Abstract
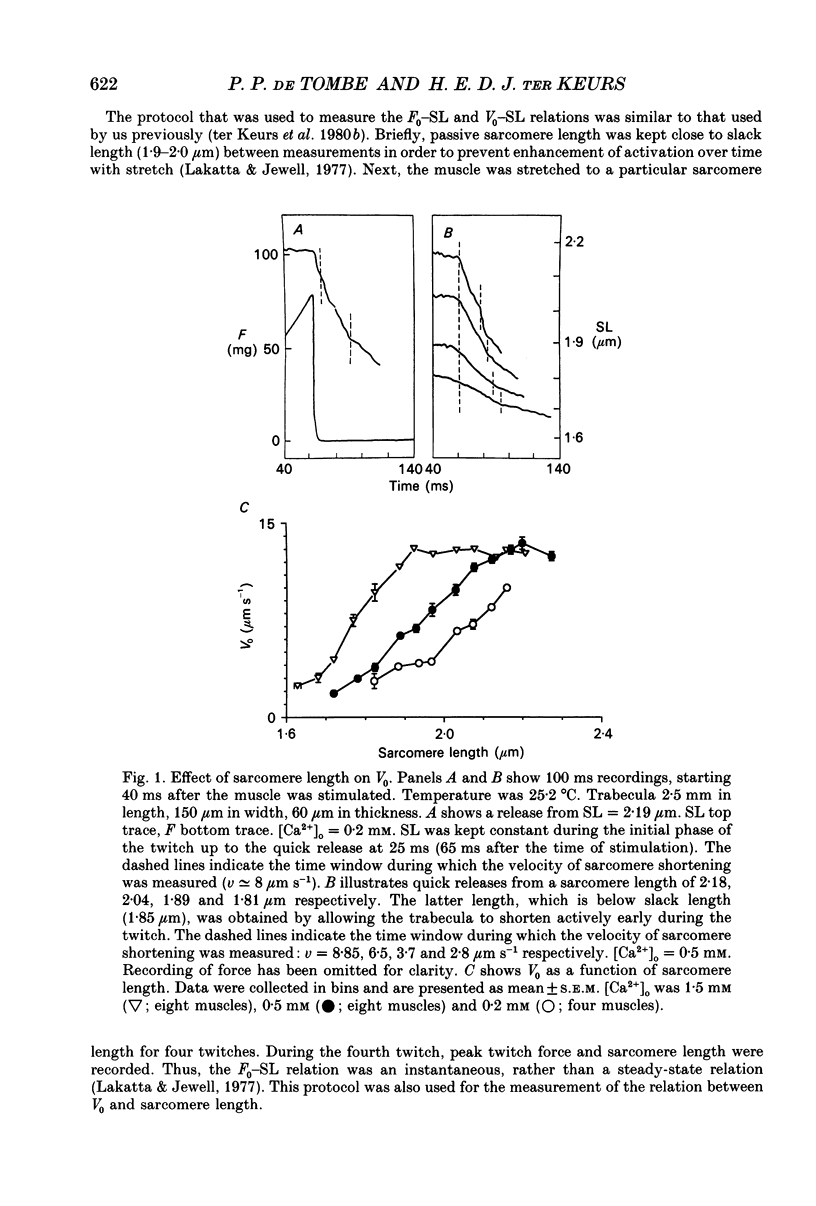
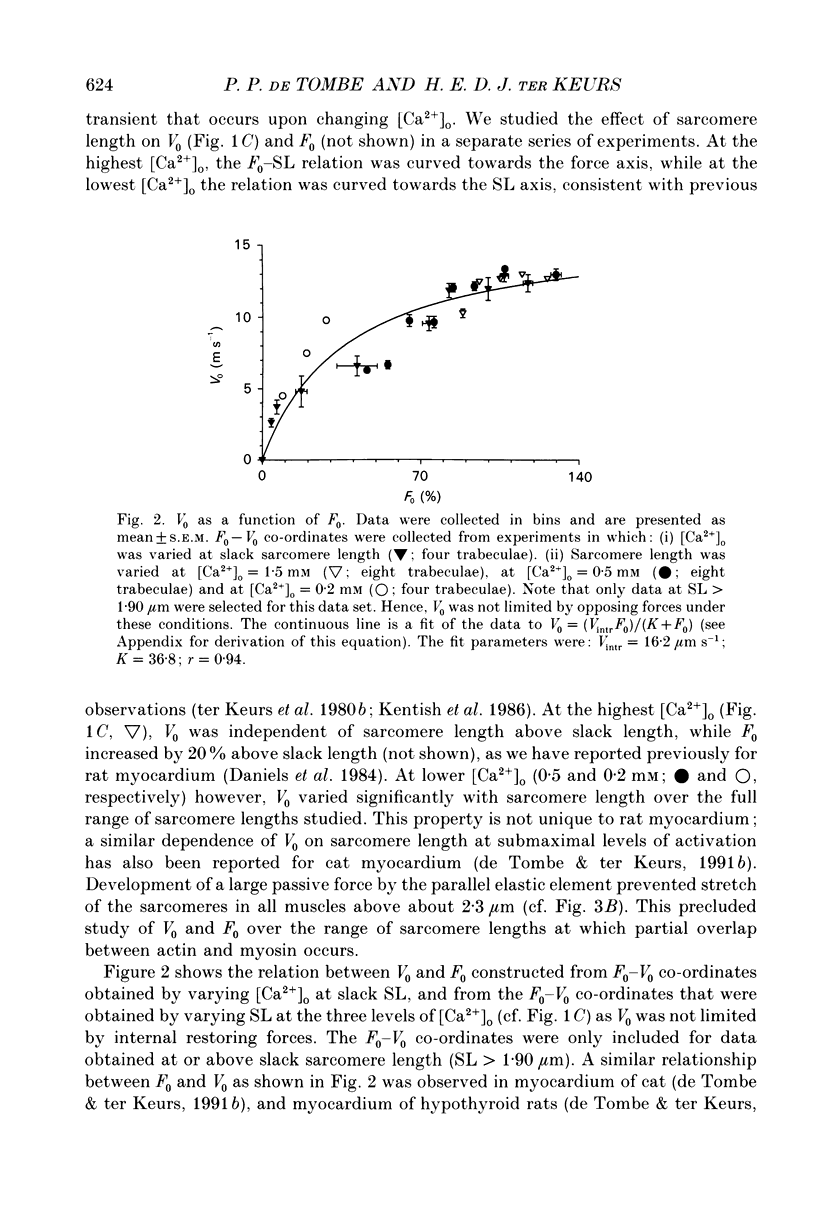
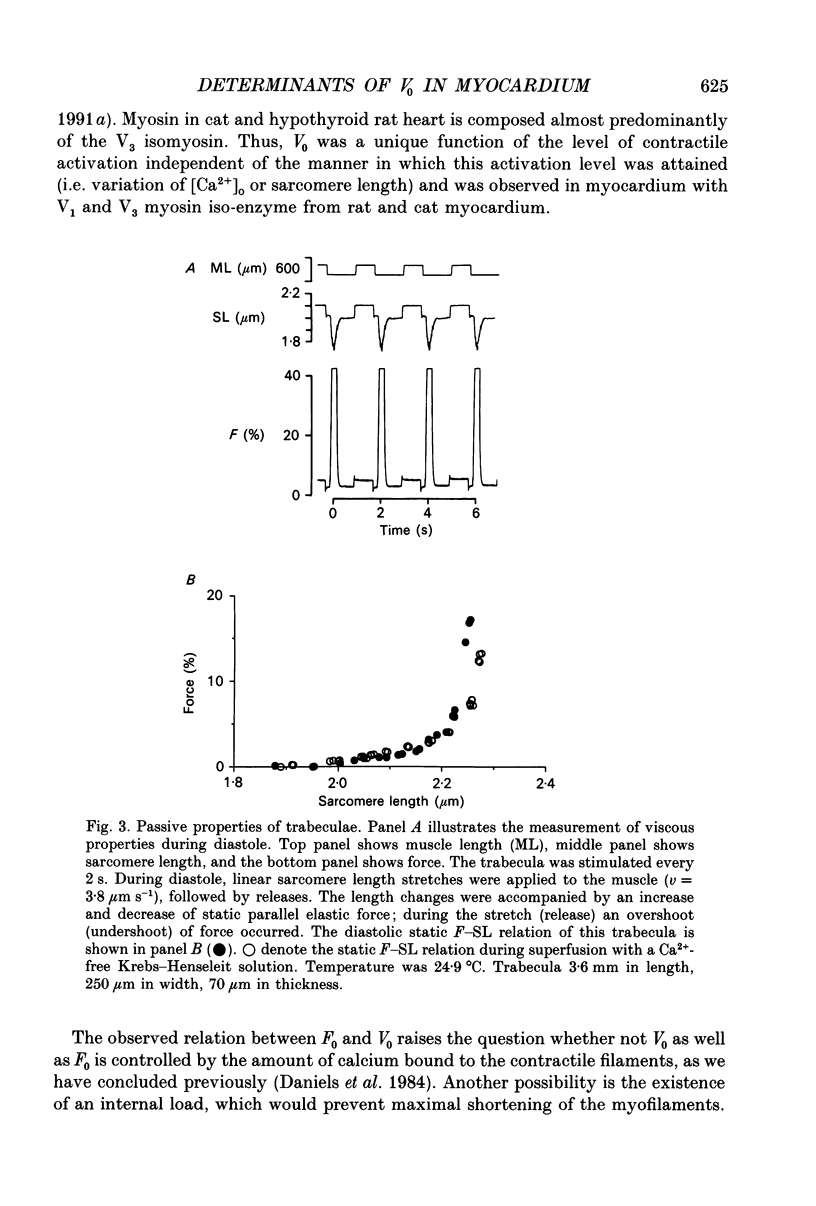
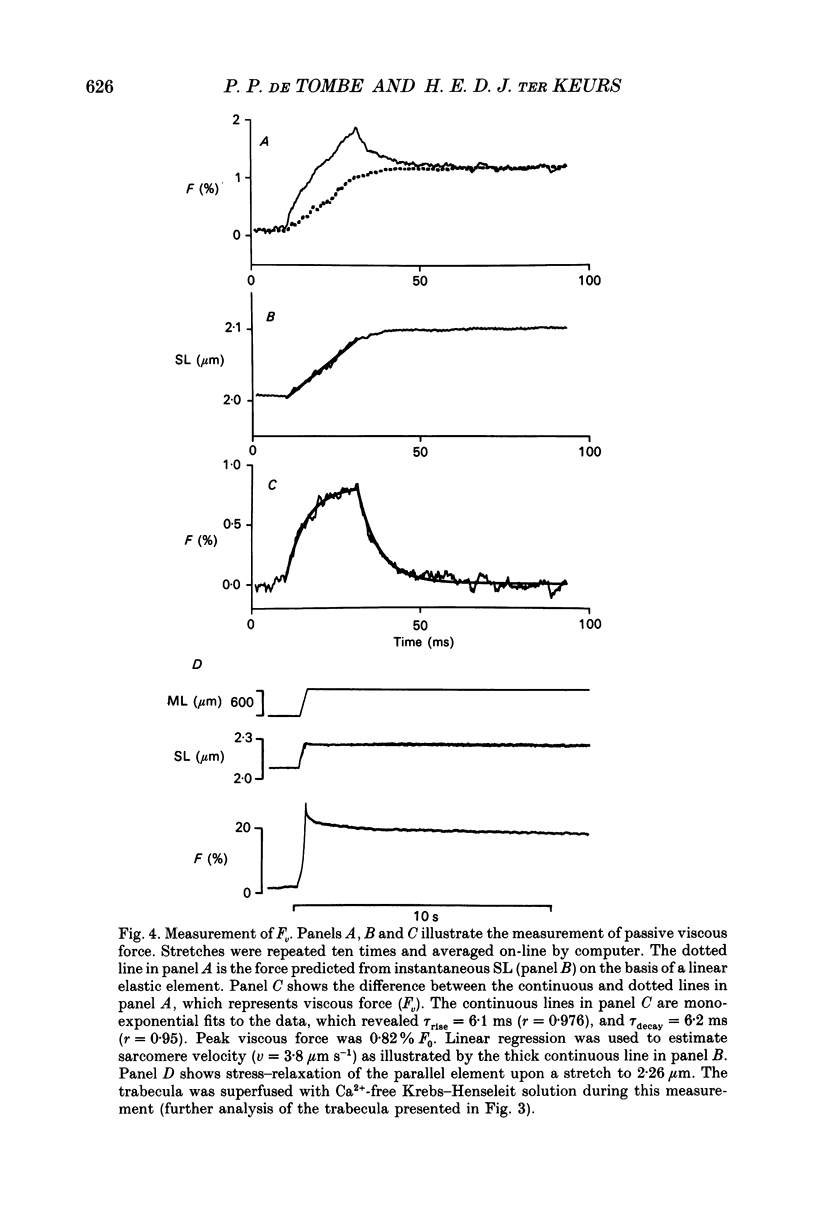
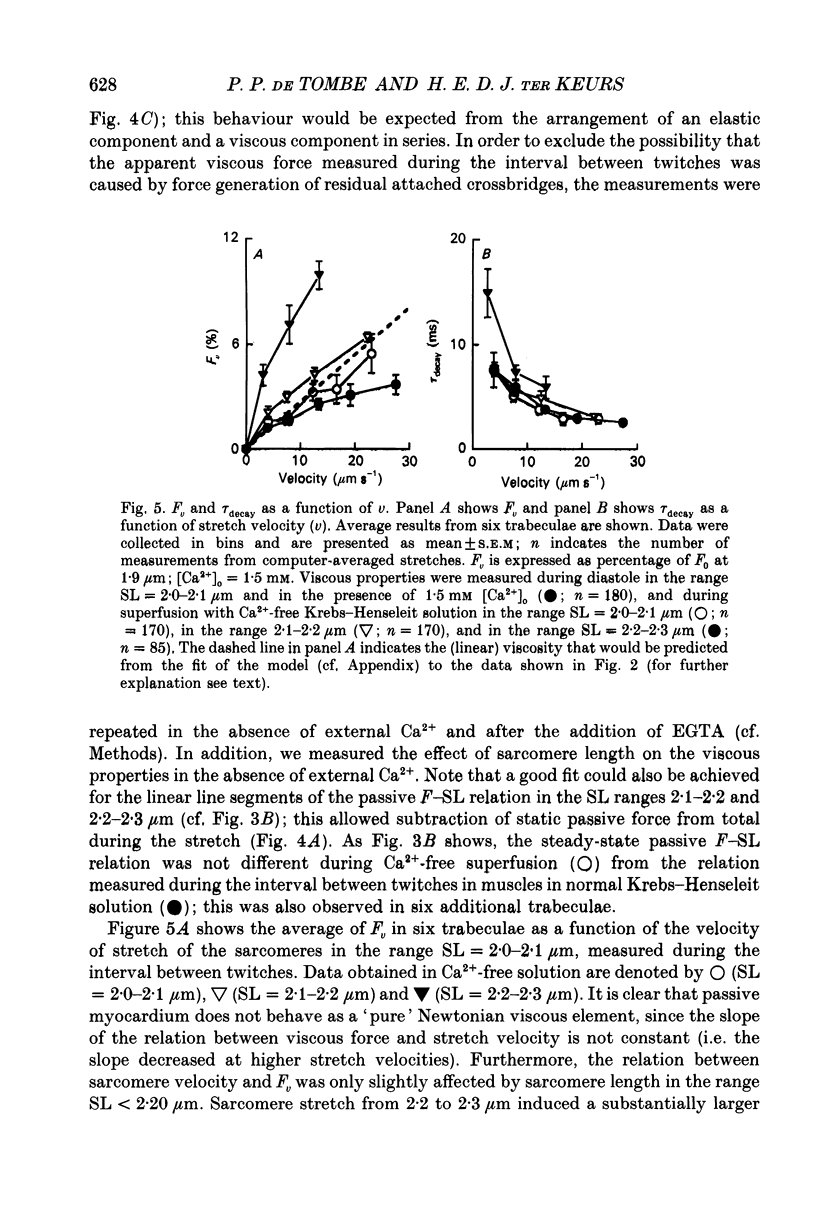
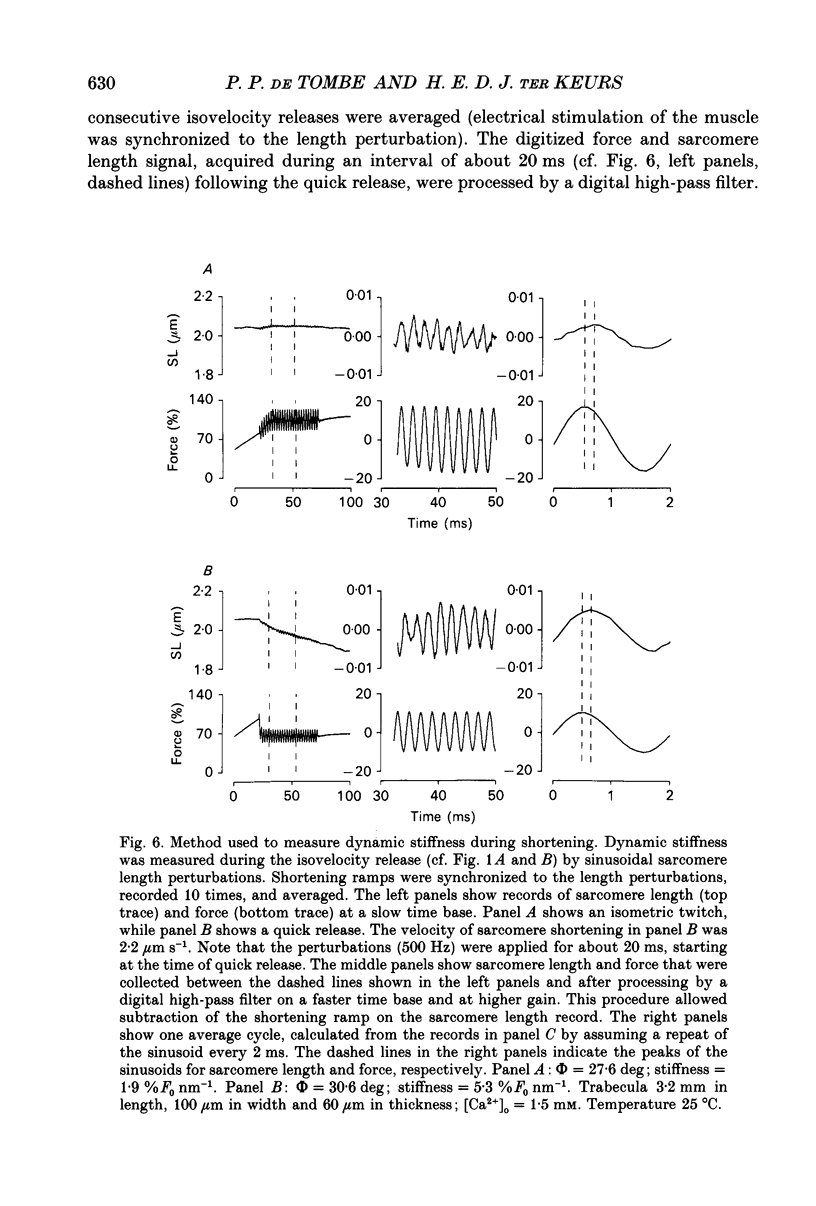
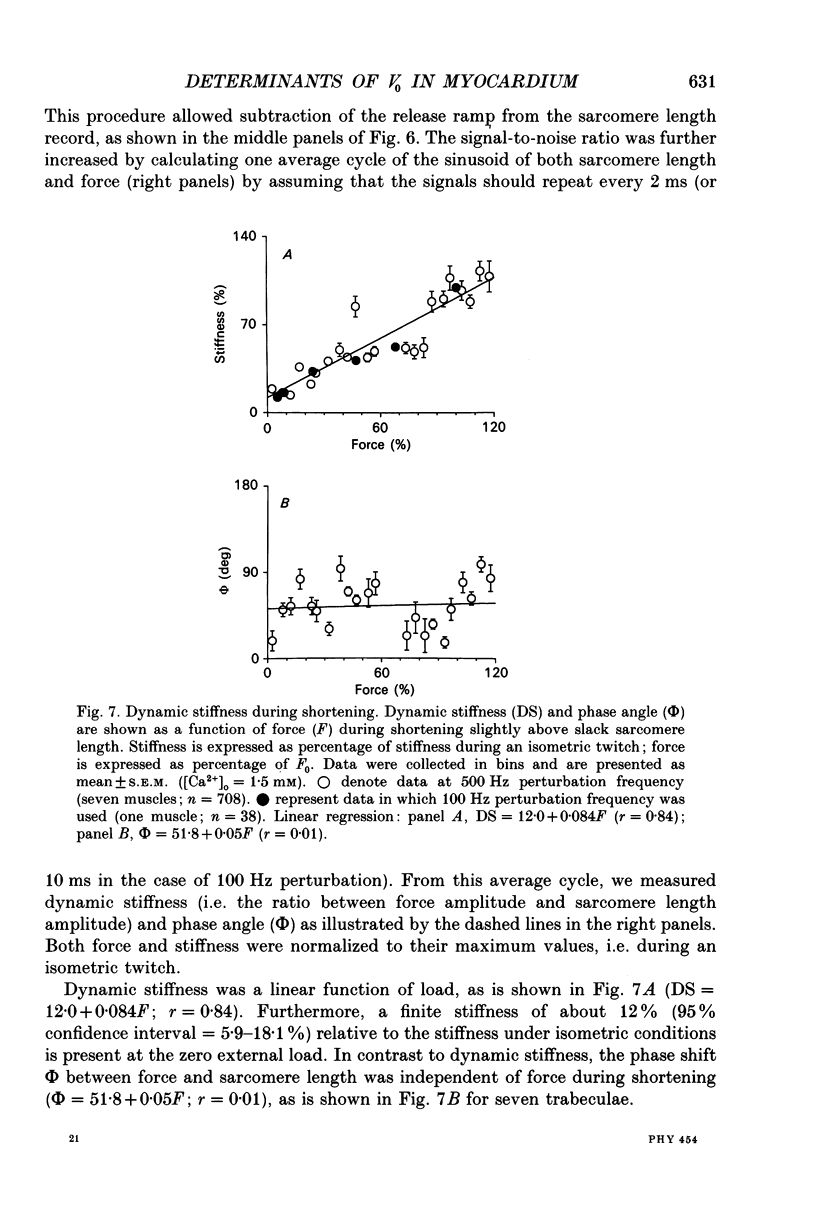
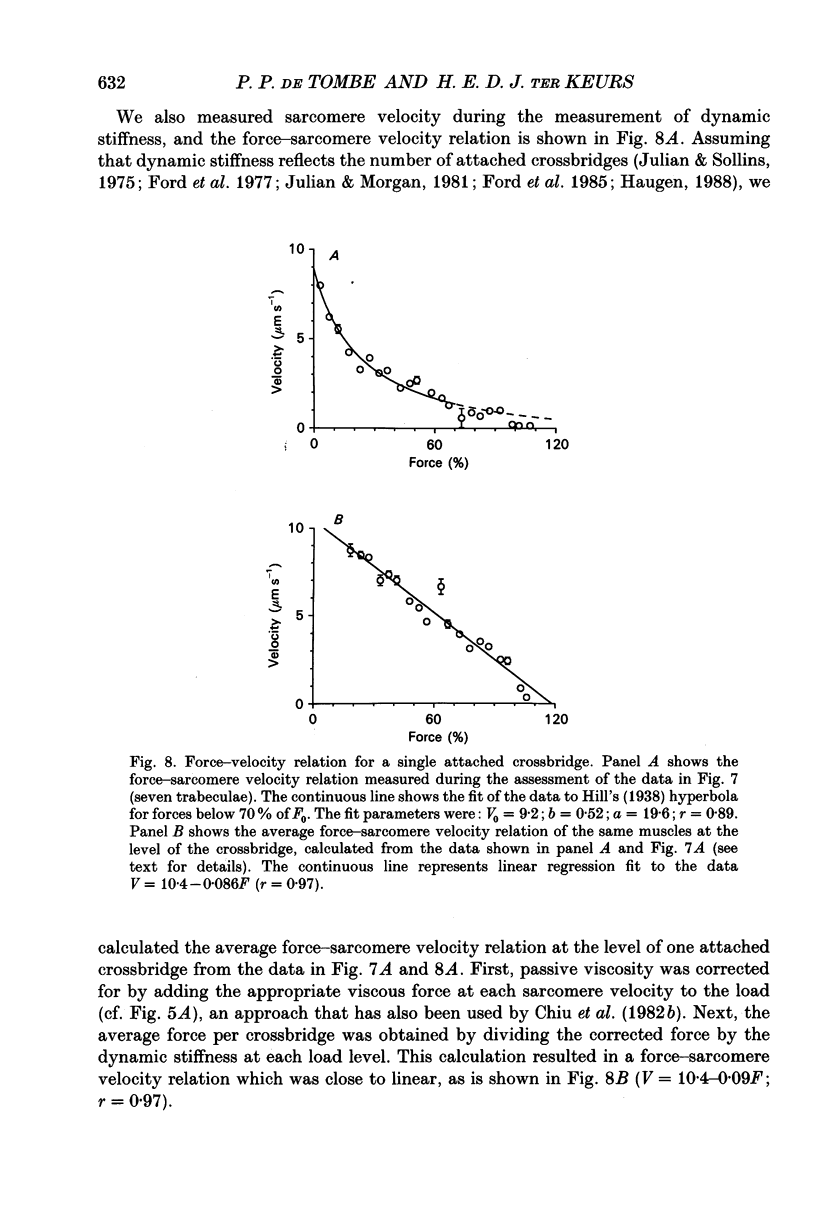
1. Peak twitch force (F0) and sarcomere length (SL) were measured in trabeculae that had been dissected from the right ventricle of rat heart and that were superfused with a modified Krebs-Henseleit solution at 25 degrees C. Sarcomere length was measured by laser diffraction techniques. Force was measured with a silicone strain gauge. Unloaded velocity of sarcomere shortening (V0) was measured by the 'isovelocity release' technique. 2. At [Ca2+]o = 1.5 mM and SL below 1.9 microns, V0 increased in proportion to SL, while V0 was independent of SL above 1.9 microns. At [Ca2+]o = 0.5 mM, V0 was proportional to SL up to 2.2 microns. At [Ca2+]o = 0.2 mM, V0 was proportional to SL up to 2.3 microns which is the longest SL that we were able to study in our trabeculae. 3. A unique relationship was observed between V0 and F0, irrespective of whether F0 was altered by variation of [Ca2+]o or sarcomere length above slack length. 4. Passive viscosity (Fv) was measured during the pause between contractions in the presence of 1.5 mM [Ca2+bdo and in the range SL = 2.0-2.1 microns by applying 0.1 micron stretches at various velocities up to v = 30 microns s-1. The force response to stretch, corrected for the contribution of parallel elastic force, showed viscoelastic characteristics with an exponential increase to a maximum (Fv) during stretch and an exponential decline after the end of the stretch. Fv increased, by 0.3%F0 microns-1 s-1, in proportion to v < 5 microns s-1; the increase of Fv was smaller at higher v, suggesting non-Newtonian viscous properties. 5. The time constant of the increase of force during the stretch decreased (tau rise congruent to 7 ms to tau rise congruent to 4 ms) with increases in v (congruent to 4 microns s-1 to v congruent to 10 microns s-1; P = 0.02). The time constant of decay of force at the end of the stretch also decreased with increases in v (tau decay congruent to 8 ms at v congruent to 4 microns s-1 to tau decay congruent to 3 ms at v congruent to 30 microns s-1; P < 0.001). Calculated stiffness of the elastic term of the viscoelastic element was independent of v, i.e. 45-50 N mm-3.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kurihara S. Calcium transients in mammalian ventricular muscle. Eur Heart J. 1980;Suppl A:5–15. doi: 10.1093/eurheartj/1.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Brady A. J. Mechanical properties of isolated cardiac myocytes. Physiol Rev. 1991 Apr;71(2):413–428. doi: 10.1152/physrev.1991.71.2.413. [DOI] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert D. L., Claes V. A., Sonnenblick E. H. Velocity of shortening of unloaded heart muscle and the length-tension relation. Circ Res. 1971 Jul;29(1):63–75. doi: 10.1161/01.res.29.1.63. [DOI] [PubMed] [Google Scholar]
- Chiu Y. C., Ballou E. W., Ford L. E. Force, velocity, and power changes during normal and potentiated contractions of cat papillary muscle. Circ Res. 1987 Mar;60(3):446–458. doi: 10.1161/01.res.60.3.446. [DOI] [PubMed] [Google Scholar]
- Chiu Y. L., Ballou E. W., Ford L. E. Internal viscoelastic loading in cat papillary muscle. Biophys J. 1982 Nov;40(2):109–120. doi: 10.1016/S0006-3495(82)84465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y. L., Ballou E. W., Ford L. E. Velocity transients and viscoelastic resistance to active shortening in cat papillary muscle. Biophys J. 1982 Nov;40(2):121–128. doi: 10.1016/S0006-3495(82)84466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M., Noble M. I., ter Keurs H. E., Wohlfart B. Velocity of sarcomere shortening in rat cardiac muscle: relationship to force, sarcomere length, calcium and time. J Physiol. 1984 Oct;355:367–381. doi: 10.1113/jphysiol.1984.sp015424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog. J Physiol. 1975 Mar;246(1):255–275. doi: 10.1113/jphysiol.1975.sp010889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
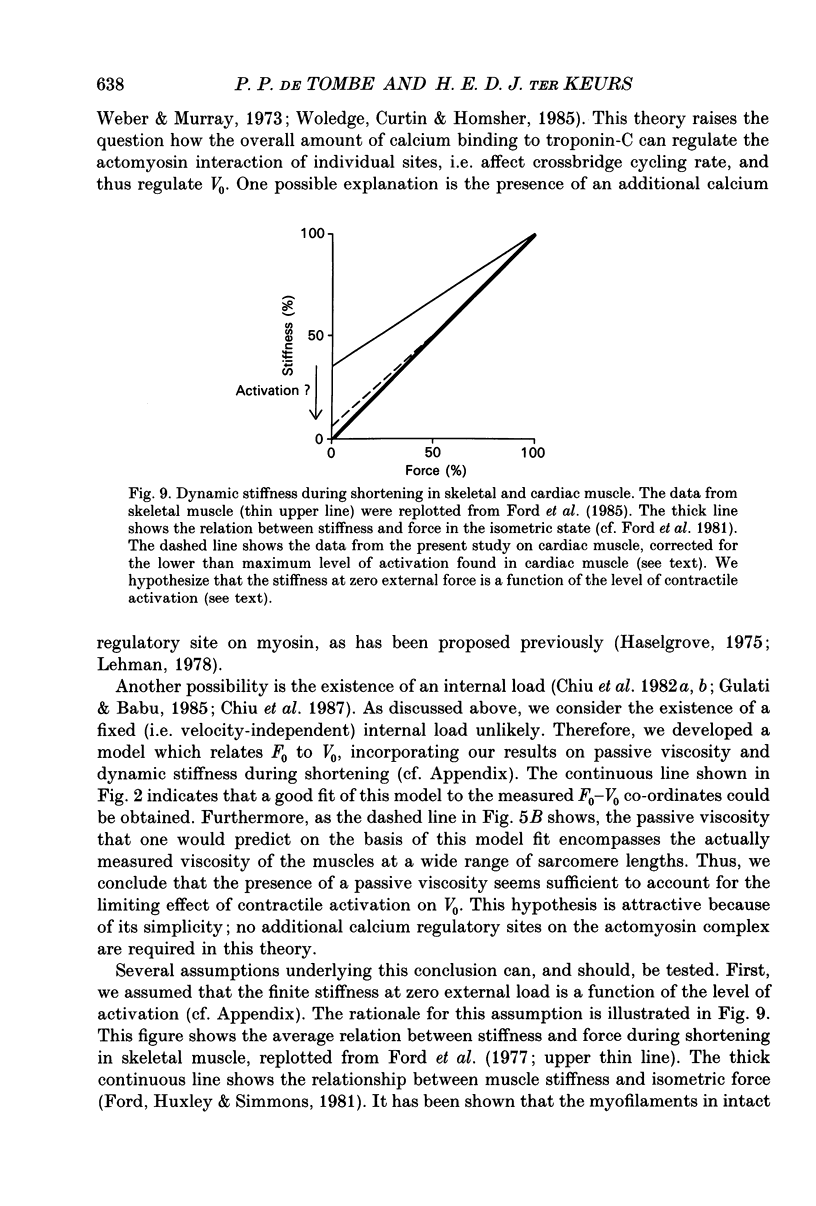
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension transients during steady shortening of frog muscle fibres. J Physiol. 1985 Apr;361:131–150. doi: 10.1113/jphysiol.1985.sp015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Contraction kinetics of intact and skinned frog muscle fibers and degree of activation. Effects of intracellular Ca2+ on unloaded shortening. J Gen Physiol. 1985 Oct;86(4):479–500. doi: 10.1085/jgp.86.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Haselgrove J. C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J Mol Biol. 1975 Feb 15;92(1):113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Jewell B. R. Calcium- and length-dependent force production in rat ventricular muscle. J Physiol. 1982 Aug;329:527–540. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann P. A., Fuchs F. Bound calcium and force development in skinned cardiac muscle bundles: effect of sarcomere length. J Mol Cell Cardiol. 1988 Aug;20(8):667–677. doi: 10.1016/s0022-2828(88)80012-9. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Morgan D. L. Variation of muscle stiffness with tension during tension transients and constant velocity shortening in the frog. J Physiol. 1981;319:193–203. doi: 10.1113/jphysiol.1981.sp013901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Sollins M. R. Variation of muscle stiffness with force at increasing speeds of shortening. J Gen Physiol. 1975 Sep;66(3):287–302. doi: 10.1085/jgp.66.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J. C., ter Keurs H. E., Ricciardi L., Bucx J. J., Noble M. I. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986 Jun;58(6):755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- Lakatta E. G., Jewell B. R. Length-dependent activation: its effect on the length-tension relation in cat ventricular muscle. Circ Res. 1977 Mar;40(3):251–257. doi: 10.1161/01.res.40.3.251. [DOI] [PubMed] [Google Scholar]
- Lehman W. Thick-filament-linked calcium regulation in vertebrate striated muscle. Nature. 1978 Jul 6;274(5666):80–81. doi: 10.1038/274080a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Gwathmey J. K., Allen P. D., Briggs G. M., Morgan J. P. Modulation by the thyroid state of intracellular calcium and contractility in ferret ventricular muscle. Circ Res. 1988 Dec;63(6):1080–1089. doi: 10.1161/01.res.63.6.1080. [DOI] [PubMed] [Google Scholar]
- Martyn D. A., Rondinone J. F., Huntsman L. L. Myocardial segment velocity at a low load: time, length, and calcium dependence. Am J Physiol. 1983 May;244(5):H708–H714. doi: 10.1152/ajpheart.1983.244.5.H708. [DOI] [PubMed] [Google Scholar]
- Noble M. I. The diastolic viscous properties of cat papillary muscle. Circ Res. 1977 Mar;40(3):288–292. doi: 10.1161/01.res.40.3.288. [DOI] [PubMed] [Google Scholar]
- Podolin R. A., Ford L. E. The influence of calcium on shortening velocity of skinned frog muscle cells. J Muscle Res Cell Motil. 1983 Jun;4(3):263–282. doi: 10.1007/BF00711996. [DOI] [PubMed] [Google Scholar]
- SONNENBLICK E. H. INSTANTANEOUS FORCE-VELOCITY-LENGTH DETERMINANTS IN THE CONTRACTION OF HEART MUSCLE. Circ Res. 1965 May;16:441–451. doi: 10.1161/01.res.16.5.441. [DOI] [PubMed] [Google Scholar]
- Schouten V. J., Bucx J. J., de Tombe P. P., ter Keurs H. E. Sarcolemma, sarcoplasmic reticulum, and sarcomeres as limiting factors in force production in rat heart. Circ Res. 1990 Oct;67(4):913–922. doi: 10.1161/01.res.67.4.913. [DOI] [PubMed] [Google Scholar]
- Wang K., Wright J. Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J Cell Biol. 1988 Dec;107(6 Pt 1):2199–2212. doi: 10.1083/jcb.107.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]
- de Tombe P. P., ter Keurs H. E. Force and velocity of sarcomere shortening in trabeculae from rat heart. Effects of temperature. Circ Res. 1990 May;66(5):1239–1254. doi: 10.1161/01.res.66.5.1239. [DOI] [PubMed] [Google Scholar]
- de Tombe P. P., ter Keurs H. E. Lack of effect of isoproterenol on unloaded velocity of sarcomere shortening in rat cardiac trabeculae. Circ Res. 1991 Feb;68(2):382–391. doi: 10.1161/01.res.68.2.382. [DOI] [PubMed] [Google Scholar]
- de Tombe P. P., ter Keurs H. E. Sarcomere dynamics in cat cardiac trabeculae. Circ Res. 1991 Feb;68(2):588–596. doi: 10.1161/01.res.68.2.588. [DOI] [PubMed] [Google Scholar]
- ter Keurs H. E., Rijnsburger W. H., van Heuningen R., Nagelsmit M. J. Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ Res. 1980 May;46(5):703–714. doi: 10.1161/01.res.46.5.703. [DOI] [PubMed] [Google Scholar]


