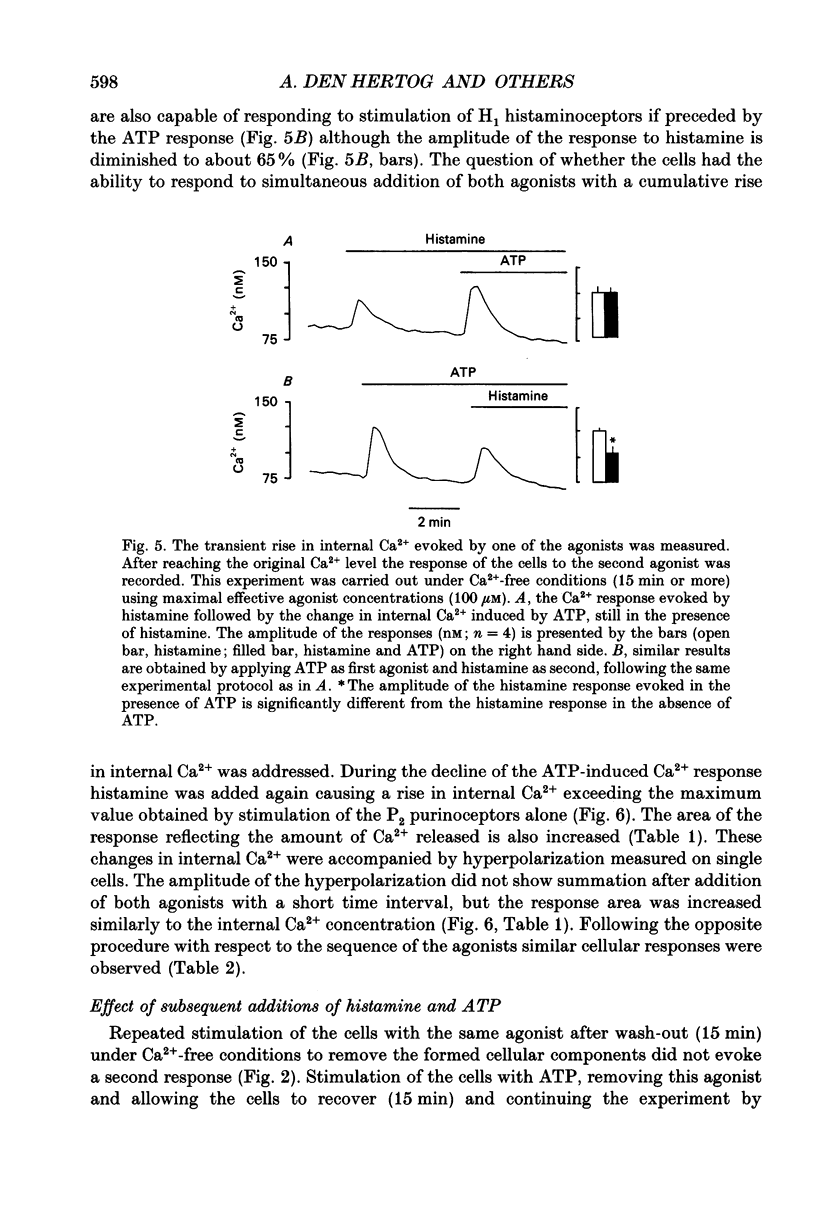
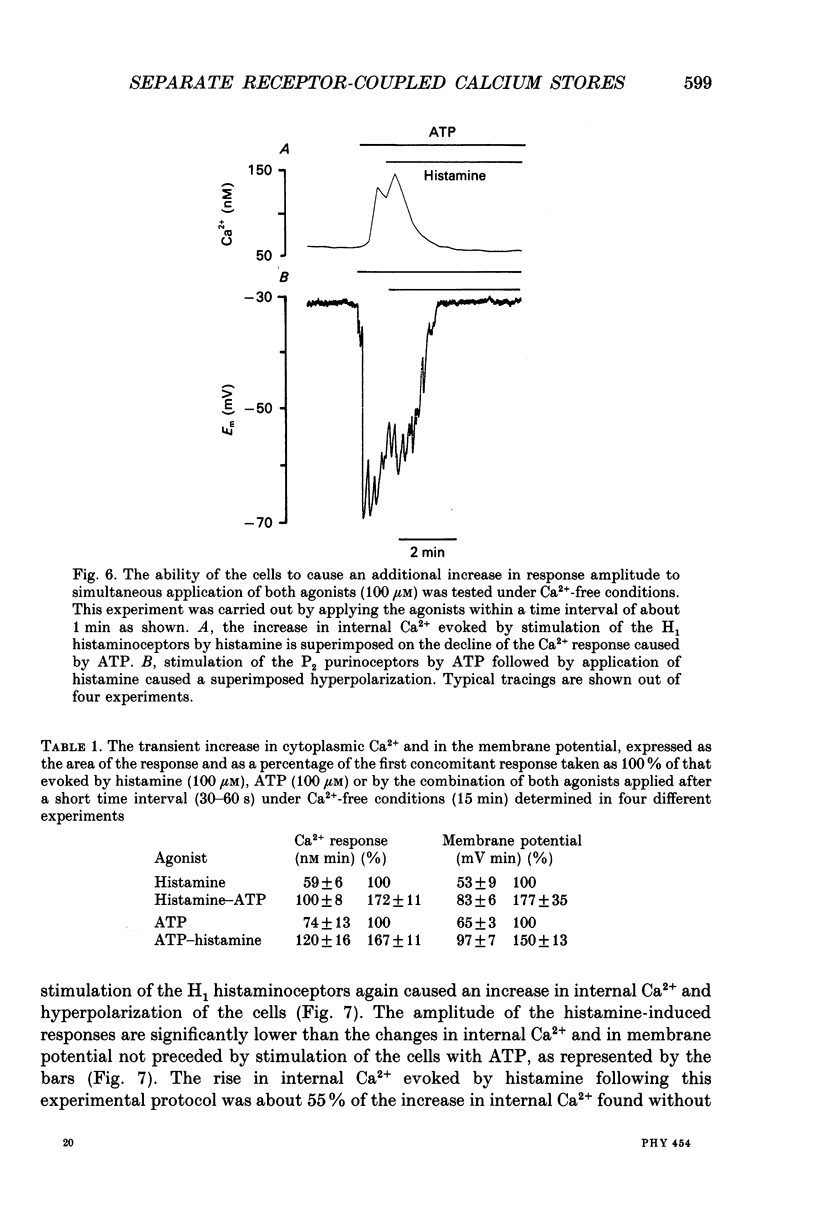
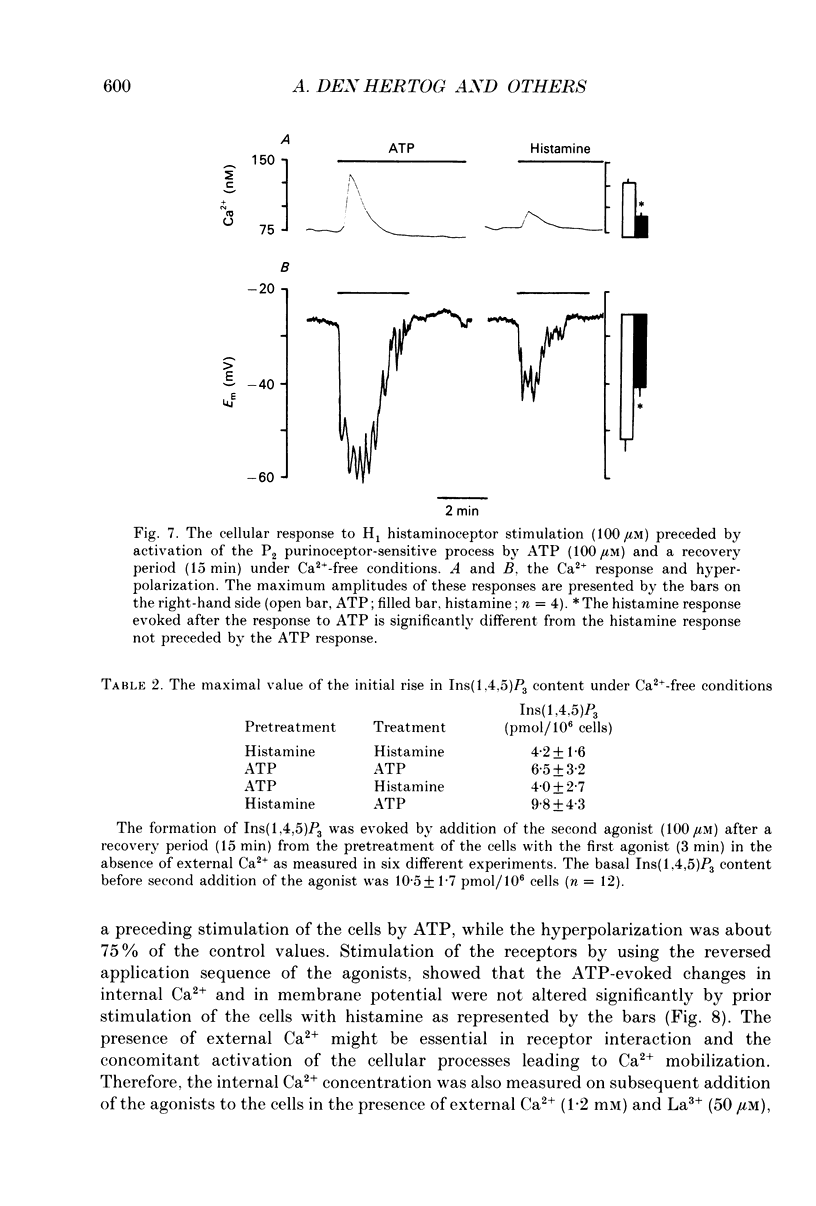
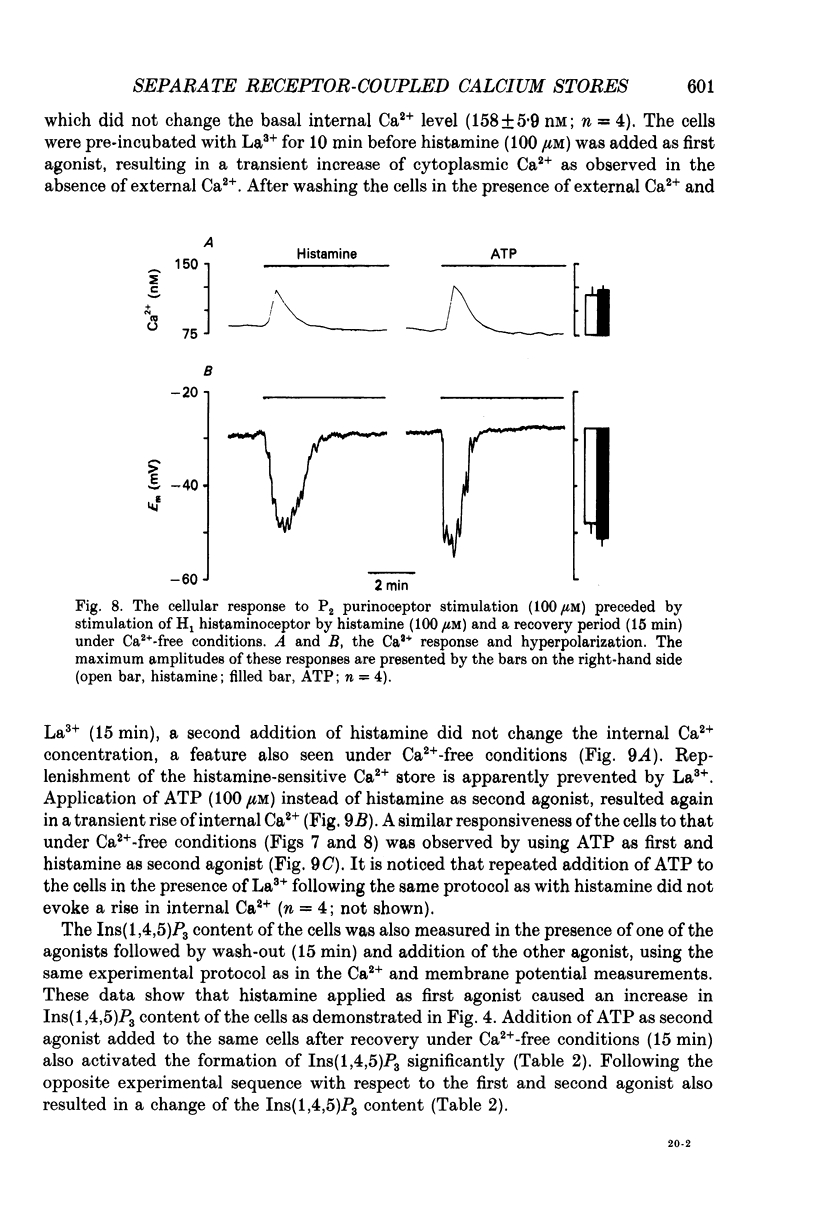
Abstract
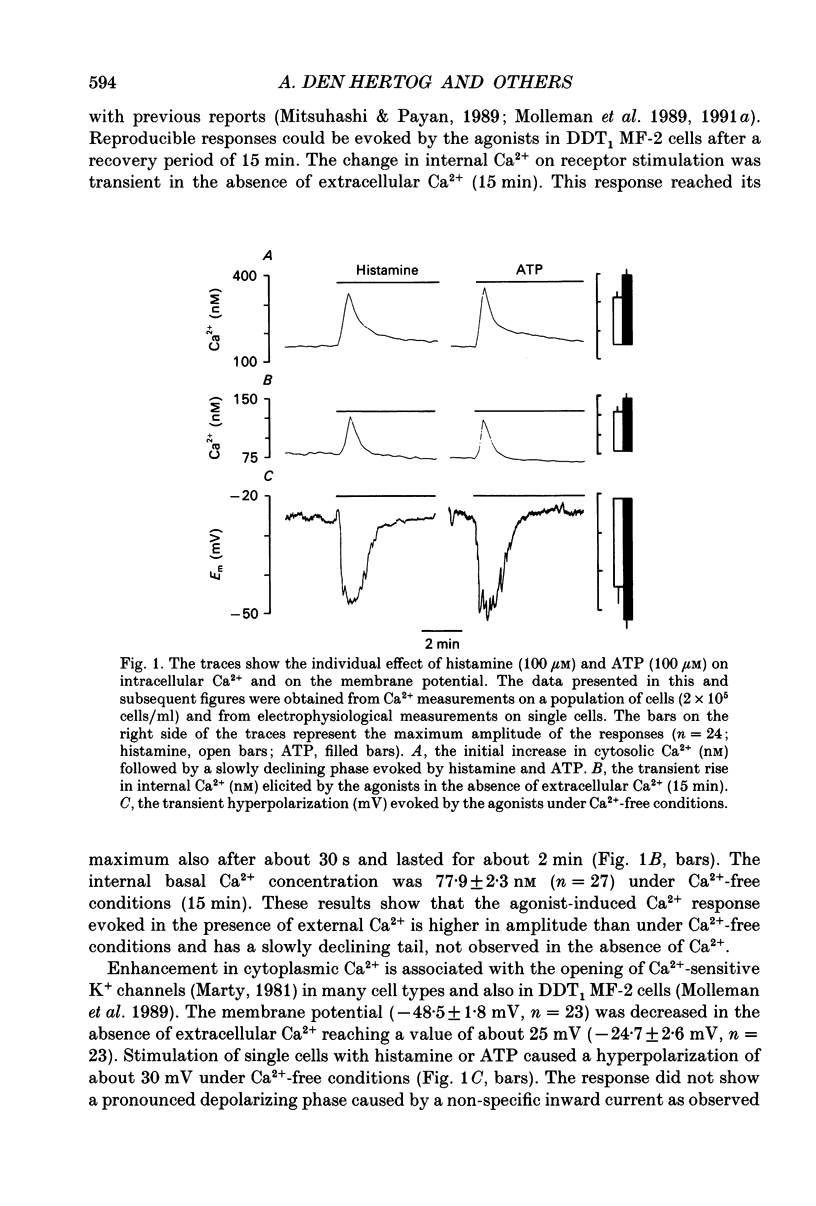
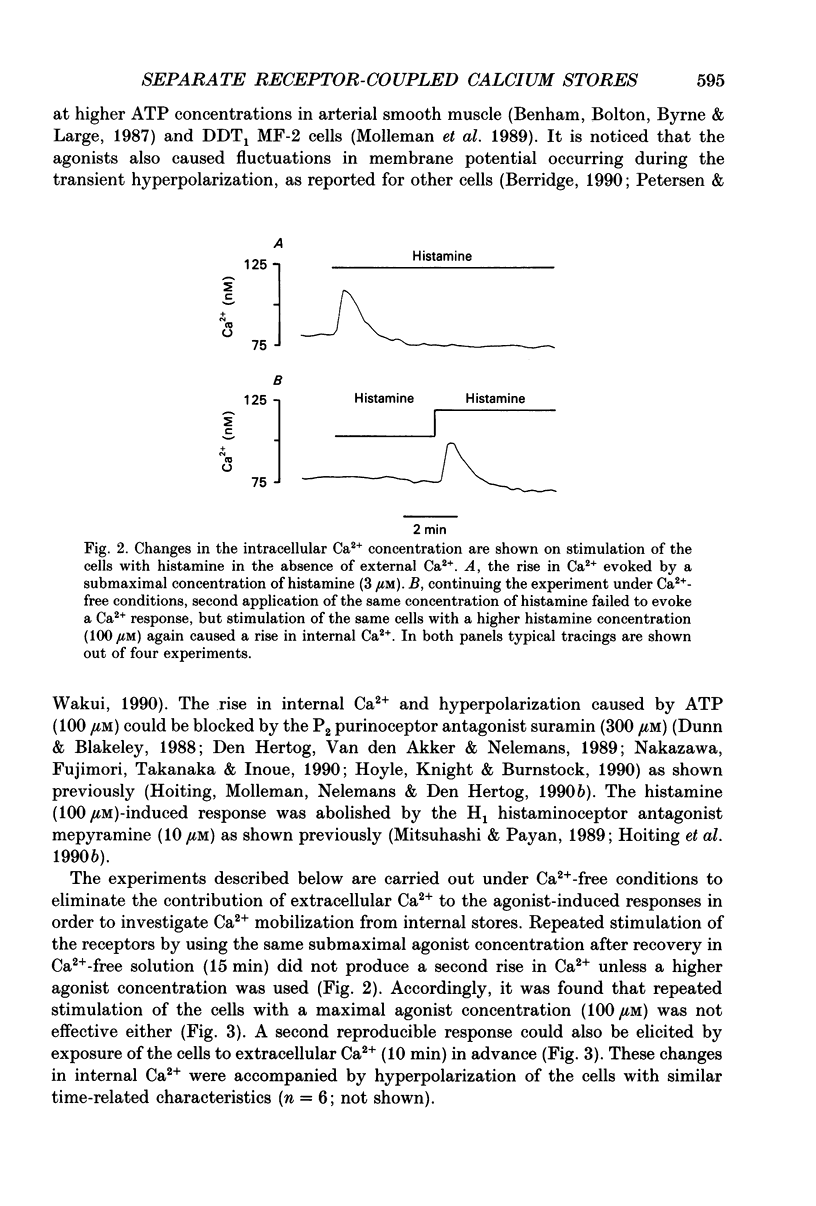
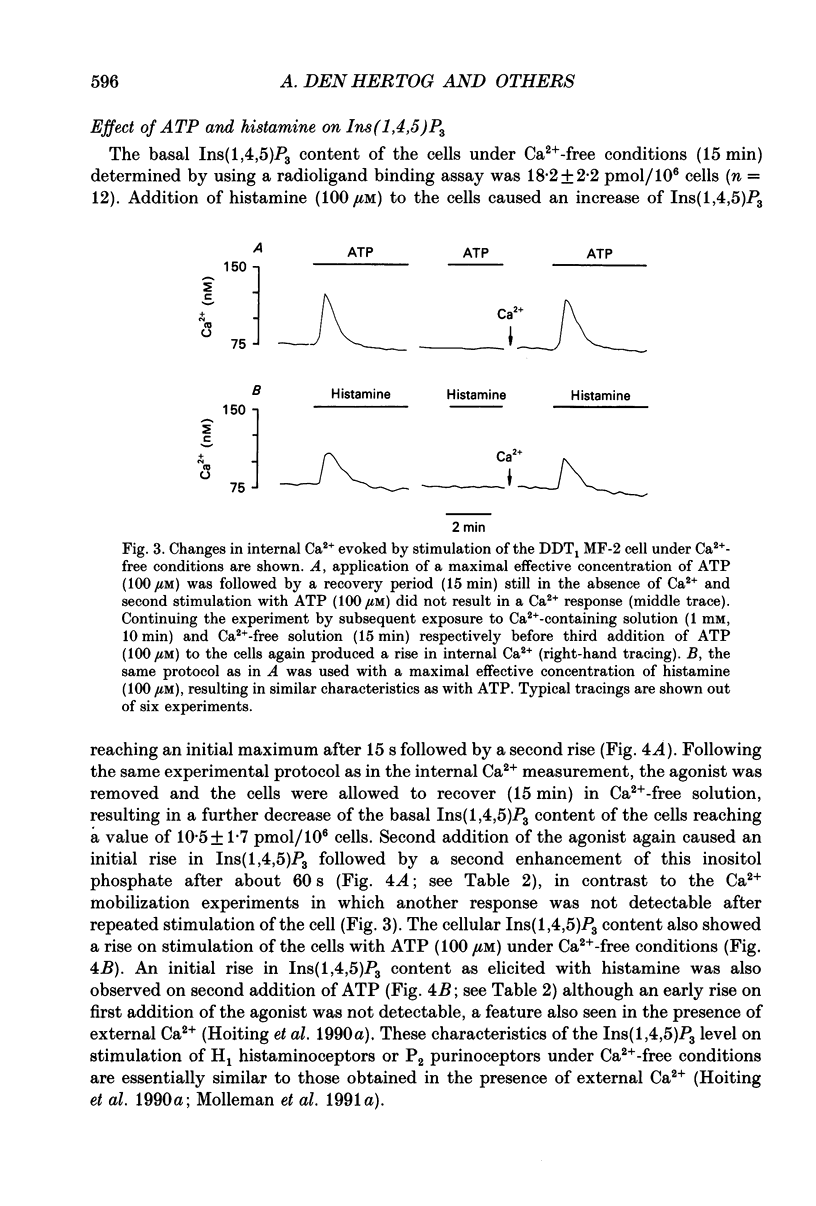
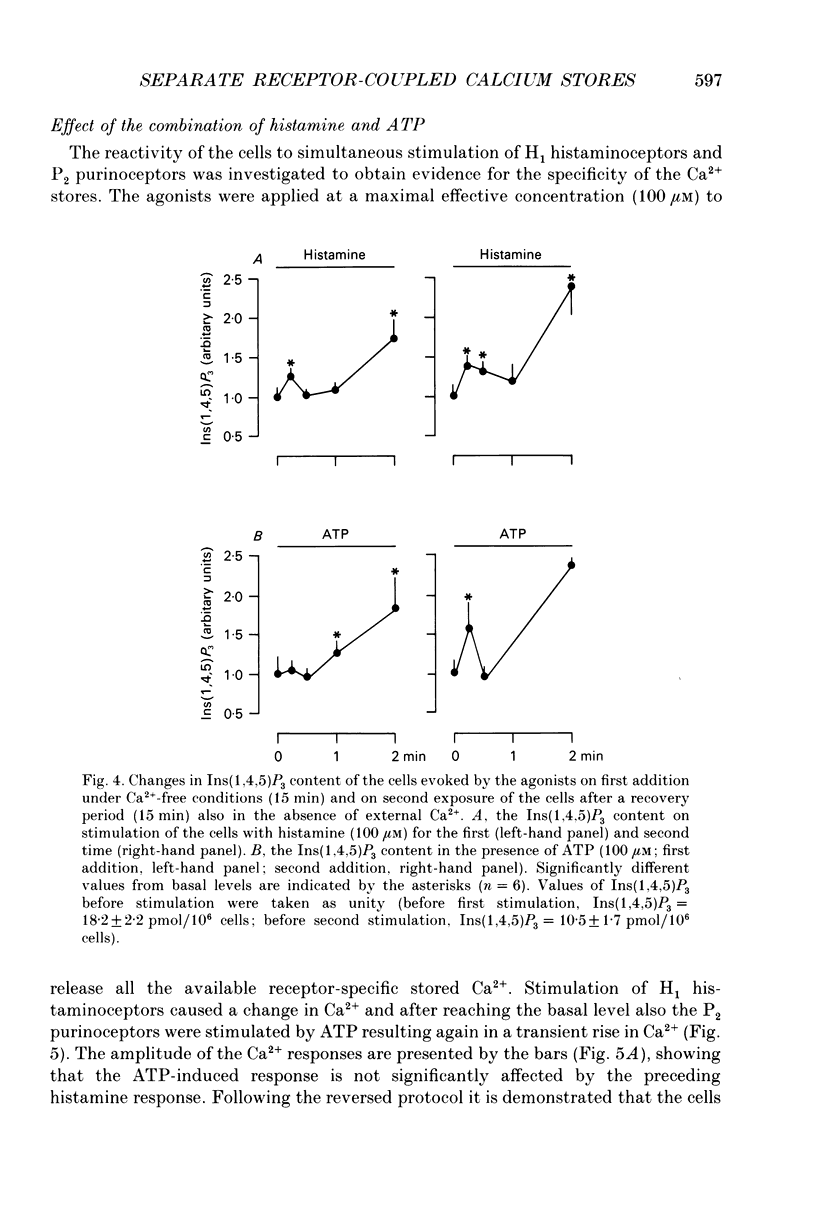
1. The specificity of intracellular Ca2+ stores to Ca(2+)-mobilizing agonists was studied in DDT1 MF-2 vas deferens cells of the Syrian hamster. 2. Application of histamine (100 microM) or ATP (100 microM) to the DDT1 MF-2 cells caused an initial increase of intracellular Ca2+ followed by a lower phase as measured by using Indo-1 as fluorescent probe at 22 degrees C. The basal Ca2+ level (146 nM) was enhanced to 309 nM by histamine and to 379 nM by ATP. 3. A transient rise in intracellular Ca2+ lasting for about 2 min was measured in the presence of histamine or ATP in the absence of extracellular Ca2+. The basal Ca2+ level (78 nM) was increased to 128 nM by histamine and to 145 nM by ATP. 4. A transient hyperpolarization was elicited in single cells as measured with microelectrodes by both agonists under Ca(2+)-free conditions with a similar time course as the change in internal Ca2+. The hyperpolarization observed in the presence of histamine amounted to 23 mV and 31 mV with ATP. The histamine-induced responses were abolished by the H1 histaminoceptor antagonist mepyramine (10 microM) and the responses evoked by ATP were blocked by the P2 purinoceptor antagonist suramin (300 microM). 5. A second internal Ca2+ response could only be evoked under Ca(2+)-free conditions by applying a higher agonist concentration or after replenishing the intracellular stores with Ca2+ from the extracellular space. 6. A second addition of an optimal concentration (100 microM) of the agonist to the cells under Ca(2+)-free conditions did not evoke mobilization of internal Ca2+ or hyperpolarization, but resulted in a rise of the cellular inositol (1,4,5)-trisphosphate content (Ins(1,4,5)P3) as determined by a radioligand binding assay. 7. The cells responded to both agonists (100 microM) with a transient Ca2+ response if successively applied at a maximal effective concentration (100 microM) under Ca(2+)-free conditions. 8. Simultaneous stimulation of H1 histaminoceptors and P2 purinoceptors resulted in the absence of external Ca2+ in an additional increase in internal Ca2+ represented by the amplitude and area of the response and in an increased response area of the hyperpolarization.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Action of externally applied adenosine triphosphate on single smooth muscle cells dispersed from rabbit ear artery. J Physiol. 1987 Jun;387:473–488. doi: 10.1113/jphysiol.1987.sp016585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J., Cobbold P. H., Cuthbertson K. S. Spatial and temporal aspects of cell signalling. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):325–343. doi: 10.1098/rstb.1988.0080. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Bielkiewicz-Vollrath B., Carpenter J. R., Schulz R., Cook D. A. Early production of 1,4,5-inositol trisphosphate and 1,3,4,5-inositol tetrakisphosphate by histamine and carbachol in ileal smooth muscle. Mol Pharmacol. 1987 May;31(5):513–522. [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate and inositol 1,4,5-trisphosphate act by different mechanisms when controlling Ca2+ in mouse lacrimal acinar cells. FEBS Lett. 1989 Jul 17;251(1-2):43–48. doi: 10.1016/0014-5793(89)81425-5. [DOI] [PubMed] [Google Scholar]
- Chilvers E. R., Challiss R. A., Barnes P. J., Nahorski S. R. Mass changes of inositol(1,4,5)trisphosphate in trachealis muscle following agonist stimulation. Eur J Pharmacol. 1989 May 30;164(3):587–590. doi: 10.1016/0014-2999(89)90269-0. [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Irvine R. F., Dawson A. P. Synergistic control of Ca2+ mobilization in permeabilized mouse L1210 lymphoma cells by inositol 2,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate. Biochem J. 1990 Oct 15;271(2):549–553. doi: 10.1042/bj2710549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A. Calcium and the action of adrenaline, adenosine triphosphate and carbachol on guinea-pig taenia caeci. J Physiol. 1982 Apr;325:423–439. doi: 10.1113/jphysiol.1982.sp014160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A. Calcium and the alpha-action of catecholamines on guinea-pig taenia caeci. J Physiol. 1981 Jul;316:109–125. doi: 10.1113/jphysiol.1981.sp013776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hertog A., Van den Akker J., Nelemans A. Suramin and the inhibitory junction potential in taenia caeci of the guinea-pig. Eur J Pharmacol. 1989 Dec 7;173(2-3):207–209. doi: 10.1016/0014-2999(89)90522-0. [DOI] [PubMed] [Google Scholar]
- Donié F., Reiser G. A novel, specific binding protein assay for quantitation of intracellular inositol 1,3,4,5-tetrakisphosphate (InsP4) using a high-affinity InsP4 receptor from cerebellum. FEBS Lett. 1989 Aug 28;254(1-2):155–158. doi: 10.1016/0014-5793(89)81029-4. [DOI] [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Hoiting B., Molleman A., Duin M., den Hertog A., Nelemans A. P2 purinoceptor-mediated inositol phosphate formation in relation to cytoplasmic calcium in DDT1 MF-2 smooth muscle cells. Eur J Pharmacol. 1990 Jul 31;189(1):31–39. doi: 10.1016/0922-4106(90)90227-o. [DOI] [PubMed] [Google Scholar]
- Hoiting B., Molleman A., Nelemans A., Den Hertog A. P2-purinoceptor-activated membrane currents and inositol tetrakisphosphate formation are blocked by suramin. Eur J Pharmacol. 1990 May 31;181(1-2):127–131. doi: 10.1016/0014-2999(90)90253-3. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutzenhiser R., van Breemen C. Mechanism of activation of isolated rabbit aorta by PGH2 analogue U-44069. Am J Physiol. 1981 Nov;241(5):C243–C249. doi: 10.1152/ajpcell.1981.241.5.C243. [DOI] [PubMed] [Google Scholar]
- Manolopoulos V. G., Pipili-Synetos E., Den Hertog A., Nelemans A. Inositol phosphates formed in rat aorta after alpha 1-adrenoceptor stimulation are inhibited by forskolin. Eur J Pharmacol. 1991 May 25;207(1):29–36. doi: 10.1016/s0922-4106(05)80034-3. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. Regulation of cytosolic free calcium in fura-2-loaded rat parotid acinar cells. J Biol Chem. 1987 Dec 25;262(36):17362–17369. [PubMed] [Google Scholar]
- Missiaen L., Declerck I., Droogmans G., Plessers L., De Smedt H., Raeymaekers L., Casteels R. Agonist-dependent Ca2+ and Mn2+ entry dependent on state of filling of Ca2+ stores in aortic smooth muscle cells of the rat. J Physiol. 1990 Aug;427:171–186. doi: 10.1113/jphysiol.1990.sp018166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi M., Payan D. G. Solubilization and characterization of the pyrilamine-binding protein from cultured smooth muscle cells. Mol Pharmacol. 1989 Jun;35(6):751–759. [PubMed] [Google Scholar]
- Molleman A., Hoiting B., Duin M., van den Akker J., Nelemans A., Den Hertog A. Potassium channels regulated by inositol 1,3,4,5-tetrakisphosphate and internal calcium in DDT1 MF-2 smooth muscle cells. J Biol Chem. 1991 Mar 25;266(9):5658–5663. [PubMed] [Google Scholar]
- Molleman A., Nelemans A., Den Hertog A. P2-purinoceptor-mediated membrane currents in DDT1 MF-2 smooth muscle cells. Eur J Pharmacol. 1989 Oct 4;169(1):167–174. doi: 10.1016/0014-2999(89)90829-7. [DOI] [PubMed] [Google Scholar]
- Molleman A., Nelemans A., van den Akker J., Duin M., den Hertog A. Voltage-dependent sodium and potassium, but no calcium conductances in DDT1 MF-2 smooth muscle cells. Pflugers Arch. 1991 Jan;417(5):479–484. doi: 10.1007/BF00370943. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989 Jan 5;264(1):205–212. [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. Reversible and selective antagonism by suramin of ATP-activated inward current in PC12 phaeochromocytoma cells. Br J Pharmacol. 1990 Sep;101(1):224–226. doi: 10.1111/j.1476-5381.1990.tb12117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelemans A., Hoiting B., Molleman A., Duin M., den Hertog A. Alpha-adrenoceptor regulation of inositol phosphates, internal calcium and membrane current in DDT1 MF-2 smooth muscle cells. Eur J Pharmacol. 1990 Jul 31;189(1):41–49. doi: 10.1016/0922-4106(90)90228-p. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Does inositol tetrakisphosphate play a role in the receptor-mediated control of calcium mobilization? Cell Calcium. 1989 Jul;10(5):375–383. doi: 10.1016/0143-4160(89)90063-8. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Wakui M. Oscillating intracellular Ca2+ signals evoked by activation of receptors linked to inositol lipid hydrolysis: mechanism of generation. J Membr Biol. 1990 Nov;118(2):93–105. doi: 10.1007/BF01868467. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Reynolds E. E., Dubyak G. R. Agonist-induced calcium transients in cultured smooth muscle cells: measurements with fura-2 loaded monolayers. Biochem Biophys Res Commun. 1986 May 14;136(3):927–934. doi: 10.1016/0006-291x(86)90421-3. [DOI] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V., Jr Characterization of calcium-activated potassium channels in single smooth muscle cells using the patch-clamp technique. Pflugers Arch. 1987 Feb;408(2):98–111. doi: 10.1007/BF00581337. [DOI] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]


