Abstract
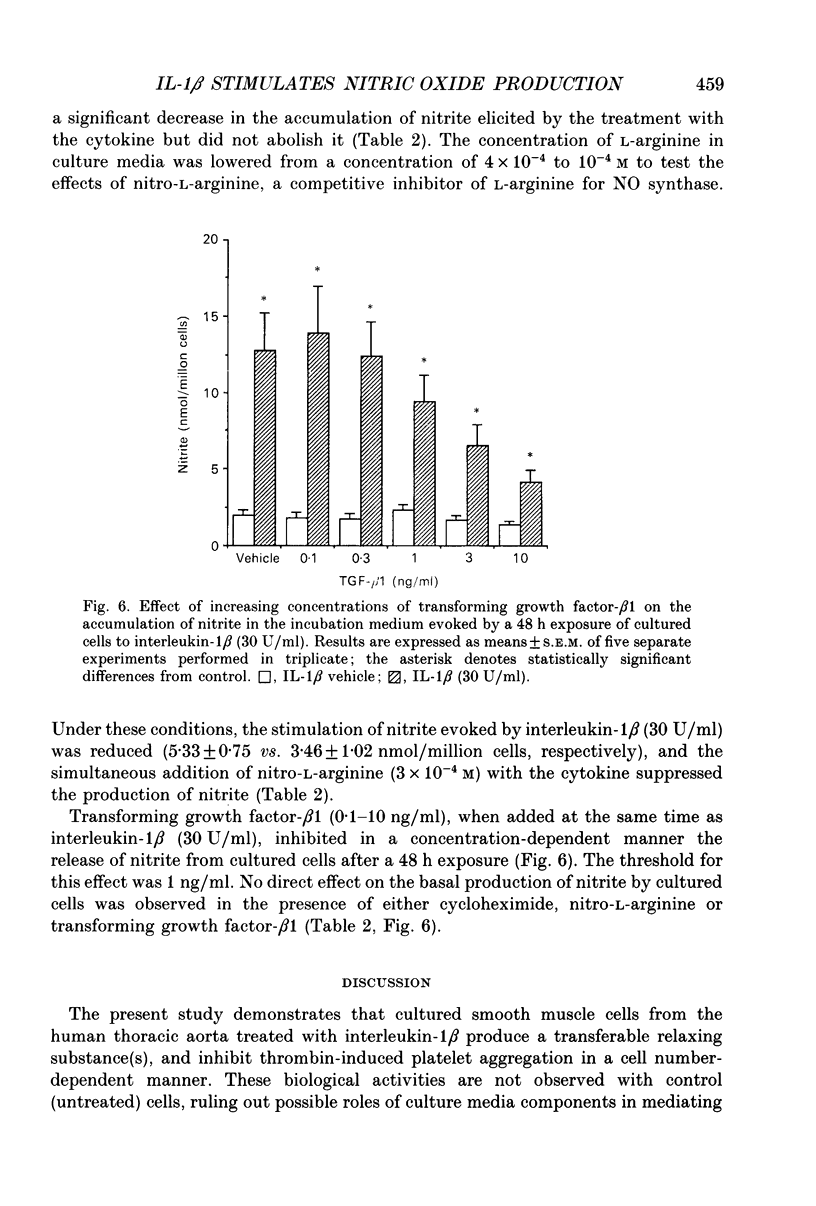
1. Experiments were performed to investigate the effects of human recombinant interleukin-1 beta on the production of vasoactive substances by human aortic smooth muscle cells in culture. Smooth muscle cells were cultured either on microcarrier beads for bioassay experiments, or in multiwell plates for the determination of nitrite levels. 2. Cells were grown on microcarrier beads, treated with interleukin-1 beta or vehicle (control) for 24 h, and packed in a column which was perfused with oxygenated Krebs-Ringer solution in the presence of indomethacin. The activity of the perfusates was bioassayed by measuring the changes in tension of a contracted ring of Wistar rat aorta without endothelium, and by evaluating the modulation of thrombin-induced platelet aggregation. 3. Perfusates from interleukin-1 beta treated cells evoked relaxations of the contracted detector tissues, and microcarrier beads covered with treated cells inhibited thrombin-induced platelet aggregation. Superoxide dismutase enhanced these effects whereas Methylene Blue abolished them. Control cells evoke neither relaxation nor inhibition of platelet aggregation. Interleukin-1 beta induced a time- and concentration-dependent production of nitrite. Cycloheximide and nitro-L-arginine inhibited the relaxations and the production of nitrite evoked by interleukin-1 beta-treated cells. L-Arginine but not D-arginine overcame the blockade elicited by nitro-L-arginine. Transforming growth factor-beta 1 reduced the interleukin-1 beta-dependent generation of nitrite by cultured smooth muscle cells and relaxation of contracted bioassay tissues. 4. Interleukin-1 beta, transforming growth factor-beta 1, Methylene Blue and L-arginine-related compounds did not induce significant variations of tension of the detector rings. 5. These data demonstrate that the inflammatory and immunological mediator interleukin-1 can stimulate the production of a nitric oxide-like substance(s) in cultured human smooth muscle cells leading to the activation of soluble guanylate cyclase. Liberation of transforming growth factor-beta by activated platelets may inhibit these reactions.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Assoian R. K., Sporn M. B. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986 Apr;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley D., Cohen R. A., Levinsky N. G. Interleukin 1 inhibits contraction of vascular smooth muscle. J Clin Invest. 1989 Jan;83(1):331–335. doi: 10.1172/JCI113879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley D., Schwartz J. H., Brenner B. M. Interleukin 1 induces prolonged L-arginine-dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest. 1991 Feb;87(2):602–608. doi: 10.1172/JCI115036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Lückhoff A., Bassenge E. Endothelium-derived relaxant factor inhibits platelet activation. Naunyn Schmiedebergs Arch Pharmacol. 1987 Nov;336(5):566–571. doi: 10.1007/BF00169315. [DOI] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Durante W., Schini V. B., Scott-Burden T., Junquero D. C., Kroll M. H., Vanhoutte P. M., Schafer A. I. Platelet inhibition by an L-arginine-derived substance released by IL-1 beta-treated vascular smooth muscle cells. Am J Physiol. 1991 Dec;261(6 Pt 2):H2024–H2030. doi: 10.1152/ajpheart.1991.261.6.H2024. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Pollock J. S., Schmidt H. H., Heller M., Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L. V., Majack R. A. Vascular smooth muscle cells express distinct transforming growth factor-beta receptor phenotypes as a function of cell density in culture. J Biol Chem. 1989 Mar 25;264(9):5241–5244. [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Green S. J., Mellouk S., Hoffman S. L., Meltzer M. S., Nacy C. A. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol Lett. 1990 Aug;25(1-3):15–19. doi: 10.1016/0165-2478(90)90083-3. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Groeneveld A. B., Nauta J. J., Thijs L. G. Peripheral vascular resistance in septic shock: its relation to outcome. Intensive Care Med. 1988;14(2):141–147. doi: 10.1007/BF00257468. [DOI] [PubMed] [Google Scholar]
- Kadota K., Yui Y., Hattori R., Uchizumi H., Kawai C. A new relaxing factor in supernatant of incubated rat peritoneal neutrophils. Am J Physiol. 1991 Mar;260(3 Pt 2):H967–H972. doi: 10.1152/ajpheart.1991.260.3.H967. [DOI] [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Majack R. A. Beta-type transforming growth factor specifies organizational behavior in vascular smooth muscle cell cultures. J Cell Biol. 1987 Jul;105(1):465–471. doi: 10.1083/jcb.105.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Marsden P. A., Ballermann B. J. Tumor necrosis factor alpha activates soluble guanylate cyclase in bovine glomerular mesangial cells via an L-arginine-dependent mechanism. J Exp Med. 1990 Dec 1;172(6):1843–1852. doi: 10.1084/jem.172.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McKenna T. M. Prolonged exposure of rat aorta to low levels of endotoxin in vitro results in impaired contractility. Association with vascular cytokine release. J Clin Invest. 1990 Jul;86(1):160–168. doi: 10.1172/JCI114679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Nelson B. J., Ralph P., Green S. J., Nacy C. A. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J Immunol. 1991 Mar 15;146(6):1849–1857. [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Pignat W., Leighton J., Märki F., Vosbeck K., Alkan S. Transforming growth factor beta 2 differentially modulates interleukin-1 beta- and tumour-necrosis-factor-alpha-stimulated phospholipase A2 and prostaglandin E2 synthesis in rat renal mesangial cells. Biochem J. 1990 Aug 15;270(1):269–271. doi: 10.1042/bj2700269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J., Vosbeck K. Transforming growth factor beta 2 inhibits interleukin 1 beta- and tumour necrosis factor alpha-induction of nitric oxide synthase in rat renal mesangial cells. Biochem Biophys Res Commun. 1991 Mar 15;175(2):372–379. doi: 10.1016/0006-291x(91)91574-v. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Lorenz R. R., Vanhoutte P. M. Bioassay of endothelium-derived relaxing factor(s): inactivation by catecholamines. Am J Physiol. 1985 Jul;249(1 Pt 2):H95–101. doi: 10.1152/ajpheart.1985.249.1.H95. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Junquero D. C., Scott-Burden T., Vanhoutte P. M. Interleukin-1 beta induces the production of an L-arginine-derived relaxing factor from cultured smooth muscle cells from rat aorta. Biochem Biophys Res Commun. 1991 Apr 15;176(1):114–121. doi: 10.1016/0006-291x(91)90897-g. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Vanhoutte P. M. L-arginine evokes both endothelium-dependent and -independent relaxations in L-arginine-depleted aortas of the rat. Circ Res. 1991 Jan;68(1):209–216. doi: 10.1161/01.res.68.1.209. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Baur U., Bürgin M., Bühler F. R. Epidermal growth factor responsiveness in smooth muscle cells from hypertensive and normotensive rats. Hypertension. 1989 Apr;13(4):295–304. doi: 10.1161/01.hyp.13.4.295. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Hahn A. W., Baur U., Box R. J., Bühler F. R. Induction of growth-related metabolism in human vascular smooth muscle cells by low density lipoprotein. J Biol Chem. 1989 Jul 25;264(21):12582–12589. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayeh M. A., Marletta M. A. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989 Nov 25;264(33):19654–19658. [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Walsh P. N., Mills D. C., White J. G. Metabolism and function of human platelets washed by albumin density gradient separation. Br J Haematol. 1977 Jun;36(2):287–296. doi: 10.1111/j.1365-2141.1977.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Wolin M. S., Cherry P. D., Rodenburg J. M., Messina E. J., Kaley G. Methylene blue inhibits vasodilation of skeletal muscle arterioles to acetylcholine and nitric oxide via the extracellular generation of superoxide anion. J Pharmacol Exp Ther. 1990 Sep;254(3):872–876. [PubMed] [Google Scholar]
- Wood K. S., Buga G. M., Byrns R. E., Ignarro L. J. Vascular smooth muscle-derived relaxing factor (MDRF) and its close similarity to nitric oxide. Biochem Biophys Res Commun. 1990 Jul 16;170(1):80–88. doi: 10.1016/0006-291x(90)91243-l. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]


