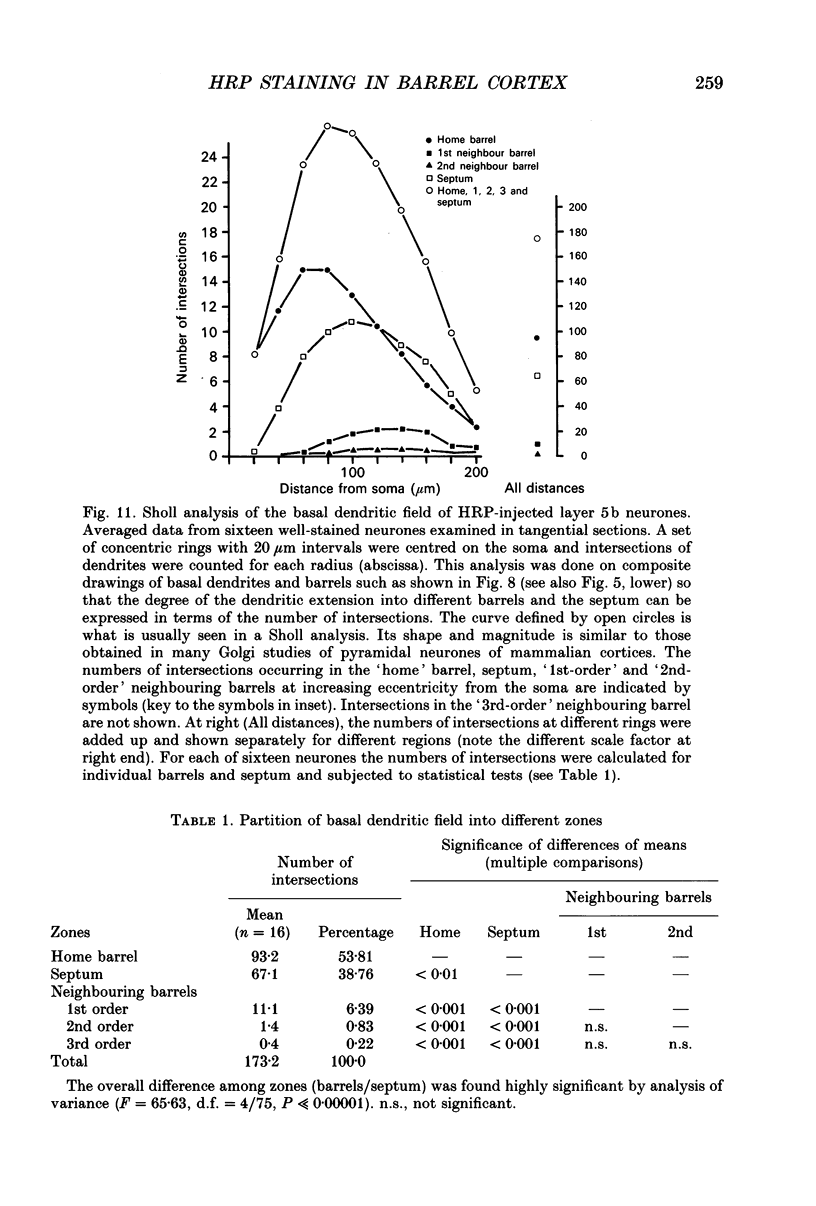
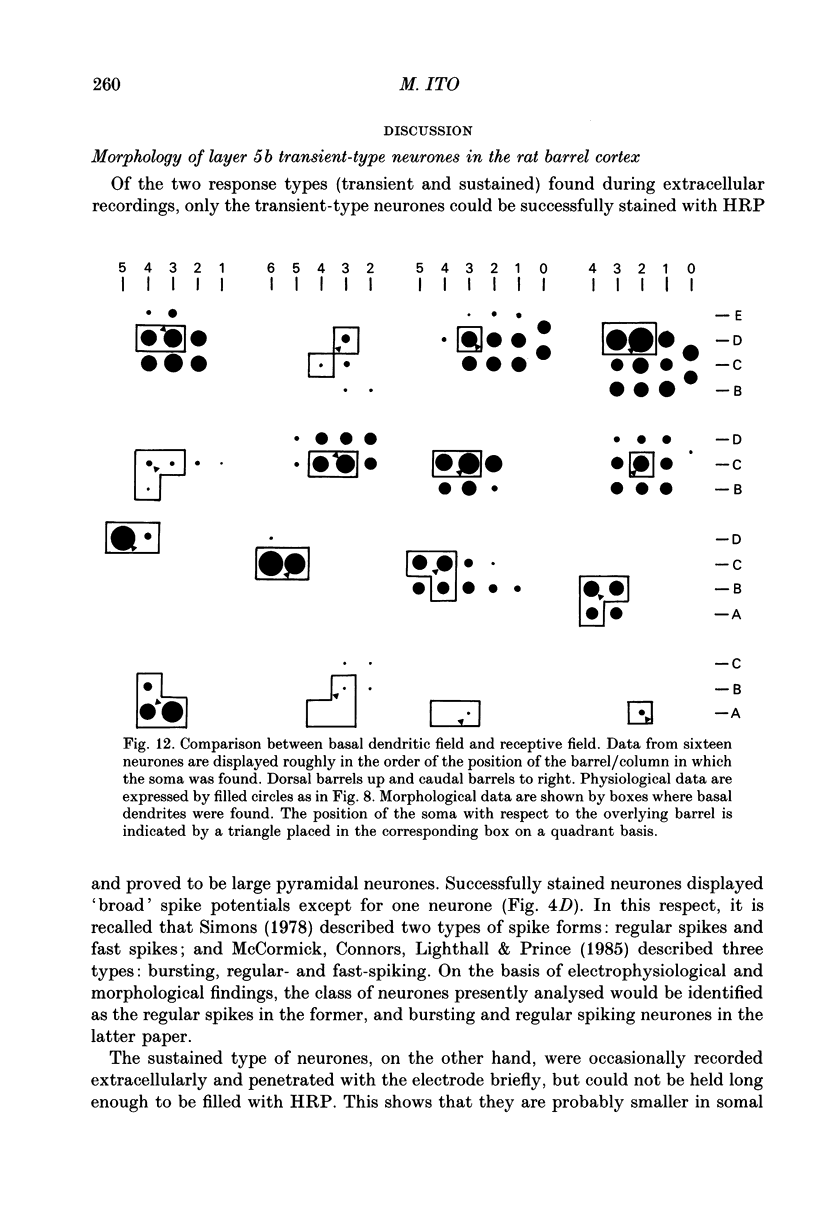
1. Using diaminobenzidine (DAB) as a chromagen, horseradish peroxidase-injected neurones and cytochrome oxidase-stained barrels were visualized simultaneously in the rat vibrissa cortex. Neurones were initially tested during extracellular recording for responses to whisker deflections. This was followed by intracellular injection of the soma with horseradish peroxidase (HRP) and histological processing to visualize the HRP-stained neurone in an incubation solution which contained, in addition to DAB, cytochrome C for cytochrome oxidase (CO) reaction of the barrels. 2. Recording and intracellular staining were made in layer 5b under urethane anaesthesia. CO-stained barrels were observed in layer 4. Physiologically and morphologically characterized neurones were mostly large pyramidal neurones that responded to more than one whisker and displayed transient-type responses. 3. In tangential sections, the apical dendrite of the HRP-filled neurone was followed from the soma level upward as it ascended through the barrelfield in layer 4. The cross-section of the apical dendrite was found in the periphery of the CO-stained barrel. Using the apical dendrite as a guide, the basal dendritic field of the layer 5b pyramidal neurone was aligned on the pattern of layer 4 barrels. The soma was seen to project basal dendrites in all directions, involving one or two neighbouring barrels/columns. 4. In sixteen neurones examined in tangential sections, a complete spatial tuning map constructed by measuring sensitivity of the neurone to different whiskers could be compared to the basal dendritic field in relation to the pattern of overlying layer 4 barrels. The mean receptive field size in terms of the number of effective whiskers was 5.8 whereas the mean dendritic field size in terms of the number of barrels/columns involved was 2.2. In addition to the well-documented role of intracortical connectivity in elaboration of multi-whisker receptor fields in the cortical neurones, the role played by direct inputs from multi-whisker thalamic ventrobasal neurones was discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981 Jun;29(6):775–775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Agmon A., Connors B. W. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41(2-3):365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Callahan C. A., Friedman M. A. Thalamo-cortical processing of vibrissal information in the rat. I. Intracortical origins of surround but not centre-receptive fields of layer IV neurones in the rat S1 barrel field cortex. J Comp Neurol. 1991 Jan 8;303(2):193–210. doi: 10.1002/cne.903030203. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Callahan C. A. Thalamo-cortical processing of vibrissal information in the rat. II. spatiotemporal convergence in the thalamic ventroposterior medial nucleus (VPm) and its relevance to generation of receptive fields of S1 cortical "barrel" neurones. J Comp Neurol. 1991 Jan 8;303(2):211–224. doi: 10.1002/cne.903030204. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Fox K. Spatiotemporal convergence and divergence in the rat S1 "barrel" cortex. J Comp Neurol. 1987 Sep 8;263(2):265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- Bernardo K. L., McCasland J. S., Woolsey T. A., Strominger R. N. Local intra- and interlaminar connections in mouse barrel cortex. J Comp Neurol. 1990 Jan 8;291(2):231–255. doi: 10.1002/cne.902910207. [DOI] [PubMed] [Google Scholar]
- Bernardo K. L., Woolsey T. A. Axonal trajectories between mouse somatosensory thalamus and cortex. J Comp Neurol. 1987 Apr 22;258(4):542–564. doi: 10.1002/cne.902580406. [DOI] [PubMed] [Google Scholar]
- Chapin J. K., Lin C. S. Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol. 1984 Oct 20;229(2):199–213. doi: 10.1002/cne.902290206. [DOI] [PubMed] [Google Scholar]
- Chmielowska J., Carvell G. E., Simons D. J. Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. J Comp Neurol. 1989 Jul 15;285(3):325–338. doi: 10.1002/cne.902850304. [DOI] [PubMed] [Google Scholar]
- Crandall J. E., Korde M., Caviness V. S., Jr Somata of layer V projection neurons in the mouse barrelfield cortex are in preferential register with the sides and septa of the barrels. Neurosci Lett. 1986 Jun 6;67(1):19–24. doi: 10.1016/0304-3940(86)90201-6. [DOI] [PubMed] [Google Scholar]
- Erzurumlu R. S., Jhaveri S., Benowitz L. I. Transient patterns of GAP-43 expression during the formation of barrels in the rat somatosensory cortex. J Comp Neurol. 1990 Feb 15;292(3):443–456. doi: 10.1002/cne.902920310. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Chang F. L. Dendritic pattern formation involves both oriented regression and oriented growth in the barrels of mouse somatosensory cortex. Brain Res. 1988 Sep 1;471(1):148–152. doi: 10.1016/0165-3806(88)90160-5. [DOI] [PubMed] [Google Scholar]
- Gutnick M. J., Prince D. A. Dye coupling and possible electrotonic coupling in the guinea pig neocortical slice. Science. 1981 Jan 2;211(4477):67–70. doi: 10.1126/science.7444449. [DOI] [PubMed] [Google Scholar]
- Ito M. Processing of vibrissa sensory information within the rat neocortex. J Neurophysiol. 1985 Sep;54(3):479–490. doi: 10.1152/jn.1985.54.3.479. [DOI] [PubMed] [Google Scholar]
- Ito M. Response properties and topography of vibrissa-sensitive VPM neurons in the rat. J Neurophysiol. 1988 Oct;60(4):1181–1197. doi: 10.1152/jn.1988.60.4.1181. [DOI] [PubMed] [Google Scholar]
- Ito M. Some quantitative aspects of vibrissa-driven neuronal responses in rat neocortex. J Neurophysiol. 1981 Oct;46(4):705–715. doi: 10.1152/jn.1981.46.4.705. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Killackey H. P. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. I. The normal morphology of specific thalamocortical afferents. J Neurosci. 1987 Nov;7(11):3529–3543. doi: 10.1523/JNEUROSCI.07-11-03529.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralek K. A., Jensen K. F., Killackey H. P. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res. 1988 Nov 1;463(2):346–351. doi: 10.1016/0006-8993(88)90408-8. [DOI] [PubMed] [Google Scholar]
- Kristt D. A., Waldman J. V. Late postnatal changes in rat somatosensory cortex. Temporal and spatial relationships of GABA-T and AChE histochemical reactivity. Anat Embryol (Berl) 1986;174(1):115–122. doi: 10.1007/BF00318343. [DOI] [PubMed] [Google Scholar]
- Landry P., Wilson C. J., Kitai S. T. Morphological and electrophysiological characteristics of pyramidal tract neurons in the rat. Exp Brain Res. 1984;57(1):177–190. doi: 10.1007/BF00231144. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Whitteridge D. Form, function and intracortical projections of spiny neurones in the striate visual cortex of the cat. J Physiol. 1984 Aug;353:463–504. doi: 10.1113/jphysiol.1984.sp015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985 Oct;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- SHOLL D. A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953 Oct;87(4):387–406. [PMC free article] [PubMed] [Google Scholar]
- Simons D. J., Carvell G. E. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol. 1989 Feb;61(2):311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Simons D. J. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978 May;41(3):798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Simons D. J. Temporal and spatial integration in the rat SI vibrissa cortex. J Neurophysiol. 1985 Sep;54(3):615–635. doi: 10.1152/jn.1985.54.3.615. [DOI] [PubMed] [Google Scholar]
- Simons D. J., Woolsey T. A. Morphology of Golgi-Cox-impregnated barrel neurons in rat SmI cortex. J Comp Neurol. 1984 Nov 20;230(1):119–132. doi: 10.1002/cne.902300111. [DOI] [PubMed] [Google Scholar]
- Steffen H., Van der Loos H. Early lesions of mouse vibrissal follicles:: their influence on dendrite orientation in the cortical barrelfield. Exp Brain Res. 1980;40(4):419–431. doi: 10.1007/BF00236150. [DOI] [PubMed] [Google Scholar]
- Sugitani M., Yano J., Sugai T., Ooyama H. Somatotopic organization and columnar structure of vibrissae representation in the rat ventrobasal complex. Exp Brain Res. 1990;81(2):346–352. doi: 10.1007/BF00228125. [DOI] [PubMed] [Google Scholar]
- Van der Loos H., Woolsey T. A. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973 Jan 26;179(4071):395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Waite P. M. Somatotopic organization of vibrissal responses in the ventro-basal complex of the rat thalamus. J Physiol. 1973 Jan;228(2):527–540. doi: 10.1113/jphysiol.1973.sp010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. L. Thalamocortical synaptic relations: a review with emphasis on the projections of specific thalamic nuclei to the primary sensory areas of the neocortex. Brain Res. 1979 Dec;180(3):275–311. doi: 10.1016/0165-0173(79)90008-0. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Woolsey T. A., Dierker M. L., Wann D. F. Mouse SmI cortex: qualitative and quantitative classification of golgi-impregnated barrel neurons. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2165–2169. doi: 10.1073/pnas.72.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey T. A., Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970 Jan 20;17(2):205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]