Abstract
Autophagic activation in immune cells, gut microbiota dysbiosis, and metabolic abnormalities have been reported separately as characteristics of systemic lupus erythematosus (SLE). Elucidating the crosstalk among the immune system, commensal microbiota, and metabolites is crucial to understanding the pathogenesis of autoimmune diseases. Emerging evidence shows that basophil activation plays a critical role in the pathogenesis of SLE; however, the underlying mechanisms remain largely unknown. Here, we investigated the effects of autophagic inhibition on the pathogenesis of basophils in SLE using Autophagy-related gene 5 (Atg5) knockout (Atg5−/−) as an autophagic inhibitor. Specifically, we knocked out basophilic Atg5 in vivo to investigate its impact on lupus metabolism. Furthermore, Atg5−/− basophils were transferred to basophil-depleted MRL/MpJ-Faslpr (MRL/lpr) mice to study their effect on disease metabolism. Metagenomic and targeted metabolomic sequencing results indicated considerable reduction in the levels of plasma autoantibodies and inflammatory cytokines in the Atg5−/− basophil transfer group compared with that in the control group. Transplanting Atg5−/− basophils improved the gut microbiota balance in MRL/lpr mice, increasing the abundance of beneficial bacteria, such as Ligilactobacillus murinus and Faecalitalea rodentium, and reducing that of potentially pathogenic bacteria such as Phocaeicola salanitronis. The transplantation of Atg5-deficient basophils improved lupus symptoms by modulating lipid and amino acid metabolism. This improvement was linked to changes in the gut microbiota, particularly an increase in Ligilactobacillus murinus and Faecalitalea rodentium populations. These microbial shifts are believed to promote the production of beneficial metabolites, such as γ-linolenic acid and oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine, while reducing the levels of harmful metabolites such as arginine. These alterations in the metabolic profile contribute to the alleviation of lupus symptoms. Collectively, these findings reveal a novel role of basophil autophagy in SLE, highlighting its potential as a therapeutic target.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-025-02041-1.
Keywords: Basophils, Atg5, Lupus, Gut microbiota, Metabolism, Autoantibodies, Inflammatory cytokines
Introduction
Systemic lupus erythematosus (SLE) is characterized by the loss of autoimmune tolerance, which causes imbalanced immune cell responses [1]. The pathogenesis of SLE remains unclear, with major challenges in its prevention and treatment, including poor clinical outcomes and limited therapeutic options [2]. Accordingly, there is an urgent need to clarify its pathogenesis and develop new strategies for its prevention and treatment [3]. The mammalian intestine contains a dense, complex microbial community known as the microbiota. Diverse microbial communities in the gastrointestinal tract are pivotal for maintaining organismal homeostasis and physiological stability [4]. Although the gut microbiome-related disease list is expanding, the underlying mechanisms remain elusive. SLE, characterized by the loss of self-tolerance and persistent self-attack, results in chronic inflammation, autoantibody production, and multisystem injury and is mostly incurable to date [5]. The gut microbiota and their metabolites, which are key environmental triggers of immune responses, are implicated in SLE progression through mechanisms such as niche translocation, molecular mimicry, epitope spreading, bystander activation, and enhanced systemic inflammation [6]. Individual variations in the gut microbiota may affect the metabolism and biotransformation of disease-modifying anti-rheumatic drugs, thereby impacting their efficacy and toxicity and contributing to heterogeneous therapeutic responses. Modulating the gut microbiota through diet, probiotics, antibiotics, fecal transplantation, or helminths to restore immune tolerance and homeostasis is a promising neoadjuvant therapy for SLE [7]. Altering metabolite profiles is another crucial mechanism by which the gut bacteria influence host immune homeostasis. Whether derived solely from bacteria or through co-metabolism with the host, changes in metabolites alter local or systemic immune responses [8]. Elucidating the crosstalk among commensal microbiota, metabolites, and the immune system may be crucial for understanding the immunopathology of SLE [9].
Basophils, a subgroup of leukocytes in the peripheral blood, have garnered increasing attention for their potent functions [10]. Recent studies have highlighted their crucial role in both innate and acquired immunity, particularly in immune regulation, which has substantially advanced the field [11–13]. Our previous study demonstrated that basophil activation, which led to autoantibody and pro-inflammatory cytokine production, exacerbated SLE pathogenesis in both patients and MRL/lpr mice [14]. Additionally, studies using models such as Lyn−/− mice and Pristane-induced lupus mice have shown the involvement of basophil activation in SLE pathogenesis [15]. These findings suggest that inhibiting basophil activation could offer a new theoretical foundation for SLE treatment and prevention; hence, regulating basophil activation and elucidating its pathogenic mechanisms may facilitate the development of new strategies for this cause.
Autophagy-related gene 5 (Atg5) is essential for autophagy, a lysosomal degradation pathway for removing proteins and organelles [16]. Abnormal autophagy in the immune cells of patients with SLE is implicated in the pathogenesis of various diseases [17, 18]. Increased T-cell autophagy with age has been observed in lupus mouse models (MRL/lpr), potentially regulating autoreactive T-cell survival [19]. CD4+ naive T cells from patients with SLE show higher autophagy levels than healthy controls, a phenotype induced by SLE IgG antibodies [20]. Early B-cell development in lupus mice results in autophagy activation that increases with age; additionally, in patients with SLE, enhanced autophagy in naive B cells correlates with disease activity [21]. Autophagy is crucial for plasma cell maturation. Macrophages from mice with lupus and mononuclear cells from patients with SLE display increased expression of autophagy-related genes (Atg5, Atg12, and Beclin 1), and aberrant autophagy activation may worsen SLE by promoting macrophage production of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)−6 [22]. Our study found elevated autophagy in Th17 and Treg cells in patients with SLE and in MRL/lpr mice, which was corrected through chloroquine treatment [23]. Reducing autophagy in T and B cells has been shown to ameliorate SLE progression [19, 24]. Collectively, increased autophagy across various immune cells is a common feature in SLE, with enhanced basophil activation potentially driving T- and B-cell differentiation and antibody release. However, the specific role of basophil autophagy in SLE and its effect on disease progression remain unexplored. Therefore, we hypothesized that modulating autophagy in basophils could provide insights into SLE disease mechanisms. This research aimed to investigate how Atg5 deficiency in basophils influences inflammation, autoantibody production, cytokine levels, and metabolic abnormalities in lupus-prone MRL/lpr mice, focusing on its effects on gut microbiota composition and function. Additionally, it explored the therapeutic potential of gut microbiota modulation, including transplantation of Ligilactobacillus murinus (L. murinus) and Faecalitalea rodentium (F. rodentium), in improving metabolism and reducing the severity of lupus.
Materials and methods
Mice
Female MRL/MpJ-Faslpr (MRL/lpr), MRL/MpJ, Atg5 knockout (Atg5 flox/flox Cre+/−, Atg5−/−), and Atg5 flox/flox Cre+/−, Atg5+/+ mice were acquired from the Shanghai SLAC Laboratory Animal Company (License No: SCXK (Hu) 2017–0005) and housed in a pathogen-free facility at the Laboratory Animal Center of the Affiliated Hospital of Guangdong Medical University. The maintenance of these mice was approved by the Ethics Committee for Experimental Animals of the Affiliated Hospital of Guangdong Medical University (Approval no. GDY2202248). All experiments were conducted according to the national animal welfare guidelines.
Basophil depletion and adoptive transfer in MRL/lpr mice
Basophil depletion in female MRL/lpr mice was achieved via intraperitoneal injection of 5 μg anti-mouse FcεRIα (eBioscience, USA) twice daily for three days. The control group (non-depleted) mice received an isotype control antibody (eBioscience, USA). For basophil adoptive transfer, basophils were isolated from the bone marrow-induced cells of age-matched Atg5-knockout mice (Atg flox/flox Cre+/−, Atg5−/−) and Atg flox/flox Cre+/−, Atg5+/+ mice. FcεRIα+ CD117− CD11c− CD49b+ basophils were further purified via sorting (Becton Dickinson, USA), yielding a purity of > 90%. Subsequently, 10,000 basophils per mouse were adoptively transferred via the tail vein. To investigate the impact of basophil autophagy deficiency on lupus progression, MRL/lpr mice were divided into three groups—a control, an Atg5+/+ basophil adoptive transfer, and an Atg5−/− basophil adoptive transfer groups. Additionally, MRL/MpJ mice served as a separate control group for MRL/lpr mice. Both the control and MRL/MpJ mice received tail vein injections of normal saline. The Atg5+/+ basophil adoptive transfer group underwent basophil depletion and subsequent transfer of Atg5+/+ basophils at 6, 8, 10, 12, and 14 weeks. Similarly, the Atg5−/− basophil adoptive transfer group underwent basophil depletion, followed by the transfer of Atg5−/− basophils at the same intervals. All mice were euthanized at 16 weeks for assessing disease progression.
Western blotting
Western blotting was used to detect the target protein expression in cells. The protein samples were subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred onto polyvinylidene fluoride membranes. The membrane was blocked using 5% bovine serum albumin in TBST, incubated with the primary antibodies, washed, and then incubated with a horseradish peroxidase-conjugated secondary antibody. Luminol-generated chemiluminescence was visualized using the Azure C500 Western Blot Imaging System (Azure Biosystems, CA, USA) and analyzed using ImageJ software.
Pathological assessment of colon and liver tissues
Colon and liver tissues were harvested, fixed in 4% paraformaldehyde in phosphate-buffered saline for 24 h, embedded in paraffin, and sectioned at a 3 μm thickness. The pathological assessment of colon tissues involved histological examination using hematoxylin and eosin staining to identify morphological changes. Tissues were scored based on criteria such as inflammatory cell infiltration, epithelial integrity, and crypt damage. The degree of colonic inflammatory cell infiltration was rated on a scale from normal (0) to dense inflammatory infiltrate (10). The degree of liver hepatocyte degeneration and necrosis was rated on a scale from normal (0) to dense (10).
Plasma analysis
At 16 weeks of age, all mice were sacrificed under anesthesia, and their blood samples were collected via cardiac puncture. The blood samples were centrifuged at 3,000 rpm for 10 min to separate the plasma, which was then immediately stored at −80 °C.
Plasma levels of autoantibodies, including anti-nuclear antibody (ANA) and anti-double-stranded DNA antibody (anti-dsDNA), were measured using a mouse ELISA Kit (Alpha Diagnostic Intl. Inc., TX, USA) and a mouse anti-dsDNA ELISA Kit (Alpha Diagnostic Intl. Inc.), respectively, as per the manufacturer’s instructions.
Plasma levels of inflammatory cytokines, such as TNF-α, interferon (IFN)-γ, IL-1β, IL-2, IL-4, IL-6, IL-13, and IL-17, were measured using a Milliplex® MAP kit (Millipore, MA, USA), as per the manufacturer’s instructions.
Plasma levels of biochemical markers such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine kinase (CK), α-hydroxybutyrate dehydrogenase (α-HBDH), cholesterol, and lipoproteins (high and low-density; HDL and LDL), and those of bile acid, albumin, globulin, and A/G ratio, were analyzed using Roche cobas 8000 modular analyzer (Roche Diagnostics, Basel, Switzerland).
Metagenomic sequencing
Fecal samples collected at 16 weeks of age were processed and subjected to metagenomic sequencing at Wuhan MetWare Biotechnology Co., Ltd. (Wuhan, China). Each group was evaluated using four independent samples. The experimental procedure consisted of three main phases: sample testing, library construction, and sequencing. During testing, DNA samples were assessed using two methods. Initially, DNA degradation and potential contamination were monitored using 1% agarose gel electrophoresis. Subsequently, the DNA concentration was measured with the Qubit® dsDNA Assay Kit using a Qubit® 2.0 Fluorometer (Thermo Fisher Scientific, MA, USA). Samples with an optical density (OD) value between 1.8 and 2.0 and DNA content exceeding 1 μg were chosen for library construction. From each sample, 1 μg of DNA was taken as the input material. Sequencing libraries were generated using the NEBNext® Ultra™ DNA Library Prep Kit (New England Biolabs, MA, USA), as per the manufacturer’s instructions. Index codes were assigned to link sequences to their respective samples. DNA was fragmented to 350 bp via sonication, followed by end-polishing, A-tailing, and ligation with full-length adaptors for Illumina sequencing. DNA was amplified via polymerase chain reaction (PCR), and the products were purified using the AMPure XP system (Beckman Coulter, CA, USA). Library size distribution was analyzed using an Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA) and quantified using real-time PCR. Sequencing involved clustering index-coded samples using a cBot Cluster Generation System (Illumina Inc., CA, USA), as per the manufacturer’s instructions. Following cluster generation, the libraries were sequenced on an Illumina NovaSeq platform to produce paired-end reads. Metagenomic data were analyzed via preprocessing the sequencing results, followed by metagenome assembly. Gene prediction, abundance analysis, species annotation, and functional annotation were then conducted, using common databases.
Widely targeted metabolomics
Plasma samples collected at 16 weeks of age were processed and analyzed using targeted metabolomics at Wuhan MetWare Biotechnology Co., Ltd. Each group consisted of four independent samples. Samples were thawed from −80 °C and ground in liquid nitrogen (20 mg each). After adding 400 µL of 70% methanol/water internal standard, the mixture was then vortexed and centrifuged (16,260 × g, 4 °C) for 10 min. The supernatant was collected for analysis, following storage at −20 °C for 30 min and centrifugation (16,260 × g, 4 °C, 3 min). Metabolites were detected and identified using a liquid-chromatography-mass spectrometry system with an electrospray ionization source at Wuhan MetWare Biotechnology Co., Ltd. Data were analyzed using the Analyst 1.63 software and a proprietary MetWare database. Orthogonal partial least squares-discriminant analysis (OPLS-DA) was performed using the R package (V3.5.1) and MetaboAnalystR (V1.0.1) to obtain the variable importance in projection (VIP) values. Differential metabolites for the two-group analysis were identified using VIP values > 1 and P-values < 0.05 (Student’s t-test). Data were log-transformed (log2) and mean-centered before OPLS-DA, and a permutation test with 200 iterations was conducted to prevent overfitting. Identified metabolites were annotated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) Compound database and mapped to the KEGG Pathway database. Significantly enriched pathways were identified using a hypergeometric test based on the P-values for listed metabolites. Spearman's rank correlation and redundancy analyses were performed using the OmicStudio tools and Wekemo BioIncloud, respectively.
Fecal microbiota transplantation (FMT)
MRL/lpr lupus mice were stratified into distinct experimental groups—control (untreated lupus controls), antibiotic (antibiotic treatment to deplete gut microbiota), F. rodentium FMT post-antibiotic clearance, L. murinus FMT post-antibiotic clearance, Phocaeicola salanitronis (P. salanitronis) FMT post-antibiotic clearance, and L. murinus + F. rodentium FMT (co-transplantation post-antibiotic clearance). Normal control mice, MRL/MpJ, were included for a comparative analysis.
At eight weeks of age, mice in the antibiotic group received a broad-spectrum antibiotic cocktail to deplete their endogenous gut microbiota. The cocktail, comprising ampicillin (0.2 g/L), metronidazole (0.2 g/L), neomycin (0.2 g/L), and vancomycin (0.1 g/L), was administered via drinking water for two weeks, with replacement every three days.
Mice designated for FMT underwent the same antibiotic pretreatment as did the antibiotic group. Subsequently, they received specific bacterial strains via gavage at a concentration of 108 CFU/200 μL. The strains were sourced from Beina Chuanglian Biotechnology Co., Ltd., with the following strain numbers: F. rodentium (BNCC363015), L. murinus (BNCC194688), and P. salanitronis (BNCC358144). For the L. murinus + F. rodentium-FMT group, equal volumes of the two strains were mixed to achieve the total dose. Gavage was performed thrice weekly, and mice were sacrificed for tissue collection after six weeks.
Metabolite treatment
MRL/lpr mice were divided into the following groups: a control (untreated lupus controls), a γ-linolenic acid (GLA) (GLPBIO, USA) monotherapy, an oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine (OPPC) (GLPBIO, USA) monotherapy, an arginine (GLPBIO, USA) monotherapy, and a combination therapy groups (OPPC and GLA). Concurrently, MRL/MpJ mice served as the normal controls for MRL/lpr mice. Mice in the GLA monotherapy group were orally administered GLA every other day, starting from week eight, at a dosage of 20 mg/kg using a vehicle. Mice in the OPPC monotherapy group were orally administered OPPC every other day, starting from week eight, at a dosage of 20 mg/kg using a vehicle. Mice in the arginine monotherapy group were orally administered arginine every other day, starting from week eight, at a dosage of 20 mg/kg using a vehicle. In the combination therapy group, GLA and OPPC were mixed equally, and mice were orally administered the mixture every other day, starting from week eight, at a total dosage of 20 mg/kg using a vehicle. All mice receiving metabolite treatment were sacrificed after six weeks for disease assessment.
Statistical analysis
Data are presented as mean ± SEM, based on a minimum of three independent experiments. To assess the significant differences, two-tailed unpaired Student's t tests were used to compare two independent groups, and one-way ANOVA was used to compare three or more groups. The threshold for statistical significance was set at P < 0.05. Data analysis and graphical generation were performed using GraphPad Prism 8.0.2 (GraphPad Software, CA, USA).
Results
Atg5 deficiency in basophils alleviates colon inflammation
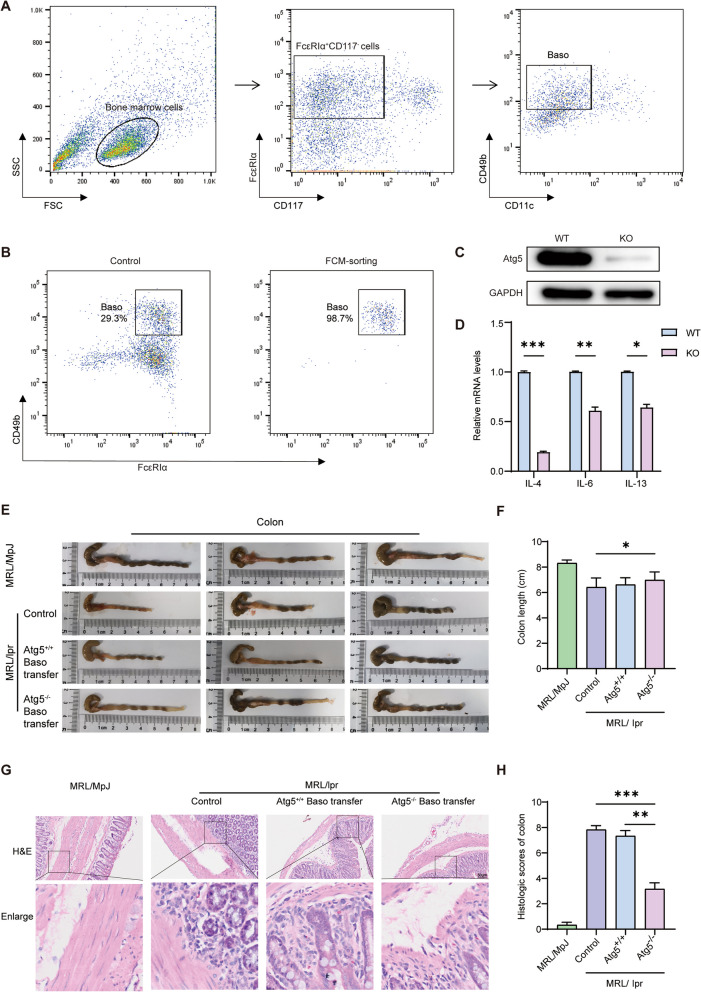
We extracted the bone marrow from Atg5 knockout mice (Atg5 flox/flox Cre±, Atg5−/−), induced basophils using recombinant mouse IL-3, and further improved their purity using flow cytometry. Basophils were sorted using the markers FcεRIα+ CD117− CD11c− CD49b+ (Fig. 1A). After sorting, basophil purity, as confirmed by flow cytometry, reached 98.7% (Fig. 1B). Western blotting results indicated a significant decrease in Atg5 expression in the knockout group compared to that in the wild-type group (Fig. 1C). In addition, we found that the expression level of inflammatory cytokines (IL-4, IL-6, and IL-13) in the knockout group was significantly decreased compared to the wild-type group (Fig. 1D). We evaluated the effects of basophil depletion and adoptive transfer levels in MRL/lpr mice. The experimental design and timelines are shown in Fig. S1. The results showed that one-time basophil depletion led to a significant decrease in the percentage of basophils in the peripheral blood of MRL/lpr mice and remained low for over 10 days (Fig. S2). Meanwhile, one-time basophil transfer led to a significant increase in the percentage of basophils in the peripheral blood, which persisted for over 10 days (Fig. S3). To investigate the impact of basophil autophagy deficiency on lupus progression, MRL/lpr mice were divided into three groups—a control, an Atg5+/+ basophil adoptive transfer, and an Atg5−/− basophil adoptive transfer groups. Additionally, MRL/MpJ mice served as a separate control group for the MRL/lpr mice. Both the control group and the MRL/MpJ mice received tail vein injections of normal saline. The Atg5+/+ basophil adoptive transfer group underwent basophil depletion, followed by the transfer of Atg5+/+ basophils at 6, 8, 10, 12, and 14 weeks. Similarly, the Atg5−/− basophil adoptive transfer group underwent basophil depletion, followed by the transfer of Atg5−/− basophils at the same intervals.
Fig. 1.
Atg5 deficiency in basophils improves colon inflammation in MRL/lpr mice. A Flow cytometry sorting of bone marrow-induced basophils. B Flow cytometry verification of basophil purity. C Western blotting analysis of Atg5 levels in basophils. D qPCR analysis of IL-4, IL-6, and IL-13 levels in basophils. E Morphological observation of colonic tissues in mice. F Statistical analysis of colonic tissues in mice. G Hematoxylin and Eosin (H&E) staining for assessing colonic inflammation in mice. H Statistical analysis of colonic inflammation in mice. *P < 0.05; ** P < 0.01; *** P < 0.001
All mice were euthanized at 16 weeks of age to assess disease progression. Colon length was found to be significantly longer in the Atg5−/− group than in the control group (Fig. 1E, F). Colonic pathological staining revealed significantly fewer inflammatory cells in the Atg5−/− group than in the control group, suggesting that Atg5−/− basophils ameliorate colonic inflammation in mice with lupus (Fig. 1G, H).
Atg5 deficiency in basophils reduces autoantibody levels, inflammatory cytokine expression, and improves metabolism
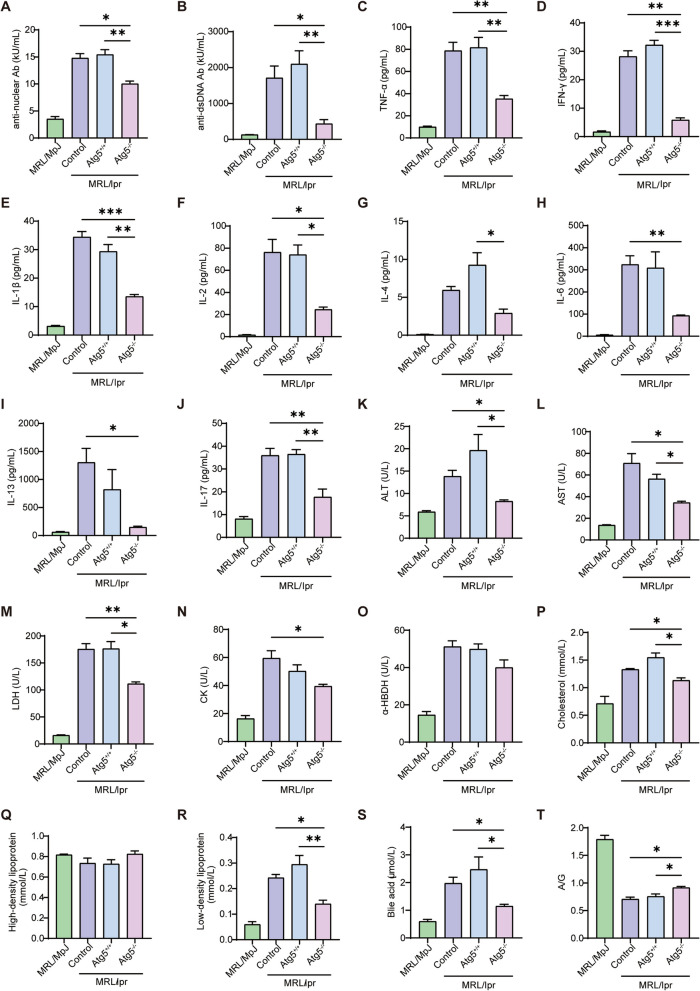
Autoantibodies and inflammatory cytokines are key metrics for assessing lupus severity [25]. Autoantibody detection revealed significantly lower levels of anti-nuclear and anti-dsDNA antibodies in the Atg5−/− basophil adoptive transfer group than that in the control and Atg5+/+ basophil adoptive transfer groups (Fig. 2A, B). Subsequent cytokine analysis showed that the TNF-α, IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-13, and IL-17 levels were lower in the Atg5−/− basophils adoptive transfer group compared to that in the control and Atg5+/+ basophil adoptive transfer groups (Fig. 2C–J). These findings suggest that Atg5 deficiency in basophils reduces in vivo autoantibody and inflammatory cytokine production.
Fig. 2.
Atg5 deficiency in basophils decreases autoantibody expression, reduces inflammatory cytokine levels, and improves metabolism in MRL/lpr mice. A, B Statistical analysis of plasma anti-nuclear antibodies and anti-dsDNA antibodies, respectively. C–J Statistical analysis of plasma cytokines, including tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)−1β, IL-2, IL-4, IL-6, IL-13, and IL-17. K–T Levels of plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine kinase (CK), α-hydroxybutyrate dehydrogenase (α-HBDH), cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), bile acids, and A/G ratio. *P < 0.05; ** P < 0.01
To explore the metabolic changes in SLE, we measured the plasma levels of enzymes, proteins, and lipids. The results indicated significantly lower plasma levels of ALT, AST, and LDH in the Atg5−/− basophil adoptive transfer group than in the control and the Atg5+/+ basophil adoptive transfer groups (Fig. 2K–M), reflecting an improved liver metabolism. Plasma CK levels showed a decreasing trend (Fig. 2N), indicating that the damage to the muscle tissue of the mice was alleviated. These findings suggest that basophil autophagy deficiency inhibits amino acid metabolism. α-HBDH has been reported to be closely associated with SLE and may serve as a predictive marker [26]. However, our results showed no significant changes in its levels (Fig. 2O). Our results indicated decreased plasma levels of cholesterol, LDL, and bile acids (Fig. 2P, R, S); however, HDL and the A/G ratio levels increased in the Atg5−/− basophil adoptive transfer group compared to those in the control and Atg5+/+ basophil adoptive transfer groups (Fig. 2Q, T). These findings suggest that an Atg5 deficiency in basophils improves metabolism in vivo.
Atg5 deficiency in basophils improves gut microbiota dysbiosis
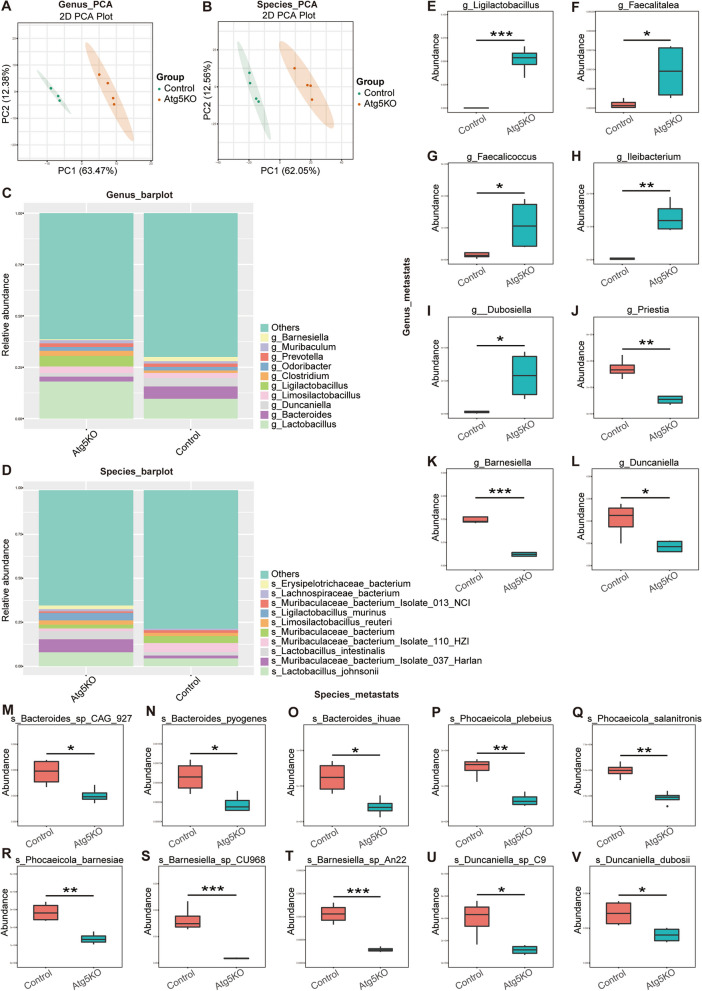
We collected fecal samples from the control (Atg5+/+) and Atg5−/− basophil transplanted MRL/lpr mice for metagenomic sequencing to examine the impact of Atg5 deficiency in basophils on the gut microbiota composition in MRL/lpr mice. Principal component analysis revealed significant separation at the genus and species levels between the control and Atg5−/− groups (Fig. 3A–D).
Fig. 3.
Metagenomic analysis of fecal samples from the control (Atg5+/+) and Atg5−/− basophil transplanted MRL/lpr mice. A, B Principal component analysis. C, D Taxonomic abundance plots. E–L Statistical analysis using Metastats at the genus level in control and Atg5−/− groups of mice. (M–V) Statistical analysis using Metastats at the species level in control and Atg5−/− groups of mice. *P < 0.05; ** P < 0.01
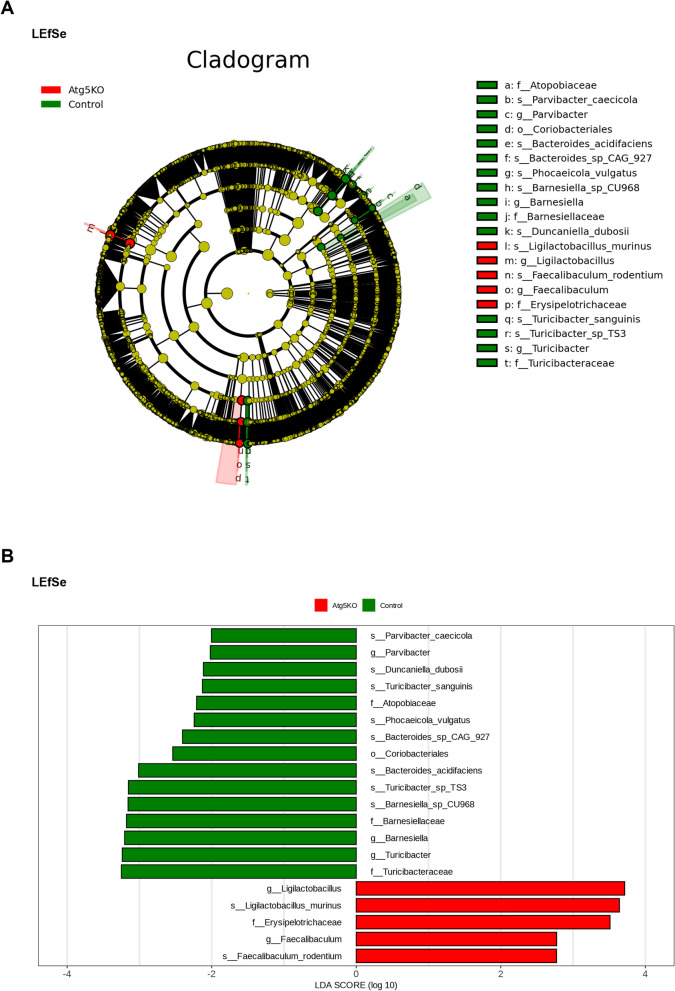
At the genus level, Ligilactobacillus and Faecalitalea were more abundant in Atg5−/− mice than that in control mice (Fig. 3E–L). At the species level, the abundance of Bacteroides_sp_CAG_927, Bacteroides_pyogenes, Bacteroides_ihuae, Phocaeicola_plebeius, Phocaeicola_salanitronis, Phocaeicola_barnesiae, Barnesiella_sp_CU968, Barnesiella_sp_An22, Duncaniella_sp_C9, and Duncaniella dubosii decreased in the Atg5−/− group compared to that in the control mice (Fig. 3M–V). The linear discriminant analysis confirmed similar trends. In the control group, Bacteroides_sp_CAG_927, Barnesiella_sp_CU968, and Duncaniella dubosii were significantly more abundant. Conversely, the abundance of Ligilactobacillus and Faecalitalea was significantly increased following basophil transplantation in Atg5-knockout mice (Fig. 4A, B). We analyzed the species-level changes in fecal metagenome sequencing results to identify potential microbial markers of SLE.
Fig. 4.
Linear discriminant analysis Effect Size (LEfSe) analysis of fecal metagenomics in the control (Atg5+/+) and Atg5−/− basophil transplanted MRL/lpr mice. (A, B) Cladograms and bar plots generated via LEfSe identify significantly different microbiota taxa between groups (Linear discriminant analysis > 2.5, P < 0.05). The diameter of each node in the cladograms is proportional to the relative abundance of the taxonomic unit. Red indicates taxa enriched in the Atg5−/− group and green indicates taxa enriched in the control group
These results indicate that transplanting basophils with Atg5 deficiency can correct the gut microbiota imbalance in MRL/lpr mice. This procedure increased the abundance of beneficial bacteria such as Ligilactobacillus and Faecalitalea while significantly reducing the abundance of potentially pathogenic bacteria, including Phocaeicola_salanitronis, Bacteroides_sp_CAG_927, Barnesiella_sp_CU968, and Duncaniella dubosii.
Atg5 deficiency in basophils affects gut microbiota function
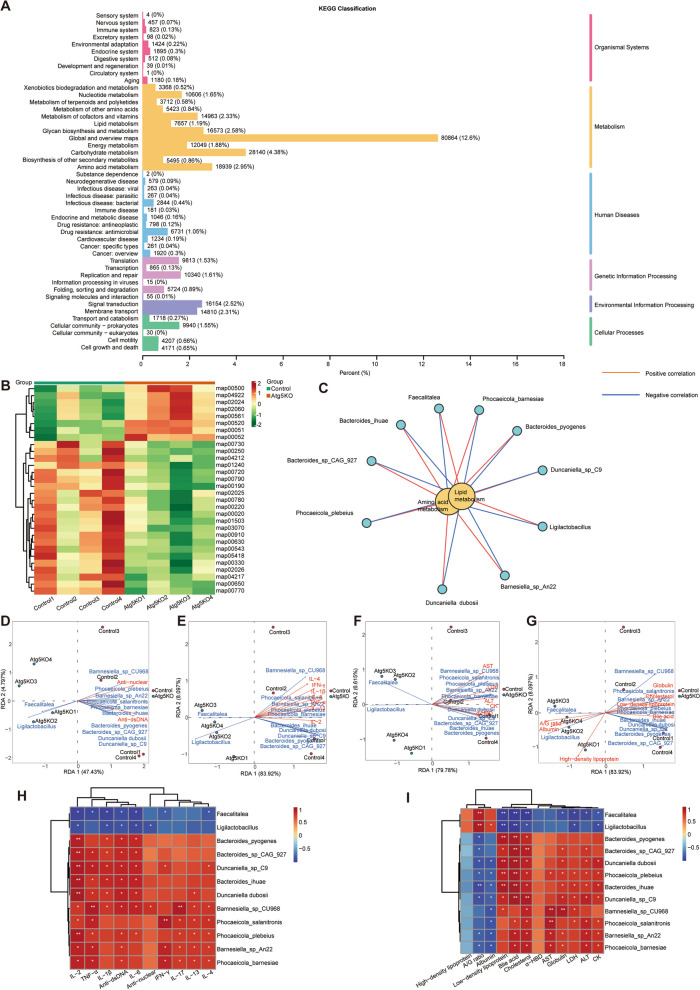
To assess the effects of transplanting basophils with Atg5 deficiency on gut microbiota function, we annotated our fecal metagenomics sequencing data using the KEGG database. Pathway profiles were generated for microbial communities at each sampling site. A total of 308,190 annotated Unigenes were mapped to canonical KEGG reference pathways, distributed across six signaling cascades (Fig. 5A). These genes were categorized into several groups: “Carbohydrate metabolism” (28,140 members), “Amino acid metabolism” (18,939 members), “Energy metabolism” (12,049 members), “Endocrine system” (1,895 members), “Endocrine and metabolic disease” (1,046 members), “Immune system” (823 members), and “Immune disease” (181 members). We observed significant upregulation in the “Starch and sucrose metabolism (map00500),” “Fructose and mannose metabolism (map00051),” and “Glycerolipid metabolism (map00561)” pathways in the Atg5−/− group compared to that in the control group. Conversely, the pathways for “Alanine, aspartate, and glutamate metabolism (map00250),” “Oxidative phosphorylation (map00190),” and “arginine biosynthesis (map00220)” were significantly downregulated in the Atg5−/− group (Fig. 5B).
Fig. 5.
Atg5 deficiency in basophils affects the gut microbiota function in MRL/lpr mice. A Predicted Kyoto Encyclopedia of Genes and Genomes (KEGG) functional classification of metagenomic Unigenes. The numbers on the bar chart represent the count of annotated Unigenes. B Predicted KEGG level 3 functional classification. C Network diagram of the correlation between different bacteria and KEGG pathways. Red lines indicate positive correlations, whereas blue lines indicate negative correlations (Spearman's rank correlation test). D–G Distribution of microbiota species and disease severity indexes in redundancy analysis (RDA). Arrow length indicates the magnitude of the correlation between disease severity indexes and sample distribution, with longer lines indicating greater correlation. The acute angle between arrow lines and axes indicated positive correlation, whereas the obtuse angle indicates negative correlation. H, I Spearman's rank correlation coefficient heatmap. H Correlation analysis between microbiota species abundance and specific metabolites. I Correlation analysis between specific indexes and lupus disease severity indexes (plasma levels of autoantibodies, pro-inflammatory cytokines, enzymes, proteins, lipids). R-values are represented by different colors, with red squares indicating a positive correlation, blue squares indicating a negative correlation, and darker shades indicating a stronger positive/negative correlation. Numbers in the heatmap cells represent P-values. *P < 0.05, **P < 0.01
In the Atg5−/− group, significantly enriched bacteria such as Ligilactobacillus and Faecalitalea positively correlated with the “Starch and sucrose metabolism (map00500),” “Fructose and mannose metabolism (map00051),” and “Glycerolipid metabolism (map00561)” pathways. These bacteria negatively correlated with the “Alanine, aspartate and glutamate metabolism (map00250),” “Oxidative phosphorylation (map00190),” and “arginine biosynthesis (map00220)” pathways. Conversely, in the control group, bacteria such as Bacteroides_sp_CAG_927, Bacteroides_pyogenes, Bacteroides_ihuae, and others showed positive correlations with “Alanine, aspartate, and glutamate metabolism (map00250),” “Oxidative phosphorylation (map00190),” and “arginine biosynthesis (map00220)” pathways. These bacteria also negatively correlated with “Starch and sucrose metabolism (map00500),” “Fructose and mannose metabolism (map00051),” and “Glycerolipid metabolism (map00561)” pathways (Fig. 5C).
To explore the role of gut microbiota in lupus pathogenesis, we conducted Spearman's correlation analyses between various gut microbiota profiles and lupus severity indices, including plasma levels of autoantibodies, inflammatory cytokines, and other metabolic markers. Elevated levels of Ligilactobacillus and Faecalitalea were negatively correlated with plasma autoantibodies (anti-dsDNA, anti-nuclear), inflammatory cytokines (TNF-α, IFN-γ, IL-1β), and metabolic indices (HDL, albumin, A/G ratio). Conversely, Bacteroides_sp_CAG_927, Bacteroides_pyogenes, and other bacteria showed positive correlations with plasma autoantibodies, inflammatory cytokines, and metabolic indices such as AST, ALT, CK, and LDL (Fig. 5D–I). Consequently, transplanting basophils with Atg5 deficiency may alleviate lupus via modulating gut microbiota populations, particularly those of Ligilactobacillus and Faecalitalea. These bacteria may ameliorate lupus symptoms by enhancing carbohydrate metabolism and suppressing amino acid metabolism, potentially reducing autoantibodies and inflammatory cytokines, thereby improving plasma metabolism.
Atg5 deficiency in basophil transplantation improves plasma metabolic abnormalities
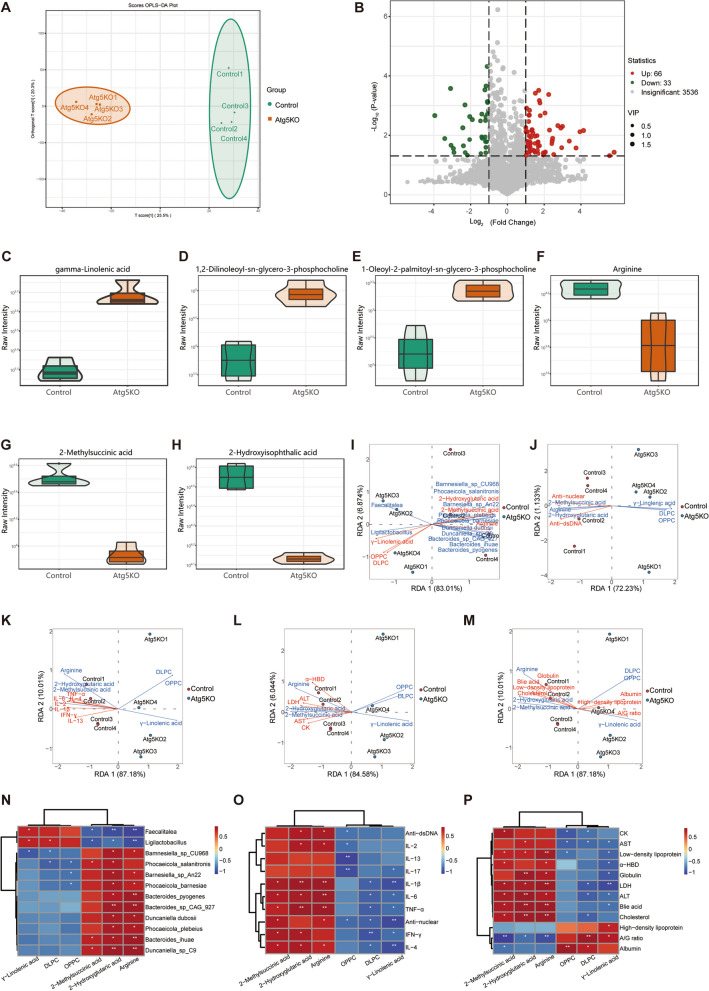
We employed widely targeted metabolomics to analyze the plasma metabolome of MRL/lpr mice and investigated the association between gut microbiota and plasma metabolites. OPLS-DA revealed a distinct difference between the control and Atg5−/− groups (Fig. 6A). A volcano plot revealed 66 upregulated and 33 downregulated metabolites in the Atg5−/− group (Fig. 6B). Our metabolomic analysis revealed increased levels of GLA, 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC), and OPPC, whereas the levels of arginine, methylmalonic acid, and 2-hydroxyglutaric acid decreased in the Atg5−/− group compared to those in the control group (Fig. 6C–H).
Fig. 6.
Atg5 deficiency in basophil transplantation improves plasma metabolic abnormalities in MRL/lpr mice. A Orthogonal partial least squares-discriminant analysis (OPLS-DA) score chart for the control (green) and Atg5−/− (red) mice groups. B Volcano plot of differential metabolites. Selection criteria for differential metabolites are variable importance in projection (VIP) > 1 and P-value < 0.05. C–H Violin plots of specific metabolites in correlation analysis. The box within the violin plot center represents the interquartile range. The thin black lines extending from it represent the 95% confidence interval. The central black horizontal line corresponds to the median, whereas the external shapes depict the data distribution density. I–M RDA between microbiota species abundance and disease severity indexes. Distribution of samples and lupus disease severity indexes. The figure shows the microbiota species distribution and disease severity indexes in RDA. Arrow length indicates the magnitude of the correlation between disease severity indexes and sample distribution, with longer lines indicating greater correlation. The acute angle between arrow lines and axes indicated a positive correlation, whereas the obtuse angle indicates a negative correlation. (N–P) Spearman's rank correlation coefficient heatmap. N Correlation analysis between microbiota species abundance and specific metabolites. O, P Correlation analysis between specific metabolites and lupus disease severity indexes. R-values are represented by different colors, with red squares indicating a positive correlation, blue squares indicating a negative correlation, and darker shades indicating a stronger positive/negative correlation. Numbers in the heatmap cells represent P-values. *P < 0.05, **P < 0.01
We conducted a correlation analysis to explore the functional relationship between the gut microbiota and differential plasma metabolites. The results of the redundancy analysis and Spearman's correlation analyses between these metabolites and lupus severity indices revealed that in the Atg5−/− group, enriched species, such as Ligilactobacillus and Faecalitalea, showed positive correlations with beneficial metabolites (GLA, DLPC, and OPPC) and negative correlations with harmful metabolites (arginine, methylmalonic acid, 2-hydroxyglutaric acid). Results of the relationship between these metabolites and autoantibodies, inflammatory cytokines, and biochemical indices indicated that GLA, DLPC, and OPPC negatively correlated with plasma autoantibodies (anti-dsDNA, anti-nuclear) and inflammatory cytokines (TNF-α, IFN-γ, and IL-1β). GLA, DLPC, and OPPC were negatively correlated with enzymes (AST, ALT, LDH, CK, and α-HBDH) and lipids (globulin, cholesterol, LDL, bile acid), but positively with HDL, albumin, and the A/G ratio (Fig. 6I–P). Therefore, transplanting basophils with Atg5 deficiency could improve the lupus condition by modulating lipid and amino acid metabolism through alterations in gut microbiota populations, particularly those of Ligilactobacillus and Faecalitalea. These bacteria may ameliorate lupus by promoting beneficial metabolites (GLA, DLPC, and OPPC) and reducing harmful metabolites (arginine, methylmalonic acid, and 2-hydroxyglutaric acid), thus lowering autoantibody and inflammatory cytokine levels and improving biochemical metabolism (Fig. 6Q).
Transplantation of L. murinus and F. rodentium improves metabolism through the increased production of GLA and OPPC
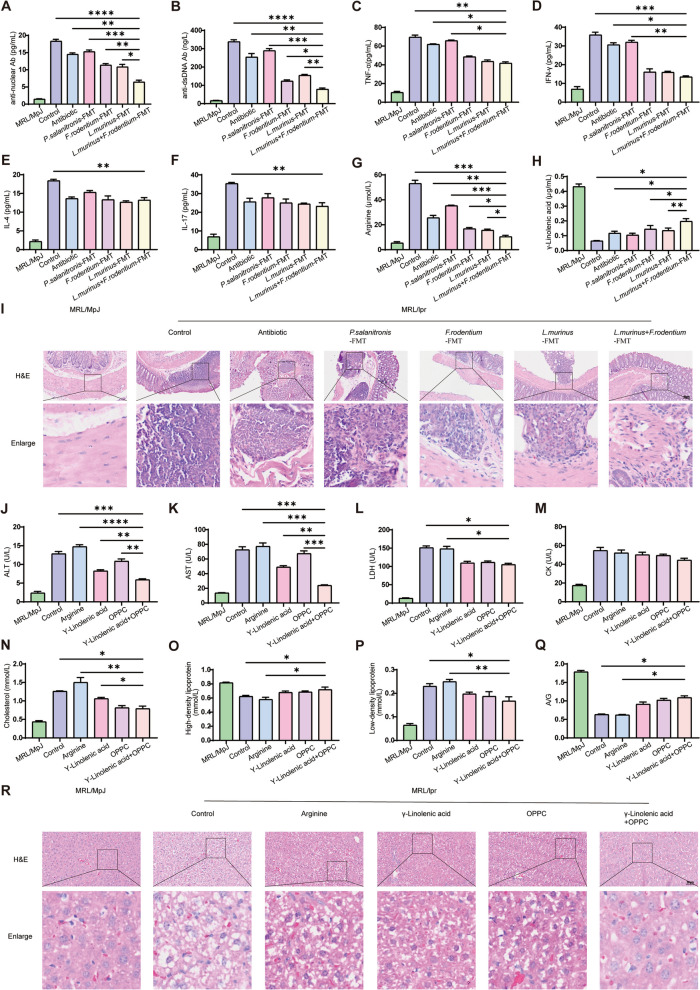
Based on fecal metagenomic sequencing of the control (Atg5+/+) and Atg5−/− basophil transplanted MRL/lpr mice, we conducted FMT targeting the most enriched taxa in Atg5−/− basophil groups, L. murinus and F. rodentium, and the control group's P. salivarius. Plasma autoantibody levels in MRL/lpr mice showed significantly lower anti-nuclear and anti-dsDNA antibodies in the F. rodentium FMT and L. murinus FMT groups compared to that in the controls and P. salivarius FMT, with the combined group exhibiting further reduction (Fig. 7A, B). This suggests that F. rodentium and L. murinus caused a reduction in autoantibody expression. Inflammatory cytokines TNF-α and IFN-γ were significantly lower in the L. murinus FMT, F. rodentium FMT, and combined groups versus controls and P. salivarius FMT, with no differences among the treatment groups (Fig. 7C, D). IL-4 and IL-17 levels were also reduced in the treatment groups compared to that in the controls, without significant variations between the groups (Fig. 7E, F). Intestinal pathology indicated less inflammatory cell deposition in the F. rodentium FMT and L. murinus FMT groups than that in controls and P. salivarius FMT, with the combined group showing further reduction (Fig. 7I, S4). These findings indicate that P. salivarius exacerbates intestinal inflammation in MRL/lpr mice, whereas F. rodentium and L. murinus ameliorate it, with a combined approach offering superior therapeutic benefits.
Fig. 7.
Transplantation of Ligilactobacillus. murinus and Faecalibaculum. rodentium improves metabolism through the increased production of GLA and OPPC. A, B Statistical analysis of plasma anti-nuclear and anti-dsDNA antibodies in mice. C–F Statistical analysis of plasma cytokines, including TNF-α, IFN-γ, IL-4, and IL-17, in mice. G–H Statistical analysis of plasma arginine and γ-linolenic acid in mice. I H&E staining analysis of colon pathology in mice. J–Q Statistical analysis of plasma ALT, AST, LDH, CK, cholesterol, HDL, LDL, and A/G ratio in mice. R H&E staining analysis of liver pathology in mice. * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001
Notably, in our analysis of amino acid and lipid profiles in MRL/lpr mice, we noted significantly lower arginine levels in the F. rodentium FMT and L. murinus FMT groups relative to the control and P. salanitronis FMT groups. The L. murinus + F. rodentium FMT group exhibited even lower arginine levels (Fig. 7G). In contrast, GLA levels were markedly higher in the F. rodentium FMT and L. murinus FMT groups, with the L. murinus + F. rodentium FMT group showing a further increase (Fig. 7H). These findings align with our plasma metabolomic data, suggesting that Atg5 deficiency in basophils enriches L. murinus and F. rodentium, promoting GLA production through lipid metabolism and improving overall metabolism, whereas basophil enrichment of P. salanitronis and other microbes through amino acid metabolism leads to arginine production and disease exacerbation. We then administered the most enriched metabolites from the control group (arginine) and the Atg5−/− basophil group (GLA and OPPC) to MRL/lpr mice to assess their impact on disease progression. The GLA and OPPC treatments significantly reduced AST, ALT, and LDH levels compared to the control and arginine groups, with the combined treatment showing further reductions in AST and ALT (Fig. 7J–L). CK levels remained unchanged across all groups (Fig. 7M). These results indicate that arginine increases transaminases in MRL/lpr mice, while GLA and OPPC ameliorate this increase, with the combined treatment showing superior therapeutic effects. Protein and lipid analyses revealed that GLA and OPPC interventions decreased cholesterol levels in MRL/lpr mice, probably by elevating HDL and reducing LDL (Fig. 7N–P). The A/G ratio improved with GLA and OPPC treatments, with no significant differences observed between combined and individual treatments (Fig. 7Q). Pathological assessments of livers from MRL/lpr mice treated with GLA and OPPC showed significant improvements in hepatocyte degeneration and necrosis compared to the control and arginine groups, with the combined treatment group showing further improvements (Fig. 7R, S5). In conclusion, Atg5 deficiency in basophils improves liver function and metabolism by enriching L. murinus and F. rodentium to produce GLA and OPPC.
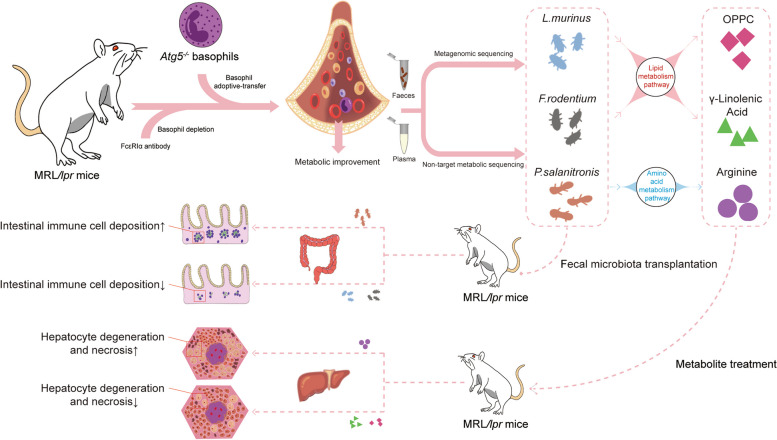
Discussion
SLE, a chronic autoimmune disease, triggers an excessive immune response, causing significant autoantibody and pro-inflammatory cytokine production. This damages multiple organs and systems; however, their underlying mechanisms remain need further study [27]. The impact of an Atg5 deficiency on intestinal flora, metabolism changes, and disease progression in SLE, mediated by basophils, warrants further investigation. We investigated the effect of Atg5-deficient basophils on the gut microbiota and their impact on disease progression (Fig. 8). Colonic pathological staining revealed significantly fewer inflammatory cells in the Atg5−/− group than that in the control group, indicating that Atg5−/− basophils could alleviate colonic inflammation in mice with lupus. Various autoantibodies characteristically occur in SLE. Autoantibodies against nuclear proteins such as anti-nuclear and anti-dsDNA antibodies serve as diagnostic markers for SLE [28]. The origin, specificity, and pathogenicity of these antibodies have been studied extensively, and they may contribute to multiple end-organ injuries in patients with SLE. Anti-dsDNA antibodies may also contribute to SLE pathogenesis by catalyzing the hydrolysis of specific DNA molecules or peptides within cells [29]. In our study, the levels of anti-nuclear and anti-dsDNA antibodies significantly reduced in basophil autophagy-deficient lupus mice. Inflammatory cytokines are immunomodulatory molecules produced by immune cells that are associated with SLE disease activity [30]. Levels of TNF-α, IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-13, and IL-17 significantly decreased in basophil autophagy-deficient lupus mice. In conclusion, defects in basophil autophagy reduce autoantibody and inflammatory cytokine production in mice with lupus. During plasma biochemical metabolism, HDL transports cholesterol to various organs, improves immunity, and prevents atherosclerosis [31]. However, LDLs lead to further deposition of cholesterol in the blood vessel wall, which aggravates atherosclerosis. Bile acids are the end-products of cholesterol metabolism in the liver [32]. In our results on plasma biochemical metabolism, the levels of plasma cholesterol, bile acids, and LDL decreased in basophil autophagy-deficient lupus mice; however, the levels of HDL did not change significantly. Plasma albumin increased, globulin decreased, and the A/G ratio improved, indicating that basophil autophagy defects improved metabolic levels in vivo. The observed increase in the levels of IL-4, LDL, and ALT in MRL/lpr mice following adoptive transfer from Atg5+/+ basophils raises concerns about potential Th2-mediated immune response. Indeed, Th2 cells reportedly play a crucial role in lupus pathogenesis, particularly in B-cell activation and antibody production, which can contribute to immune complex deposition and subsequent tissue damage, including in the liver [33]. The increased LDL observed in MRL/lpr mice following adoptive transfer could be associated with Th2-mediated immune activation. Th2 cells can influence lipid metabolism, as seen in conditions such as atherosclerosis, where Th2 cytokines contribute to plaque formation and lipid deposition. In the context of lupus, dysregulated Th2 responses could potentially lead to altered lipid profiles, including increased LDL levels, which are known to contribute to cardiovascular disease risk in patients with lupus [34, 35]. The increase in the levels of ALT, a marker of liver injury, following adoptive transfer could be a consequence of the pro-inflammatory environment induced by Th2-associated immunity. While Th2 responses are generally considered to be anti-inflammatory, they can also contribute to tissue damage in certain contexts. For example, IL-4, a Th2 cytokine, has been shown to induce hepatic inflammation in certain models. In lupus, the interaction between Th2-associated immunity and the liver can lead to hepatocyte necrosis or apoptosis, ultimately resulting in liver injury. The liver is a crucial organ for detoxification and immune responses, and its dysfunction can be a direct result of the inflammatory cytokines secreted by activated immune cells, including those of the Th2 lineage [36, 37]. In summary, the observed increase in IL-4, LDL, and ALT levels in MRL/lpr mice following adoptive transfer from Atg5+/+ basophils could indeed be related to Th2-mediated immune activation. Th2-associated immunity, with its influence on B-cell activation, antibody production, and cytokine profiles, can contribute to both lipid dysregulation and liver injury in lupus. This highlights the complexity of immune responses in lupus and the potential of Th2-associated immunity to play a pathogenic role in certain contexts. Other studies have reported that α-HBD is closely related to SLE and may be a predictive marker for lupus [26], but there is no significant change in our results.
Fig. 8.
Atg5 deficiency in basophils improves metabolism in MRL/lpr mice by regulating gut microbiota dysbiosis
Metagenomic sequencing revealed a significant increase in Ligilactobacillus and Faecalitalea at the genus level in Atg5−/− mice. At the species level, the abundance of Bacteroides_sp_CAG_927, Bacteroides_pyogenes, and others was significantly reduced in the Atg5−/− group. Ligilactobacillus, formerly a part of the Lactobacillus genus, is now classified as a distinct genus. Current research indicates a link between an imbalance in the gut microbiota and autoimmune diseases. Lactobacillus spp. may influence autoimmune conditions, such as lupus, via modulating the gut microbiota. Recent studies have suggested that probiotics and synbiotics could be alternative treatments with potential clinical benefits for autoimmune diseases such as lupus. Previous studies have demonstrated that Lactobacillus supplementation alleviates autoimmune diseases in mice [38]. Ligilactobacillus may mitigate SLE by potentially regulating immune responses and remodeling gut microbiota [39]. Faecalibaculum, a probiotic, plays a crucial role in maintaining the gut microbiome balance and promoting intestinal health. Studies indicate that Faecalibaculum negatively correlates with IFN-γ and IL-4 levels. Post-treatment colitis is often characterized by an abundance of Faecalibaculum. Therefore, basophils with Atg5 deficiency may alleviate lupus symptoms by increasing Faecalibaculum levels. Bacteroides sp. CAG 927 is a member of Bacteroides. Variations in the number and types of Bacteroides spp. are reportedly linked to autoimmune diseases. Upon analyzing the metagenomes of 307 fecal samples from 102 patients with autoimmune diseases and 86 healthy controls, the metagenomes of patients with autoimmune diseases were found to significantly differ from those of healthy controls [39]. Autoimmune diseases exhibit an increased relative abundance of Bacteroides. Barnesiella_sp_CU968 is a member of the genus Barnesiella. Barnesiella spp. can exacerbate pathogenic autoimmunity and inflammation in SLE [7]. Another study reported an increased Barnesiella abundance in patients with SLE [40]. Its abundance correlates with various immunoregulatory cells, including B cells and invariant natural killer T cells, in the spleen and liver marginal zones [41]. Duncaniella dubosii is a member of the genus Duncaniella. Research identifying gut microbial species associated with disease variability in a common mouse model of colitis found that Duncaniella dubosii, the closest phylogenetic neighbor according to 16S rRNA gene analysis, shared 94.04% sequence identity. Therefore, Duncaniella dubosii infection may be associated with autoimmune diseases. Basophils with Atg5 deficiency may alleviate lupus symptoms by increasing Ligilactobacillus and Faecalibaculum and reducing various Bacteroides and Phocaeicola species. In the Atg5−/− group, enriched bacteria such as Ligilactobacillus and Faecalitalea were positively correlated with pathways such as “Starch and sucrose metabolism,” “Fructose and mannose metabolism,” and “Glycerolipid metabolism,” but negatively correlated with “Alanine, aspartate, and glutamate metabolism,” “Oxidative phosphorylation,” and “Arginine biosynthesis.” Conversely, in the control group, bacteria such as Bacteroides_sp_CAG_927 and others showed a positive correlation with “Alanine, aspartate, and glutamate metabolism,” “Oxidative phosphorylation,” and “Arginine biosynthesis” pathways, while showing a negative correlation with “Starch and sucrose metabolism,” “Fructose and mannose metabolism,” and “Glycerolipid metabolism.”
Lipid metabolism is vital for cellular functions and immune responses. Lipids synthesized de novo or acquired from exogenous sources are crucial for energy production, cell growth, membrane formation, and cell signaling [42]. Current therapies for autoimmune diseases often target lipid metabolic pathways for therapeutic effects. Lipid metabolism may contribute to the pathogenesis of autoimmune diseases and related comorbidities [43]. Consequently, basophils with Atg5 deficiency may mitigate disease by correcting gut microbiota imbalances and enhancing glycolytic and lipid metabolism. In addition to carbohydrates, amino acids are essential for immune cell development, particularly for effector functions [44]. Arginine, a non-essential amino acid, serves as a precursor of ornithine, citrulline, and nitrite [45]. De novo synthesis of arginine uses aspartate and scavenges excess citrulline to produce arginine for nitric oxide species (NOS) generation [46]. The conversion of citrulline to arginine is an energy-intensive process involving the hydrolysis of ATP to AMP. Once it becomes the reactive intermediate citrulline adenylate with ATP, it undergoes a rate-limiting step catalyzed by arginosuccinate synthetase, which requires aspartate and releases AMP to form arginosuccinate. Arginosuccinate is converted into arginine by arginosuccinate lyase, which releases fumarate as a byproduct. Once formed, arginine is catabolized into ornithine and urea by arginase-1, or NO and citrulline by iNOS. Ornithine and citrulline are involved in downstream metabolic pathways, including the uric acid cycle. The upregulation of iNOS and arginase-1 correlates strongly with immune cell activation and is closely linked to immune responses in both healthy and diseased states [47]. Consequently, basophil autophagy deficiency may suppress arginase-1 expression by influencing gut microbiota imbalance and inhibiting amino acid metabolism. We found that elevated levels of Ligilactobacillus and Faecalitalea were negatively correlated with plasma autoantibodies, pro-inflammatory cytokines, and metabolic indices such as HDL, albumin, and A/G ratio. Metabolomic analysis revealed increases in GLA, DLPC, and OPPC and decreased levels of arginine, methylmalonic acid, and 2-hydroxyglutaric acid in the Atg5−/− group compared to that in the control group. GLA is an essential unsaturated fatty acid. Previous research showed that GLA significantly inhibited lymphocyte proliferation and reduced TNF-α and IL-2 secretion by stimulating human T cells in vitro. Low fatty acid levels in the plasma phospholipids of patients with SLE may cause unchecked T cell proliferation and increased TNF-α, IL-1, and IL-2 production, contributing to inflammation because fatty acids such as GLA can inhibit T cell proliferation and reduce TNF-α, IL-1, and IL-2 production [48]. Another study suggested that evening primrose oil, probably owing to its GLA content, extended the survival of autoimmune mice [49]. Additionally, GLA derivatives may inhibit the conversion of arachidonic acid into pro-inflammatory leukotrienes. DLPC, a form of phosphatidylcholine with lauroyl groups at positions 1 and 2, is a key component of polyenylphosphatidylcholine, which is a polyunsaturated phosphatidylcholine mixture. DLPC inhibits NLRP3 inflammasome activation, reducing IL-1β, IL-18, IL-6, and TNF-α secretion in lipopolysaccharide-primed bone marrow-derived macrophages when activated by ATP or free fatty acids [50, 51]. OPPC is an amphoteric phospholipid. Studies on LPLA2 null mice, which show lymphoid hypertrophy, glomerulonephritis, and abnormal serologies, suggest that low OPPC levels contribute to autoimmunity via phospholipidosis [52]. Thus, an Ag5 deficiency in basophils may mitigate lupus by enriching the Ligilactobacillus population and enhancing lipid metabolism. We found that in the Atg5−/− group, Ligilactobacillus and Faecalitalea were positively correlated with beneficial metabolites such as GLA, DLPC, and OPPC and negatively correlated with arginine, methylmalonic acid, and 2-hydroxyglutaric acid. GLA, DLPC, and OPPC negatively correlated with plasma autoantibodies and inflammatory cytokines. Additionally, these metabolites were negatively associated with enzymes and lipids, including AST, ALT, LDH, CK, globulin, cholesterol, and LDL, but positively associated with HDL, albumin, and A/G ratio. In conclusion, knocking out basophilic Atg5 improves metabolism in MRL/lpr mice by modulating gut microbiota dysbiosis.
The interplay among the immune system, gut microbiota, and metabolites in SLE has been a growing area of interest, with our study offering novel insights into this complex dynamic. Our findings highlight several innovative aspects, including the use of Atg5−/− basophils as an autophagic inhibitor to investigate its impact on lupus metabolism, which, to our knowledge, has not been previously explored. These results reveal that autophagic inhibition in basophils significantly reduces the levels of plasma autoantibodies and inflammatory cytokines, suggesting a potential immunomodulatory role of basophils in SLE. This is a significant advancement from previous studies that have reported on the presence of autoantibodies and cytokines in SLE by demonstrating a direct effect of modulating basophil autophagy on these markers. Moreover, our study is the first to demonstrate that the transplantation of Atg5−/− basophils can improve gut microbiota balance in MRL/lpr mice, a model of lupus, by enriching beneficial bacteria and reducing potentially pathogenic ones. This finding underscores the importance of the gut-immune axis in SLE and suggests that targeting basophil autophagy could be a viable therapeutic strategy to modulate this axis. However, our study is not without limitations. The use of a single lupus-prone mouse model may limit the generalizability of our findings to other SLE subsets or genetic backgrounds. In our study, we have taken steps to control for genetic background differences by using Atg5+/+ mice with the same genetic background (B6) as controls for the Atg5−/− mice. We observed significant differences between the Atg5−/− and Atg5+/+ basophil transplant groups, while no significant differences were found between the Atg5+/+ basophil transplant group and the control group. These observations suggest that the genetic background itself does not significantly confound the immune response differences we are investigating.
Future studies should include a broader range of models to validate our results and explore the impact of basophil autophagy across different SLE phenotypes. Additionally, while our study provides evidence for the role of basophil autophagy in modulating gut microbiota and metabolism in SLE, the precise molecular mechanisms remain to be fully elucidated. Further research is needed to dissect the signaling pathways involved and to understand how changes in the microbiota translate into metabolic improvements. Nevertheless, our study opens new avenues for research into the role of basophils in autoimmune diseases. The therapeutic potential of targeting basophil autophagy appears promising and warrants further investigation. Future work should focus on translating these findings into clinical applications and exploring the efficacy and safety of modulating basophil autophagy in patients with SLE. In conclusion, our study revealed a novel role of basophil autophagy in SLE, highlighting its potential as a therapeutic target. While there are limitations to our work, the innovative findings provide a foundation for future research aimed at understanding the complex interactions among immune cells, the microbiome, and metabolism in autoimmune diseases.
Supplementary Information
Acknowledgements
We gratefully acknowledge the services provided by the Wuhan MetWare Biotechnology Co., Ltd. (Wuhan, China).
Abbreviations
- SLE
Systemic lupus erythematosus
- Baso
Basophil
- Atg5
Autophagy-related gene 5
- ANA
Anti-nuclear antibody
- anti-dsDNA
Anti-double-stranded DNA antibody
- TNF
Tumor necrosis factor
- IFN
Interferon
- IL
Interleukin
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- LDH
Lactate dehydrogenase
- CK
Creatine kinase
- HBDH
Hydroxybutyrate dehydrogenase
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- A
Albumin
- G
Globulin
- FMT
Fecal microbiota transplantation
- WT
Wild-type
- KO
Knockout
- GLA
Gamma-linolenic acid
- DLPC
1,2-Dilauroyl-sn-glycero-3-phosphocholine
- OPPC
Oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine
- MMA
Methylmalonic acid
Authors’ contributions
Jiaxuan Chen, Hua-feng Liu, and Qingjun Pan designed the experiments. Jiaxuan Chen, Quanren Pan, Lu Lu, and Xiaorong Huang performed the experiments. Jiaxuan Chen, Shuting Wang, Xiaoxian Liu, and Jiaqi Lun analyzed the data. Jiaxuan Chen, Xiaowei Xu, Hongyong Su, Fengbiao Guo, Lawei Yang, Liuyong You, Haiyan Xiao, and Wenying Luo prepared the figures. Jiaxuan Chen and Quanren Pan wrote the paper. All authors reviewed the manuscript. All authors have read and approved the article.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82070757, 82270770) (Q-J.P.), Guangdong Provincial Key Laboratory of Autophagy and Major Chronic Noncommunicable Diseases (2022B1212030003) (H-F.L.), Science and Technology Planning Project of Zhanjiang City (2021A05067) (H.S.).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All experiments using animals were approved by the Ethics Committee for Experimental Animals of the Affiliated Hospital of Guangdong Medical University (Approval no. GDY2202248). All experiments were conducted according to the national animal welfare guidelines.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaxuan Chen, Quanren Pan, Lu Lu and Xiaorong Huang contributed equally to this work.
Contributor Information
Hua-feng Liu, Email: liuhf@gdmu.edu.cn.
Qingjun Pan, Email: pqj@gdmu.edu.cn.
References
- 1.Perez RK, Gordon MG, Subramaniam M, Kim MC, Hartoularos GC, Targ S, Sun Y, Ogorodnikov A, Bueno R, Lu A, et al: Single-cell RNA-seq reveals cell type-specific molecular and genetic associations to lupus. Science. 2022;376:eabf1970. [DOI] [PMC free article] [PubMed]
- 2.Mori S, Kohyama M, Yasumizu Y, Tada A, Tanzawa K, Shishido T, Kishida K, Jin H, Nishide M, Kawada S, et al. Neoself-antigens are the primary target for autoreactive T cells in human lupus. Cell. 2024;187:6071-6087.e6020. [DOI] [PubMed] [Google Scholar]
- 3.Durcan L, O’Dwyer T, Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet. 2019;393:2332–43. [DOI] [PubMed] [Google Scholar]
- 4.Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 2016;30:1589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christovich A, Luo XM. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front Immunol. 2022;13:946248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiao Y, Wu L, Huntington ND, Zhang X. Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front Immunol. 2020;11:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Lin J, Xiao L, Zhang X, Zhao L, Wang M, Li L. Gut microbiota in systemic lupus erythematosus: A fuse and a solution. J Autoimmun. 2022;132:102867. [DOI] [PubMed] [Google Scholar]
- 8.Tong Y, Marion T, Schett G, Luo Y, Liu Y. Microbiota and metabolites in rheumatic diseases. Autoimmun Rev. 2020;19:102530. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Qing P, Yang H, Wu Y, Liu Y, Luo Y. Gut Microbiome and Metabolites in Systemic Lupus Erythematosus: Link. Mechanisms and Intervention Front Immunol. 2021;12:686501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol. 2009;9:9–13. [DOI] [PubMed] [Google Scholar]
- 11.Tchen J, Simon Q, Chapart L, Thaminy MK, Vibhushan S, Saveanu L, Lamri Y, Saidoune F, Pacreau E, Pellefigues C, et al. PD-L1- and IL-4-expressing basophils promote pathogenic accumulation of T follicular helper cells in lupus. Nat Commun. 2024;15:3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dossybayeva K, Abdukhakimova D, Poddighe D. Basophils and Systemic Lupus Erythematosus in Murine Models and Human Patients. Biology (Basel). 2020;9(10):308. 10.3390/biology9100308. [DOI] [PMC free article] [PubMed]
- 14.Pan Q, Gong L, Xiao H, Feng Y, Li L, Deng Z, Ye L, Zheng J, Dickerson CA, Ye L, et al. Basophil Activation-Dependent Autoantibody and Interleukin-17 Production Exacerbate Systemic Lupus Erythematosus. Front Immunol. 2017;8:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dema B, Lamri Y, Pellefigues C, Pacreau E, Saidoune F, Bidault C, Karasuyama H, Sacré K, Daugas E, Charles N. Basophils contribute to pristane-induced Lupus-like nephritis model. Sci Rep. 2017;7:7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galluzzi L, Green DR. Autophagy-Independent Functions of the Autophagy Machinery. Cell. 2019;177:1682–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan Q, Gao C, Chen Y, Feng Y, Liu WJ, Liu HF. Update on the role of autophagy in systemic lupus erythematosus: A novel therapeutic target. Biomed Pharmacother. 2015;71:190–3. [DOI] [PubMed] [Google Scholar]
- 18.Dang J, Li J, Xin Q, Shan S, Bian X, Yuan Q, Liu N, Ma X, Li Y, Liu Q. Gene-gene interaction of ATG5, ATG7, BLK and BANK1 in systemic lupus erythematosus. Int J Rheum Dis. 2016;19:1284–93. [DOI] [PubMed] [Google Scholar]
- 19.Gros F, Arnold J, Page N, Décossas M, Korganow AS, Martin T, Muller S. Macroautophagy is deregulated in murine and human lupus T lymphocytes. Autophagy. 2012;8:1113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alessandri C, Barbati C, Vacirca D, Piscopo P, Confaloni A, Sanchez M, Maselli A, Colasanti T, Conti F, Truglia S, et al. T lymphocytes from patients with systemic lupus erythematosus are resistant to induction of autophagy. Faseb j. 2012;26:4722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke AJ, Ellinghaus U, Cortini A, Stranks A, Simon AK, Botto M, Vyse TJ. Autophagy is activated in systemic lupus erythematosus and required for plasmablast development. Ann Rheum Dis. 2015;74:912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B, Yue Y, Dong C, Shi Y, Xiong S. Blockade of macrophage autophagy ameliorates activated lymphocytes-derived DNA induced murine lupus possibly via inhibition of proinflammatory cytokine production. Clin Exp Rheumatol. 2014;32:705–14. [PubMed] [Google Scholar]
- 23.An N, Chen Y, Wang C, Yang C, Wu ZH, Xue J, Ye L, Wang S, Liu HF, Pan Q. Chloroquine Autophagic Inhibition Rebalances Th17/Treg-Mediated Immunity and Ameliorates Systemic Lupus Erythematosus. Cell Physiol Biochem. 2017;44:412–22. [DOI] [PubMed] [Google Scholar]
- 24.Weindel CG, Richey LJ, Bolland S, Mehta AJ, Kearney JF, Huber BT. B cell autophagy mediates TLR7-dependent autoimmunity and inflammation. Autophagy. 2015;11:1010–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seelen MA, Trouw LA, Daha MR. Diagnostic and prognostic significance of anti-C1q antibodies in systemic lupus erythematosus. Curr Opin Nephrol Hypertens. 2003;12:619–24. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Han H, Li J, Li D, Jiang L. Alpha-hydroxybutyrate dehydrogenase as a biomarker for predicting systemic lupus erythematosus with liver injury. Int Immunopharmacol. 2019;77:105922. [DOI] [PubMed] [Google Scholar]
- 27.Morand EF, Fernandez-Ruiz R, Blazer A, Niewold TB. Advances in the management of systemic lupus erythematosus. BMJ. 2023;383:e073980. [DOI] [PubMed] [Google Scholar]
- 28.Bossuyt X, De Langhe E, Borghi MO, Meroni PL. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat Rev Rheumatol. 2020;16:715–26. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Xiao S, Xia Y, Wang H. The Therapeutic Strategies for SLE by Targeting Anti-dsDNA Antibodies. Clin Rev Allergy Immunol. 2022;63:152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rönnblom L, Leonard D. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med. 2019;6:e000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding WY, Protty MB, Davies IG, Lip GYH. Relationship between lipoproteins, thrombosis, and atrial fibrillation. Cardiovasc Res. 2022;118:716–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu H, Shao W, Liu Q, Liu N, Wang Q, Xu J, Zhang X, Weng Z, Lu Q, Jiao L, et al. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat Commun. 2022;13:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gieseck RL 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62–76. [DOI] [PubMed] [Google Scholar]
- 34.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348–58. [DOI] [PubMed] [Google Scholar]
- 35.Toubi E, Shoenfeld Y. Predictive and protective autoimmunity in cardiovascular diseases: is vaccination therapy a reality? Lupus. 2005;14:665–9. [DOI] [PubMed] [Google Scholar]
- 36.Hong J, Wang L, Zhao X, Yu X, Sheng L, Xu B, Liu D, Zhu Y, Long Y, Hong F. Th2 factors may be involved in TiO₂ NP-induced hepatic inflammation. J Agric Food Chem. 2014;62:6871–8. [DOI] [PubMed] [Google Scholar]
- 37.Reißing J, Berres M, Strnad P, Wree A, Inzaugarat ME, Trautwein C, Bruns T, Zimmermann HW. Th2 Cell Activation in Chronic Liver Disease Is Driven by Local IL33 and Contributes to IL13-Dependent Fibrogenesis. Cell Mol Gastroenterol Hepatol. 2024;17:517–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim DS, Park Y, Choi JW, Park SH, Cho ML, Kwok SK. Lactobacillus acidophilus Supplementation Exerts a Synergistic Effect on Tacrolimus Efficacy by Modulating Th17/Treg Balance in Lupus-Prone Mice via the SIGNR3 Pathway. Front Immunol. 2021;12:696074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu Y, Sun S, Huang Y, Gao Q, Xie X, Wang P, Li J, Liang L, He X, Jiang Y, et al. Metagenomic analysis revealed the potential role of gut microbiome in gout. NPJ Biofilms Microbiomes. 2021;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li BZ, Zhou HY, Guo B, Chen WJ, Tao JH, Cao NW, Chu XJ, Meng X. Dysbiosis of oral microbiota is associated with systemic lupus erythematosus. Arch Oral Biol. 2020;113:104708. [DOI] [PubMed] [Google Scholar]
- 41.Presley LL, Wei B, Braun J, Borneman J. Bacteria associated with immunoregulatory cells in mice. Appl Environ Microbiol. 2010;76:936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han X. Lipidomics for studying metabolism. Nat Rev Endocrinol. 2016;12:668–79. [DOI] [PubMed] [Google Scholar]
- 43.Robinson G, Pineda-Torra I, Ciurtin C, Jury EC. Lipid metabolism in autoimmune rheumatic disease: implications for modern and conventional therapies. J Clin Invest. 2022;132(2):e148552. 10.1172/JCI148552. [DOI] [PMC free article] [PubMed]
- 44.Lemos H, Huang L, Prendergast GC, Mellor AL. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat Rev Cancer. 2019;19:162–75. [DOI] [PubMed] [Google Scholar]
- 45.Hibbs JB Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–6. [DOI] [PubMed] [Google Scholar]
- 46.Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paredes A, Justo-Méndez R, Jiménez-Blasco D, Núñez V, Calero I, Villalba-Orero M, Alegre-Martí A, Fischer T, Gradillas A, Sant’Anna VAR, et al. γ-Linolenic acid in maternal milk drives cardiac metabolic maturation. Nature. 2023;618:365–73. [DOI] [PubMed] [Google Scholar]
- 49.Brown AC. Lupus erythematosus and nutrition: a review of the literature. J Ren Nutr. 2000;10:170–83. [DOI] [PubMed] [Google Scholar]
- 50.Lee WJ, Weng SH, Su NW. Individual Phosphatidylcholine Species Analysis by RP-HPLC-ELSD for Determination of Polyenylphosphatidylcholine in Lecithins. J Agric Food Chem. 2015;63:3851–8. [DOI] [PubMed] [Google Scholar]
- 51.Liu B, Li X, Yu H, Shi X, Zhou Y, Alvarez S, Naldrett MJ, Kachman SD, Ro SH, Sun X, et al. Therapeutic potential of garlic chive-derived vesicle-like nanoparticles in NLRP3 inflammasome-mediated inflammatory diseases. Theranostics. 2021;11:9311–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abe A, Hiraoka M, Shayman JA. Positional specificity of lysosomal phospholipase A2. J Lipid Res. 2006;47:2268–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.