Abstract
Despite significant strides in medical treatments and surgical procedures for cardiovascular diseases, these conditions continue to be a major global health concern. The persistent need for innovative therapeutic approaches to mend damaged heart tissue highlights the complexity and urgency of this medical challenge. In recent years, stem cells have emerged as a promising tool for tissue regeneration, but challenges such as graft rejection and tumor formation have limited their clinical application. Exosomes, extracellular vesicles containing a diverse array of biomolecules, have garnered significant attention for their potential in regenerative medicine. The cardioprotective and reparative properties of mesenchymal stem cell-derived exosomes hold promise for the treatment of heart diseases. These exosomes can modulate various cellular processes, including angiogenesis, apoptosis, and inflammation, thereby enhancing cardiac function. Despite the growing interest, there remains a lack of comprehensive reviews synthesizing the molecular mechanisms, preclinical, and clinical evidence related to the specific role of MSC-derived exosomes in cardiac therapies. This review aims to fill that gap by exploring the potential of MSC-derived exosomes as a therapeutic strategy for cardiac diseases. This review explores the potential of mesenchymal stem cell-derived exosomes as a therapeutic strategy for cardiac diseases. We discuss the molecular mechanisms underlying their cardioprotective effects, summarize preclinical and clinical studies investigating their efficacy, and address the challenges and future perspectives of exosome-based therapies. The collective evidence suggests that MSC-derived exosomes hold promise as a novel and effective therapeutic approach for cardiac diseases.
Graphical abstract
Keywords: Exosomes, Mesenchymal stem cells, Cardiovascular diseases, Tissue repair, Angiogenesis
Introduction
In recent years, extensive research has been conducted on new therapeutic methods for repairing damaged heart tissue. One of the most promising approaches in this field is the use of mesenchymal stem cells (MSCs) and their derived products. MSCs were first discovered in 1968 and have been used in preclinical research for many years [1]. MSCs are multipotent cells derived from embryonic mesoderm and have the potential to differentiate into various types of connective tissue cells such as osteoblasts (bone cells), chondrocytes (cartilage cells), adipocytes (fat cells), and myocytes (muscle cells). These cells have attracted the attention of many researchers due to their ability to repair damaged tissues and their important role in regulating the immune system [2–5]. One of the prominent features of MSCs is their high ability to self-renew and differentiate into various types of connective tissue cells. This feature has made MSCs a powerful tool for tissue engineering and the treatment of various diseases [6]. In addition, MSCs have anti-inflammatory and immunosuppressive properties that make them very suitable for the treatment of autoimmune and inflammatory diseases [7]. The main sources of MSCs include bone marrow (one of the oldest and best-known sources of MSCs, adipose tissue (extracting these cells from adipose tissue is a less invasive method than bone marrow), umbilical cord (Wharton’s jelly and umbilical cord blood), dental pulp, skeletal muscle, amniotic fluid, and other sources (such as synovium, fetal liver, and some tumor tissues) [8–10]. Each of these sources has its own advantages and disadvantages, and the choice of the appropriate source depends on the type of disease and the patient’s needs. In recent years, extensive research has been conducted on the clinical applications of MSCs. These cells have been investigated in the treatment of diseases such as cardiovascular diseases, neurological diseases, bone and joint diseases, and autoimmune diseases. The results of initial studies indicate the effectiveness of MSCs in improving the function of damaged organs and reducing disease symptoms [11].
In recent years, researchers have focused on the role of extracellular vesicles (EVs), and especially exosomes, in mediating the paracrine effects of stem cells. Exosomes are membrane-bound nanoparticles that contain a wide range of biomolecules such as proteins, lipids, and nucleic acids. These molecules can be transferred to target cells and alter their function [12]. MSC-derived exosomes have emerged as a novel therapeutic tool for cardiac injuries due to their high potential for tissue repair, modulation of the immune response, and reduction of inflammation. These exosomes can be directly delivered to the site of injury and contribute to improved cardiac function by stimulating angiogenesis, reducing apoptosis, and promoting tissue repair. This method has several advantages over direct stem cell transplantation [13, 14]. One of the main concerns in stem cell transplantation is the possibility of tumor formation. Exosomes, due to their inability to proliferate, significantly reduce the risk of tumorigenesis and increase the safety of using exosomes, addressing concerns related to the formation of abnormal cell masses [15, 16]. Stem cell transplantation may be accompanied by a host immune response and lead to graft rejection [17]. Exosomes, as membrane-bound nanoparticles, typically elicit a lower immune response than stem cells, which increases the biocompatibility of exosomes and improves the likelihood of treatment success [18]. Exosomes can be easily produced from cultured stem cells and stored for long periods under appropriate conditions, enabling the mass production of exosomes and their use in the treatment of various patients. Moreover, exosomes can be engineered to specifically target damaged heart tissue, increasing the efficacy of treatment and reducing side effects [19, 20]. Exosomes contain a wide range of biomolecules that can affect target cells and stimulate various processes such as angiogenesis, reduction of inflammation, and tissue repair. These extensive paracrine effects lead to an improvement in overall cardiac function. These advantages include greater safety, ease of production and storage, more precise targeting, broader paracrine effects, and the absence of the need for complex surgery [21, 22].
Cardiac diseases are recognized as one of the leading causes of death worldwide. These diseases, often of atherosclerotic origin, lead to restricted blood flow to the heart muscle and ultimately to heart damage and failure [23]. Heart attack, stable and unstable angina, and heart failure are among the most common types of these diseases [24]. Damage to the heart muscle, whether due to an acute heart attack or chronic diseases, can lead to significant structural and functional changes in the heart. These changes include the death of heart cells (apoptosis and necrosis), the formation of scar tissue, impaired heart pump function, and ultimately heart failure [25, 26]. Heart failure is defined as a complex clinical syndrome in which the heart is unable to pump enough blood to meet the metabolic needs of the body [27].
As a vital organ in the body, the heart has a limited ability to self-repair. These limitations are primarily due to the specialized nature of heart cells (cardiomyocytes) and the body’s inflammatory response to injury [28]. Cardiomyocytes are highly specialized cells with a very limited ability to divide and replace damaged cells [29]. After injury, heart tissue is primarily replaced by scar tissue, which lacks contractile function. In addition, the body’s severe inflammatory response to heart damage can lead to the formation of more extensive scar tissue and impaired heart pump function. These limitations in the heart’s spontaneous repair lead to long-term complications such as heart failure and reduced quality of life for patients [30, 31]. For this reason, researchers are looking for new therapeutic methods to repair damaged heart tissue. Cell therapy, tissue engineering, and the use of growth factors are among the methods that have been investigated in this area. However, these methods are also associated with challenges such as graft rejection, tumor formation, and high costs.
In recent years, extensive research has been conducted on the pathophysiological mechanisms of heart damage and the development of new treatment methods for these diseases. Current treatments include drug therapy, lifestyle changes, and, in more severe cases, heart transplantation. However, these treatments are often associated with limitations, and the need for the development of new therapeutic methods is felt [32]. One of the most promising approaches in the treatment of heart damage is the use of stem cells and their derived products. Stem cells, due to their unique ability to differentiate into various types of cells and repair damaged tissues, have attracted the attention of many researchers. Numerous studies have shown that stem cell transplantation can lead to improved heart function, reduced scar tissue size, and increased blood flow to the heart muscle [33].
Exosomes derived from MSCs have shown great potential as an emerging therapeutic tool for repairing damaged heart tissue. These exosomes can play a role in the treatment of a wide range of heart injuries such as heart attacks, ischemia/reperfusion, cardiomyopathy, heart failure, and more by stimulating repair processes, reducing inflammation, and improving heart function [14, 34]. These exosomes can help repair heart tissue damaged by heart attack by stimulating angiogenesis (blood vessel formation), reducing apoptosis (programmed cell death), and improving myocardial function. Also, exosomes can modulate the immune response, preventing inflammation and scar tissue formation [35]. MSC exosomes have high potential in the treatment of a wide range of heart injuries [36]. However, more research is still needed to investigate the efficacy and long-term safety of these treatments in humans.
In this review article, we will focus on the application of MSC-derived exosomes in heart injuries. First, we will review the characteristics of exosomes and their mechanisms of action in heart tissue repair. Then, we will review the preclinical and clinical studies conducted in this area and evaluate the therapeutic potential of these exosomes. Finally, we will discuss the existing challenges and future prospects of using MSC-derived exosomes in the treatment of heart injuries.
Exosomes: Biogenesis and characteristics
Intercellular communication is a hallmark of multicellular organisms, which can occur through direct cell-cell contact or the transfer of secreted molecules [37]. In the last two decades, a third mechanism for intercellular communication has been introduced, involving the transport of EVs. In cell-cell interactions, various soluble factors such as chemokines, cytokines, growth factors, and extracellular matrix proteins are involved [38]. Recent research has shown that cells also utilize another form of communication via membrane-bound EVs. These vesicles contain bioactive molecules such as proteins, nucleic acids, and lipids, which are horizontally transferred between cells and induce a wide range of activities in the recipient cell [39, 40]. In general, these EVs are released into the extracellular space by various mammalian cell types under both physiological and pathological conditions. In addition to the production and release of these vesicles under physiological conditions, increased levels have been observed in various diseases such as infections and cardiac diseases [41]. EVs are secreted by a variety of cell types including adipocytes, stem cells, dendritic cells, B and T lymphocytes, mast cells, platelets, neurons, epithelial cells, and endothelial cells [42, 43]. There are three types of EVs, including apoptotic bodies, microvesicles, and exosomes, based on their size and intracellular origin [44].
Apoptotic bodies range in size from 50 to 5000 nanometers and contain nucleic acids such as DNA, RNA, and histone proteins, which are produced during apoptosis and rapidly engulfed and degraded by macrophages [45]. Apoptotic bodies play a highly varied role in cell-cell communication. Some of these roles include: transmitting inflammatory signals, inducing immune responses, transferring genetic material, and regulating cell growth and proliferation [46, 47]. For example, apoptotic bodies can carry “eat-me” signals on their surface, which help phagocytic cells identify and eliminate them [48]. Additionally, these bodies can transfer their antigens to antigen-presenting cells (APCs), thereby stimulating an immune response. However, in some cases, apoptotic bodies can lead to the release of toxic cell contents and chronic inflammation (Table 1) [49, 50].
Table 1.
Characteristics of different types of EVs
| Feature | Exosomes | Microvesicles | Apoptotic Bodies |
|---|---|---|---|
| Origin | Multivesicular bodies (MVBs) within the cell | Direct budding from the plasma membrane | Fragmented apoptotic cells |
| Size | 30–160 nm | 100–1000 nm | 1000–5000 nm |
| Density | High (1.17–1.20 g/mL) | Low (1.07–1.17 g/mL) | Low (1.07–1.17 g/mL) |
| Cargo | Proteins, lipids, nucleic acids (mRNA, miRNA) | Proteins, lipids, nucleic acids | Cellular contents (organelles, DNA fragments) |
| Biogenesis | Inward budding of the endosomal membrane, forming intraluminal vesicles. Fusion of MVBs with the plasma membrane releases exosomes. | Outward budding of the plasma membrane, resulting in the shedding of vesicles. | Cell death and fragmentation of the plasma membrane. |
| Function | Cell-to-cell communication, intercellular signaling, immune response | Cell-to-cell communication, coagulation, angiogenesis | Clearance of apoptotic cells, immune response |
| Protein Composition | Enriched in tetraspanins (CD9, CD63, CD81), heat shock proteins (HSPs), and tumor susceptibility gene 101 (TSG101) | Varying depending on the cell of origin, but often contain integrins, selectins, and growth factors | Contain proteins associated with apoptosis, such as annexins and phosphatidylserine |
| Markers | CD63, CD9, CD81, TSG101, Alix | Annexin V, phosphatidylserine | Annexin V, phosphatidylserine, DNA fragmentation |
| RNA Content | Contain a diverse array of RNAs, including mRNAs, miRNAs, and lncRNAs | Can contain RNAs, but typically in lower abundance compared to exosomes | May contain fragmented RNAs from the apoptotic cell |
| References | [44, 54–56] | [44, 57, 58] | [59–61] |
The term “exosome” was first coined in 1970 by Rose Johnstone and her colleagues who were studying the maturation of sheep reticulocytes into erythrocytes and tracking transferrin receptors. They observed intracellular vesicles filled with small, membrane-bound structures of uniform size that released their contents outside the cell, a process opposite to endocytosis. Thus, the vesicles formed inside the cell were named exosomes. Initially, they believed that exosome secretion served as a mechanism to eliminate unwanted proteins from the cell [51]. Subsequent studies have revealed the significant and fundamental role of these vesicles in a number of biological processes, including cell repair, immune response induction, carcinogenesis, and genetic reprogramming [52, 53].
Exosomes are a specific type of small, secreted vesicles of endosomal origin that are released by many types of cells. These vesicles are spherical particles with a lipid bilayer and range in size from 30 to 160 nanometers. Exosomes also possess surface marker proteins that reflect their endosomal origin. Exosomes have numerous functions, most importantly establishing intercellular communication over both short and long distances within the body. These particles carry a set of receptor ligands, enzymatic contents, cytokines, and genetic material from the parent cell, which upon binding and entering the target cell can lead to the sending of inhibitory or stimulatory signals, phenotypic changes, and genetic reprogramming in the target cells. Among all EVs, exosomes have received the most attention from researchers in the past two decades [44, 54–56]. A comparative table of the characteristics of different types of EVs will be presented below.
Unlike other EVs such as microvesicles, ectosomes, and shedding vesicles, exosomes do not originate from direct budding of the plasma membrane but rather from multivesicular bodies (MVBs) [62]. Various factors such as cell type, physiological conditions, and environmental factors can influence exosome biogenesis [63]. Generally, exosome formation occurs in four stages [64, 65]:
-
i.
Formation of early endosomes: The process begins with the formation of early endosomes. These endosomes are portions of the cell membrane that fold inwards and form small vesicles.
-
ii.
Formation of late endosomes: Early endosomes gradually mature into late endosomes. At this stage, the endosomes become more acidic and specific proteins and lipids are added to their membrane.
-
iii.
Formation of multivesicular bodies (MVBs): Late endosomes gradually mature into multivesicular bodies. Inside these bodies, smaller vesicles called intraluminal vesicles (ILVs) are formed. These ILVs are actually the future exosomes.
-
iv.
Exosome release: Multivesicular bodies move towards the cell membrane and fuse with it. As a result of this fusion, the intraluminal vesicles are released into the extracellular environment as exosomes.
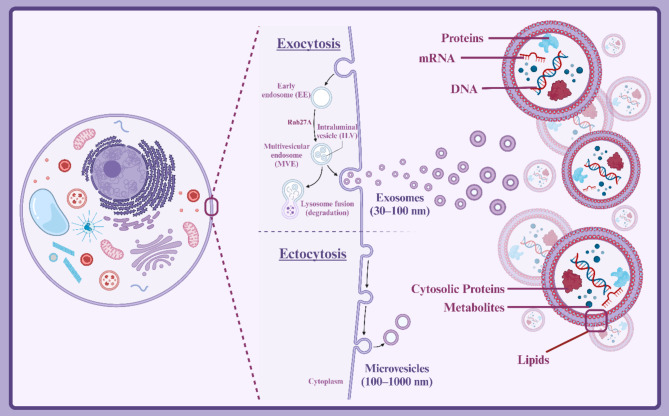
The protein content of EVs and exosomes from different origins has been analyzed using SDS-PAGE followed by staining, proteomic analysis, or immunoblotting (Western blotting) [66]. The proteins found in exosomes are highly diverse and can vary depending on the cell of origin, physiological, and pathological conditions [19, 67]. Generally, exosome proteins can be categorized into three main groups: membrane proteins, cytosolic proteins, and proteins associated with cellular organelles [68]. Membrane proteins are embedded in the exosome membrane and serve as surface markers to identify the origin of exosomes [69]. Cytosolic proteins, as a major component of exosome content, play a crucial role in various biological functions [70]. Located within the exosome lumen, these proteins perform a wide range of functions including enzymatic, structural, and signaling activities [71]. The roles of cytosolic proteins in exosomes encompass facilitating exosome formation and maturation, protecting exosome contents, generating intercellular signals, and modulating target cell phenotype [71]. By carefully studying the protein composition of exosomes and identifying proteins associated with different organelles, valuable information can be obtained about exosome biosynthetic pathways, selective mechanisms for packaging exosome contents, and the role of exosomes in intercellular communication (Fig. 1) [72].
Fig. 1.
Biogenesis and structure of exosomes. Created with BioRender.com
Mechanisms of effect exosomes in heart damage
Following cardiac injury, we observe reduced blood flow to the heart and consequently cardiomyocyte apoptosis or necrosis [73]. In ischemia/reperfusion (I/R) injury, events such as endothelial inflammation, oxidative stress, and myocardial cell death are observed [74]. Impaired cardiac angiogenesis can suppress repair and restoration of normal function [75]. In recent years, it has been shown that various types of exosomes secreted by cells, such as adipose-derived mesenchymal stem cells (ADMSCs), bone marrow mesenchymal stem cells (BMMSCs), and coronary serum exosomes (Exosomes released from cardiac cells, particularly coronary endothelial cells, into circulation, especially in response to myocardial ischemia), can reduce myocardial damage due to their anti-inflammatory, antioxidant, anti-apoptotic, anti-fibrotic, and pro-angiogenic properties [76–78]. Therefore, the use of exosomes has become a promising cell-free approach for cardiac repair after injury, which will be further explored in this study.
Anti-inflammation
Inflammation is a biological process that the body uses to protect itself from external influences such as bacteria or physical injury. However, chronic inflammation can lead to damage to tissues and organs, including the heart after injuries such as myocardial infarction (MI) and ischemic injury [79]. Acute inflammation typically occurs after a cardiac injury, such as a heart attack, and serves as a defense mechanism [80]. Immune cells migrate to the damaged site, eliminate pathogens, and initiate the repair process. However, if acute inflammation is not fully resolved, it can transition into chronic inflammation [81]. The primary goal of treating inflammation in heart diseases is to reduce its severity and duration, and improve cardiac function. Various treatments exist to reduce inflammation in heart diseases, including anti-inflammatory drugs, antioxidants, renin-angiotensin system inhibitors, and lifestyle modifications [82]. A thorough understanding of the molecular and cellular mechanisms of inflammation in heart diseases can contribute to the development of new and effective treatments for these conditions [83].
Studies have shown that exosomes can affect inflammation through various mechanisms. One of these mechanisms is the delivery of anti-inflammatory molecules such as interleukin 10 (IL-10) and transforming growth factor beta (TGF-β) to target cells by exosomes [84]. These molecules can inhibit the expression of inflammatory cytokines and reduce the activity of immune cells. Exosomes can also inhibit the activation of immune cells such as macrophages and T lymphocytes, which occurs through interaction with cell surface receptors and regulation of inflammation-related gene expression [85]. Exosomes can carry antioxidant enzymes and small antioxidant molecules that protect cells from oxidative stress [86].
Dysregulation of the immune system can lead to autoimmune and inflammatory diseases [87]. Exosomes can prevent damage to cardiac tissues by modulating the immune response. This effect is achieved through two mechanisms: modulation of the innate immune response and regulation of the adaptive immune response [88]. Exosomes can regulate the activity of innate immune cells such as dendritic cells and natural killer cells, which can help reduce inflammation and promote tissue repair [89]. Exosomes can influence the activity of T and B lymphocytes, modulating the adaptive immune response, and can help prevent graft rejection and reduce the risk of atherosclerosis [90].
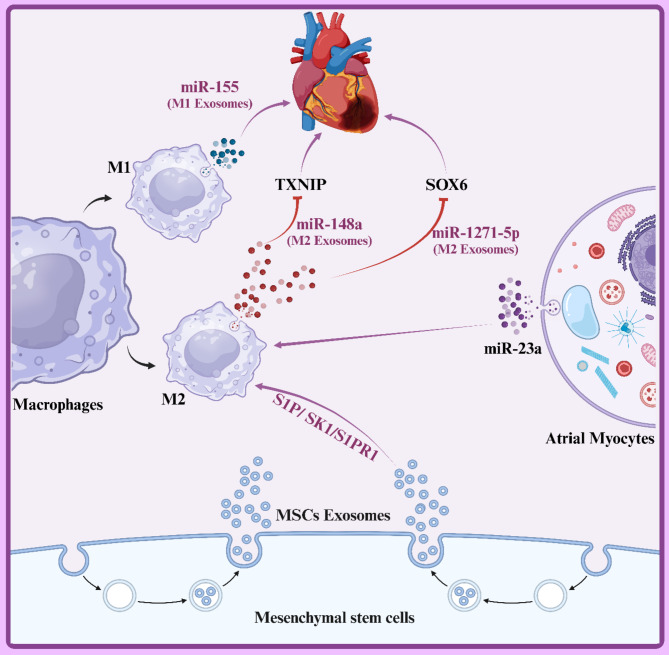
Systemic inflammation is a complex immune response characterized by increased levels of pro-inflammatory cytokines such as TNF-α and decreased levels of anti-inflammatory cytokines such as IL-10. This cytokine imbalance can lead to tissue damage, including the heart [91, 92]. Studies have shown that a deficiency or dysfunction of IL-10 can impact endothelial progenitor cell (EPC)-derived exosomes, leading to increased expression of the ILK protein and activation of the NF-κB pathway. This process is associated with exacerbated inflammation. Conversely, decreasing ILK activity in exosomes can help to inhibit inflammation. Additionally, adipose-derived stromal cell (ADSC)-derived exosomes containing miR-93-5p can protect against myocardial injury by targeting TLR4-mediated inflammatory responses [84, 93]. Furthermore, miR-181a found in human umbilical cord blood-derived mesenchymal stem cell (MSCs-exo) exosomes can suppress inflammation and increase the proportion of regulatory T cells (Tregs) by inhibiting the c-FOS protein. This leads to significant improvement in myocardial ischemia/reperfusion injury [94]. Macrophages are categorized into two main phenotypes: M1-like and M2-like [95]. M1-like macrophages produce large amounts of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, while M2-like macrophages produce anti-inflammatory cytokines like IL-10 [96]. During myocardial infarction (MI), M1 macrophages are initially recruited to the infarcted area, followed by the recruitment of M2 macrophages for anti-inflammatory activities [97]. Therefore, the balance between M1 and M2 macrophages is a potential therapeutic target for heart injury. Exosomes derived from MSCs exhibit anti-inflammatory properties by promoting the polarization of pro-inflammatory M1 macrophages into anti-inflammatory M2 macrophages [98, 99]. Additionally, M2 exosomes inhibit cardiomyocyte apoptosis by negatively regulating SOX6 expression, a result of miR-1271-5p suppression [100]. Adipose-derived MSC exosomes (ADMSC) promote the polarization of M2 macrophages, and the suppressive effects of ADMSC exosomes on MI-induced myocardial apoptosis and fibrosis were reversed by sphingosine-1-phosphate receptor 1 (S1PR1) [101]. Overall, exosomes ameliorate cardiac injury by stimulating the S1P/SK1/S1PR1 signaling pathway and facilitating M2 macrophage polarization. Furthermore, exosomes derived from atrial myocytes treated with angiotensin II promote M2 macrophage polarization by transferring miR-23a (Fig. 2) [102]. In contrast, M1-derived exosomes exacerbate myocardial injury by inhibiting angiogenesis and cardiac healing through the suppression of Sirt1, PAK2, Rac1, and AMPKα2 signaling pathways [103].
Fig. 2.
This diagram illustrates the anti-inflammatory properties of exosomes in cardiovascular diseases. It highlights the role of exosomal microRNAs (miR-155, miR-148a, miR-1271-5p) derived from M1 and M2 macrophages in modulating inflammation. The interaction with key proteins like TXNIP and SOX6 demonstrates how exosomes can influence inflammatory responses in heart cells, promoting a potential therapeutic pathway through mesenchymal stem cell (MSC) exosomes to mitigate inflammation in cardiovascular conditions. Created with BioRender.com
One of the crucial mechanisms by which MSC-derived exosomes influence atherosclerosis is through the regulation of macrophage polarization. Studies have revealed that the miR-let7/HMGA2/NF-κB pathway plays a pivotal role in polarizing macrophages towards the M2 phenotype [104]. miR-let7, a microRNA, induces M2 polarization by inhibiting the expression of HMGA2 (a transcription factor) and reducing NF-κB activation (a key transcription factor in inflammatory responses). MSC-derived exosomes contain abundant miR-let7. When these exosomes interact with macrophages, the encapsulated miR-let7 is transferred into the macrophages, inhibiting HMGA2 expression and reducing NF-κB activation, thereby promoting M2 polarization [105]. Consequently, inflammation within atherosclerotic plaques is reduced, slowing disease progression. MSC-derived exosomes can also inhibit macrophage infiltration into atherosclerotic plaques. This effect is mediated through the inhibition of the miR-let7/IGF2BP1/PTEN pathway. IGF2BP1 is a protein that prevents the degradation of PTEN mRNA. PTEN is an enzyme that inhibits the PI3K/Akt pathway. The PI3K/Akt pathway plays a crucial role in cell survival and proliferation [83, 84]. Therefore, by inhibiting the miR-let7/IGF2BP1/PTEN pathway, MSC-derived exosomes can reduce macrophage infiltration and survival in atherosclerotic plaques [104]. Studies have shown that miR-129-5p found in MSC-derived exosomes can target the TRAF3 gene and inhibit the NF-κB signaling pathway, thereby reducing inflammation in cardiac tissue and improving cardiac function in animal models of heart failure. This mechanism suggests that miR-129-5p can act as an anti-inflammatory agent and prevent damage to cardiac cells [106].
Sepsis, a severe inflammatory response to infection, can lead to severe heart damage. Recent studies have shown that microRNAs, particularly miR-223, play an important role in protecting the heart from sepsis-induced injury [107]. MSCs are capable of producing exosomes that contain high levels of miR-223. These exosomes can exert their protective effects by transferring miR-223 to other cells, including cardiac cells and macrophages [78]. miR-223 reduces inflammation in cardiac tissue by decreasing the expression of inflammation-related genes such as IL-6, IL-1β, and ICAM-1. This reduction in inflammation, in turn, prevents damage to cardiac cells [108]. Additionally, miR-223 regulates macrophage function to prevent an excessive inflammatory response [109]. Two important proteins, Sema3A and Stat3, play a role in causing inflammation and heart damage in sepsis. Studies have shown that miR-223 protects the heart by decreasing the expression of these two proteins [110]. Clinical studies have shown that miR-223 levels in the blood of septic patients are significantly lower than in healthy individuals. Additionally, septic patients with lower miR-223 levels have a higher mortality rate. These findings indicate that decreased miR-223 levels are associated with disease severity and a worse prognosis in septic patients [111, 112].
Anti-oxidative stress
Injury to the heart muscle, or myocardium, often leads to the overproduction of reactive oxygen species (ROS). This imbalance between ROS production and elimination results in damage to biological molecules such as proteins, lipids, and DNA, causing lipid peroxidation, protein degradation, and DNA mutations. This process is known as oxidative stress [113, 114]. In myocardial injury, oxidative stress plays a significant role in inflammation, apoptosis, fibrosis, and ultimately, heart failure. ROS produced under these conditions can directly damage cardiomyocytes or exacerbate the inflammatory response by activating inflammatory signaling pathways. Additionally, oxidative stress can worsen injury by decreasing the activity of antioxidant enzymes and reducing the cell’s ability to combat free radicals. Consequently, inhibiting oxidative stress has emerged as a potential therapeutic strategy to reduce myocardial damage and improve cardiac function [115–117].
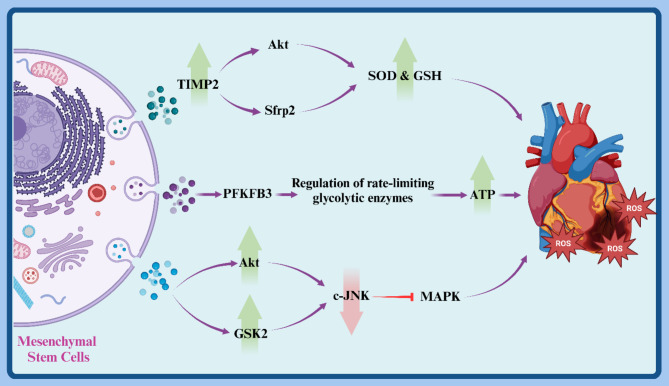
Research findings have shown that exosomes derived from human umbilical cord mesenchymal stem cells (hUCMSCs) limit extracellular matrix (ECM) remodeling. Moreover, exosomes from TIMP2-overexpressing hUCMSCs (hUCMSCs-exoTIMP2) increased superoxide dismutase (SOD) and glutathione (GSH) levels while reducing malondialdehyde (MDA) in a mouse model of myocardial infarction (MI). Furthermore, pretreatment with hUCMSCs-exoTIMP2 increased Akt phosphorylation in the infarcted myocardium, suggesting that hUCMSCs-exoTIMP2 alleviates MI-induced oxidative stress and ECM remodeling, improving cardiac function partly through the Akt/Sfrp2 pathway [118].
MSC-derived exosomes carry five enzymes involved in the glycolytic ATP production phase, along with phosphorylated PFKFB3, which upregulates rate-limiting glycolytic enzymes. In I/R injury, MSC-derived exosomes contribute to reduced infarct size and adverse remodeling by increasing tissue ATP production in the heart, which can supplement depleted cellular antioxidants in I/R myocardium due to the presence of peroxidase and glutathione S-transferase. Additionally, MSC-derived exosomes decreased pro-apoptotic c-JNK phosphorylation by increasing AKT and GSK2 phosphorylation (Fig. 3) [119].
Fig. 3.
This diagram illustrates the anti-oxidative stress mechanisms of mesenchymal stem cell (MSC)-derived exosomes in myocardial injury. It highlights how TIMP2 enhances Akt activation, leading to increased superoxide dismutase (SOD) and glutathione (GSH) levels, as well as the regulation of glycolytic enzymes through PFKFB3. These processes contribute to reducing reactive oxygen species (ROS) and improving ATP production, thereby alleviating oxidative stress and protecting cardiac cells after myocardial infarction. Created with BioRender.com
Reducing cell death
Apoptosis, a form of programmed cell death, is characterized by structural changes such as cell shrinkage, nuclear pyknosis, and fragmentation. Myocardial apoptosis is initiated by short-term ischemia following prolonged myocardial ischemia or reperfusion [120]. Apoptosis begins at the infarct border within hours to days post-acute infarction. An increasing number of studies have demonstrated that exosomes restore cell survival and cardiac function by protecting against cardiomyocyte apoptosis. Inhibiting cardiomyocyte death can improve cardiac function and reverse adverse myocardial remodeling [121, 122]. Apoptosis contributes to cardiomyocyte death after MI under pathophysiological conditions such as oxidative stress [123]. As mentioned, exosomes reduce cardiomyocyte apoptosis through anti-inflammatory and anti-oxidative stress pathways, and also reduce cell death by targeting autophagy.
Exosomes secreted by ADMSCs (ADMSCs-exo) in a MI model showed a significant increase in miR-671 expression after exosome treatment, while decreased miR-671 expression reduced the protective properties of exosomes. Exosomal miR-671 reduces myocardial cell apoptosis, myocardial fibrosis, and inflammation by targeting the transforming growth factor beta receptor 2 (TGFBR2) and inhibiting Smad2 phosphorylation [124]. Exosomal miR-146a interacts with the 3’-untranslated region of EGR1 to inhibit post-transcriptional expression of EGR1 and reverse AMI activation and hypoxia-induced TLR4/NFκB signaling, indicating that miR-146a from adipose-derived stem cell (ADSC)-derived exosomes can ameliorate AMI-induced apoptosis, inflammation, and fibrosis [125].
miR-320a is another significant microRNA found in MSC-derived exosomes. The level of this microRNA is elevated in patients with heart failure and correlates with disease severity. Studies have shown that miR-320a stimulates cardiac fibroblast proliferation by activating the PI3K/Akt/mTOR signaling pathway. This can lead to cardiac fibrosis and worsening heart function. Therefore, inhibiting the activity of miR-320a could be an effective therapeutic strategy to reduce cardiac fibrosis in patients with heart failure [126].
Myocarditis, an inflammation of the heart muscle, is often caused by viral infections such as coxsackievirus B3 (CVB3). This inflammation can lead to severe damage to heart cells and eventually heart failure [127]. Autophagy is a natural process in cells where they break down damaged or unnecessary components for recycling or removal. Activation of autophagy can help cells cope with various stresses, such as infection [128].
A study showed that hucMSC exosomes can protect cardiomyocytes from CVB3 virus-induced damage by activating the autophagy pathway [129]. hucMSC exosomes stimulate the autophagy pathway by activating AMP-activated protein kinase (AMPK) and inhibiting the mechanistic target of rapamycin (mTOR). AMPK is a cellular energy sensor that is activated in response to decreased cellular energy levels. Activation of AMPK leads to the inhibition of mTOR, a major regulator of cell growth and autophagy [130]. Inhibition of mTOR, in turn, stimulates autophagy [131]. These findings suggest the potential of using hucMSC exosomes as a novel therapy for viral myocarditis.
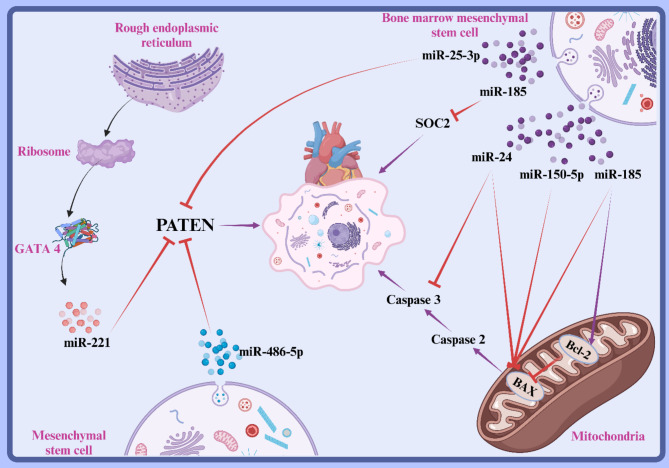
Bax is a crucial pro-apoptotic protein in the Bcl-2 family and its stability is crucial for the regulation of mitochondrial apoptotic pathways and is also involved in myocardial apoptosis in MI [132]. Myocardial tissue of MI models showed decreased expression of miR-150-5p and increased expression of Bax, and BMMSCs-exo was associated with increased expression of miR-150-5p and decreased expression of Bax in MI models. Consequently, exosomal miR-150-5p can improve cardiac function in MI models by reducing myocardial pathological alterations and decreasing apoptosis through targeting Bax [133]. Levels of miR-24 were significantly increased by injection of hypoxic BMMSCs-exo in AMI models, reducing infarct size and improving cardiac function [134]. A study showed that cardiac function in MI models was significantly improved after intramuscular injection of exosomes overexpressing miR-338, and exosomal miR-338 could suppress myocardial cell apoptosis in MI models and improve myocardial function by regulating the JNK pathway through targeting MAP3K2 [135].
Tumor suppressor phosphatase and tensin homology (PTEN) is also associated with apoptosis, and its downregulation can regulate cell proliferation and promote cell apoptosis [136]. Exocytosis of BMMSCs resulted in a significant increase in miR-25-3p in cardiomyocytes, which directly reduced the expression of pro-apoptotic genes FASL and PTEN. Moreover, miR-25-3p decreases the levels of enhancer of zeste homolog 2 (EZH2) and H3K27me3, leading to downregulation of the cardioprotective gene eNOS and the anti-inflammatory gene SOCS3. MSC co-culture can reduce OGD-induced cardiomyocyte apoptosis and inflammatory responses, thereby driving the overexpression of miR-25-3p [137]. miR-486-5p in bone marrow stromal cell-derived exosomes inhibited H/R-induced apoptosis of H9c2 cells. Bone marrow stromal cell-derived exosomes inhibited PTEN expression in H9c2 cells via miR-486-5p and also activated the PI3K/AKT signaling pathway in vitro. In vivo, the PI3K/Akt signaling pathway can repair myocardial damage (Fig. 4) [138].
Fig. 4.
This diagram demonstrates how mesenchymal stem cell (MSC) exosomes reduce cardiomyocyte apoptosis after myocardial injury. Key microRNAs, such as miR-25-3p and miR-150-5p, inhibit pro-apoptotic proteins like PTEN and Bax, promoting cell survival. The activation of anti-apoptotic pathways and regulation of caspases highlight the therapeutic potential of exosomal treatments in improving cardiac function and mitigating adverse remodeling following ischemic events. Created with BioRender.com
Anti-fibrosis
Cardiac fibrosis, the replacement of heart muscle tissue with scar tissue, commonly occurs in response to heart injuries such as myocardial infarction. This process impairs the heart’s pumping function and can lead to heart failure. After myocardial infarction, the proliferation of fibroblasts leads to the formation of non-contractile scar tissue, which can compromise cardiac function. Myocardial fibrosis is typically the result of irreversible damage to the myocardial tissue caused by MI [139, 140]. Anti-fibrotic effects are crucial for cardiac repair. By inhibiting the fibrosis process, it is possible to improve cardiac function, reduce the risk of heart failure, and increase patient lifespan [141]. Exosomes have shown the potential to aid in heart tissue repair and improve function by reducing inflammation, inhibiting fibroblast activation, and remodeling the extracellular matrix.
Research has shown that exo-miR-218-5p or exo-miR-363-3p, which are enriched in exosomes derived from endothelial progenitor cells (EPC-Exos), promote the transformation of mesenchymal endothelial cells. They also inhibit myocardial fibrosis by increasing p53 and decreasing the expression of a junctional regulatory protein [142]. Expression of miR-30e in the myocardial tissue of MI models is weak, and overexpression of exo-miR-30e can alleviate pathological damage, apoptosis, and fibrosis in myocardial tissue because miR-30e negatively regulates the expression of LOX1. Further treatment with exosomes inhibits the expression of LOX1. Additionally, results showed that overexpression of exo-miR-30e inhibited the NF-κB P65/Caspase-9 signaling pathway in the myocardial tissue of MI models, inhibiting myocardial apoptosis and fibrosis [143]. Hypoxic stress leads to increased apoptosis and decreased survival of cardiac cells, while FNDC5-OV can reduce this damage. Furthermore, treatment with FNDC5-MSC significantly reduced cardiac fibrosis and improved cardiac function [144].
Exosomes secreted by cardiac progenitor cells (CPCs) under hypoxic conditions facilitate tube formation by endothelial cells, and exosome injection reduces the expression of profibrotic genes in TGF-β-stimulated fibroblasts. Exosomes derived from hypoxic CPCs improved cardiac function and reduced fibrosis after I/R injury [145]. Additionally, exosomes derived from atrial myocytes treated with angiotensin II inhibit M2 macrophage polarization and suppress the expression of atrial fibroblast fibrosis markers by transferring miR-23a [146].
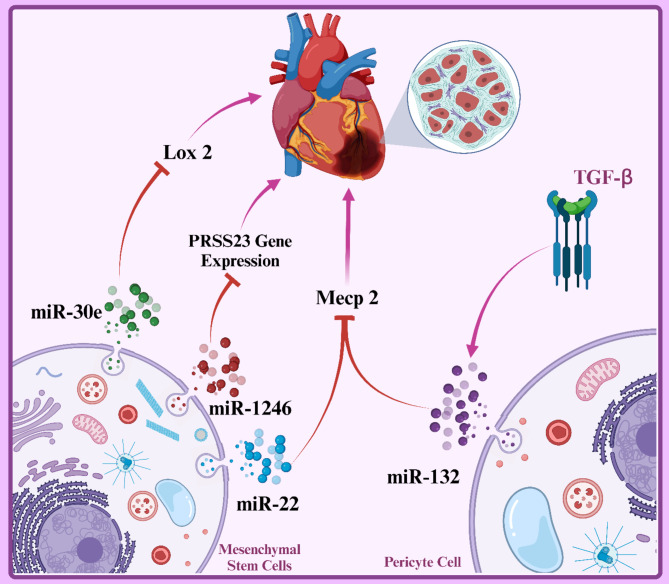
The PRSS23 gene encodes a protein known as a protease. Proteases are enzymes that break down other proteins. PRSS23 is recognized as a detrimental factor for the heart due to its role in inflammation and tissue degradation. Increased activity of this protease can lead to damage of heart cells, inflammation, and ultimately, heart failure [147–149]. miR-1246 is specifically found in exosomes derived from MSCs. This microRNA inhibits the translation of specific mRNAs by binding to their 3’ untranslated region (UTR). In simpler terms, miR-1246 acts like an off switch, regulating cellular activity by silencing specific genes. In the case of miR-1246, this microRNA targets the mRNA of the PRSS23 gene, preventing the production of the PRSS23 protein, which is a protease and inflammatory agent. Reduced levels of PRSS23 mean decreased inflammation and damage to heart tissue. By lowering PRSS23 levels, tissue degradation and inflammation in the heart are reduced, leading to improved cardiac function [149].
Studies have shown that under hypoxic conditions, commonly found in heart diseases such as myocardial infarction (MI), MSCs can help improve cardiac function by secreting exosomes enriched with protective microRNAs. One such important microRNA is miR-22 [20, 150]. When cardiac cells are subjected to ischemic conditions, they undergo apoptosis due to lack of oxygen. However, research has shown that MSC-derived exosomes enriched with miR-22 can prevent apoptosis in cardiac cells. In other words, these exosomes act as a protective shield and protect cardiac cells from damage [151]. miR-22 works by downregulating the Mecp2 gene. Mecp2 is a protein that plays a significant role in the process of cardiac fibrosis. By decreasing Mecp2 gene expression, miR-22 prevents cardiac fibrosis and promotes the repair of damaged tissue. Additionally, miR-22 enhances angiogenesis in the heart, improving blood supply to the cardiac tissue and reducing damage [152, 153].
Pericytes are cells that surround blood vessel walls and play a crucial role in maintaining vascular integrity and regulating repair processes [154]. Exosomes derived from Bristol pericytes (a type of human pericyte) contain miR-132, which can be transferred to endothelial cells (ECs) under ischemic conditions [155]. When miR-132 is transferred to endothelial cells, it prevents the formation of cardiac scar tissue by decreasing the expression of the Mecp2 gene, a profibrotic gene. This means reduced cardiac fibrosis and improved heart function. Transforming growth factor beta (TGF-β) is an important cytokine that is secreted in response to tissue injury. This factor can increase the expression of miR-132 in pericytes. Therefore, TGF-β enhances cardiac tissue repair processes by activating miR-132 (Fig. 5) [156, 157].
Fig. 5.
The illustration shows how microRNAs (miRNAs) from mesenchymal stem cells (MSCs) and pericytes help reduce cardiac fibrosis after injury. After cardiac damage, MSCs release miRNAs like miR-30e, miR-1246, and miR-22, which target fibrosis-related genes (e.g., LOX1, PRSS23) to promote tissue repair. TGF-β increases miR-132 expression in pericytes, inhibiting the profibrotic Mecp2 gene in endothelial cells. This highlights the potential of exosome-derived miRNAs for therapeutic strategies in heart repair post-myocardial infarction. Created with BioRender.com
Angiogenesis
Angiogenesis, the process of forming new blood vessels from pre-existing microvasculature, relies on the migration and proliferation of vascular endothelial cells and is essential for repairing an ischemic microenvironment [85]. Exosomes protect against MI/IR by promoting angiogenesis. Vascular endothelial growth factor (VEGF) is the primary driving factor of cardiac angiogenesis post-MI [86]. VEGF genes can significantly improve myocardial perfusion, increase vascular density, and enhance ventricular function [87]. Hypoxia-inducible factor-1α (HIF-1α), an upstream regulator of VEGF, is induced by UCMSCs and thus plays a crucial role in angiogenesis and cardiac repair [88]. In MI mice, injection of exosomes derived from BMMSCs, ADMSCs, and UCMSCs around the MI border zone enhanced angiogenesis by increasing VEGF [60]. Pi-sen Huang and colleagues [89] demonstrated that exosomes from atorvastatin pre-treated MSCs (MSCATV-exo) administration prevent H/SD-induced endothelial cell apoptosis. MSCATV-exo enhances angiogenesis in the infarct border zone and inhibits the increase of IL-6 and TNF-α. MSCATV-exo uses LncRNA H19 to control the levels of miR-675, which in turn affects the activity of VEGF and vascular cell adhesion molecule-1. In another study, DEXs were shown to significantly increase the tube-forming capacity of cardiac microvascular endothelial cells (CMECs) by increasing the expression of VEGF, CD31, and miR-494-3p in CMECs. DEX-miR-494-3p increased CMEC tube formation and angiogenesis in mice post-MI. Research has shown that exosomes from BMMSC transduced with lentiviral CXCR4 (Exo(CR4)) enhance cardiac function, reduce infarct size, and improve cardiac remodeling by increasing angiogenesis through increased VEGF. However, Akt inhibitors and the knockdown of CXCR4 abolished the protective effects of Exo (CR4). Wnt4 induces β-catenin activation in endothelial cells and exerts pro-angiogenic effects, and exosomes derived from hucMSC enhance angiogenesis by regulating the Wnt4/β-catenin signaling pathway [91]. Also, hucMSCs-exoTIMP2 enhances HUVEC proliferation and migration and increases the number and length of tube-like structures formed in vitro, and administration of huc-exoTIMP2 into the infarct border zone in MI mice significantly increased endogenous CD31 expression [32]. It has been demonstrated that young MSCs-exo are superior to old MSCs-exo in promoting intravascular formation, reducing fibrosis, and inhibiting apoptosis. miR-221-3p is significantly decreased in old MSCs-exo, and when miR-221-3p was overexpressed in old Exos, old MSCs were activated, and their ability to repair the heart was restored. The effects of miR-221-3p are mediated by inhibiting PTEN to increase Akt kinase activity.
As mentioned in the previous section, miR-132 is an important microRNA that plays a role in various cellular processes, including angiogenesis, and pericytes secrete exosomes containing miR-132. These exosomes stimulate angiogenesis by transferring miR-132 to endothelial cells (cells lining the inner surface of blood vessels) [154]. One of the most important mechanisms of miR-132 function in stimulating angiogenesis is the reduction of the expression of a protein called p120RasGAP. This protein inhibits the Ras pathway, preventing the activation of genes that are essential for blood vessel growth. Therefore, the decrease in p120RasGAP expression by miR-132 leads to the activation of the Ras pathway and, consequently, the stimulation of new blood vessel growth [158, 159]. Additionally, the Ras pathway is an important cell signaling pathway that plays a role in regulating cell growth, proliferation, and survival. Activation of this pathway by miR-132 leads to increased expression of genes that are essential for the formation of new blood vessels [160].
Exosomes derived from BM-MSCs enhance the angiogenic activity of endothelial cells (ECs) by transferring the inducer of extracellular matrix metalloproteinase (EMMPRIN) [161]. EMMPRIN promotes angiogenesis by activating and upregulating vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP9), as well as acting as a co-receptor for VEGFR2 [162]. Additionally, BM-MSC-derived exosomes enriched with miR-126 induce tube formation in ischemia/reperfusion-injured ECs by activating the PI3K/Akt/eNOS signaling pathway [163]. STAT3 is a key transcription factor in the angiogenesis process and promotes angiogenesis by inducing the expression of VEGF, bFGF, MMP-2, and MMP-9 [164]. BM-MSC-derived exosomes enriched with STAT3 enhance the angiogenic capacity of human umbilical vein endothelial cells (HUVECs) by regulating STAT3 translocation [165, 166]. Furthermore, exosomes derived from AD-MSCs overexpressing SIRT1 significantly increase the migration and tube formation of endothelial progenitor cells (EPCs) through Nrf2 involvement and activation of CXCL12/CXCR7 signaling in EPCs [167].
The PTEN gene acts as a potent inhibitor of angiogenesis by inactivating the PI3K/Akt pathway and increasing the production of the anti-angiogenic factor TSP-1, thereby preventing the formation of new blood vessels [168]. Studies have shown that exosomes derived from BM-MSCs containing miR-221-3p can increase VEGF levels and consequently enhance angiogenesis by decreasing the expression of the PTEN gene and activating the Akt/eNOS/VEGF pathway [169]. Additionally, under conditions of oxygen and glucose deprivation, the levels of miR-29b-3p and Akt protein decrease and the expression of the PTEN gene increases in neurons and brain microvascular endothelial cells [170, 171]. However, BM-MSC exosomes enriched with miR-29b-3p can improve angiogenesis in a mouse model of ischemic stroke by decreasing PTEN expression and increasing the expression of VEGF and its receptor (VEGFR-2) and activating the Akt protein [172].
Similar to other pro-angiogenic factors, increased angiopoietin and HIF-1α have a positive effect on angiogenesis. It has been shown that miR-21-5p, which is abundant in exosomes extracted from endometrial-derived MSCs (EnMSCs), increases angiopoietin levels in HUVECs by suppressing PTEN and subsequently increasing Akt phosphorylation, leading to increased VEGF regulation [173]. DLL4 is an angiostatic factor that suppresses angiogenesis by inhibiting the formation of endothelial tip cells. Exosomes extracted from AD-MSCs promote EC migration and tube formation by inhibiting FIH1 via miR-31, which leads to increased activation of HIF-1α [174]. HIF-1α can increase the migration of EPCs to ischemic areas by increased expression of CXCL12/CXCR4 and creating a concentration gradient that all leads to improved angiogenesis of EPC migration. Exosomes improve the angiogenic capacity of ECs by regulating the upregulation of eNOS and subsequently increasing NO production under I/R injury [175]. It has been shown that co-culturing EPCs with exosomes derived from AD-MSCs with overexpression of Nrf2 leads to increased levels of senescence marker protein 30 (SMP30), an anti-aging molecule, and decreased levels of Nox-1&4, oxidative stress factors. Activated Nrf2 appears to be transported to the nucleus to activate the antioxidant response element (ARE), which induces the activity of antioxidant enzymes [176].
Angiogenic growth factors such as angiopoietin and HIF-1α play a crucial role in the formation of new blood vessels [177]. Studies have shown that miR-21-5p found in exosomes of endometrial-derived mesenchymal stem cells (EnMSCs) increases angiopoietin levels and consequently VEGF production by decreasing PTEN gene activity and increasing Akt protein activity. This leads to the enhancement of the angiogenesis process [173]. On the other hand, the DLL4 protein slows down the angiogenesis process by inhibiting the formation of endothelial tip cells [178]. However, exosomes derived from adipose-derived mesenchymal stem cells (AD-MSCs) increase HIF-1α activity by decreasing FIH1 gene activity through miR-31 [174]. HIF-1α also enhances the migration of endothelial progenitor cells (EPCs) to damaged areas by increasing the expression of CXCL12 and CXCR4 proteins, thereby improving angiogenesis [179, 180]. In addition, exosomes increase the angiogenic capacity of endothelial cells by increasing the production of nitric oxide (NO) through the activation of the eNOS enzyme [181]. In Table 2, We summarize the findings from various studies regarding the impact of mesenchymal stem cell-derived exosomes on cardiac injuries.
Table 2.
A review of studies on the effects of mesenchymal stem cell-derived exosomes in cardiac injuries
| Effect | Exosomes from | Related miRNA | Mechanism | Reference |
|---|---|---|---|---|
| Anti-inflammation | human umbilical cord MSCs | miR-181a | Inhibiting inflammatory response | [94] |
| miR-24–3p | Inhibit Plcb3, NF-κB | [182] | ||
| - | PP2A/p-Akt/Foxo3 | [183] | ||
| adipose- mesenchymal stem cells | miR-93-5p |
Inhibiting inflammatory response Atg7 pathway |
[93] | |
| - |
Inhibiting inflammatory response S1P/SK1/S1PR1 pathway |
[184] | ||
| Bone Marrow Mesenchymal Stem Cells | - |
Promoting M2 macrophage polarization AKT1/AKT2 pathway |
[185] | |
| miR-let7 |
Inhibits IGF2BP1, preventing PTEN degradation, leading to inhibition of PI3K/Akt pathway Inhibits HMGA2 and NF-κB, promoting M2 macrophage polarization |
[104] | ||
| Mesenchymal stem cells | miR-129-5p | Inhibition of NF-κB signaling pathway by targeting the TRAF3 gene | [106] | |
| miR-1246 | Binds to the 3’ UTR of PRSS23 mRNA, preventing its translation and reducing PRSS23 protein levels | [149] | ||
| miR-223 | decreasing expression of IL-6, IL-1β, and ICAM-1; regulates macrophage function; decreases Sema3A and Stat3 expression | [186] | ||
| miR-25–3p | Inhibit EZH2 | [137] | ||
| miR-129–5p | Inhibit HMGB1 | [187] | ||
| Anti-oxidative stress | umbilical cord MSCs | - | Akt/Sfrp2 pathway | [188] |
| - | Increases SOD and GSH levels, reduces MDA, activates Akt/Sfrp2 pathway | [118] | ||
| Mesenchymal stem cells | - | Increases tissue ATP production by carrying glycolytic enzymes and phosphorylated PFKFB3; reduces pro-apoptotic c-JNK phosphorylation by increasing AKT and GSK2 phosphorylation | [119] | |
| Anti-Fibrosis | Mesenchymal stem cells | miR-1246 | Binds to the 3’ UTR of PRSS23 mRNA, preventing its translation and reducing PRSS23 protein levels | [149] |
| miR-22 | Downregulates Mecp2 gene, preventing cardiac fibrosis and promoting angiogenesis | [189, 190] | ||
| miR-30e | Negatively regulates LOX1 expression, inhibits NF-κB P65/Caspase-9 signaling pathway | [143] | ||
| Endothelial progenitor cells | miR-218-5p | Increases p53 expression, decreases expression of a junctional regulatory protein | [142] | |
| miR-363-3p | Increases p53 expression, decreases expression of a junctional regulatory protein | [142] | ||
| Bristol pericytes | miR-132 | transferred to endothelial cells, decreases Mecp2 expression, and reduces cardiac fibrosis | [191] | |
| Bone Marrow Mesenchymal Stem Cells | miR-210 | ALFM3/p53, PI3K/Akt pathway | [192] | |
| Reducing cell death | Mesenchymal stem cells | miR-1246 | Activates the PI3K/Akt/mTOR signaling pathway | [126] |
| miR-223 | decreasing expression of IL-6, IL-1β, and ICAM-1 | [186] | ||
| miR-25–3p | Downregulate apoptotic proteins FASL and PTEN | [137] | ||
| miR-338 | Suppresses myocardial cell apoptosis by regulating the JNK pathway through targeting MAP3K2 | [135] | ||
| Adipose-derived mesenchymal stem cells | miR-671 | Targets TGFBR2, inhibiting Smad2 phosphorylation | [124] | |
| miR-146a | Targets EGR1 mRNA, inhibiting its expression and downregulating TLR4/NFκB signaling | [125] | ||
| Bone Marrow Mesenchymal Stem Cells | miR-210 | ALFM3/p53, PI3K/Akt pathway | [192] | |
| miR-21a-5p | Downregulate pro-apoptotic gene PDCD4, PTEN, Peli1 and FasL | [193] | ||
| miR-338 | MAP3K2/JNK pathway | [194] | ||
| miR-150-5p | Increases miR-150-5p expression, decreases Bax expression, reduces myocardial apoptosis and pathological alterations | [133] | ||
| miR-24 | Reduces infarct size and improves cardiac function | [134] | ||
| miR-25-3p | Directly reduces expression of FASL and PTEN; decreases EZH2 and H3K27me3, leading to downregulation of eNOS and SOCS3 | [137] | ||
| miR-486-5p | Inhibits PTEN expression, activates PI3K/Akt signaling pathway | [138] | ||
| human umbilical cord MSCs | - | ctivates autophagy pathway by stimulating AMPK and inhibiting mTOR | [129] | |
| miR-19a | target SOX6, activate AKT, inhibit JNK3/caspase-3 | [195] | ||
| miR-150–5p | Inhibit Bax | [196] | ||
| Adipose-derived mesenchymal stem cells | miR-671 | Inhibit TGFBR2/Smad2 | [197] | |
| Angiogenesis | Mesenchymal stem cells | miR-22 | Downregulates Mecp2 gene, preventing cardiac fibrosis and promoting angiogenesis | [189, 190] |
| miR-1956 |
Activate ERK1/2 and VEGF, downregulate Notch-1 Enhanced ADMSCs proliferation |
[198] | ||
| miR-543 | Downregulate COL4A1 | [199] | ||
| Bristol pericytes | miR-132 | Transfers to endothelial cells, reduces p120RasGAP expression, activates Ras pathway | [191] |
Clinical trial using MSC-derived exosomes for cardiac disease
Previous sections highlighted the remarkable potential of mesenchymal stem cell-derived exosomes in repairing cardiac injuries. In vitro and in vivo studies have demonstrated their significant capacity to mend damaged heart tissue, reduce inflammation, and enhance cardiac function. However, despite these promising findings, the number of clinical trials investigating the efficacy and safety of these exosomes in humans remains limited. The following table provides an evaluation of clinical trials conducted using these exosomes in cardiac injuries (Table 4).
Table 4.
Clinical trials registered on ClinicalTrials.gov on the topic of MSC exosomes in cardiac injuries
| Exosomes from | Related miRNA | Cardiac Disease | NO | Year/ Country |
|---|---|---|---|---|
| Mesenchymal Stem Cells | - | Multiple Organ Dysfuntion Syndrome After Surgical Repaire of Acute Type an Aortic Dissection | NCT04356300 |
2020 China |
| Epicardial fat-derived exosomes | miR-126 | Atrial fibrillation | NCT03478410 |
2021 Israel |
| Mesenchymal Stem Cells | - | Heart failure (HF) and acute myocardial infarction | NCT05669144 |
2022 Iran |
| Placental tissue | - | Preeclampsia Patients | NCT04154332 |
2024 United States |
Challenges and future directions
Despite significant advancements in utilizing mesenchymal stem cell-derived exosomes (MSC-Exos) for cardiac injury treatment, substantial challenges persist that require further investigation and resolution. A primary challenge lies in standardizing the production and purification methods for exosomes. The diversity of existing methods and the absence of a standardized protocol hinder comparisons between different studies and complicate data interpretation. Additionally, determining the optimal dosage, injection route, and timing for exosome administration remains unanswered.
Another challenge is the incomplete understanding of the exact mechanisms by which exosomes enhance cardiac function. Although numerous studies have examined the molecular content of exosomes and their effects on target cells, the precise mechanisms underlying exosome interactions with the injured cardiac environment and the induction of tissue repair remain elusive. Moreover, long-term safety assessments of exosome use in clinical trials are another crucial consideration. Furthermore, the variability in exosome source and isolation methods can lead to significant differences in their therapeutic properties, which complicates efforts to establish consistent treatment protocols. Additionally, the immune response to exosome administration in various patient populations remains poorly understood, raising questions about the biocompatibility of exosome therapies.
One major concern is the variability in biodistribution, which can be influenced by the chosen route. Intravenous, intramuscular, or local injections can lead to inconsistent localization of exosomes at the target site, potentially resulting in suboptimal therapeutic effects or unintended side effects. The clearance rate of exosomes is another factor to consider. Intravenous administration may lead to rapid clearance by the mononuclear phagocyte system, limiting bioavailability at the target site. Conversely, local injections, while potentially offering better retention, can induce inflammatory responses that may hinder efficacy. Furthermore, the formulation and stability of exosomes can be compromised by the administration route. Direct tissue injection may damage both the exosomes and surrounding tissue, while encapsulation strategies, such as hydrogel formulations, can complicate the preparation and administration process. Patient-specific factors, including anatomical variations and underlying health conditions, can further influence the efficacy and safety of different administration routes. Additionally, patient comfort and compliance may be affected by factors like pain associated with intramuscular or subcutaneous injections. Regulatory and standardization issues can arise when developing protocols for various administration routes, potentially leading to variability in clinical outcomes. While exosome-based therapies offer significant potential, careful consideration of administration routes and their associated challenges is essential to optimize therapeutic efficacy and minimize adverse effects.
Given the substantial potential of MSC-Exos in treating cardiac injuries, future research directions should focus on addressing these challenges. Developing standardized methods for exosome production and purification, designing robust clinical trials with adequate sample sizes, and conducting comprehensive preclinical studies to investigate exosome mechanisms of action are among the top priorities. Moreover, employing advanced techniques such as high-throughput sequencing to analyze the RNA and protein contents of exosomes may reveal additional insights into their function and therapeutic potential. Furthermore, collaboration between academic and industry partners can accelerate the translation of research findings into clinical applications. Finally, regulatory frameworks need to be established to guide the safe and effective use of exosome-based therapies in clinical settings. Furthermore, engineering exosomes to enhance their efficacy and target them to the injured cardiac tissue could serve as an innovative strategy for treating these diseases.
Conclusion
Mesenchymal stem cell-derived exosomes (MSC-Exos) have garnered significant attention as a novel generation of cell-based therapies. These nano-sized vesicles carry a wide range of biomolecules that can directly or indirectly influence their surrounding environment. Numerous studies have demonstrated the substantial potential of MSC-Exos in repairing cardiac damage.
One of the most critical mechanisms of MSC-Exo action is their anti-inflammatory properties. By regulating the immune response and reducing the production of inflammatory cytokines, these exosomes contribute to minimizing tissue damage and promoting repair. Moreover, MSC-Exos exhibit potent angiogenic properties and can stimulate the growth of new blood vessels, a crucial process for repairing damaged cardiac tissue. Additionally, MSC-Exos possess antioxidant and anti-apoptotic properties, protecting cardiac cells from oxidative stress and cell death. However, challenges persist in utilizing MSC-Exos for treating heart diseases. A primary challenge lies in standardizing the production and purification methods for exosomes. Furthermore, the precise mechanisms by which MSC-Exos interact with cardiac cells and surrounding tissues require further investigation. Additionally, long-term safety assessments of MSC-Exo use in clinical trials are another crucial consideration.
Given the high potential of MSC-Exos in treating heart diseases, future research should focus on addressing these challenges. Developing standardized methods for exosome production and purification, designing robust clinical trials with adequate sample sizes, and conducting comprehensive preclinical studies to investigate MSC-Exo mechanisms of action are among the top priorities. Additionally, engineering exosomes to enhance their efficacy and target them to the injured cardiac tissue could serve as an innovative strategy for treating these diseases.
In conclusion, MSC-Exos, as an emerging therapeutic tool, hold promising prospects for treating heart diseases. With further research and the resolution of existing challenges, these nano-sized particles can be utilized to develop effective and safe therapies for cardiac patients.
Acknowledgements
Not applicable.
Author contributions
MS, and HR designed the review study and contributed to writing the manuscript draft. HR searched the literature and contributed to writing the manuscript. MS contributed to the literature search and edited the manuscript. All authors have confirmed the final version of the manuscript and are accountable for the contents of all parts of the work. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
All data is available in this article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Artificial Intelligence (AI)
The authors declare that they have not use AI-generated work in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Y, et al. Opportunities and challenges: mesenchymal stem cells in the treatment of multiple sclerosis. Int J Neurosci. 2023;133(9):1031–44. [DOI] [PubMed] [Google Scholar]
- 2.Li B, et al. Dental-derived mesenchymal stem cells: state of the art. Front Cell Dev Biol. 2021;9:654559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malekpour K, et al. The potential use of mesenchymal stem cells and their derived exosomes for Orthopedic diseases Treatment. Stem Cell Rev Rep. 2022;18(3):933–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, et al. Clinical application of mesenchymal stem cells in rheumatic diseases. Stem Cell Res Ther. 2021;12(1):567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu M, et al. Role of hedgehog signaling pathways in multipotent mesenchymal stem cells differentiation. Cell Transpl. 2024;33:9636897241244943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Q, et al. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res Ther. 2020;11(1):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaripova LN et al. Mesenchymal stem cells in the Pathogenesis and therapy of Autoimmune and Autoinflammatory diseases. Int J Mol Sci, 2023. 24(22). [DOI] [PMC free article] [PubMed]
- 8.Diotallevi F et al. Mesenchymal stem cells and psoriasis: systematic review. Int J Mol Sci, 2022. 23(23). [DOI] [PMC free article] [PubMed]
- 9.Gholami Farashah MS, et al. Bone marrow mesenchymal stem cells’ osteogenic potential: superiority or non-superiority to other sources of mesenchymal stem cells? Cell Tissue Bank. 2023;24(3):663–81. [DOI] [PubMed] [Google Scholar]
- 10.Kangari P, et al. Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res Ther. 2020;11(1):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu X, et al. Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Front Endocrinol (Lausanne). 2023;14:1099310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen CM, et al. Placental exosomes as biomarkers for maternal diseases: current advances in isolation, characterization, and detection. ACS Sens. 2023;8(7):2493–513. [DOI] [PubMed] [Google Scholar]
- 13.Feng X, et al. Effects of human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation in situ on primary ovarian insufficiency in SD rats. Reprod Sci. 2020;27(7):1502–12. [DOI] [PubMed] [Google Scholar]
- 14.Pan Y, et al. Mesenchymal stem cell-derived exosomes in cardiovascular and cerebrovascular diseases: from mechanisms to therapy. Biomed Pharmacother. 2023;163:114817. [DOI] [PubMed] [Google Scholar]
- 15.Akhavan Rahnama M, et al. The Effect of exosomes derived from unrestricted somatic stem cells on murine model of Sepsis. Cells Tissues Organs. 2023;212(2):164–75. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran A, Dhar R, Devi A. Stem cell-derived exosomes: an Advanced Horizon to Cancer Regenerative Medicine. ACS Appl Bio Mater. 2024;7(4):2128–39. [DOI] [PubMed] [Google Scholar]
- 17.Ho BX, Teo AKK, Ng NHJ. Innovations in bio-engineering and cell-based approaches to address immunological challenges in islet transplantation. Front Immunol. 2024;15:1375177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hade MD, Suire CN, Suo Z. An effective peptide-based platform for efficient Exosomal Loading and Cellular Delivery of a microRNA. ACS Appl Mater Interfaces. 2023;15(3):3851–66. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, et al. Therapeutic strategies and enhanced production of Stem Cell-Derived exosomes for tissue regeneration. Tissue Eng Part B Rev. 2023;29(2):151–66. [DOI] [PubMed] [Google Scholar]
- 20.Koohsarian P, et al. Reviewing the role of cardiac exosomes in myocardial repair at a glance. Cell Biol Int. 2021;45(7):1352–63. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, et al. Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Front Cell Dev Biol. 2023;11:1029671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou J, et al. Exosomes derived from odontogenic stem cells: its role in the dentin-pulp complex. Regen Ther. 2023;24:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barungi S, et al. Clinical implications of inflammation in atheroma formation and novel therapies in cardiovascular diseases. Front Cell Dev Biol. 2023;11:1148768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdulfattah SY, Samawi FT. Estimating the role of single-nucleotide polymorphism (rs1800629)-308 G/A of TNF-alpha gene as genetic marker associated with angina pectoris in a sample of Iraqi patients. J Genet Eng Biotechnol. 2023;21(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ismail TF et al. Hypertensive Heart Disease-The Imaging Perspective. J Clin Med, 2023. 12(9). [DOI] [PMC free article] [PubMed]
- 26.Sayers JR, Riley PR. Heart regeneration: beyond new muscle and vessels. Cardiovasc Res. 2021;117(3):727–42. [DOI] [PubMed] [Google Scholar]
- 27.Damy T. [Heart failure, progress and challenges]. Soins. 2017;62(820):20–1. [DOI] [PubMed] [Google Scholar]
- 28.Tenreiro MF, et al. Next generation of heart regenerative therapies: progress and promise of cardiac tissue engineering. NPJ Regen Med. 2021;6(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omatsu-Kanbe M et al. Atypically shaped cardiomyocytes (ACMs): the identification, characterization and New insights into a subpopulation of Cardiomyocytes. Biomolecules, 2022. 12(7). [DOI] [PMC free article] [PubMed]
- 30.Peterson EA, Sun J, Wang J. Leukocyte-mediated Cardiac Repair after myocardial infarction in non-regenerative vs. Regenerative systems. J Cardiovasc Dev Dis, 2022. 9(2). [DOI] [PMC free article] [PubMed]
- 31.Ul Haq A et al. Extrinsically conductive nanomaterials for Cardiac tissue Engineering Applications. Micromachines (Basel), 2021. 12(8). [DOI] [PMC free article] [PubMed]
- 32.Hutt E, Desai MY. Medical treatment strategies for hypertrophic cardiomyopathy. Am J Cardiol. 2024;212s:S33–41. [DOI] [PubMed] [Google Scholar]
- 33.Hatani T, Yoshida Y. Transplantation of Human Induced Pluripotent Stem Cell-Derived cardiomyocytes in a mouse myocardial infarction model. Methods Mol Biol. 2021;2320:285–93. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Z, et al. Mesenchymal stem cell-derived exosomes: a possible therapeutic strategy for repairing heart injuries. Front Cell Dev Biol. 2023;11:1093113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tariq U, et al. Role of Biomaterials in Cardiac Repair and Regeneration: therapeutic intervention for myocardial infarction. ACS Biomater Sci Eng. 2022;8(8):3271–98. [DOI] [PubMed] [Google Scholar]
- 36.Ashique S, et al. Unraveling the emerging Niche Role of Extracellular vesicles (EVs) in traumatic Brain Injury (TBI). CNS Neurol Disord Drug Targets. 2024;23(11):1357–70. [DOI] [PubMed] [Google Scholar]
- 37.He M, et al. Cell-cell communication in kidney fibrosis. Nephrol Dial Transpl. 2024;39(5):761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M, Pedersen LC, Xu D. Targeting heparan sulfate-protein interactions with oligosaccharides and monoclonal antibodies. Front Mol Biosci. 2023;10:1194293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fyfe J, et al. Role of lipid signalling in extracellular vesicles-mediated cell-to-cell communication. Cytokine Growth Factor Rev. 2023;73:20–6. [DOI] [PubMed] [Google Scholar]
- 40.Patel NJ, Ashraf A, Chung EJ. Extracell Vesicles as Regulators Extracell Matrix Bioeng (Basel), 2023. 10(2). [DOI] [PMC free article] [PubMed]
- 41.Kisielewska M et al. Utilizing Extracellular Vesicles for Eliminating ‘Unwanted Molecules’: Harnessing Nature’s Structures in Modern Therapeutic Strategies. Molecules, 2024. 29(5). [DOI] [PMC free article] [PubMed]
- 42.Carvalho-Silva LT, et al. Extracellular vesicles in carcinoma microenvironment. Biochem Soc Trans. 2023;51(2):771–81. [DOI] [PubMed] [Google Scholar]
- 43.Nail HM, et al. Exosomal miRNA-mediated intercellular communications and immunomodulatory effects in tumor microenvironments. J Biomed Sci. 2023;30(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papakonstantinou E et al. Milk exosomes and a new way of communication between mother and child. EMBnet J, 2024. 29. [DOI] [PMC free article] [PubMed]
- 45.Tang H et al. Mesenchymal stem cell-derived apoptotic bodies: Biological functions and therapeutic potential. Cells, 2022. 11(23). [DOI] [PMC free article] [PubMed]
- 46.Wang L, Sun Z, Wang H. Extracellular vesicles and the regulation of tumor immunity: current progress and future directions. J Cell Biochem. 2021;122(7):760–9. [DOI] [PubMed] [Google Scholar]
- 47.Yan Z, et al. Regulatory roles of extracellular vesicles in immune responses against Mycobacterium tuberculosis infection. World J Clin Cases. 2021;9(25):7311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du R et al. Glioblastoma phagocytic cell death: balancing the opportunities for therapeutic manipulation. Cells, 2024. 13(10). [DOI] [PMC free article] [PubMed]
- 49.Baljon JJ, Wilson JT. Bioinspired vaccines to enhance MHC class-I antigen cross-presentation. Curr Opin Immunol. 2022;77:102215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang F, et al. Delivery of nanoparticle antigens to antigen-presenting cells: from extracellular specific targeting to intracellular responsive presentation. J Control Release. 2021;333:107–28. [DOI] [PubMed] [Google Scholar]
- 51.Johnstone RM, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20. [PubMed] [Google Scholar]
- 52.Peng Q, et al. The potential roles of cigarette smoke-induced extracellular vesicles in oral leukoplakia. Eur J Med Res. 2023;28(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefanius K, Servage K, Orth K. Exosomes in cancer development. Curr Opin Genet Dev. 2021;66:83–92. [DOI] [PubMed] [Google Scholar]
- 54.Lu Z, et al. Role of circulating exosomes in Cerebrovascular diseases: a Comprehensive Review. Curr Neuropharmacol. 2023;21(7):1575–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefanska K et al. The role of exosomes in Human Carcinogenesis and Cancer Therapy-recent findings from Molecular and Clinical Research. Cells, 2023. 12(3). [DOI] [PMC free article] [PubMed]
- 56.Yu J et al. Exosomes as a source of biomarkers for gastrointestinal cancers. Cancers (Basel), 2023. 15(4). [DOI] [PMC free article] [PubMed]
- 57.Clancy JW, Schmidtmann M, D’Souza-Schorey C. The ins and outs of microvesicles. FASEB Bioadv. 2021;3(6):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fayyazpour P, et al. The role of exosomes in cancer biology by shedding light on their lipid contents. Pathol Res Pract. 2023;250:154813. [DOI] [PubMed] [Google Scholar]
- 59.Costigan A, Hollville E, Martin SJ. Discriminating between apoptosis, necrosis, Necroptosis, and ferroptosis by Microscopy and Flow Cytometry. Curr Protoc. 2023;3(12):e951. [DOI] [PubMed] [Google Scholar]
- 60.Mahani M, et al. Carbon quantum dots-annexin V probe: photoinduced electron transfer mechanism, phosphatidylserine detection, and apoptotic cell imaging. Mikrochim Acta. 2022;189(2):69. [DOI] [PubMed] [Google Scholar]
- 61.Pisko J, et al. Apoptotic cells in mouse blastocysts are eliminated by neighbouring blastomeres. Sci Rep. 2021;11(1):9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meldolesi J. Unconventional protein secretion dependent on two Extracellular vesicles: exosomes and ectosomes. Front Cell Dev Biol. 2022;10:877344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sundar V, Saraswathi V. Effect of ethanol on Exosome Biogenesis: possible mechanisms and therapeutic implications. Biomolecules, 2023. 13(2). [DOI] [PMC free article] [PubMed]
- 64.Perrin P, et al. Retrofusion of intralumenal MVB membranes parallels viral infection and coexists with exosome release. Curr Biol. 2021;31(17):3884–e38934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rincon-Riveros A et al. Regulation of Antitumor Immune responses by Exosomes Derived from Tumor and Immune cells. Cancers (Basel), 2021. 13(4). [DOI] [PMC free article] [PubMed]
- 66.Yang K, et al. [Proteomic analysis of serum and serum exosomes, and their application in intrahepatic cholangiocarcinoma]. Se Pu. 2021;39(11):1191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huo L, et al. The emerging role of neural cell-derived exosomes in Intercellular Communication in Health and neurodegenerative diseases. Front Neurosci. 2021;15:738442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palomar-Alonso N, Lee M, Kim M. Exosomes: membrane-associated proteins, challenges and perspectives. Biochem Biophys Rep. 2024;37:101599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, et al. The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer. Exp Mol Med. 2022;54(9):1390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El Safadi D et al. Exosome-mediated Antigen Delivery: unveiling novel strategies in viral infection control and Vaccine Design. Vaccines (Basel), 2024. 12(3). [DOI] [PMC free article] [PubMed]
- 71.Lee YJ, Shin KJ, Chae YC. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp Mol Med. 2024;56(4):877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bano A, et al. Exosomics in oral cancer diagnosis, prognosis, and therapeutics - an emergent and imperative non-invasive natural nanoparticle-based approach. Crit Rev Oncol Hematol. 2022;178:103799. [DOI] [PubMed] [Google Scholar]
- 73.Huang A et al. Bardoxolone Methyl Ameliorates Myocardial Ischemia/Reperfusion Injury by Activating the Nrf2/HO-1 Signaling Pathway. Cardiovasc Ther, 2023. 2023: p. 5693732. [DOI] [PMC free article] [PubMed]
- 74.Liu Y, et al. Aucubin protects against myocardial ischemia-reperfusion injury by regulating STAT3/NF-kappaB/HMGB-1 pathway. Int J Cardiol. 2024;400:131800. [DOI] [PubMed] [Google Scholar]
- 75.Gladka MM, et al. Cardiomyocytes stimulate angiogenesis after ischemic injury in a ZEB2-dependent manner. Nat Commun. 2021;12(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu M, et al. Exosomes derived from MSC pre-treated with oridonin alleviates myocardial IR injury by suppressing apoptosis via regulating autophagy activation. J Cell Mol Med. 2021;25(12):5486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nejabati HR, Nikzad S, Roshangar L. Therapeutic potential of mesenchymal stem cells in PCOS. Curr Stem Cell Res Ther. 2024;19(2):134–44. [DOI] [PubMed] [Google Scholar]
- 78.Xu H, et al. Bone marrow mesenchymal stem cells-derived exosomes ameliorate LPS-induced acute lung injury by mir-223-regulated alveolar macrophage M2 polarization. J Biochem Mol Toxicol. 2024;38(1):e23568. [DOI] [PubMed] [Google Scholar]
- 79.Tjandra PM, Ripplinger CM, Christiansen BA. The heart-bone connection: relationships between myocardial infarction and osteoporotic fracture. Am J Physiol Heart Circ Physiol. 2024;326(3):H845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu SC, Wu W, Zhang SY. Manifestations and mechanism of SARS-CoV2 mediated Cardiac Injury. Int J Biol Sci. 2022;18(7):2703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, et al. Recent progress in therapeutic strategies and biomimetic nanomedicines based on neutrophils for inflammation treatment. Nanomed (Lond). 2023;18(5):485–500. [DOI] [PubMed] [Google Scholar]
- 82.Ismaiel A, Dumitrascu DL. How to Reduce Cardiovascular risk in nonalcoholic fatty liver disease. Am J Ther. 2023;30(3):e242–56. [DOI] [PubMed] [Google Scholar]
- 83.Sapna F, et al. Advancements in heart failure management: a Comprehensive Narrative Review of emerging therapies. Cureus. 2023;15(10):e46486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356(6342):1026–30. [DOI] [PubMed] [Google Scholar]
- 85.Wang B, et al. Mutual regulation of PD-L1 immunosuppression between tumor-associated macrophages and tumor cells: a critical role for exosomes. Cell Commun Signal. 2024;22(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen W, et al. The emerging role of exosomes in the pathogenesis, prognosis and treatment of necrotizing enterocolitis. Am J Transl Res. 2020;12(11):7020–33. [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H, et al. Intestinal fungi and systemic autoimmune diseases. Autoimmun Rev. 2023;22(2):103234. [DOI] [PubMed] [Google Scholar]
- 88.Hu X, et al. Exosomes reveal the dual nature of radiotherapy in tumor immunology. Cancer Sci. 2022;113(4):1105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khan FH, et al. Role of exosomes in lung cancer: a comprehensive insight from immunomodulation to theragnostic applications. Biochim Biophys Acta Rev Cancer. 2022;1877(5):188776. [DOI] [PubMed] [Google Scholar]
- 90.Saravanan PB, et al. Exosomes in transplantation: role in allograft rejection, diagnostic biomarker, and therapeutic potential. Life Sci. 2023;324:121722. [DOI] [PubMed] [Google Scholar]
- 91.Arora S, et al. Potential application of immunotherapy for modulation of pulp inflammation: opportunities for vital pulp treatment. Int Endod J. 2021;54(8):1263–74. [DOI] [PubMed] [Google Scholar]
- 92.Wang X, et al. Serum cytokine profiles predict response to systemic glucocorticoid in active vitiligo. Postepy Dermatol Alergol. 2024;41(2):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu J, et al. Mir-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol Therapy-Nucleic Acids. 2018;11:103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei Z, et al. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci. 2019;232:116632. [DOI] [PubMed] [Google Scholar]
- 95.Liu H, Yao M, Ren J. Codonopsis pilosula-derived glycopeptide dCP1 promotes the polarization of tumor-associated macrophage from M2-like to M1 phenotype. Cancer Immunol Immunother. 2024;73(7):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lethen I, et al. Tofacitinib affects M1-like and M2-like polarization and tissue factor expression in macrophages of healthy donors and IBD patients. Inflamm Bowel Dis. 2024;30(7):1151–63. [DOI] [PubMed] [Google Scholar]
- 97.Niu XH, et al. Activating alpha7nAChR helps post-myocardial infarction healing by regulating macrophage polarization via the STAT3 signaling pathway. Inflamm Res. 2023;72(4):879–92. [DOI] [PubMed] [Google Scholar]
- 98.Arabpour M, Saghazadeh A, Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97:107823. [DOI] [PubMed] [Google Scholar]
- 99.Ma T, et al. Enhanced osteolysis targeted therapy through Fusion of exosomes derived from M2 macrophages and bone marrow mesenchymal stem cells: modulating macrophage polarization. Small. 2024;20(7):e2303506. [DOI] [PubMed] [Google Scholar]
- 100.Long R, et al. M2 macrophage-derived exosomes carry mir-1271-5p to alleviate cardiac injury in acute myocardial infarction through down-regulating SOX6. Mol Immunol. 2021;136:26–35. [DOI] [PubMed] [Google Scholar]
- 101.Dai Y, et al. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome signaling pathway. J Mol Cell Cardiol. 2020;142:65–79. [DOI] [PubMed] [Google Scholar]
- 102.Li J, Zhang Q, Jiao H. LncRNA NRON promotes M2 macrophage polarization and alleviates atrial fibrosis through suppressing exosomal miR-23a derived from atrial myocytes. J Formos Med Assoc. 2021;120(7):1512–9. [DOI] [PubMed] [Google Scholar]
- 103.Hu H, et al. Exosomes derived from regulatory T cells ameliorate acute myocardial infarction by promoting macrophage M2 polarization. IUBMB Life. 2020;72(11):2409–19. [DOI] [PubMed] [Google Scholar]
- 104.Li J, et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE–/-mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 2019;510(4):565–72. [DOI] [PubMed] [Google Scholar]
- 105.Ma J, et al. Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2 macrophage polarization and reduces macrophage infiltration to attenuate atherosclerosis. Acta Biochim Biophys Sin (Shanghai). 2021;53(9):1227–36. [DOI] [PubMed] [Google Scholar]
- 106.Yan F, Cui W, Chen Z. Mesenchymal stem cell-derived exosome-loaded microRNA-129-5p inhibits TRAF3 expression to alleviate apoptosis and oxidative stress in heart failure. Cardiovasc Toxicol. 2022;22(7):631–45. [DOI] [PubMed] [Google Scholar]
- 107.Ghafouri-Fard S, et al. Regulatory Role of non-coding RNAs on Immune responses during Sepsis. Front Immunol. 2021;12:798713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L, et al. MiR-223-3p affects myocardial inflammation and apoptosis following myocardial infarction via targeting FBXW7. J Thorac Dis. 2022;14(4):1146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yuan S, et al. miR-223: an Immune Regulator in Infectious disorders. Front Immunol. 2021;12:781815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li G, et al. Mir-223-3p contributes to suppressing NLRP3 inflammasome activation in Streptococcus equi ssp. zooepidemicus infection. Vet Microbiol. 2022;269:109430. [DOI] [PubMed] [Google Scholar]
- 111.Gao L, et al. Changes and clinical value of serum miR-24 and miR-223 levels in patients with severe pneumonia. Int J Gen Med. 2023;16:3797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu G, et al. Low serum miR-223 expression predicts poor outcome in patients with acute myeloid leukemia. J Clin Lab Anal. 2020;34(3):e23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Byrne NJ, et al. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radic Biol Med. 2021;169:317–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tirichen H, et al. Mitochondrial reactive oxygen species and their contribution in chronic kidney Disease Progression through oxidative stress. Front Physiol. 2021;12:627837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Agraharam G, Girigoswami A, Girigoswami K. Myricetin: a multifunctional flavonol in Biomedicine. Curr Pharmacol Rep. 2022;8(1):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Briyal S, Ranjan AK, Gulati A. Oxidative stress: a target to treat Alzheimer’s disease and stroke. Neurochem Int. 2023;165:105509. [DOI] [PubMed] [Google Scholar]
- 117.Sadasivam N et al. Oxidative stress, Genomic Integrity, and Liver diseases. Molecules, 2022. 27(10). [DOI] [PMC free article] [PubMed]
- 118.Ni J, et al. Exosomes Derived from TIMP2-Modified human umbilical cord mesenchymal stem cells enhance the Repair Effect in Rat Model with myocardial infarction possibly by the Akt/Sfrp2 pathway. Oxid Med Cell Longev. 2019;2019:p1958941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arslan F, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301–12. [DOI] [PubMed] [Google Scholar]
- 120.Han X, et al. Targeting ferroptosis: a novel insight against myocardial infarction and ischemia-reperfusion injuries. Apoptosis. 2023;28(1–2):108–23. [DOI] [PubMed] [Google Scholar]
- 121.Tan Y, et al. PHLPP1 deficiency ameliorates cardiomyocyte death and cardiac dysfunction through inhibiting Mcl-1 degradation. Cell Signal. 2022;92:110281. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y, et al. The Role of Regulatory T Cells in Heart Repair after myocardial infarction. J Cardiovasc Transl Res. 2023;16(3):590–7. [DOI] [PubMed] [Google Scholar]
- 123.Zhang L, Zhao S, Wang Y. Diannexin alleviates myocardial ischemia-reperfusion injury by orchestrating cardiomyocyte oxidative damage, macrophage polarization and fibrotic process by TLR4-NF-kB-mediated inactivation of NLRP3 inflammasome. Int Immunopharmacol. 2024;130:111668. [DOI] [PubMed] [Google Scholar]
- 124.Wang X, et al. Adipose-derived mesenchymal stem cells-derived exosomes carry MicroRNA-671 to alleviate myocardial infarction through inactivating the TGFBR2/Smad2 Axis. Inflammation. 2021;44(5):1815–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pan J, et al. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J Cell Biochem. 2019;120(3):4433–43. [DOI] [PubMed] [Google Scholar]
- 126.Wang Q-G, et al. miR-320a in serum exosomes promotes myocardial fibroblast proliferation via regulating the PIK3CA/Akt/mTOR signaling pathway in HEH2 cells. Experimental Therapeutic Med. 2021;22(2):873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu T, et al. MicroRNA-324-3p plays a protective role against Coxsackievirus B3-Induced viral myocarditis. Virol Sin. 2021;36(6):1585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Belyaeva E, et al. The spectrum of cell death in sarcoma. Biomed Pharmacother. 2023;162:114683. [DOI] [PubMed] [Google Scholar]
- 129.Gu X, et al. Exosomes derived from umbilical cord mesenchymal stem cells alleviate viral myocarditis through activating AMPK/mTOR-mediated autophagy flux pathway. J Cell Mol Med. 2020;24(13):7515–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spengler K et al. VEGF Triggers Transient Induction of Autophagy in endothelial cells via AMPKalpha1. Cells, 2020. 9(3). [DOI] [PMC free article] [PubMed]
- 131.Li B, et al. Hydroquinone-induced endoplasmic reticulum stress affects TK6 cell autophagy and apoptosis via ATF6-mTOR. Environ Toxicol. 2023;38(8):1874–90. [DOI] [PubMed] [Google Scholar]
- 132.Guedes JP, et al. Acetic acid triggers cytochrome c release in yeast heterologously expressing human Bax. Apoptosis. 2022;27(5–6):368–81. [DOI] [PubMed] [Google Scholar]
- 133.Wu Z, et al. RETRACTED:BMSCs-derived exosomal microRNA-150-5p attenuates myocardial infarction in mice. Int Immunopharmacol. 2021;93:107389. [DOI] [PubMed] [Google Scholar]
- 134.Zhang CS, et al. Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur Rev Med Pharmacol Sci. 2019;23(15):6691–9. [DOI] [PubMed] [Google Scholar]
- 135.Fu DL, et al. MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur Rev Med Pharmacol Sci. 2020;24(19):10107–17. [DOI] [PubMed] [Google Scholar]
- 136.Liu X, et al. Phosphatase and Tensin Homology deleted on chromosome 10 inhibitors promote neural stem cell proliferation and differentiation. Front Pharmacol. 2022;13:907695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Peng Y, et al. Exosomal mir-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis. 2020;11(5):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sun XH, et al. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via mir-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res. 2019;177:23–32. [DOI] [PubMed] [Google Scholar]
- 139.Ricketts SN, Qian L. The heart of cardiac reprogramming: the cardiac fibroblasts. J Mol Cell Cardiol. 2022;172:90–9. [DOI] [PubMed] [Google Scholar]
- 140.Tan Y, et al. Murine neonatal cardiac B cells promote cardiomyocyte proliferation and heart regeneration. NPJ Regen Med. 2023;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neuber S, Emmert MY, Nazari-Shafti TZ. Hopes and hurdles of employing mesenchymal stromal cells in the treatment of Cardiac Fibrosis. Int J Mol Sci, 2021. 22(23). [DOI] [PMC free article] [PubMed]
- 142.Ke X, et al. Exosomal miR-218-5p/miR-363-3p from endothelial progenitor cells ameliorate myocardial infarction by targeting the p53/JMY signaling pathway. Oxid Med Cell Longev. 2021;2021:p5529430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pu L, et al. Exosomes released from mesenchymal stem cells overexpressing microRNA-30e ameliorate heart failure in rats with myocardial infarction. Am J Transl Res. 2021;13(5):4007–25. [PMC free article] [PubMed] [Google Scholar]
- 144.Deng J, et al. FNDC5/irisin improves the therapeutic efficacy of bone marrow-derived mesenchymal stem cells for myocardial infarction. Stem Cell Res Ther. 2020;11(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gray WD, et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116(2):255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sánchez-Alonso S, et al. Extracellular vesicle-mediated Immune Regulation of tissue remodeling and Angiogenesis after myocardial infarction. Front Immunol. 2018;9:2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cwilichowska N, et al. Diagnostic and therapeutic potential of protease inhibition. Mol Aspects Med. 2022;88:101144. [DOI] [PubMed] [Google Scholar]
- 148.Rai M, et al. Contribution of proteases to the hallmarks of aging and to age-related neurodegeneration. Aging Cell. 2022;21(5):e13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Z, et al. Exosomal microRNA-1246 from human umbilical cord mesenchymal stem cells potentiates myocardial angiogenesis in chronic heart failure. Cell Biol Int. 2021;45(11):2211–25. [DOI] [PubMed] [Google Scholar]
- 150.Wang X, et al. The application potential and advance of mesenchymal stem cell-derived exosomes in myocardial infarction. Stem Cells Int. 2021;2021:p5579904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu H, et al. MSC-derived exosomes protect auditory hair cells from neomycin-induced damage via autophagy regulation. Biol Res. 2024;57(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hu R, et al. Transplantation of human umbilical cord blood mononuclear cells promotes functional endometrium reconstruction via downregulating EMT in damaged endometrium. Regen Ther. 2024;27:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Qu X, et al. ONO-1301 enhances post-transplantation survival of human induced pluripotent stem cell-derived cardiac tissue sheet by promoting angiogenesis. J Heart Lung Transpl. 2023;42(6):716–29. [DOI] [PubMed] [Google Scholar]
- 154.Hattori Y. The multiple roles of Pericytes in vascular formation and microglial functions in the brain. Life (Basel), 2022. 12(11). [DOI] [PMC free article] [PubMed]
- 155.Wang YC, et al. Exosomal miR-107 antagonizes profibrotic phenotypes of pericytes by targeting a pathway involving HIF-1alpha/Notch1/PDGFRbeta/YAP1/Twist1 axis in vitro. Am J Physiol Heart Circ Physiol. 2021;320(2):H520–34. [DOI] [PubMed] [Google Scholar]
- 156.Xiang Z, et al. MeCP2 epigenetically regulates alpha-smooth muscle actin in human lung fibroblasts. J Cell Biochem. 2020;121(7):3616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang M, et al. LncRNA CFAR promotes cardiac fibrosis via the miR-449a-5p/LOXL3/mTOR axis. Sci China Life Sci. 2023;66(4):783–99. [DOI] [PubMed] [Google Scholar]
- 158.Goulet MR et al. Regulation of cellular communication network factor 1 by Ras homolog family member A in bovine steroidogenic luteal cells. J Anim Sci, 2022. 100(7). [DOI] [PMC free article] [PubMed]
- 159.Zheng G, et al. SCAP contributes to embryonic angiogenesis by negatively regulating KISS-1 expression in mice. Cell Death Dis. 2023;14(4):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ren LX, et al. A novel ZNF304/miR-183-5p/FOXO4 pathway regulates cell proliferation in Clear Cell Renal Carcinoma. Front Oncol. 2021;11:710525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jackson HK et al. Extracellular vesicles Potentiate Medulloblastoma Metastasis in an EMMPRIN and MMP-2 Dependent Manner. Cancers (Basel), 2023. 15(9). [DOI] [PMC free article] [PubMed]
- 162.Kim HS, et al. EMMPRIN expression is associated with metastatic progression in osteosarcoma. BMC Cancer. 2021;21(1):1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pan Q, et al. Exosomes derived from mesenchymal stem cells ameliorate Hypoxia/Reoxygenation-Injured ECs via transferring MicroRNA-126. Stem Cells Int. 2019;2019:p2831756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mohan CD, et al. Crocetin imparts antiproliferative activity via inhibiting STAT3 signaling in hepatocellular carcinoma. IUBMB Life. 2021;73(11):1348–62. [DOI] [PubMed] [Google Scholar]
- 165.Du W, et al. Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials. 2017;133:70–81. [DOI] [PubMed] [Google Scholar]
- 166.Wang W, et al. Exosomes secreted from mesenchymal stem cells mediate the regeneration of endothelial cells treated with rapamycin by delivering pro-angiogenic microRNAs. Exp Cell Res. 2021;399(1):112449. [DOI] [PubMed] [Google Scholar]
- 167.Huang H, et al. Exosomes from SIRT1-Overexpressing ADSCs restore cardiac function by improving angiogenic function of EPCs. Mol Ther Nucleic Acids. 2020;21:737–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Aziz MNM et al. Anti-metastatic and anti-angiogenic effects of Curcumin Analog DK1 on Human Osteosarcoma cells in Vitro. Pharmaceuticals (Basel), 2021. 14(6). [DOI] [PMC free article] [PubMed]
- 169.Ning W, et al. Blocking exosomal miRNA-153-3p derived from bone marrow mesenchymal stem cells ameliorates hypoxia-induced myocardial and microvascular damage by targeting the ANGPT1-mediated VEGF/PI3k/Akt/eNOS pathway. Cell Signal. 2021;77:109812. [DOI] [PubMed] [Google Scholar]
- 170.Kong LY, et al. Mir-666-3p mediates the Protective effects of mesenchymal stem cell-derived Exosomes Against Oxygen-glucose deprivation and reoxygenation- induced cell Injury in Brain Microvascular endothelial cells via Mitogen-activated protein kinase pathway. Curr Neurovasc Res. 2021;18(1):20–77. [DOI] [PubMed] [Google Scholar]
- 171.Zhang M, et al. Exosomal Mir-3613-3p derived from oxygen-glucose deprivation-treated brain microvascular endothelial cell promotes microglial M1 polarization. Cell Mol Biol Lett. 2023;28(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang J, et al. Extracellular vesicles-encapsulated microRNA-29b-3p from bone marrow-derived mesenchymal stem cells promotes fracture healing via modulation of the PTEN/PI3K/AKT axis. Exp Cell Res. 2022;412(2):113026. [DOI] [PubMed] [Google Scholar]
- 173.Wang K, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by Exosomal MicroRNA-21. Stem Cells Transl Med. 2017;6(1):209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhu D, et al. Exosomes from adipose-derived stem cells alleviate myocardial infarction via microRNA-31/FIH1/HIF-1alpha pathway. J Mol Cell Cardiol. 2022;162:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang C, et al. ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway. J Cell Mol Med. 2018;22(3):1873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li X, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Parmar D, Apte M. Angiopoietin inhibitors: a review on targeting tumor angiogenesis. Eur J Pharmacol. 2021;899:174021. [DOI] [PubMed] [Google Scholar]
- 178.Jaud M et al. The PERK Branch of the unfolded protein response promotes DLL4 expression by activating an alternative translation mechanism. Cancers (Basel), 2019. 11(2). [DOI] [PMC free article] [PubMed]
- 179.Fu H, et al. Effect of heparan sulfate mimetics from Escherichia coli K5 polysaccharide on SDF-1/CXCL12-induced endothelial progenitor cells in vitro. Int J Biol Macromol. 2018;107(Pt B):2492–500. [DOI] [PubMed] [Google Scholar]
- 180.Mause SF et al. Engagement of the CXCL12-CXCR4 Axis in the Interaction of endothelial progenitor cell and smooth muscle cell to promote phenotype control and guard vascular homeostasis. Int J Mol Sci, 2022. 23(2). [DOI] [PMC free article] [PubMed]
- 181.Berkoz M, et al. Myricetin inhibits angiotensin converting enzyme and induces nitric oxide production in HUVEC cell line. Gen Physiol Biophys. 2020;39(3):249–58. [DOI] [PubMed] [Google Scholar]
- 182.Zhu F et al. Human umbilical cord mesenchymal stem cells derived exosomes attenuate injury of myocardial infarction by miR-24-3p-promoted M2 macrophage polarization. 2021. [DOI] [PubMed]
- 183.Zhu D, et al. Intrapericardial exosome therapy dampens cardiac injury via activating Foxo3. Circul Res. 2022;131(10):e135–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Deng S, et al. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114:105564. [DOI] [PubMed] [Google Scholar]
- 185.Xu R, et al. Exosomes derived from pro-inflammatory bone marrow‐derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J Cell Mol Med. 2019;23(11):7617–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang X, et al. Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci Rep. 2015;5(1):13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang S, et al. Exosomes derived from mir-129‐5p modified bone marrow mesenchymal stem cells represses ventricular remolding of mice with myocardial infarction. J Tissue Eng Regen Med. 2022;16(2):177–87. [DOI] [PubMed] [Google Scholar]
- 188.Ni J, et al. Exosomes derived from TIMP2-modified human umbilical cord mesenchymal stem cells enhance the repair effect in rat model with myocardial infarction possibly by the Akt/Sfrp2 pathway. Volume 2019. Oxidative Medicine and Cellular Longevity; 2019. p. 1958941. 1. [DOI] [PMC free article] [PubMed]
- 189.Sun H-J, et al. Functional roles of exosomes in cardiovascular disorders: a systematic review. Volume 21. European Review for Medical & Pharmacological Sciences; 2017. 22. [DOI] [PubMed]
- 190.Zhao W, Zheng X-L, Zhao S-P. Exosome and its roles in cardiovascular diseases. Heart Fail Rev. 2015;20:337–48. [DOI] [PubMed] [Google Scholar]
- 191.Chistiakov DA, Orekhov AN, Bobryshev YV. Cardiac extracellular vesicles in normal and infarcted heart. Int J Mol Sci. 2016;17(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fu D-L, et al. MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Volume 24. European Review for Medical & Pharmacological Sciences; 2020. 19. [DOI] [PubMed]
- 193.Wang S, et al. miR-19a/19b-loaded exosomes in combination with mesenchymal stem cell transplantation in a preclinical model of myocardial infarction. Regen Med. 2020;15(6):1749–59. [DOI] [PubMed] [Google Scholar]
- 194.Li C-X, et al. Circular RNA 0001273 in exosomes derived from human umbilical cord mesenchymal stem cells (UMSCs) in myocardial infarction. Volume 24. European Review for Medical & Pharmacological Sciences; 2020. 19. [DOI] [PubMed]
- 195.Wu Z, et al. RETRACTED: BMSCs-derived exosomal microRNA-150-5p attenuates myocardial infarction in mice. Elsevier; 2021. [DOI] [PubMed]
- 196.Xu H, et al. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem. 2020;121(3):2089–102. [DOI] [PubMed] [Google Scholar]
- 197.Patil M, et al. Novel mechanisms of exosome-mediated phagocytosis of dead cells in injured heart. Circul Res. 2021;129(11):1006–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Takov K, et al. Small extracellular vesicles secreted from human amniotic fluid mesenchymal stromal cells possess cardioprotective and promigratory potential. Basic Res Cardiol. 2020;115:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Han C, et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater Sci Engineering: C. 2019;99:322–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is available in this article.