Abstract
Neutrophil extracellular traps (NETs) are intricate, web-like formations composed of DNA, histones, and antimicrobial proteins, released by neutrophils. These structures participate in a wide array of physiological and pathological activities, including immune rheumatic diseases and damage to target organs. Recently, the connection between NETs and cancer has garnered significant attention. Within the tumor microenvironment and metabolism, NETs exhibit multifaceted roles, such as promoting the proliferation and migration of tumor cells, influencing redox balance, triggering angiogenesis, and driving metabolic reprogramming. This review offers a comprehensive analysis of the link between NETs and tumor metabolism, emphasizing areas that remain underexplored. These include the interaction of NETs with tumor mitochondria, their effect on redox states within tumors, their involvement in metabolic reprogramming, and their contribution to angiogenesis in tumors. Such insights lay a theoretical foundation for a deeper understanding of the role of NETs in cancer development. Moreover, the review also delves into potential therapeutic strategies that target NETs and suggests future research directions, offering new perspectives on the treatment of cancer and other related diseases.
Keywords: Neutrophil Extracellular traps, Tumor metabolism, Mitochondria, Redox reaction, Metabolic reprogramming, Angiogenesis, ROS
Introduction
Neutrophils, being the most abundant type of white blood cells in the human body, play a central role in immune defense. They combat invading microorganisms through various strategies, including phagocytosis, granule secretion, and the formation of NETs. Although the discovery of NETs is relatively recent, their role in infectious diseases has been widely recognized and extensively studied. However, recent research has revealed that NETs also play a significant role in non-infectious inflammatory diseases, particularly in cancer [1–4].
On one hand, in terms of tumor development and progression, cytokines in the tumor microenvironment can stimulate neutrophils to produce NETs. The inflammatory factors released by NETs, in turn, promote tumor growth and metastasis, forming a complex positive feedback loop. Additionally, NETs not only participate in tumor growth and metastasis but also profoundly affect tumor metabolism. By interacting with mitochondria, NETs influence redox states and mediate metabolic reprogramming. Tumor metabolic reprogramming supports cancer cell survival, invasion, and drug resistance, making it an important target for cancer therapy.
Therefore, this review will systematically elucidate the specific mechanisms of NETs in tumor development and progression, with a focus on the interaction between NETs and tumor metabolism. We will explore how NETs affect tumor metabolism by influencing mitochondrial activity, redox states, metabolic reprogramming, and angiogenesis, providing a theoretical foundation for this understanding. Moreover, we will discuss potential therapeutic strategies targeting NETs and explore future research directions, aiming to provide new ideas and methods for the treatment of tumors and other diseases.
Neutrophil extracellular traps
Overview of NETs
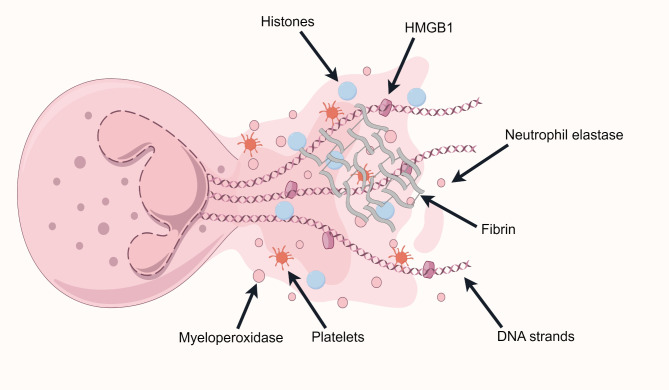
In 2004, NETs were first described as a novel immune defense mechanism. This pioneering work was published in the journal Science [5].NETs are extracellular fibrous structures composed of a DNA scaffold along with histones and neutrophil-derived granular enzymes, such as neutrophil elastase (NE) and myeloperoxidase (MPO), among other related proteins (Figure 1). The formation process of NETs, known as NETosis, can occur through various mechanisms, depending on the stimuli received by neutrophils. These stimuli typically include cytokines secreted by tumors, pathogenic infections, or drug-induced triggers. They activate neutrophils, causing them to undergo a series of stress responses, ultimately releasing large amounts of nuclear DNA or mitochondrial DNA (mtDNA) and various proteases, thereby forming NETs. Currently, three known mechanisms of NETs formation are Lytic NETs Formation, Viable NETs Formation, and Mitochondrial NETs Formation [6]. However, it is worth noting that existing studies primarily focus on Lytic NETs, and research on Viable NETs and Mitochondrial NETs is relatively limited.
Fig. 1.
Structure of neutrophil extracellular traps (NETs). Neutrophils release their decondensed chromatin along with the contents of their azurophilic granules, such as neutrophil elastase, myeloperoxidase, and high-mobility group box 1, into the extracellular space. NETs also have procoagulant properties, where activated platelets and fibrin can be found
Lytic NETs Formation is the earliest identified mechanism of NETs formation. Neutrophils undergoing Lytic NETs Formation exhibit chromatin decondensation, nuclear membrane dissolution, and mixing of nuclear and cytoplasmic contents. Eventually, the cell membrane ruptures, releasing NETs into the extracellular space. This classical mechanism specifically involves stimuli such as phorbol 12-myristate 13-acetate, lipopolysaccharides (LPS), or IL-8, which act on neutrophils and PKC-Raf/MERK/ERK signaling pathway. This activation leads to the activation of the NADPH oxidase (NOX) complex, producing reactive oxygen species (ROS), which in turn activate peptidylarginine deiminase 4 (PAD4). PAD4 converts arginine residues on histones to citrulline, promoting chromatin decondensation. Simultaneously, NE and MPO are activated and translocate to the nucleus, further facilitating chromatin decondensation. Eventually, the nuclear and granular membranes rupture, allowing the chromatin to mix with granular contents. The cell membrane then ruptures, releasing NETs and leading to the death of the neutrophil [7–10].Additionally, Gasdermin D (GSDMD) plays a crucial role in Lytic NETs Formation, and its activation also depends on ROS [11–13].Therefore, Lytic NETs Formation is highly dependent on the generation of ROS.
Neutrophils can also form NETs by releasing mtDNA. Under the induction of stimuli such as GM-CSF, LPS, or C5a, neutrophils rapidly (within about 30 min) release mtDNA, which co-localizes with granular proteins such as MPO and NE to form NETs. This process is known as Mitochondrial NETs Formation. The generation of ROS is crucial for this process [6, 14, 15].Mitochondrial NETs Formation occurs more rapidly and does not lead to cell death.In addition, neutrophils that remain alive and continue to perform functions such as phagocytosis after releasing NETs undergo a process known as Viable NETs Formation [16, 17].This mode of NETs formation is distinct from Lytic NETs Formation. It is a unique, ROS-independent mechanism of NETs release that plays an important role in host defense(Figure Figure 2, Table 1). [10, 18–20].
Fig. 2.
Mechanisms of neutrophil extracellular traps (NETs) formation. There are three known mechanisms of NETs formation: lytic NETs formation, viable NETs formation, and mitochondrial NETs formation. These mechanisms differ in terms of the inducing stimuli, dependence on ROS, the extent of PAD4 enzyme involvement, and the cellular state
Table 1.
Comparison of three mechanisms of NETs Generation
| Lytic NETs Formation | Viable NETs Formation | Mitochondrial NETs Formation | |
|---|---|---|---|
| Stimuli | PMA LPS IL-8 TNF-α | IL-6 IL-1β ATP GM-CSF | LPS C5a IL-5 PAF |
| PAD4 | PAD4-dependent | PAD4-dependent | PAD4-dependent |
| ROS | ROS-dependent | ROS-independent | ROS-dependent |
| Cell States | Neutrophil Death | Neutrophil Survival | Neutrophil Survival |
Does the production of different types of NETs also imply that they have different functional effects? In various disease contexts, NETs exhibit different trends, which may be due to differences in their types. Among the three mechanisms, except for Viable NETs Formation, ROS play a very crucial role in the formation of the other types of NETs. Therefore, future research can focus on ROS to explore its specific role in the formation and function of NETs, as well as its potential therapeutic applications in different pathological states.
Association of NETs with diseases
Initially, research on NETs primarily focused on their antimicrobial functions. However, it has since been discovered that NETs also play significant roles in various diseases (Table 2). In ischemia-reperfusion injury, the formation of NETs is closely associated with distal organ damage. This may be due to the high-ROS environment created by NETs formation, which leads to abnormal cell death, such as ferroptosis, and subsequently causes microcirculatory dysfunction [21–24].Therefore, clearing NETs or inhibiting their formation can alleviate distal organ damage. For instance, targeting the inhibition of NETs formation can improve Fundc1-dependent mitochondrial autophagy, which suppresses ferroptosis in normal cells, thereby improving microcirculatory dysfunction [2].NETs are also considered key factors in promoting inflammation and disease progression. In autoimmune diseases such as systemic lupus erythematosus, the excessive release of inflammatory mediators and autoantigens by NETs not only exacerbates the inflammatory response but also may trigger autoimmune reactions, creating a vicious cycle [3, 25, 26].Additionally, NETs play an important role in chronic inflammatory diseases such as chronic obstructive pulmonary disease and asthma. The accumulation of NETs-associated ROS and inflammatory mediators in the airways can exacerbate airway inflammation and damage normal airway epithelium, thereby promoting disease progression [4, 27, 28].
Table 2.
Association of NETs with diseases
| Types of Diseases | Names of Diseases | The Role of NETs |
|---|---|---|
| Autoimmune Diseases | SLE | Promotes the formation of immune complexes[25, 26, 232] |
| Rheumatoid Arthritis | Induces inflammation and joint damage [232, 233] | |
| Infectious Diseases | Bacterial Infections | Combating bacterial infections [5] |
| Cardiovascular Diseases | Stroke | Promotes inflammatory response and exacerbates brain damage[234, 235] |
| Chronic Inflammatory Diseases | Asthma and COPD | Induces airway inflammation and tissue damage[4, 27] |
| Tumor Diseases | Lung Cancer, Breast Cancer, Liver Cancer | Promotes tumorigenesis, metastasis, and drug resistance[31, 42, 43, 48, 49] |
In cancer, the multifaceted roles of NETs have garnered increasing attention. NETs act as accelerators in tumor progression by promoting tumor cell metastasis and creating a pro-inflammatory microenvironment [29–31]. As a key component of the immune response, NETs also play a crucial role in host defense against pathogen invasion and in the pathogenesis of various diseases. NETs can capture and kill invading microorganisms and are also involved in inflammatory responses, tissue damage, autoimmunity, and tumor development. The damaging effects of NETs on tissues and cells suggest potential links between NETs and various forms of cell death, such as ferroptosis, pyroptosis, and autophagy. This raises the question of whether NETs produced by neutrophils in response to microenvironmental stimuli might influence the death processes of surrounding cells, thereby forming a positive feedback loop or triggering other pathological effects.
Therefore, understanding the mechanisms of NETs formation and their roles in different diseases is of great significance for developing targeted therapeutic strategies against NETs. This could provide new treatment approaches for inflammatory diseases, autoimmune diseases, and cancer.
Impact of NETs on tumors
In recent years, neutrophils have garnered significant attention due to their complex roles in tumor development and progression. An elevated neutrophil-to-lymphocyte ratio is considered an important prognostic indicator for cancer patients, suggesting that neutrophils are not mere bystanders in tumor progression. It has gradually been unveiled that neutrophils have diverse functions and plasticity, highlighting that they should no longer be seen as functionally simplistic innate immune cells. Instead, neutrophils play a crucial role in shaping the tumor microenvironment and regulating tumor biology [29, 32–36].
Studies have indicated that NETs can inhibit tumor progression. In melanoma, head and neck squamous cell carcinoma, and ovarian cancer, NETs can inhibit tumor cell proliferation through adhesion or exert anti-tumor effects by inducing apoptosis or necrosis [37–39].Recent studies have discovered that in colorectal cancer (CRC) with PIK3CA mutations, NETs can be induced by a combination of chemotherapy drugs (glutaminase inhibitor CB-839 and 5-FU) and effectively kill these cancer cells [40]. Chemotherapy drugs lead to the recruitment of neutrophils and the formation of NETs, which then kill cancer cells by inducing apoptosis in tumor cells. Further molecular mechanism studies suggest that this effect is closely related to the production of ROS [41].Moreover, it is well known that Bacillus Calmette-Guérin (BCG) is crucial for the treatment of bladder cancer. BCG activates human bladder cancer cells, leading to increased production of NETs, which then promote the recruitment of T cells, monocytes, and macrophages, thereby exerting a cytotoxic effect on tumor cells [30].These studies suggest that NETs have a direct positive impact on tumor treatment.
While some studies suggest that NETs have a direct positive impact on tumor treatment, a larger body of literature supports the notion that NETs promote tumorigenesis [31, 42–49], invasion, metastasis [45, 48–62] and drug resistance (Figure 3) [53, 55, 63–65]. NETs attract cancer cells via the CCDC25 receptor, and the levels of NETs in the serum can predict liver metastasis in early-stage breast cancer patients [50]. Recent studies on chronic stress and tumor development have also confirmed the role of NETs. Specifically, chronic stress alters the lung microenvironment, promoting the formation of NETs and thereby facilitating metastasis [66].
Fig. 3.
Interactions between NETs and tumors. In the tumor microenvironment, neutrophils promote the migration and invasion of tumor cells by releasing factors such as elastase. Tumor cells, in turn, recruit more neutrophils by releasing G-CSF and CXCL and induce them to generate NETs. The NETs produced secrete molecules such as neutrophil elastase, myeloperoxidase, and high-mobility group box 1, which enhance EMT and activate pathways like TLR 4/9-COX 2, CCDC 25-ILK-b-parvin, and lncRNA MIR 503 HG-NLRP 3, promoting tumor metastasis, immune evasion, and primary tumor growth. During vascular metastasis, NETs form a mesh-like structure that encapsulates CTCs, protecting them from immune system recognition, while also activating platelets to adhere to the surface of CTCs, forming a protective layer and releasing factors that inhibit immune cell activity. This synergy enhances the survival of tumor cells in the bloodstream, facilitating their metastasis. Ultimately, CTCs migrate from the primary tumor microenvironment to new organs (such as the brain, lungs, liver, etc.), completing the entire process of tumor metastasis
Under what conditions do NETs exert tumor-suppressive effects, and when do they promote tumor development? Why do these two opposing phenomena occur? Specifically, NETs in the tumor microenvironment can both enhance immune cell cytotoxicity and inhibit immune cell function, even promoting their apoptosis. So, what factors determine the role of NETs in the tumor microenvironment? In most cases, elevated ROS can promote NETs formation, and these ROS can either kill tumor cells or protect them. Is this difference related to ROS levels, and what causes it? Therefore, exploring the role of NETs in the tumor microenvironment becomes particularly important. In fact, NETs and tumor metabolism share common regulatory factors, such as ROS and hypoxia-inducible factor (HIF-1), providing important clues for the interaction between NETs and tumor metabolism. Multiple studies have shown that NETs affect tumor metabolism, thereby influencing subsequent tumor development, which undoubtedly enhances the research value of NETs [1, 31, 49, 67–73]. Next, the impact of NETs on these aspects will be discussed separately.
Mitochondrial dynamics
Role of mitochondria in cancer cells
Tumor metabolism refers to the distinct metabolic characteristics exhibited by tumor cells during their processes of proliferation, growth, and invasion [74]. Compared to normal cells, tumor cells typically undergo metabolic reprogramming to meet their rapid growth and high energy demands, with one of the most prominent features being the Warburg effect [75, 76]. Even under aerobic conditions, tumor cells tend to produce energy through glycolysis rather than the more efficient oxidative phosphorylation. This effect allows tumor cells to use glucose as their primary energy source and produce large amounts of lactate, even in the presence of sufficient oxygen [77, 78].Additionally, tumor cells show a significantly increased dependence on other nutrients, such as glutamine and lipids. Through glutamine metabolism, tumor cells are able to maintain redox balance and meet biosynthetic demands [79, 80]. To support their rapid growth and proliferation, tumor cells also have a greatly increased need for lipids. Lipid metabolism not only provides the necessary raw materials for biosynthesis but also generates a large amount of ROS [81–84].
Mitochondria play a crucial role in the processes mentioned above [77, 78, 85–87]. Moreover, the structure and function of mitochondria in tumor cells may undergo changes, impacting cellular energy metabolism, apoptosis, and other physiological processes, thereby promoting tumor growth and metastasis [88–90]. Mitochondrial RNA methylation shapes the metabolic reprogramming of tumor cells, thereby promoting metastasis [91].Lastly, mitochondria are the primary sites of ROS production in the body. During the process of ATP generation, electrons can leak from the electron transport chain and combine with oxygen to form superoxide anions (O2-), which subsequently generate ROS. In tumor progression, moderate levels of ROS can aid tumor growth by activating various signaling pathways related to cell proliferation, survival, and metastasis, such as MAPK and PI3K/Akt [92–95].In advanced triple-negative breast cancer (TNBC), MYC and MCL1 are often co-amplified and synergistically contribute to ROS production, maintaining chemoresistant cancer stem cells. They highlight the critical role of mitochondria and ROS in the drug resistance of TNBC [96].
Overall, mitochondria are pivotal in the development and progression of tumors, with their dysfunction being closely linked to the metabolic reprogramming and redox imbalance observed in cancer cells. Disrupting mitochondrial function in tumors could potentially alter tumor metabolism in various ways, thereby providing new avenues for cancer treatment. To explore this possibility, several key questions need to be addressed: How can we precisely modulate mitochondrial function? What are the broader implications of impairing mitochondrial function on other facets of tumor metabolism? Addressing these questions could lead to novel strategies in cancer therapy.
NETs and tumor mitochondria
There are multiple factors, mechanisms, and pathways that can stimulate the formation of NETs. Apart from Viable NETs Formation and immune complex-induced NETs release, which are independent of ROS generation, other NETs formation processes typically rely on ROS [97]. NETosis can also be induced by stimuli such as calcium ionophores, GM-CSF, TNFα, or IL-1β, under conditions that do not significantly affect ROS production [98].However, overall, the formation of most NETs, whether through Lytic NETs Formation or Mitochondrial NETs Formation, involves the production and elevation of ROS. ROS are directly related to the formation and function of NETs in most cases. As one of the primary sites of ROS production in the body, mitochondria are undoubtedly closely linked to NETs. Although research in this area is still limited, there is growing interest in the interactions between NETs and mitochondria [99–101].
Studies have found that neutrophils in patients with hepatocellular carcinoma (HCC) contain high levels of mitochondrial ROS and form NETs rich in oxidized mtDNA through mitochondrial adhesion. These NETs play a significant role in promoting inflammation and metastasis [102]. SIRT1 can open the neutrophil mitochondrial permeability transition pore to release mtDNA, inducing Mitochondrial NETs Formation. Therapeutic intervention targeting this pathway can effectively reduce lung metastasis in breast cancer [103]. Conversely, the impact of NETs on cells in the tumor microenvironment, including tumor cell mitochondria, has also been studied. Some literature indicates that NETs upregulate genes related to mitochondrial biogenesis in tumor cells, increase mitochondrial density and ATP production, enhance the percentage of cancer cells with reduced mitochondrial membrane potential, and increase oxygen consumption rates. Mechanistically, NE and HMGB1 released from NETs can bind to TLR-4 and TLR9, respectively, activating tumor cell proliferation, increasing mitochondrial biogenesis, and promoting the release of cytokines such as IL-6 and IL-8. This also leads to more NETs release by neutrophils, forming a positive feedback loop that further enhances mitochondrial function and directly stimulates cancer cell proliferation [48, 104].
In summary, there may be interactions between NETs and mitochondria that play an important role in tumor progression. Mitochondria-generated ROS are crucial in the formation of NETs and can also feedback to influence mitochondrial function. It is reasonable to hypothesize that the interaction between ROS formation and NETs contributes to their dual effects. When NETs excessively impact tumor mitochondria, they may cause hyperactivity in mitochondrial function, thereby promoting tumor progression. However, in the early stages of tumors, when mitochondria have not yet produced large amounts of ROS and the tumor has not secreted many factors, the small amount of NETs formed may exhibit tumor-suppressive functions in the tumor microenvironment.
Understanding the specific mechanisms of NETosis and its interactions with other factors in the tumor microenvironment will help answer this question. Additionally, NETs can enrich mtDNA, and the oxidation state of mtDNA is closely related to NETs formation, but what are the specific regulatory mechanisms? Only by clarifying these mechanisms can targeting the content and oxidation state of mtDNA in the NETs environment become a potential strategy for treating cancer and related inflammation. Precisely interfering with these pathological mitochondrial processes could bring new breakthroughs in cancer treatment.
Redox reactions
The dual role of redox reactions in cancer
Under normal physiological conditions, redox reactions are the basis for maintaining normal physiological and metabolic functions of organisms. However, if oxidative stress occurs, the redox balance within cells may be disturbed, leading to the excessive production of ROS. Therefore, maintaining redox balance is crucial for cell growth, differentiation, and even cell death [105–107].In cancer, redox reactions exhibit a complex dual role [106]. On one hand, excessive free radicals and ROS accumulate in the body, causing damage to cellular structures, including proteins, lipids, and nucleic acids, leading to gene mutations that promote the formation and proliferation of cancer cells [107–109]. Additionally, oxidative stress can activate various signal transduction pathways, such as NF-kB and MAPK, which support the survival and proliferation of cancer cells [59, 83, 84, 108–110].Recent studies have found that when TNBC cells are co-cultured with macrophages, the ROS levels in some TNBC cells significantly increase, enhancing their metastatic ability by upregulating IL1α expression. Therefore, preventing ROS elevation can serve as a new strategy to reduce TNBC metastasis [83]. Moreover, peroxidation is associated with epithelial-mesenchymal transition (EMT), which affects cell migration and metastasis [109, 111–115].Recent research demonstrated that in clear cell renal cell carcinoma, eliminating ROS reduces the migration and invasion capabilities of clear cell renal cell carcinoma cells and inhibits EMT, thereby suppressing tumor progression [113].
On the other hand, high levels of oxidative stress may surpass the antioxidant defenses of cancer cells, leading to cell death, such as apoptosis or necrosis [108, 109]. The prostate cancer susceptibility gene LanCL1 can protect tumor cells from oxidative stress damage, ultimately leading to tumor progression [108]. Certain anticancer treatment strategies aim to kill cancer cells by increasing oxidative stress levels within the cells [107, 116].Recent perspectives have highlighted that ascorbate can make pancreatic cancer cells more susceptible to killing via oxidative stress mechanisms, thereby enhancing their sensitivity to radiotherapy [116]. The authors suggest that the cytotoxic effect may be related to the regulation of glutathione peroxidase 4 activity. This implies that by modulating specific redox-sensitive molecules, such as glutathione and thioredoxin, the redox state of cells can be adjusted, offering new strategies for cancer treatment. An important related concept is “ferroptosis,” in which redox-regulating molecules like glutathione and thioredoxin play key roles. Their expression levels and activities directly affect the catalytic activity of iron ions and the degree of lipid peroxidation, thus regulating the occurrence of ferroptosis. ROS are deeply connected to cell death and ferroptosis. Consequently, NETs, which are closely related to ROS, might also have a significant impact on the redox balance and cell death processes in tumors. Thoroughly analyzing the interactions and regulatory mechanisms of these cell death modes will not only help elucidate cellular biological processes but also provide a theoretical basis for optimizing cancer treatment strategies [117–119].
NETs and redox reactions in tumors
It is noteworthy that NETs and ROS are intricately linked [1, 6, 54, 120, 121].Major components of NETs, such as MPO, play a crucial role in the generation of ROS. In the KEGG database, several genes closely related to NETs formation are also associated with ROS production, such as NCF1 and RAC1. The activity of these genes is critical in regulating ROS generation and intracellular oxidative stress responses, and they also influence the formation of NETs.
Tumor-derived substances such as IL-8 and HMGB1 in the tumor microenvironment may trigger oxidative stress reactions, thereby promoting the release of NETs, a process closely related to tumor progression [59, 84, 122–124].Additionally, research has found that HCC reduces the secretion of histidine-rich glycoprotein, leading to the activation of PI3K and NF-κB, the secretion of IL-8, the recruitment of neutrophils, and the production of ROS, ultimately promoting the formation of NETs [59].This process can be inhibited by antioxidants, such as free thiol-containing agents, which can prevent NETosis. As the largest thiol pool in the human plasma, albumin determines the redox state of plasma. The oxidation of albumin-derived free thiols is sufficient to cause the accumulation of ROS in neutrophils, leading to an imbalance in plasma redox state, resulting in non-inflammatory NETosis, and promoting lung metastasis. These results suggest that the redox balance of plasma can trigger NETosis and tumor lung metastasis, providing new therapeutic and diagnostic opportunities to combat cancer progression [45].
Similarly, the formation of NETs influences tumor behavior by affecting ROS levels. In recent years, the interplay between microorganisms, such as bacteria, and tumors has received growing interest. Elevated NETs and ROS within tumors can diminish the effectiveness of antibacterial therapies. To address this, scientists have developed a neutrophil-loaded nanoparticle known as SPPS, which reduces ROS levels indirectly by reprogramming NETosis, thereby enhancing the efficacy of bacterial treatments. This study offers valuable insights into how antibacterial therapies can be leveraged for cancer treatment and highlights the significant role NETs play in oxidative stress [84].
So, does the increase in NETs-related ROS have antitumor effects? On one hand, NETs-related ROS may have antitumor activity [41]. Other studies also imply that the cytotoxicity of ROS itself may inhibit tumor growth [30, 37–39, 41]. On the other hand, given the close relationship between NETs and mitochondria and ROS, as well as the potential link between NETs and cell death pathways, NETs might promote tumors in redox reactions. The ferroptosis pathway, which is closely related to ROS, seems to be a good entry point. Studies have found that increased NETs formation in TNBC tissues is significantly associated with tumor size, Ki67 levels, and lymph node metastasis. Inhibiting NETs can suppress TNBC tumor growth and lung metastasis, and the mechanism is that NETs inhibit the phosphorylation of the tumor suppressor Merlin, leading to TNBC cells resisting ferroptosis [125].Therefore, future research focusing on the interaction between NETs and ferroptosis, as well as the role of redox reactions and mitochondria in tumors, is expected to provide new insights for developing novel cancer treatment strategies.
Metabolic reprogramming
The metabolic reprogramming of tumor cells endows cancer cells with a unique ability to survive and proliferate in the nutrient- and oxygen-limited tumor microenvironment. This reprogramming involves fundamental changes in energy production and utilization pathways, enabling cancer cells to preferentially utilize specific metabolic pathways to meet their rapid proliferation needs. The Warburg effect is the most representative example of this phenomenon. Tumor mitochondria play a central role in tumor metabolic reprogramming. By regulating the balance between oxidative phosphorylation, glycolysis, and ROS levels, mitochondria reshape energy production pathways and redox balance, allowing tumor cells to survive and thrive in harsh growth conditions [77, 78].
NETs and metabolic reprogramming
The formation of NETs is closely related to metabolic reprogramming. Both NOX-dependent and NOX-independent NETosis induction can lead to increased extracellular acidification rate, enhanced lactate dehydrogenase activity, PKM2 dimerization, and reduced pyruvate kinase M2 activity, thereby promoting lactate production. This suggests the occurrence of the Warburg effect, leading to metabolic reprogramming. Additionally, the study also demonstrated that exogenous lactate treatment can induce NETs formation [126].As a complex composed of various proteases (NE, MPO, HMGB1, etc.) and DNA, NETs and their associated components have been shown to significantly impact tumor metabolic reprogramming. The following sections will provide a detailed explanation of several key components of NETs:
Serine proteases
Serine proteases are involved in critical processes such as blood coagulation, immune response, cell signaling, and development in the body. PI3K/Akt/mTOR pathways and HIF-1 are central regulators of angiogenesis, glycolysis, cancer metabolism, and cancer cell proliferation, which are essential processes for regulating the Warburg effect [127–131].Serine proteases (such as NE and PR3) can promote glycolytic metabolism in cancer cells by activating pathways such as HIF-1α or PI3K [132, 133]. NE can degrade insulin receptor substrate-1, enhancing the interaction between PI3K and the potent mitogen platelet-derived growth factor receptor, thereby biasing the PI3K axis towards tumor cell proliferation [42]. NE released from NETs activates TLR4 on cancer cells, leading to increased expression of proteins related to cell division and fusion, as well as proteins associated with mitophagy. These changes collectively promote the metabolic reprogramming of cancer cells, providing additional energy to support their accelerated growth [48]. Therefore, we speculate that NE within NETs may indirectly promote tumor metabolic reprogramming through similar mechanisms, thereby supporting rapid tumor growth.
High-mobility group box 1 (HMGB1)
During the formation of NETs, HMGB1 is released from the nucleus of neutrophils, participating in DNA decondensation and chromatin extrusion. HMGB1 not only structurally assists in forming the network framework of NETs but also enhances the inflammatory response of NETs through interactions with other molecules. In recent years, HMGB1 has been shown to promote tumor cell metastasis and progression through processes like EMT and angiogenesis [134]. It has been shown that HMGB1 secreted by breast cancer cells can activate fibroblasts via the RAGE/aerobic glycolysis pathway, and the activated fibroblasts, in turn, promote breast cancer cell metastasis by increasing lactate production [135, 136]. In gastric cancer cells, HMGB1 promotes mitochondrial fission and autophagy through the RAGE-mediated signaling pathway, further driving chemotherapy resistance and tumor growth [137]. Therefore, HMGB1 is a potential target for tumor metabolic reprogramming.
MPO
Hypochlorous acid and ROS produced by MPO can alter the redox state in the tumor microenvironment, which affects the metabolic pathways of tumor cells, thereby promoting metabolic reprogramming. Specifically, this can cause lipid peroxidation, which triggers cell death pathways such as ferroptosis. This is not only toxic to tumor cells but also suppresses anti-tumor immune responses by reducing the effector functions of immune cells, such as CD8 + T cells, indirectly promoting the growth and survival of tumor cells [138–141]. Given that MPO is a key component of NETs, it is reasonable to hypothesize that MPO released from NETs could similarly modulate the redox environment and metabolic pathways within the tumor microenvironment, thereby influencing tumor growth and immune evasion.
Therefore, the release of NETs can create a pro-inflammatory tumor microenvironment, indirectly promoting the Warburg effect in cancer cells by activating signaling pathways such as NF-κB [142, 143].Additionally, while NETs themselves do not directly cause hypoxia, the inflammatory response and vascular damage they induce may exacerbate hypoxia in the tumor microenvironment, further promoting glycolytic metabolism [144].Although the role of NETs in metabolic reprogramming has been widely recognized, and numerous studies have focused on the independent roles of components such as HMGB1, MPO, and NE in tumor metabolic reprogramming, it is worth investigating whether these components have the same effects through their interactions and combined actions in the context of NETs.
NETs and lipid metabolic changes
Cancer cell metabolic reprogramming extends beyond changes in glycolytic pathways, encompassing significant alterations in the metabolic processing of fatty acids and amino acids as well. These metabolic shifts contribute to enhanced protein synthesis and structural modifications within cells, both of which are essential for sustaining cancer cell proliferation and metastasis [145–148]. Components of NETs, including histones, MPO, and ROS, have been associated with lipid peroxidation, suggesting that NETs might influence lipid metabolism and thereby impact tumor behavior. It has been shown that the mitochondrial protein GCN5L1 can exacerbate liver inflammation and oxidative stress by promoting NETs formation. Given that lipid metabolic dysregulation is a hallmark of non-alcoholic steatohepatitis, this indicates that NETs might play an indirect role in lipid metabolism regulation through their effects on liver inflammation and oxidative stress [149]. Furthermore, it has been demonstrated that a small poly-anion (SPA) capable of neutralizing the harmful effects of NETs on lipid bilayers has been developed [150]. Since lipid metabolism is closely linked to cell death mechanisms such as ferroptosis, it is plausible that NETs formation may interfere with cell death pathways, including ferroptosis and apoptosis.
In summary, whether it is the generation of ROS, glycolysis, or changes in lipid metabolism, these are key aspects of cellular metabolism. Although the specific mechanisms of NETs in these processes have not been fully elucidated, their effect on promoting tumor metabolic reprogramming is clear. The close relationship between substances such as MPO and HMGB1 with mitochondria and ROS not only indicates that elevated ROS and mitochondrial changes may be one of the initiating factors of tumor metabolic reprogramming but also underscores the significance of NETs in various aspects of tumor metabolism. This further illustrates the correlation between ROS and NETs. This suggests that attempts to improve tumor metabolism through stabilizing mitochondria or regulating ROS could be explored. Of course, while the role of NETs in metabolic reprogramming has been widely recognized and numerous studies have focused on the independent roles of components such as HMGB1, MPO, and NE in tumor metabolic reprogramming, it is worth investigating whether these components exert similar effects through their interactions within the context of NETs. This could reveal synergistic mechanisms by which NETs influence tumor metabolism and progression. Whether NETs are a cause or consequence in these processes will determine if tumor metabolism can be directly modulated through NETs, which is a direction for future research. These issues underscore the need for further exploration of the role of NETs in tumor metabolism. Additionally, although direct evidence linking NETs to lipid metabolic changes in cancer is limited, the association between NET components and lipid peroxidation supports the hypothesis that NETs may modulate lipid metabolism in the tumor microenvironment. Therefore, based on existing research, integrating studies on the association of NETs components with tumor metabolic reprogramming has important scientific and clinical significance.
Tumor angiogenesis
In cancer, angiogenesis is a critical step for tumor growth and metastasis. Therefore, inhibiting angiogenesis has become an important strategy for cancer treatment [151–154]. Evidence suggests that hypoxia induces the overexpression of diacylglycerol kinase γ (DGKG) in tumor endothelial cells, which then activates the ZEB2/TGF-β1 axis, promoting angiogenesis and immune suppression in HCC. Targeting DGKG can enhance the efficacy of dual blockade of PD-1 and VEGFR-2 therapies [154].Therefore, besides direct tumor-killing strategies, attempting to inhibit tumor growth by targeting tumor angiogenesis might be a more moderate, effective, and less resistance-prone approach. Additionally, ROS can promote tumor angiogenesis, providing sufficient oxygen and nutrients to the tumor, thereby facilitating its growth and metastasis [155–159].
NETs and tumor angiogenesis
Beyond their direct vascular regulation, NETs play a critical role in tumor metastasis through their interaction with platelets in the bloodstream and protection of circulating tumor cells (CTCs), thereby creating a more favorable microenvironment for tumor cell metastasis [123, 160].Increasing evidence suggests that NETs play a significant role in promoting angiogenesis within the tumor microenvironment. Components of NETs, such as MMP9 and HMGB1, can directly act on endothelial cells, stimulating their proliferation, migration, and tube formation. Additionally, NETs can indirectly affect the angiogenesis process by releasing pro-angiogenic factors and modulating immune cell functions [73, 142]. In the hypoxic environment of gastric cancer, HMGB1 mediates the formation of NETs through the TLR4/p38 MAPK signaling pathway. NETs directly induce GC cell invasion and migration and accelerate GC growth by increasing angiogenesis [43]. Multiplex immunohistochemistry analysis has shown that NETs can induce HUVEC proliferation, survival, and chemotaxis, with effects comparable to VEGF stimulation. Blocking NETs reduced microvessel density and significantly inhibited tumor growth [161].A recent study also found that elevated levels of NETs in the postoperative tumor microenvironment are closely associated with tumor angiogenesis. By constructing injectable hydrogels for the specific delivery of NETs inhibitors, researchers successfully reduced tumor microvessel density and inhibited tumor growth, confirming the role of NETs in promoting tumor angiogenesis [162].
It is evident that NETs have a direct promoting effect on tumor angiogenesis, suggesting that targeting NETs might influence the nutrient supply to tumors. Using NETs as a therapeutic target or combining it with other drugs may yield better clinical outcomes. Additionally, the presence of high ROS in the context of NETs, and the consistency between NETs and ROS in tumor angiogenesis, indicate an interaction between mitochondrial changes, metabolic reprogramming, high ROS production, NETs, and angiogenesis in tumors. Tumor metabolic reprogramming and high ROS not only provide a foundation for the production of NETs but also promote angiogenesis. The presence of these newly formed blood vessels and NETs further facilitates tumor metastasis (Figure 4).
Fig. 4.
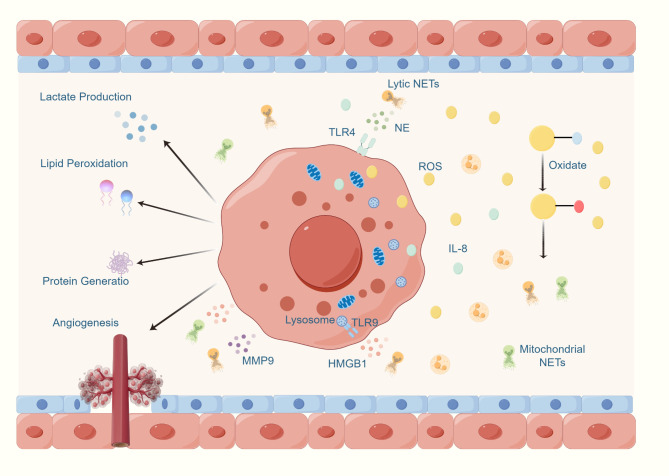
The Interaction between NETs and tumor metabolism. The mitochondria of tumor cells secrete large amounts of ROS and IL-8, which act on extracellular neutrophils, transforming them into NETs. Meanwhile, ROS oxidizes the thiol groups in plasma albumin, leading to further ROS accumulation and accelerating NETs formation. The NETs produced release neutrophil elastase and high-mobility group box 1, which bind to the TLR4 receptors on the tumor cell membrane and the TLR9 receptors on intracellular lysosomal membranes, promoting tumor cell proliferation, increasing mitochondrial density, and enhancing ATP production. In terms of tumor reprogramming, NETs not only induce lactate production but also regulate lipid metabolism through oxidative stress, affecting fatty acid oxidation. Additionally, they promote protein synthesis and structural changes in cells, thereby supporting cancer cell growth and metastasis. Finally, the high-mobility group box 1 and MMP9 secreted by NETs stimulate angiogenesis, providing additional nutrients for tumor growth
NETs as a therapeutic target
The aforementioned content highlights the critical and intricate relationships among ROS, NETs, metabolic reprogramming, redox balance, and mitochondria. Targeting NETs and ROS, along with their related metabolic pathways, holds promise for breakthroughs in cancer treatment, especially in controlling metastasis. However, despite the significant role of ROS in tumor development and progression, strategies that solely target ROS often fail to achieve substantial therapeutic effects due to the challenges in precisely regulating ROS levels [163, 164].NETs, playing pivotal roles in the tumor microenvironment and being closely related to ROS, represent a potentially more effective therapeutic target. By regulating ROS levels involved in NETs formation, it might be possible to inhibit tumor growth and metastasis while also reducing drug resistance. This approach offers a novel and potentially synergistic pathway with traditional therapies for cancer patients, providing a unique advantage over conventional treatments.
Inhibition of NETs formation
Hydroxychloroquine
Hydroxychloroquine (HCQ) is a medication widely used for the treatment of malaria, rheumatoid arthritis, and systemic lupus erythematosus. It has also gained attention for its potential role in treating COVID-19 by blocking the formation of NETs [165].HCQ primarily inhibits NETs formation through the suppression of autophagy, interference with neutrophil activation, and other pathways [25, 166, 167].Additionally, HCQ can reduce intracellular ROS accumulation by inhibiting autophagy pathways or lysosomal function, thus protecting cells from oxidative stress damage [166, 168].HCQ can also increase ROS levels, inducing apoptosis in tumor cells. This mechanism is t associated with HCQ’s anticancer activity [95, 169].
HCQ not only disrupts tumor cell energy metabolism by inhibiting autophagy, reducing glycolysis and lactate production, but also affects lipid metabolism [139, 170, 171].Furthermore, HCQ impacts mitochondrial function in tumors by disrupting mitochondrial membrane potential and reducing oxidative phosphorylation efficiency, thereby inhibiting tumor cell proliferation [95, 172].Moreover, HCQ can inhibit tumor angiogenesis by suppressing autophagy and regulating VEGF expression, thereby limiting the tumor’s blood supply [173, 174].Therefore, HCQ has multiple tumor-inhibiting capabilities, and whether its effects are exerted through the inhibition of NETs or specific aspects of NETs formation warrants further investigation.
Cl-amidine
The enzyme PAD4 is crucial in the formation of NETs, producing citrullinated histone H3, a hallmark product of NETs. Cl-amidine, an experimental drug, inhibits the formation of NETs by irreversibly inhibiting the activity of PAD4 enzyme [175–178].Furthermore, Cl-amidine can reduce ROS-mediated tumor exosome release, thereby inhibiting the invasive and metastatic capabilities of tumors [179, 180].Cl-amidine also inhibits PAD4 enzyme activity, thereby reducing autophagy, altering metabolic states, restoring normal mitochondrial function, and maintaining cellular redox balance by reducing ROS production, which inhibits tumor growth and dissemination [181–183].Lastly, Cl-amidine can inhibit tumor angiogenesis, limiting the blood supply to tumors [183].Therefore, Cl-amidine may exert antitumor effects by inhibiting NETs.
Sivelestat
Sivelestat, an NE inhibitor, is mainly used for treating acute lung injury and acute respiratory distress syndrome. By inhibiting neutrophil elastase activity, it blocks the formation of NETs [184–188]. Sivelestat has potential in cancer treatment by inhibiting NETs formation in a mouse model of lung cancer, thereby suppressing tumor metastasis [189].
Other drugs
Statins, due to their anti-inflammatory properties, ability to reduce oxidative stress, inhibit neutrophil activation, and alter cell membrane lipid composition, show potential in influencing neutrophil function and NETs formation [190–193].While most evidence suggests that statins inhibit NETs, some reports indicate that statin treatment may induce NETs formation [194].Therefore, further investigation into the effects of statins on NETs formation is warranted.
Metformin has potential anti-inflammatory and antioxidant effects, which may benefit various diseases, including cancer [195–200]. Metformin and DNase I could significantly reverse the pro-pancreatic cancer effects of NETs in vivo and in vitro, potentially by preventing the activation of NOX in neutrophils [201].
Aspirin is believed to effectively reverse pathological NETosis induced by platelets through NF-κB inhibition [202–204]. Aspirin plays a role in combating tumors and reversing drug resistance [205–207].Dual antiplatelet therapy with aspirin and ticagrelor significantly reduced micrometastases in mice by inhibiting platelet activation and NETs formation [208]. Aspirin promotes T-cell infiltration, improving the immune microenvironment [207].Whether NETs in the tumor microenvironment participate in this mechanism is a direction worth exploring further.
These three classes of drugs reduce ROS production through distinct mechanisms, influencing tumor cell metabolic reprogramming, angiogenesis, and mitochondrial function. Ultimately, they contribute to regulating the tumor microenvironment and inhibiting tumor growth and spread [209–213]. Although these drugs are not typically classified as NETs inhibitors in experimental studies, their potential connection with inflammation and ROS suggests they may also influence NETs formation. Additionally, their role in tumor metabolism underscores their potential therapeutic value in cancer treatment. However, in clinical practice, these drugs are more commonly prescribed for other conditions, raising concerns about potential side effects if their NETs-inhibiting properties are considered in isolation. Furthermore, their mechanisms of action shed light on the complex interactions among NETs, ROS, and tumor metabolism, which could be pivotal in future research exploring these drugs in the context of cancer therapy.
Degrading formed NETs
DNase are enzymes capable of hydrolyzing DNA and are widely used clinically to treat cystic fibrosis. Additionally, DNase I can hydrolyze the DNA within NETs, disrupting their structure and helping to prevent excessive inflammatory responses and tissue damage. Consequently, DNase I has shown potential therapeutic value in treating diseases such as diabetes and cancer [31, 44, 214, 215]. During the progression of NASH to HCC, there is a selective increase in regulatory T cells (Tregs) in the liver. NETs can promote the differentiation of naive CD4 + T cells into Tregs by reprogramming their metabolism, exacerbating this process. Treatment with DNase I can reduce Treg activity, thereby reversing this pathological process [31].
Heparin can bind to DNA and histones within NETs, destabilizing them and promoting their degradation [216–219]. Low molecular weight heparin has been reported to directly prevent NETs formation [216–219].However, heparin can induce NETs formation in vitro in a PAD4-independent manner [6, 220, 221].Thus, the effects of heparin on NETs can vary depending on the disease context, potentially leading to different outcomes.
Anti-histone antibodies (AHA) are a type of autoantibodies that target histones. AHA can specifically recognize and bind to histones exposed on the surface of NETs. Subsequently, the immune complexes formed between AHA and histones can activate the classical complement pathway, generating a series of effector molecules. These effector molecules are recognized by complement receptors on the surface of phagocytes like macrophages, promoting the phagocytosis and degradation of NETs [219, 222].
Streptokinase is a protein produced by streptococci and is widely used in clinical thrombolytic therapy. Additionally, streptokinase can directly degrade key components of NETs, such as DNA and histones. This dual action not only effectively reduces thrombus formation but also alleviates inflammation. This indicates that streptokinase has new therapeutic potential in thrombolytic therapy and NETs-related diseases such as sepsis, rheumatoid arthritis, vasculitis, and cancer [223–225].
In summary, although there are numerous methods and approaches to inhibit NETs, achieving significant clinical benefits requires in-depth research and comprehensive application of these methods. The widespread use of heparin and statins in other diseases and their dual effects on NETs pose significant limitations in their practical clinical application.Aside from these classes of drugs, the most commonly used inhibitors of NETs in scientific research are DNase, Cl-amidine, and hydroxychloroquine, but these drugs also have their limitations. Specifically, the efficiency of DNase in breaking down NETs may be insufficient to counteract the excessive formation of NETs in chronic inflammatory diseases. Cl-amidine, while inhibiting PAD4, may also inhibit other PAD isoforms, potentially causing nonspecific side effects, and its safety and efficacy have yet to be confirmed. Hydroxychloroquine, as an antimalarial drug, has proven toxicity to the heart and retina. Moreover, all three drugs have relatively short half-lives in biological systems, affecting their long-term efficacy. Fortunately, the use of nanomaterials or other methods to modify these drugs to extend their duration and effectiveness is being explored [226]. For instance, the design of a novel nanoplatform called AMD can target tumors and achieve on-demand release of DNase I through near-infrared second-region laser stimulation. This effectively extended the degradation time and effect of DNase I on NETs, showing potential in cancer immunotherapy and metastasis inhibition [227].
While individual responses to NETs-targeted therapies may vary, this approach has proven effective and relatively resistant to the development of drug resistance. Given the complexity of NETs, many commonly used drugs exhibit some degree of inhibitory effect on them. Therefore, it is essential to conduct in-depth studies on NETs to determine which patients are most likely to benefit from such treatments, which could have significant implications for the treatment of tumors and other diseases. Additionally, as many drugs can influence ROS, it raises the possibility that they might also impact tumor metabolism through interactions with both NETs and ROS. Future research should aim to clarify the specific roles of NETs across different tumor types, refine the application strategies of existing drugs, and develop targeted NETs inhibitors. Moreover, investigating NETs-related biomarkers could help identify patients who would respond best to NETs-targeted therapies, allowing for more personalized and precise treatment plans. Such advancements could offer new hope in clinical treatments and substantially improve patient outcomes (Figure 5).
Fig. 5.
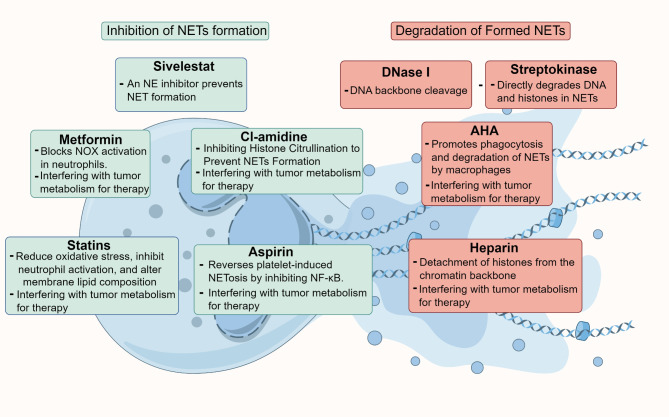
Various strategies for therapeutically targeting NETs. 1)Inhibition of NETs formation: Cl-amidine, Sivelestat, statins, metformin, aspirin. Notably, Cl-amidine, metformin, statins, and aspirin have the dual ability to target both NETs and tumor metabolism simultaneously. 2)Degradation of Formed NETs: DNase I, AHA, heparin, streptokinase. Among these, AHA and heparin can also influence tumor metabolism
Conclusions
In the past decade, advancements in modern biotechnology and molecular biology techniques, such as high-throughput sequencing, microscopic imaging, and gene editing technologies, have enabled researchers to study the structure, formation mechanisms, and functions of NETs in greater detail. This has significantly propelled the development of NETs research, resulting in a notable increase in related studies and publications. Originally studied in the context of infections, NETs are now being recognized for their critical roles in many malignant diseases. Although NETs have different formation mechanisms, understanding them in depth will help identify patients who can benefit clinically.
Tumors, as highly active pathological states, are closely associated with metabolism. Additionally, as research into the tumor microenvironment deepens, it has been found that NETs are closely related to tumor growth, invasion, metastasis, and response to treatment, forming a potential positive feedback loop that influences the tumor microenvironment. The role of this feedback mechanism and NETs in tumorigenesis and metastasis remains to be fully explored.
In fact, these metabolism-related effects do not operate independently but are part of a complex network of mutual regulation and compensatory mechanisms. For instance, when mitochondrial function is impaired, tumor cells can enhance glycolytic pathways to meet energy demands, ensuring cell growth even under energy-deficient conditions [228, 229]. While hypoxic conditions activate HIF-1α, promoting angiogenesis, metabolic reprogramming, and antioxidant mechanisms to neutralize excess ROS [230, 231]. Understanding these compensatory mechanisms could inform strategies to disrupt tumor metabolism by targeting NETs and ROS, potentially leading to more effective and resistance-free therapeutic outcomes.Future research should explore the compensatory relationships and between metabolic pathways, focusing on key regulators like HIF-1α and ROS. Understanding the complex network of tumor metabolism could develop more effective strategies to inhibit tumor growth and progression. Moreover, given the close relationships among mitochondria, redox reactions, metabolic reprogramming, NETs, and ROS, various forms of cell death such as ferroptosis may also be potential extensions of NETs research. The relationship between NETs and cell death mechanisms like ferroptosis can be dualistic. Are these differences due to tumor microenvironment-related signaling pathways like cGAS-STING also related to ferroptosis, ROS, and NETs? What roles do they play in tumors?
In conclusion, investigating whether targeting NETs can disrupt tumor metabolism and life activities is crucial for their future clinical application. This represents significant research value and offers promising directions for developing innovative cancer therapies.
Acknowledgements
Not applicable.
Abbreviations
- NETs
Neutrophil extracellular traps
- NE
Neutrophil elastase
- MPO
Myeloperoxidase
- mtDNA
Mitochondrial DNA
- LPS
Lipopolysaccharides
- NOX
NADPH oxidase
- ROS
Reactive oxygen species
- PAD4
Peptidylarginine deiminase 4
- GSDMD
Gasdermin D
- HMGB1
High-mobility group box 1
- BCG
Bacillus calmette-guérin
- CRC
Colorectal cancer
- SLE
Systemic lupus erythematosus
- HIF-1
Hypoxia-inducible factor 1
- TNBC
Triple-negative breast cancer
- HCC
Hepatocellular carcinoma
- EMT
Epithelial-mesenchymal transition
- DGKG
Diacylglycerol kinase γ
- CTCs
Circulating tumor cells
- HCQ
Hydroxychloroquine
- NASH
Non-alcoholic steatohepatitis
- Tregs
Regulatory T cells
- AHA
Anti-histone antibodies
Author contributions
Zhanrui Liu and Yong He designed the review. Yuanyao Dou conducted literature research, graphing, and manuscript writing. Manuscripts and figures were further verified by Conghua Lu, Rui Han. All authors have read and approved the article.
Funding
This study was supported by Chongqing Technology Innovation and Application Development Special Key Project (grant No. CSTB2023TIAD-STX0003); Talent Innovation Capacity Building Program of the Army Medical Center (grant no.ZXYZZZKY07);and National Science and Technology Major Project(grant no. 2024ZD0520203).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read and approved the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adrover JM, McDowell SAC, He XY, Quail DF, Egeblad M. NETworking with cancer: the bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell. 2023;41(3):505–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu C, Wang X, Yang C, Chen F, Shi L, Xu W, et al. Neutrophil extracellular traps drive intestinal microvascular endothelial ferroptosis by impairing Fundc1-dependent mitophagy. Redox Biol. 2023;67:102906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Zhang X, Shang J, Feng X, Yu L, Fan J, et al. Identification of NETs-related biomarkers and molecular clusters in systemic lupus erythematosus. Front Immunol. 2023;14:1150828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K, Liao Y, Li X, Wang R, Zeng Z, Cheng M, et al. Inhibition of neutrophil elastase prevents cigarette smoke exposure-induced formation of neutrophil extracellular traps and improves lung function in a mouse model of chronic obstructive pulmonary disease. Int Immunopharmacol. 2023;114:109537. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. [DOI] [PubMed] [Google Scholar]
- 6.Herre M, Cedervall J, Mackman N, Olsson A-K. Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases. Physiol Rev. 2023;103(1):277–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang D, Liu J. Neutrophil extracellular traps: a new player in cancer metastasis and therapeutic target. J Exp Clin Cancer Res. 2021;40(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–47. [DOI] [PubMed] [Google Scholar]
- 9.Mutua V, Gershwin LJ. A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics. Clin Rev Allergy Immunol. 2021;61(2):194–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang Q, Stehr AM, Naschberger E, Knopf J, Herrmann M, Stürzl M. No NETs no TIME: crosstalk between neutrophil extracellular traps and the tumor immune microenvironment. Front Immunol. 2022;13:1075260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Li Y, Chen Z, Yuan Y, Su Q, Ye K, et al. GSDMD-dependent neutrophil extracellular traps promote macrophage-to-myofibroblast transition and renal fibrosis in obstructive nephropathy. Cell Death Dis. 2022;13(8):693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao C, Liang F, Ye M, Wu S, Qin Y, Zhao L, et al. GSDMD promotes neutrophil extracellular traps via mtDNA-cGAS-STING pathway during lung ischemia/reperfusion. Cell Death Discov. 2023;9(1):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva CMS, Wanderley CWS, Veras FP, Sonego F, Nascimento DC, Gonçalves AV, et al. Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation. Blood. 2021;138(25):2702–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vorobjeva N, Galkin I, Pletjushkina O, Golyshev S, Zinovkin R, Prikhodko A, et al. Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils. Biochim Biophys Acta Mol Basis Dis. 2020;1866(5):165664. [DOI] [PubMed] [Google Scholar]
- 15.O’, Sullivan KM, Holdsworth SR. Neutrophil extracellular traps: a potential therapeutic target in MPO-ANCA associated vasculitis? Front Immunol. 2021;12:635188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yipp BG, Petri B, Salina D, Jenne CN, Scott BNV, Zbytnuik LD, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18(9):1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12(3):324–33. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Xiao S, Filipczak N, Yalamarty SSK, Shang H, Zhang J, et al. Role and therapeutic targeting strategies of neutrophil extracellular traps in inflammation. Int J Nanomed. 2023;18:5265–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Hu H, Tan S, Dong Q, Fan X, Wang Y, et al. The role of neutrophil extracellular traps in cancer progression, metastasis and therapy. Exp Hematol Oncol. 2022;11(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Xu Y, Yu F, Ma Z, Yu J, Zhang X. NETs: an extracellular DNA network structure with implication for cardiovascular disease and cancer. Hypertens Res. 2024;47(5):1260–72. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Zhang F, Zheng X, Zhang J, Cao P, Sun Z, et al. Identification of renal ischemia reperfusion injury subtypes and predictive strategies for delayed graft function and graft survival based on neutrophil extracellular trap-related genes. Front Immunol. 2022;13:1047367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo H, Guo H, Zhou Y, Fang R, Zhang W, Mei Z. Neutrophil extracellular traps in cerebral ischemia/reperfusion injury: friend and foe. Curr Neuropharmacol. 2023;21(10):2079–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuang S, Xia S, Huang P, Wu J, Qu J, Chen R, et al. Targeting P2RX1 alleviates renal ischemia/reperfusion injury by preserving mitochondrial dynamics. Pharmacol Res. 2021;170:105712. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, You D, Pan M, Weng M, Xie Q, Guan Y, et al. Knockout of the C3a receptor protects against renal ischemia reperfusion injury by reduction of NETs formation. Cell Mol Life Sci. 2023;80(11):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frangou E, Chrysanthopoulou A, Mitsios A, Kambas K, Arelaki S, Angelidou I, et al. REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann Rheum Dis. 2019;78(2):238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KH, Kronbichler A, Park DD-Y, Park Y, Moon H, Kim H, et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun Rev. 2017;16(11):1160–73. [DOI] [PubMed] [Google Scholar]
- 27.Keir HR, Chalmers JD. Neutrophil extracellular traps in chronic lung disease: implications for pathogenesis and therapy. Eur Respir Rev. 2022;31(163):210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King PT, Dousha L. Neutrophil extracellular traps and respiratory disease. J Clin Med. 2024;13(8):2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singhal S, Bhojnagarwala PS, O’Brien S, Moon EK, Garfall AL, Rao AS, et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30(1):120–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu K, Sun E, Lei M, Li L, Gao J, Nian X, Wang L, et al. BCG-induced formation of neutrophil extracellular traps play an important role in bladder cancer treatment. Clin Immunol. 2019;201:4–14. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Zhang H, Wang Y, Brown ZJ, Xia Y, Huang Z, et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis. J Hepatol. 2021;75(6):1271–83. [DOI] [PubMed] [Google Scholar]
- 32.Ng MSF, Kwok I, Tan L, Shi C, Cerezo-Wallis D, Tan Y, et al. Deterministic reprogramming of neutrophils within tumors. Science. 2024;383(6679):eadf6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau C-S, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong L, Cumpian AM, Caetano MS, Ochoa CE, De La Garza MM, Lapid DJ, et al. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol Cancer. 2013;12(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O’Donnell JS, Szczepanski S, et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity. 2017;47(4):789–e8029. [DOI] [PubMed] [Google Scholar]
- 36.Mollaoglu G, Jones A, Wait SJ, Mukhopadhyay A, Jeong S, Arya R, et al. The lineage-defining transcription factors SOX2 and NKX2-1 determine lung cancer cell fate and shape the tumor immune microenvironment. Immunity. 2018;49(4):764–e7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muqaku B, Pils D, Mader JC, Aust S, Mangold A, Muqaku L, et al. Neutrophil extracellular trap formation correlates with favorable overall survival in high grade ovarian cancer. Cancers. 2020;12(2):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millrud CR, Kågedal Å, Kumlien Georén S, Winqvist O, Uddman R, Razavi R, et al. NET-producing CD16high CD62Ldim neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. Int J Cancer. 2017;140(11):2557–67. [DOI] [PubMed] [Google Scholar]
- 39.Mayer-Hain 39SF, Pappelbaum S, Metze KI, Stock D, Goerge M. Evidence and impact of neutrophil extracellular traps in malignant melanoma. Pigment Cell Melanoma Res. 2020;33(1):63–73. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Wu S, Zhao Y, Dinh T, Jiang D, Selfridge JE, et al. Neutrophil extracellular traps induced by chemotherapy inhibit tumor growth in murine models of colorectal cancer. J Clin Invest. 2024;134(5):e175031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mousset A, Albrengues J. NETs unleashed: neutrophil extracellular traps boost chemotherapy against colorectal cancer. J Clin Invest. 2024;134(5):e178344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Xia Y, Sun B, Zheng N, Li Y, Pang X, et al. Neutrophil extracellular traps induced by the hypoxic microenvironment in gastric cancer augment tumour growth. Cell Commun Signal. 2023;21(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He X-Y, Gao Y, Ng D, Michalopoulou E, George S, Adrover JM, et al. Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment. Cancer Cell. 2024;42(3):474–e48612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue M, Nakashima R, Enomoto M, Koike Y, Zhao X, Yip K, et al. Plasma redox imbalance caused by albumin oxidation promotes lung-predominant NETosis and pulmonary cancer metastasis. Nat Commun. 2018;9(1):5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munir H, Jones JO, Janowitz T, Hoffmann M, Euler M, Martins CP, et al. Stromal-driven and amyloid β-dependent induction of neutrophil extracellular traps modulates tumor growth. Nat Commun. 2021;12(1):683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schoeps B, Eckfeld C, Prokopchuk O, Böttcher J, Häußler D, Steiger K, et al. TIMP1 triggers neutrophil extracellular trap formation in pancreatic cancer. Cancer Res. 2021;81(13):3568–79. [DOI] [PubMed] [Google Scholar]
- 48.Yazdani HO, Roy E, Comerci AJ, Van Der Windt DJ, Zhang H, Huang H, et al. Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. Cancer Res. 2019;79(21):5626–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhan X, Wu R, Kong X-H, You Y, He K, Sun X-Y, et al. Elevated neutrophil extracellular traps by HBV-mediated S100A9-TLR4/RAGE-ROS cascade facilitate the growth and metastasis of hepatocellular carcinoma. Cancer Commun. 2023;43(2):225–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583(7814):133–8. [DOI] [PubMed] [Google Scholar]
- 51.Zhang A, Zou X, Yang S, Yang H, Ma Z, Li J. Effect of NETs/COX-2 pathway on immune microenvironment and metastasis in gastric cancer. Front Immunol. 2023;14:1177604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. 2018; 361(6409):eaao4227. [DOI] [PMC free article] [PubMed]
- 53.Mousset A, Lecorgne E, Bourget I, Lopez P, Jenovai K, Cherfils-Vicini J, et al. Neutrophil extracellular traps formed during chemotherapy confer treatment resistance via TGF-β activation. Cancer Cell. 2023;41(4):757–e77510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao Y, Cong M, Li J, He D, Wu Q, Tian P, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39(3):423–e4377. [DOI] [PubMed] [Google Scholar]
- 55.Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 2020;52(5):856–e8718. [DOI] [PubMed] [Google Scholar]
- 56.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8(361):361ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang H, Du Y, Zhu C, Zhang Z, Liao G, Liu L, et al. Nanoparticulate cationic poly (amino acid) s block cancer metastases by destructing neutrophil extracellular traps. ACS Nano. 2023;17(3):2868–80. [DOI] [PubMed] [Google Scholar]
- 58.Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216(1):176–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin Y, Dai H, Sun X, Xi Z, Zhang J, Pan Y, et al. HRG inhibits liver cancer lung metastasis by suppressing neutrophil extracellular trap formation. Clin Transl Med. 2023;13(6):e1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Canè S, Barouni RM, Fabbi M, Cuozzo J, Fracasso G, Adamo A, et al. Neutralization of NET-associated human ARG1 enhances cancer immunotherapy. Sci Transl Med. 2023;15(687):eabq6221. [DOI] [PubMed] [Google Scholar]
- 61.Su X, Brassard A, Bartolomucci A, Dhoparee-Doomah I, Qiu Q, Tsering T, et al. Tumour extracellular vesicles induce neutrophil extracellular traps to promote lymph node metastasis. J Extracell Vesicles. 2023;12(8):e12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, Chen C, Shi C, Zhao X, Gao L, Guo F, et al. Cell membrane derived liposomes loaded with DNase I target neutrophil extracellular traps which inhibits colorectal cancer liver metastases. J Control Release. 2023;357:620–9. [DOI] [PubMed] [Google Scholar]
- 63.Shinde-Jadhav S, Mansure JJ, Rayes RF, Marcq G, Ayoub M, Skowronski R, et al. Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat Commun. 2021;12(1):2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, et al. Interleukin-17–induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med. 2020;217(12):e20190354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teijeira A, Garasa S, Ochoa MC, Villalba M, Olivera I, Cirella A, et al. IL8, neutrophils, and NETs in a collusion against Cancer Immunity and Immunotherapy. Clin Cancer Res. 2021;27(9):2383–93. [DOI] [PubMed] [Google Scholar]
- 66.He XY, Gao Y, Ng D, Michalopoulou E, George S, Adrover JM, et al. Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment. Cancer Cell. 2024;42(3):474–e48612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeng Z, Xu S, Wang F, Peng X, Zhang W, Zhan Y, et al. HAO1-mediated oxalate metabolism promotes lung pre-metastatic niche formation by inducing neutrophil extracellular traps. Oncogene. 2022;41(29):3719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cichon I, Ortmann W, Kolaczkowska E. Metabolic pathways involved in formation of spontaneous and lipopolysaccharide-induced neutrophil extracellular traps (NETs) differ in obesity and systemic inflammation. Int J Mol Sci. 2021;22(14):7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol. 2020;11:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cristinziano L, Modestino L, Antonelli A, Marone G, Simon H-U, Varricchi G, et al. Neutrophil extracellular traps in cancer. Semin Cancer Biol. 2022;79:91–104. [DOI] [PubMed] [Google Scholar]
- 71.Wang T, Zhou Y, Zhou Z, Zhang P, Yan R, Sun L, et al. Secreted protease PRSS35 suppresses hepatocellular carcinoma by disabling CXCL2-mediated neutrophil extracellular traps. Nat Commun. 2023;14(1):1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubenich DS, de Souza PO, Omizzollo N, Aubin MR, Basso PJ, Silva LM, et al. Tumor-neutrophil crosstalk promotes in vitro and in vivo glioblastoma progression. Front Immunol. 2023;14:1183465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang C, Wu D, Dong B, Liao G, Yu Y, Huang S, et al. The scaffold of neutrophil extracellular traps promotes CCA progression and modulates angiogenesis via ITGAV/NFκB. Cell Commun Signal. 2024;22(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Finley LWS. What is cancer metabolism? Cell. 2023;186(8):1670–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsai C-H, Chuang Y-M, Li X, Yu Y-R, Tzeng S-F, Teoh ST, et al. Immunoediting instructs tumor metabolic reprogramming to support immune evasion. Cell Metab. 2023;35(1):118–e1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heiden 77V, Cantley MG, Thompson LC. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeBerardinis RJ, Chandel NS. We need to talk about the Warburg effect. Nat Metab. 2020;2(2):127–9. [DOI] [PubMed] [Google Scholar]
- 79.Li S, Zeng H, Fan J, Wang F, Xu C, Li Y, et al. Glutamine metabolism in breast cancer and possible therapeutic targets. Biochem Pharmacol. 2023;210:115464. [DOI] [PubMed] [Google Scholar]
- 80.Guo C, You Z, Shi H, Sun Y, Du X, Palacios G, et al. SLC38A2 and glutamine signalling in cDC1s dictate anti-tumour immunity. Nature. 2023;620(7972):200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martin-Perez M, Urdiroz-Urricelqui U, Bigas C, Benitah SA. The role of lipids in cancer progression and metastasis. Cell Metab. 2022;34(11):1675–99. [DOI] [PubMed] [Google Scholar]
- 82.Yin X, Xu R, Song J, Ruze R, Chen Y, Wang C, et al. Lipid metabolism in pancreatic cancer: emerging roles and potential targets. Cancer Commun. 2022;42(12):1234–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hao M, Huang B, Wu R, Peng Z, Luo KQ. The interaction between macrophages and triple-negative breast cancer cells induces ROS-mediated interleukin 1α expression to enhance tumorigenesis and metastasis. Adv Sci. 2023;10(29):e2302857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao Y, Li M, Guo Y, Jin J, Pei F, Wang W, et al. Neutrophil hitchhiking nanoparticles enhance bacteria-mediated cancer therapy via NETosis reprogramming. J Control Release. 2024;367:661–75. [DOI] [PubMed] [Google Scholar]
- 85.Qiu S, Zhong X, Meng X, Li S, Qian X, Lu H, et al. Mitochondria-localized cGAS suppresses ferroptosis to promote cancer progression. Cell Res. 2023;33(4):299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borcherding N, Brestoff JR. The power and potential of mitochondria transfer. Nature. 2023;623(7986):283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang JF, Xing X, Luo L, Zhou XW, Feng JX, Huang KB, et al. Mitochondria-ER contact mediated by MFN2-SERCA2 interaction supports CD8 + T cell metabolic fitness and function in tumors. Sci Immunol. 2023;8(87):eabq2424. [DOI] [PubMed] [Google Scholar]
- 88.Song K-H, Kim J-H, Lee Y-H, Bae HC, Lee H-J, Woo SR, et al. Mitochondrial reprogramming via ATP5H loss promotes multimodal cancer therapy resistance. J Clin Invest. 2018;128(9):4098–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang G, Frederick DT, Wu L, Wei Z, Krepler C, Srinivasan S, et al. Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. J Clin Invest. 2016;126(5):1834–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feng Y, Yang Z, Wang J, Zhao H. Cuproptosis: unveiling a new frontier in cancer biology and therapeutics. Cell Commun Signal. 2024;22(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Delaunay S, Pascual G, Feng B, Klann K, Behm M, Hotz-Wagenblatt A, et al. Mitochondrial RNA modifications shape metabolic plasticity in metastasis. Nature. 2022;607(7919):593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu J, Wei Y, Jia W, Can C, Wang R, Yang X, et al. Chenodeoxycholic acid suppresses AML progression through promoting lipid peroxidation via ROS/p38 MAPK/DGAT1 pathway and inhibiting M2 macrophage polarization. Redox Biol. 2022;56:102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hou D, Hu F, Mao Y, Yan L, Zhang Y, Zheng Z, et al. Cationic antimicrobial peptide NRC-03 induces oral squamous cell carcinoma cell apoptosis via CypD-mPTP axis-mediated mitochondrial oxidative stress. Redox Biol. 2022;54:102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao X, Fu M, Bi R, Zheng X, Fu B, Tian S, et al. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere. 2021;263:128346. [DOI] [PubMed] [Google Scholar]
- 95.Qu F, Wang P, Zhang K, Shi Y, Li Y, Li C, et al. Manipulation of mitophagy by all-in-one nanosensitizer augments sonodynamic glioma therapy. Autophagy. 2020;16(8):1413–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee K-M, Giltnane JM, Balko JM, Schwarz LJ, Guerrero-Zotano AL, Hutchinson KE, et al. MYC and MCL1 cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metab. 2017;26(4):633–e6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kraaij T, Tengström FC, Kamerling SW, Pusey CD, Scherer HU, Toes RE, et al. A novel method for high-throughput detection and quantification of neutrophil extracellular traps reveals ROS-independent NET release with immune complexes. Autoimmun Rev. 2016;15(6):577–84. [DOI] [PubMed] [Google Scholar]
- 98.Tatsiy O, McDonald PP. Physiological stimuli induce PAD4-dependent, ROS-independent NETosis, with early and late events controlled by discrete signaling pathways. Front Immunol. 2018;9:2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cristinziano L, Modestino L, Loffredo S, Varricchi G, Braile M, Ferrara AL, et al. Anaplastic thyroid cancer cells induce the release of mitochondrial extracellular DNA traps by viable neutrophils. J Immunol. 2020;204(5):1362–72. [DOI] [PubMed] [Google Scholar]
- 100.Liu S, Feng M, Guan W. Mitochondrial DNA sensing by STING signaling participates in inflammation, cancer and beyond. Int J Cancer. 2016;139(4):736–41. [DOI] [PubMed] [Google Scholar]
- 101.Ingelsson B, Söderberg D, Strid T, Söderberg A, Bergh AC, Loitto V, et al. Lymphocytes eject interferogenic mitochondrial DNA webs in response to CpG and non-CpG oligodeoxynucleotides of class C. Proc Natl Acad Sci U S A. 2018;115(3):E478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang L-Y, Shen X-T, Sun H-T, Zhu W-W, Zhang J-B, Lu L. Neutrophil extracellular traps in hepatocellular carcinoma are enriched in oxidized mitochondrial DNA which is highly pro-inflammatory and pro-metastatic. J Cancer. 2022;13(4):1261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang C, Wang Z, Li L, Zhang Z, Jin X, Wu P, et al. Aged neutrophils form mitochondria-dependent vital NETs to promote breast cancer lung metastasis. J Immunother Cancer. 2021;9(10):e002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao J, Jin J. Neutrophil extracellular traps: New players in cancer research. Front Immunol. 2022;13:937565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lienhart W-D, Gudipati V, Macheroux P. The human flavoproteome. Arch Biochem Biophys. 2013;535(2):150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aboelella NS, Brandle C, Kim T, Ding Z-C, Zhou G. Oxidative stress in the tumor microenvironment and its relevance to cancer immunotherapy. Cancers. 2021;13(5):986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.George S, Abrahamse H. Redox potential of antioxidants in cancer progression and prevention. Antioxidants. 2020;9(11):1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J, Xiao Q, Chen X, Tong S, Sun J, Lv R, et al. LanCL1 protects prostate cancer cells from oxidative stress via suppression of JNK pathway. Cell Death Dis. 2018;9(2):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Y, Qi H, Liu Y, Duan C, Liu X, Xia T, et al. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11(10):4839–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen M, Hu C, Yang L, Guo Q, Liang Y, Wang W. Saikosaponin-D induces the pyroptosis of lung cancer by increasing ROS and activating the NF-κB/NLRP3/caspase-1/GSDMD pathway. J Biochem Mol Toxicol. 2023;37(8):e23444. [DOI] [PubMed] [Google Scholar]
- 111.Chatterjee R, Chatterjee J. ROS and oncogenesis with special reference to EMT and stemness. Eur J Cell Biol. 2020;99(2–3):151073. [DOI] [PubMed] [Google Scholar]
- 112.Palma FR, Coelho DR, Pulakanti K, Sakiyama MJ, Huang Y, Ogata FT, et al. Histone H3.1 is a chromatin-embedded redox sensor triggered by tumor cells developing adaptive phenotypic plasticity and multidrug resistance. Cell Rep. 2024;43(3):113897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gong J, Jiang S, Huang Y, Yang D, Zhang L, Li Z, et al. Astaxanthin suppresses the metastasis of clear cell renal cell carcinoma through ROS scavenging. J Funct Foods. 2024;116:106139. [Google Scholar]
- 114.Zhang L, Cao Y, Guo X, Wang X, Han X, Kanwore K, et al. Hypoxia-induced ROS aggravate tumor progression through HIF-1α-SERPINE1 signaling in glioblastoma. J Zhejiang Univ Sci B. 2023;24(1):32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meng L, Gao J, Mo W, Wang B, Shen H, Cao W, et al. MIOX inhibits autophagy to regulate the ROS-driven inhibition of STAT3/c-Myc-mediated epithelial-mesenchymal transition in clear cell renal cell carcinoma. Redox Biol. 2023;68:102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen GY, O’Leary BR, Du J, Carroll RS, Steers GJ, Buettner GR, et al. Pharmacologic ascorbate radiosensitizes pancreatic cancer but radioprotects normal tissue: the role of oxidative stress-induced lipid peroxidation. Antioxidants. 2024;13(3):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22(7):381–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang F, Xiao Y, Ding J-H, Jin X, Ma D, Li D-Q, et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2023;35(1):84–e1008. [DOI] [PubMed] [Google Scholar]
- 120.Erpenbeck L, Schön MP. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene. 2017;36(18):2483–90. [DOI] [PubMed] [Google Scholar]
- 121.Jaboury S, Wang K, O’Sullivan KM, Ooi JD, Ho GY. NETosis as an oncologic therapeutic target: a mini review. Front Immunol. 2023;14:1170603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abdol Razak N, Elaskalani O, Metharom P. Pancreatic cancer-induced neutrophil extracellular traps: a potential contributor to cancer-associated thrombosis. Int J Mol Sci. 2017;18(3):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ren J, He J, Zhang H, Xia Y, Hu Z, Loughran P, et al. Platelet TLR4-ERK5 Axis facilitates NET-mediated capturing of circulating tumor cells and distant metastasis after surgical stress. Cancer Res. 2021;81(10):2373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zha C, Meng X, Li L, Mi S, Qian D, Li Z, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med. 2020;17(1):154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yao L, Sheng X, Dong X, Zhou W, Li Y, Ma X, et al. Neutrophil extracellular traps mediate TLR9/Merlin axis to resist ferroptosis and promote triple negative breast cancer progression. Apoptosis. 2023;28(9–10):1484–95. [DOI] [PubMed] [Google Scholar]
- 126.Awasthi D, Nagarkoti S, Sadaf S, Chandra T, Kumar S, Dikshit M. Glycolysis dependent lactate formation in neutrophils: a metabolic link between NOX-dependent and independent NETosis. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):165542. [DOI] [PubMed] [Google Scholar]
- 127.Yeung SJ, Pan J, Lee M-H. Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65(24):3981–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Agani F, Jiang B-H. Oxygen-independent regulation of HIF-1: novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets. 2013;13(3):245–51. [DOI] [PubMed] [Google Scholar]
- 129.Luo J, Sun P, Zhang X, Lin G, Xin Q, Niu Y, et al. Canagliflozin modulates hypoxia-induced metastasis, angiogenesis and glycolysis by decreasing HIF-1α protein synthesis via AKT/mTOR pathway. Int J Mol Sci. 2021;22(24):13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wrzesinski K, Fey SJ. Metabolic reprogramming and the recovery of physiological functionality in 3D cultures in micro-bioreactors. Bioengineering. 2018;5(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Johar D, Elmehrath AO, Khalil RM, Elberry MH, Zaky S, Shalabi SA, et al. Protein networks linking Warburg and reverse Warburg effects to cancer cell metabolism. BioFactors. 2021;47(4):713–28. [DOI] [PubMed] [Google Scholar]
- 132.Updegraff BL, Zhou X, Guo Y, Padanad MS, Chen P-H, Yang C, et al. Transmembrane protease TMPRSS11B promotes lung cancer growth by enhancing lactate export and glycolytic metabolism. Cell Rep. 2018;25(8):2223–e22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ohara Y, Tang W, Liu H, Yang S, Dorsey TH, Cawley H, et al. SERPINB3-MYC axis induces the basal-like/squamous subtype and enhances disease progression in pancreatic cancer. Cell Rep. 2023;42(2):113434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guan X, Lu Y, Wang C, Zhan P, Chen Z. Role of CD61 + low-density neutrophils in promoting hepatocellular carcinoma metastasis through CCDC25 upregulation. Int Immunopharmacol. 2024;134:112272. [DOI] [PubMed] [Google Scholar]
- 135.Dong H, Zhang L, Liu S. Targeting HMGB1: an available therapeutic strategy for breast cancer therapy. Int J Biol Sci. 2022;18(8):3421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen Y, Cai L, Guo X, Li Z, Liao X, Zhang X, et al. HMGB1-activated fibroblasts promote breast cancer cells metastasis via RAGE/aerobic glycolysis. Neoplasma. 2021;68(1):71–8. [DOI] [PubMed] [Google Scholar]
- 137.Fan A, Gao M, Tang X, Jiao M, Wang C, Wei Y, et al. HMGB1/RAGE axis in tumor development: unraveling its significance. Front Oncol. 2024;14:1336191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Q, Xiang M. Metabolic reprogramming of MDSCs within tumor microenvironment and targeting for cancer immunotherapy. Acta Pharmacol Sin. 2022;43(10):1337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang K, Wang X, Song C, He Z, Wang R, Xu Y, et al. The role of lipid metabolic reprogramming in tumor microenvironment. Theranostics. 2023;13(6):1774–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y, Liu F, Chen L, Fang C, Li S, Yuan S, et al. Neutrophil extracellular traps (NETs) promote non-small cell lung cancer metastasis by suppressing lncRNA MIR503HG to activate the NF-κB/NLRP3 inflammasome pathway. Front Immunol. 2022;13:867516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yan M, Gu Y, Sun H, Ge Q. Neutrophil extracellular traps in tumor progression and immunotherapy. Front Immunol. 2023;14:1135086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baumann Z, Auf der Maur P, Bentires-Alj M. Feed-forward loops between metastatic cancer cells and their microenvironment-the stage of escalation. EMBO Mol Med. 2022;14(6):e14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med. 2021;218(1):e20201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li D, Li Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct Target Ther. 2020;5(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang G, Qiu M, Xing X, Zhou J, Yao H, Li M, et al. Lung cancer scRNA-seq and lipidomics reveal aberrant lipid metabolism for early-stage diagnosis. Sci Transl Med. 2022;14(630):eabk2756. [DOI] [PubMed] [Google Scholar]
- 148.Pascual G, Majem B, Benitah SA. Targeting lipid metabolism in cancer metastasis. Biochim Biophys Acta Rev Cancer. 2024;1879(1):189051. [DOI] [PubMed] [Google Scholar]
- 149.Lv T, Xiong X, Yan W, Liu M, Xu H, He Q. Mitochondrial general control of amino acid synthesis 5 like 1 promotes nonalcoholic steatohepatitis development through ferroptosis-induced formation of neutrophil extracellular traps. Clin Transl Med. 2023;13(7):e1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meara CHO, Coupland LA, Kordbacheh F, Quah BJC, Chang C-W, Simon Davis DA, et al. Neutralizing the pathological effects of extracellular histones with small polyanions. Nat Commun. 2020;11(1):6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hsu W-H, LaBella KA, Lin Y, Xu P, Lee R, Hsieh C-E, et al. Oncogenic KRAS drives lipofibrogenesis to promote angiogenesis and colon cancer progression. Cancer Discov. 2023;13(12):2652–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Z-L, Chen H-H, Zheng L-L, Sun L-P, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 2023;8(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Han M, Sun H, Zhou Q, Liu J, Hu J, Yuan W, et al. Effects of RNA methylation on tumor angiogenesis and cancer progression. Mol Cancer. 2023;22(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang L, Xu J, Zhou S, Yao F, Zhang R, You W, et al. Endothelial DGKG promotes tumor angiogenesis and immune evasion in hepatocellular carcinoma. J Hepatol. 2024;80(1):82–98. [DOI] [PubMed] [Google Scholar]
- 155.Zhang G, Liu B, Yang Y, Xie S, Chen L, Luo H, et al. Mitochondrial UQCC3 controls embryonic and tumor angiogenesis by regulating VEGF expression. iScience. 2023;26(8):107370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hwang SJ, Jung Y, Song Y-S, Park S, Park Y, Lee H-J. Enhanced anti-angiogenic activity of novel melatonin-like agents. J Pineal Res. 2021;71(1):e12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li S, Ding H, Chang J, Liu S, Dong S, Zyuzin MV, et al. Sm/Co-doped silica-based nanozymes reprogram tumor microenvironment for ATP-inhibited tumor therapy. Adv Healthc Mater. 2023;12(24):e2300652. [DOI] [PubMed] [Google Scholar]
- 158.Li X, Li J, He S, Luan S, Zhang H, Yang Y, et al. Self-assembled acid-responsive nanosystem for synergistic anti-angiogenic/photothermal/ferroptosis therapy against esophageal cancer. Adv Healthc Mater. 2024;13(6):e2302787. [DOI] [PubMed] [Google Scholar]
- 159.Tan G, Hou G, Qian J, Wang Y, Xu W, Luo W, et al. Hyaluronan-decorated copper-doxorubicin-anlotinib nanoconjugate for targeted synergistic chemo/chemodynamic/ antiangiogenic tritherapy against hepatocellular carcinoma. J Colloid Interface Sci. 2024;662:857–69. [DOI] [PubMed] [Google Scholar]
- 160.Ortiz-Espinosa S, Morales X, Senent Y, Alignani D, Tavira B, Macaya I, et al. Complement C5a induces the formation of neutrophil extracellular traps by myeloid-derived suppressor cells to promote metastasis. Cancer Lett. 2022;529:70–84. [DOI] [PubMed] [Google Scholar]
- 161.Yang S, Sun B, Li J, Li N, Zhang A, Zhang X, et al. Neutrophil extracellular traps promote angiogenesis in gastric cancer. Cell Commun Signal. 2023;21(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou H, Zhu C, Zhao Q, Ni J, Zhang H, Yang G, et al. Wrecking neutrophil extracellular traps and antagonizing cancer-associated neurotransmitters by interpenetrating network hydrogels prevent postsurgical cancer relapse and metastases. Bioact Mater. 2024;39:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14(11):709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10(3):175–6. [DOI] [PubMed] [Google Scholar]
- 165.Zhang R, Sun C, Han Y, Huang L, Sheng H, Wang J, et al. Neutrophil autophagy and NETosis in COVID-19: perspectives. Autophagy. 2023;19(3):758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Du X, Ren B, Li C, Li Q, Kan S, Wang X, et al. PRL2 regulates neutrophil extracellular trap formation which contributes to severe malaria and acute lung injury. Nat Commun. 2024;15(1):881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saeed M, Aitken EH, Rogerson SJ. Navigating the terrain of neutrophil extracellular traps in severe malaria pathogenesis. Trends Parasitol. 2024;40(4):278–9. [DOI] [PubMed] [Google Scholar]
- 168.Renard P, Caccavelli L, Legendre A, Tuchmann-Durand C, Balakirouchenane D, Blanchet B, et al. Hydroxychloroquine sulfate: a novel treatment for lipin-1 deficiency? Biomed Pharmacother. 2023;163:114813. [DOI] [PubMed] [Google Scholar]
- 169.Peng X, Zhang S, Jiao W, Zhong Z, Yang Y, Claret FX, et al. Hydroxychloroquine synergizes with the PI3K inhibitor BKM120 to exhibit antitumor efficacy independent of autophagy. J Exp Clin Cancer Res. 2021;40(1):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Paunovic V, Kosic M, Misirkic-Marjanovic M, Trajkovic V, Harhaji-Trajkovic L. Dual targeting of tumor cell energy metabolism and lysosomes as an anticancer strategy. Biochim Biophys Acta Mol Cell Res. 2021;1868(7):118944. [DOI] [PubMed] [Google Scholar]
- 171.Mukhopadhyay S, Mahapatra KK, Praharaj PP, Patil S, Bhutia SK. Recent progress of autophagy signaling in tumor microenvironment and its targeting for possible cancer therapeutics. Semin Cancer Biol. 2022;85:196–208. [DOI] [PubMed] [Google Scholar]
- 172.Wear D, Bhagirath E, Balachandar A, Vegh C, Pandey S. Autophagy inhibition via hydroxychloroquine or 3-methyladenine enhances chemotherapy-induced apoptosis in neuroblastoma and glioblastoma. Int J Mol Sci. 2023;24(15):12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schaaf MB, Houbaert D, Meçe O, Agostinis P. Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ. 2019;26(4):665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li N, Yu Y, Chen Q, Niu J, Gao C, Qu X, et al. A gene delivery system with autophagy blockade for enhanced anti-angiogenic therapy against Fusobacterium nucleatum-associated colorectal cancer. Acta Biomater. 2024;183:278–91. [DOI] [PubMed] [Google Scholar]
- 175.Suzuki M, Ikari J, Anazawa R, Tanaka N, Katsumata Y, Shimada A, et al. PAD4 deficiency improves bleomycin-induced neutrophil extracellular traps and fibrosis in mouse lung. Am J Respir Cell Mol Biol. 2020;63(6):806–18. [DOI] [PubMed] [Google Scholar]
- 176.Shi G, Liu L, Cao Y, Ma G, Zhu Y, Xu J, et al. Inhibition of neutrophil extracellular trap formation ameliorates neuroinflammation and neuronal apoptosis via STING-dependent IRE1α/ASK1/JNK signaling pathway in mice with traumatic brain injury. J Neuroinflammation. 2023;20(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sun S, Lv W, Li S, Zhang Q, He W, Min Z, et al. Smart liposomal nanocarrier enhanced the treatment of ischemic stroke through neutrophil extracellular traps and cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes (cGAS-STING) pathway inhibition of ischemic penumbra. ACS Nano. 2023;17(18):17845–57. [DOI] [PubMed] [Google Scholar]
- 178.Qin W, Li Y, Cui J, Yu B, Yu L, Yang C. Neutrophil extracellular traps as a unique target in the treatment of inflammatory pain. Biochem Biophys Res Commun. 2024;710:149896. [DOI] [PubMed] [Google Scholar]
- 179.Gurunathan S, Kim JH. Graphene oxide enhances biogenesis and release of exosomes in human ovarian cancer cells. Int J Nanomed. 2022;17:5697–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kosgodage US, Trindade RP, Thompson PR, Inal JM, Lange S. Chloramidine/bisindolylmaleimide-I-mediated inhibition of exosome and microvesicle release and enhanced efficacy of cancer chemotherapy. Int J Mol Sci. 2017;18(5):1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Neubert E, Bach KM, Busse J, Bogeski I, Schön MP, Kruss S, et al. Blue and long-wave ultraviolet light induce in vitro neutrophil extracellular trap (NET) formation. Front Immunol. 2019;10:2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fresneda Alarcon M, McLaren Z, Wright HL. Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: same foe different M.O. Front Immunol. 2021;12:649693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu X, Wang Y, Bauer AT, Kirschfink M, Ding P, Gebhardt C, et al. Neutrophils activated by membrane attack complexes increase the permeability of melanoma blood vessels. Proc Natl Acad Sci U S A. 2022;119(33):e2122716119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fei Y, Huang X, Ning F, Qian T, Cui J, Wang X, et al. NETs induce ferroptosis of endothelial cells in LPS-ALI through SDC-1/HS and downstream pathways. Biomed Pharmacother. 2024;175:116621. [DOI] [PubMed] [Google Scholar]
- 185.Wang C-L, Wang Y, Jiang Q-L, Zeng Y, Yao Q-P, Liu X, et al. DNase I and Sivelestat ameliorate experimental hindlimb ischemia-reperfusion injury by eliminating neutrophil extracellular traps. J Inflamm Res. 2023;16:707–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lim S, Khalmuratova R, Lee YY, Kim YS, Lee M, Lee NK, et al. Neutrophil extracellular traps promote ∆Np63 + basal cell hyperplasia in chronic rhinosinusitis. J Allergy Clin Immunol. 2024;153(3):705–17. [DOI] [PubMed] [Google Scholar]
- 187.Shi Y, Dong M, Wu Y, Gong F, Wang Z, Xue L, et al. An elastase-inhibiting, plaque-targeting and neutrophil-hitchhiking liposome against atherosclerosis. Acta Biomater. 2024;173:470–81. [DOI] [PubMed] [Google Scholar]
- 188.Okeke EB, Louttit C, Fry C, Najafabadi AH, Han K, Nemzek J, et al. Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials. 2020;238:119836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rayes RF, Mouhanna JG, Nicolau I, Bourdeau F, Giannias B, Rousseau S, et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight. 2019;5(16):e128008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.de Vries JJ, Autar ASA, van Dam-Nolen DHK, Donkel SJ, Kassem M, van der Kolk AG, et al. Association between plaque vulnerability and neutrophil extracellular traps (NETs) levels: the Plaque at RISK study. PLoS ONE. 2022;17(6):e0269805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tang Q, Liang B, Zhang L, Li X, Li H, Jing W, et al. Enhanced cholesterol biosynthesis promotes breast cancer metastasis via modulating CCDC25 expression and neutrophil extracellular traps formation. Sci Rep. 2022;12(1):17350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kessinger CW, Kim JW, Henke PK, Thompson B, McCarthy JR, Hara T, et al. Statins improve the resolution of established murine venous thrombosis: reductions in thrombus burden and vein wall scarring. PLoS ONE. 2015;10(2):e0116621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang Y, Wu D, Sun Q, Luo Z, Zhang Y, Wang B, et al. Atorvastatin combined with imipenem alleviates lung injury in sepsis by inhibiting neutrophil extracellular trap formation via the ERK/NOX2 signaling pathway. Free Radic Biol Med. 2024;220:179–91. [DOI] [PubMed] [Google Scholar]
- 194.Henneck T, Mergani A, Clever S, Seidler AE, Brogden G, Runft S, et al. Formation of neutrophil extracellular traps by reduction of cellular cholesterol is independent of oxygen and HIF-1α. Int J Mol Sci. 2022;23(6):3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhu W, Xu D, Mei J, Lu B, Wang Q, Zhu C, et al. Metformin reverses impaired osteogenesis due to hyperglycemia-induced neutrophil extracellular traps formation. Bone. 2023;176:116889. [DOI] [PubMed] [Google Scholar]
- 196.Chen CJ, Wu CC, Chang CY, Li JR, Ou YC, Chen WY, et al. Metformin mitigated obesity-driven cancer aggressiveness in tumor-bearing mice. Int J Mol Sci. 2022;23(16):9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mei J, Zhou J, Kong L, Dai Y, Zhang X, Song W, et al. An injectable photo-cross-linking silk hydrogel system augments diabetic wound healing in orthopedic surgery through spatiotemporal immunomodulation. J Nanobiotechnol. 2022;20(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li L, Han R, Xiao H, Lin C, Wang Y, Liu H, et al. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res. 2014;20(10):2714–26. [DOI] [PubMed] [Google Scholar]
- 199.Pan Y-H, Lin C-Y, Lu C-H, Li L, Wang Y-B, Chen H-Y, et al. Metformin synergistically enhances the antitumor activity of the third-generation EGFR-TKI CO-1686 in lung cancer cells through suppressing NF-κB signaling. Clin Respir J. 2018;12(12):2642–52. [DOI] [PubMed] [Google Scholar]
- 200.Chen H, Lin C, Lu C, Wang Y, Han R, Li L, et al. Metformin-sensitized NSCLC cells to osimertinib via AMPK-dependent autophagy inhibition. Clin Respir J. 2019;13(12):781–90. [DOI] [PubMed] [Google Scholar]
- 201.Wang G, Gao H, Dai S, Li M, Gao Y, Yin L, et al. Metformin inhibits neutrophil extracellular traps-promoted pancreatic carcinogenesis in obese mice. Cancer Lett. 2023;562:216155. [DOI] [PubMed] [Google Scholar]
- 202.Ortiz-, Muñoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR. Aspirin-triggered 15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice. Blood. 2014;124(17):2625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Guy A, Garcia G, Gourdou-Latyszenok V, Wolff-Trombini L, Josserand L, Kimmerlin Q, et al. Platelets and neutrophils cooperate to induce increased neutrophil extracellular trap formation in JAK2V617F myeloproliferative neoplasms. J Thromb Haemost. 2024;22(1):172–87. [DOI] [PubMed] [Google Scholar]
- 204.Wan T, Zhang Y, Yuan K, Min J, Mou Y, Jin X. Acetylsalicylic acid promotes corneal epithelium migration by regulating neutrophil extracellular traps in alkali burn. Front Immunol. 2020;11:551057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Han R, Lin C, Zhang C, Kang J, Lu C, Zhang Y, et al. The potential therapeutic regimen for overcoming resistance to osimertinib due to rare mutations in NSCLC. iScience. 2023;26(7):107105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Han R, Hao S, Lu C, Zhang C, Lin C, Li L, et al. Aspirin sensitizes osimertinib-resistant NSCLC cells in vitro and in vivo via Bim-dependent apoptosis induction. Mol Oncol. 2020;14(6):1152–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lu C, Gao Z, Wu D, Zheng J, Hu C, Huang D, et al. Understanding the dynamics of TKI-induced changes in the tumor immune microenvironment for improved therapeutic effect. J Immunother Cancer. 2024;12(6):e009165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yoshimoto M, Kagawa S, Kajioka H, Taniguchi A, Kuroda S, Kikuchi S, et al. Dual antiplatelet therapy inhibits neutrophil extracellular traps to reduce liver micrometastases of intrahepatic cholangiocarcinoma. Cancer Lett. 2023;567:216260. [DOI] [PubMed] [Google Scholar]
- 209.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105(6):739–45. [DOI] [PubMed] [Google Scholar]
- 210.Ahmadi M, Amiri S, Pecic S, Machaj F, Rosik J, Łos MJ, et al. Pleiotropic effects of statins: a focus on cancer. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165968. [DOI] [PubMed] [Google Scholar]
- 211.Demaré S, Kothari A, Calcutt NA, Fernyhough P. Metformin as a potential therapeutic for neurological disease: mobilizing AMPK to repair the nervous system. Expert Rev Neurother. 2021;21(1):45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yang F, Qin Y, Wang Y, Meng S, Xian H, Che H, et al. Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int J Biol Sci. 2019;15(5):1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kolawole OR, Kashfi K. NSAIDs and cancer resolution: new paradigms beyond cyclooxygenase. Int J Mol Sci. 2022;23(3):1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.He L, Liu R, Yue H, Zhang X, Pan X, Sun Y, et al. Interaction between neutrophil extracellular traps and cardiomyocytes contributes to atrial fibrillation progression. Signal Transduct Target Ther. 2023;8(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xiao Y, Ding T, Fang H, Lin J, Chen L, Ma D, et al. Innovative bio-based hydrogel microspheres micro-cage for neutrophil extracellular traps scavenging in diabetic wound healing. Adv Sci. 2024;11(21):e2401195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shute JK. Heparin, low molecular weight heparin, and non-anticoagulant derivatives for the treatment of inflammatory lung disease. Pharmaceuticals. 2023;16(4):584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Manfredi AA, Rovere-Querini P, D’Angelo A, Maugeri N. Low molecular weight heparins prevent the induction of autophagy of activated neutrophils and the formation of neutrophil extracellular traps. Pharmacol Res. 2017;123:146–56. [DOI] [PubMed] [Google Scholar]
- 218.Gollomp K, Kim M, Johnston I, Hayes V, Welsh J, Arepally GM, et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight. 2018;3(18):e99445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mengozzi L, Barison I, Malý M, Lorenzoni G, Fedrigo M, Castellani C, et al. Neutrophil extracellular traps and thrombolysis resistance: new insights for targeting therapies. Stroke. 2024;55(4):963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu X, Arfman T, Wichapong K, Reutelingsperger CPM, Voorberg J, Nicolaes GAF. PAD4 takes charge during neutrophil activation: impact of PAD4 mediated NET formation on immune-mediated disease. J Thromb Haemost. 2021;19(7):1607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhou Y, Chen B, Mittereder N, Chaerkady R, Strain M, An L-L, et al. Spontaneous secretion of the citrullination enzyme PAD2 and cell surface exposure of PAD4 by neutrophils. Front Immunol. 2017;8:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Michailidou D, Kuley R, Wang T, Hermanson P, Grayson PC, Cuthbertson D, et al. Neutrophil extracellular trap formation in anti-neutrophil cytoplasmic antibody-associated and large-vessel vasculitis. Clin Immunol. 2023;249:109274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Varjú I, Kolev K. Networks that stop the flow: a fresh look at fibrin and neutrophil extracellular traps. Thromb Res. 2019;182:1–11. [DOI] [PubMed] [Google Scholar]
- 224.Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost. 2020;18(7):1548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cole JN, Barnett TC, Nizet V, Walker MJ. Molecular insight into invasive group a streptococcal disease. Nat Rev Microbiol. 2011;9(10):724–36. [DOI] [PubMed] [Google Scholar]
- 226.Okeke EB, Louttit C, Snyder CM, Moon JJ. Neutrophils and neutrophil extracellular traps in cancer: promising targets for engineered nanomaterials. Drug Deliv Transl Res. 2023;13(7):1882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chen J, Hou S, Liang Q, He W, Li R, Wang H, et al. Localized degradation of neutrophil extracellular traps by photoregulated enzyme delivery for cancer immunotherapy and metastasis suppression. ACS Nano. 2022;16(2):2585–97. [DOI] [PubMed] [Google Scholar]
- 228.Moindjie H, Rodrigues-Ferreira S, Nahmias C. Mitochondrial metabolism in carcinogenesis and cancer therapy. Cancers. 2021;13(13):3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lin DS, Kao SH, Ho CS, Wei YH, Hung PL, Hsu MH, et al. Inflexibility of AMPK-mediated metabolic reprogramming in mitochondrial disease. Oncotarget. 2017;8(43):73627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hao X, Ren Y, Feng M, Wang Q, Wang Y. Metabolic reprogramming due to hypoxia in pancreatic cancer: implications for tumor formation, immunity, and more. Biomed Pharmacother. 2021;141:111798. [DOI] [PubMed] [Google Scholar]
- 231.Liu Y, Wu Z, Li Y, Chen Y, Zhao X, Wu M, et al. Metabolic reprogramming and interventions in angiogenesis. J Adv Res. 2024;S2090–1232(24):00178–4. [DOI] [PubMed] [Google Scholar]
- 232.Malamud M, Whitehead L, McIntosh A, Colella F, Roelofs AJ, Kusakabe T, et al. Recognition and control of neutrophil extracellular trap formation by MICL. Nature. 2024;633(8029):442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Denorme F, Portier I, Rustad JL, Cody MJ, de Araujo CV, Hoki C, et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest. 2022;132(10):e154225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun. 2020;11(1):2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.