Abstract
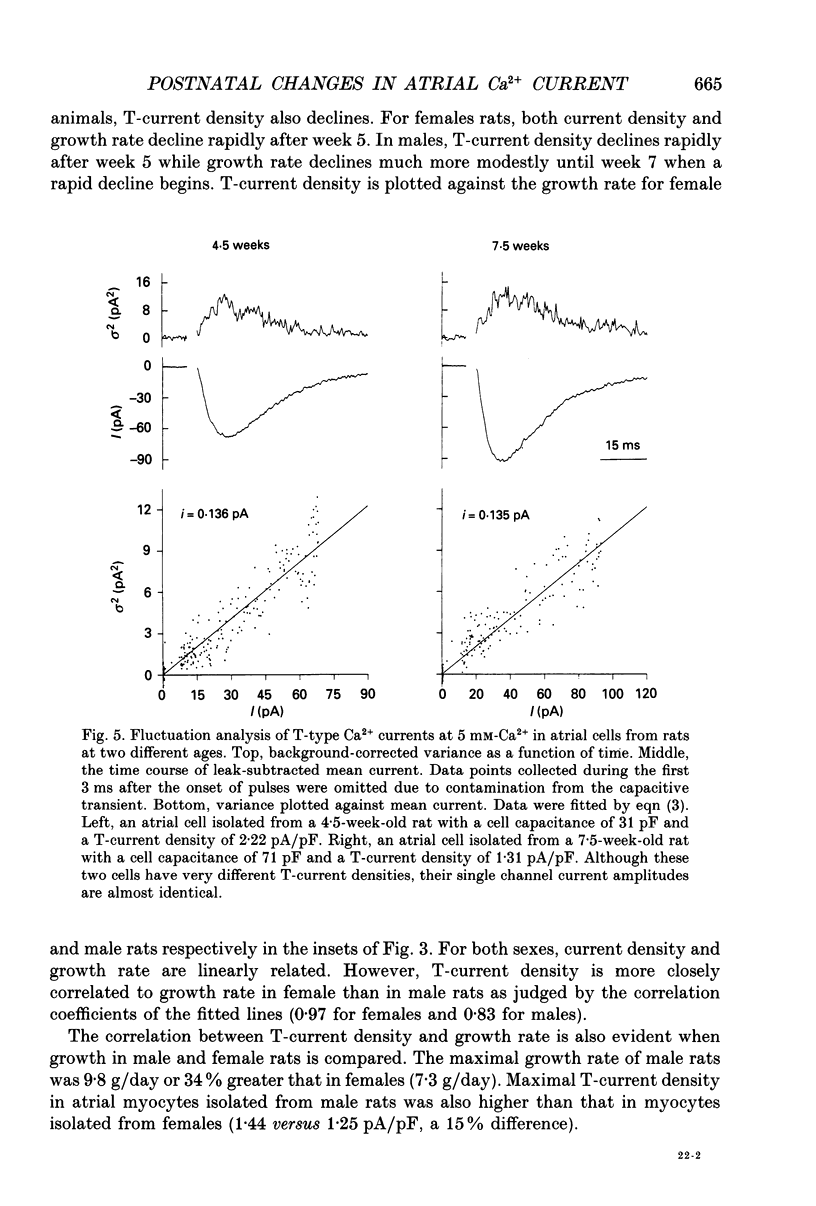
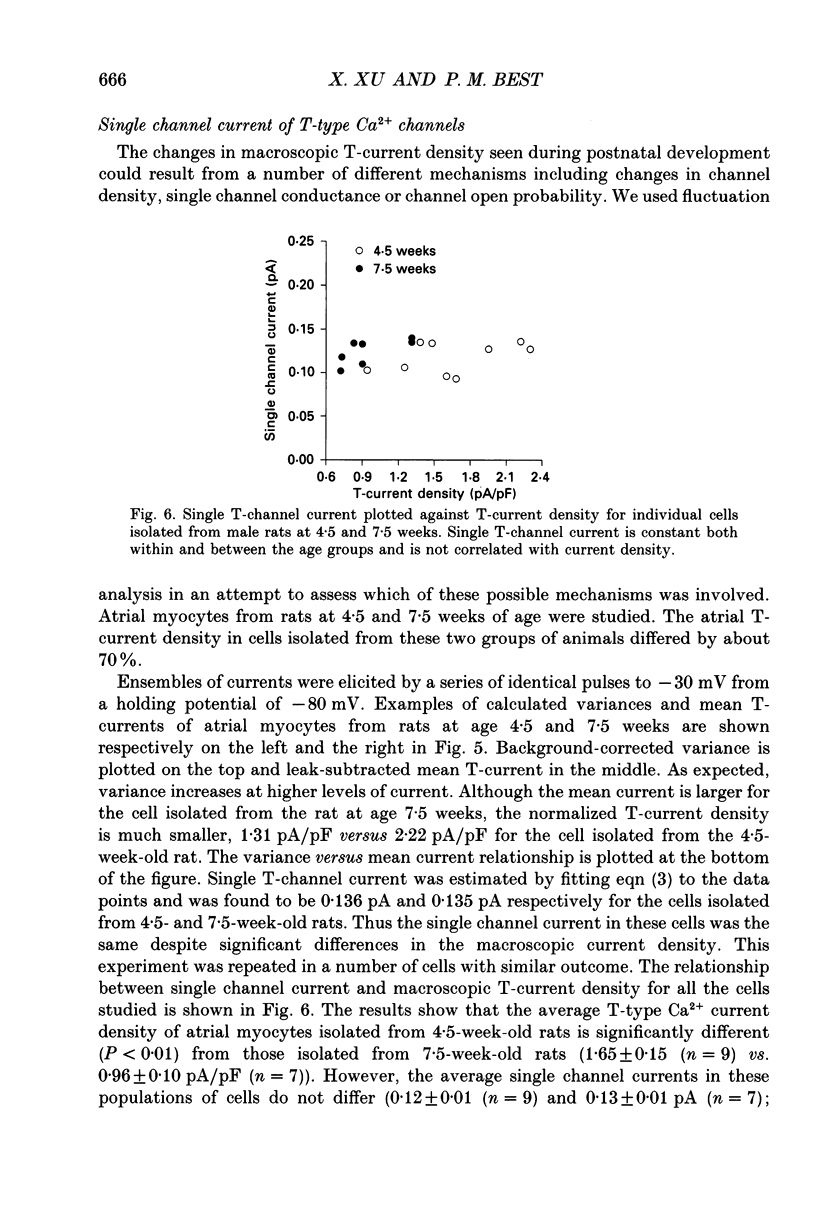
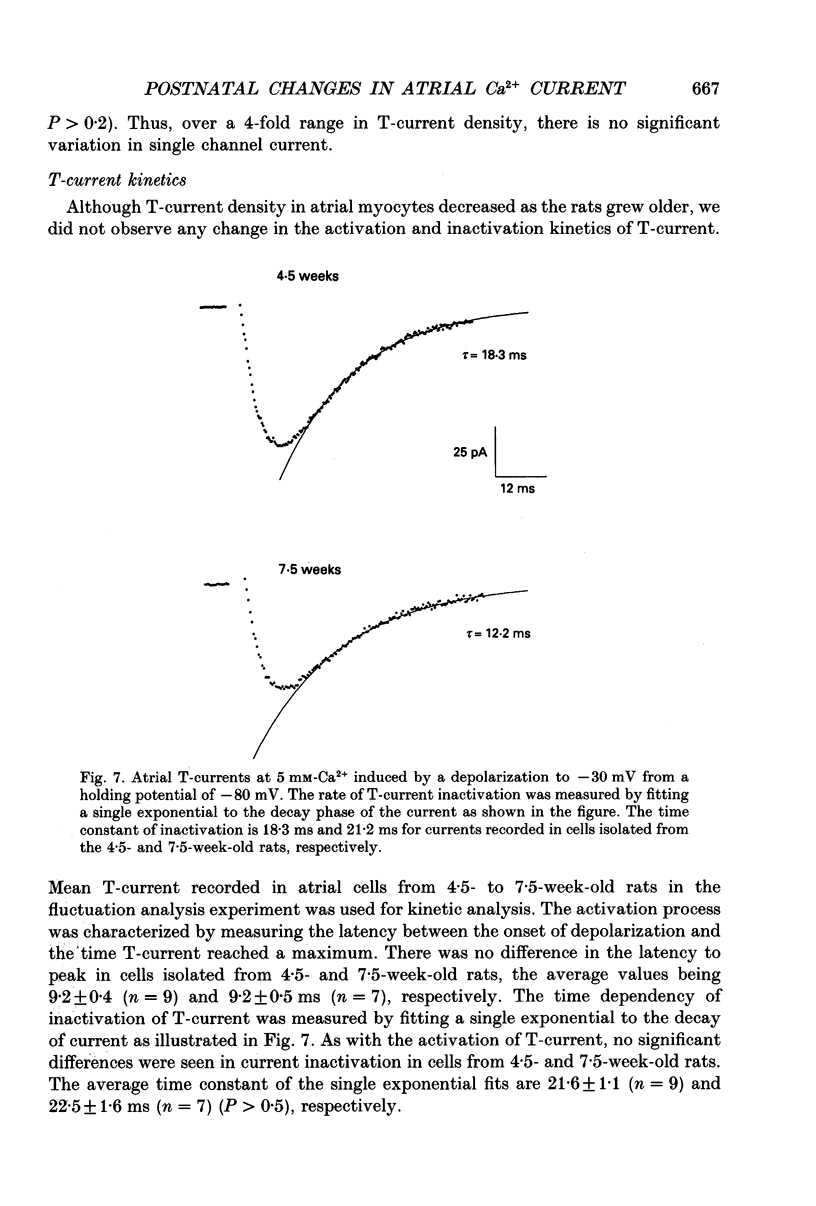
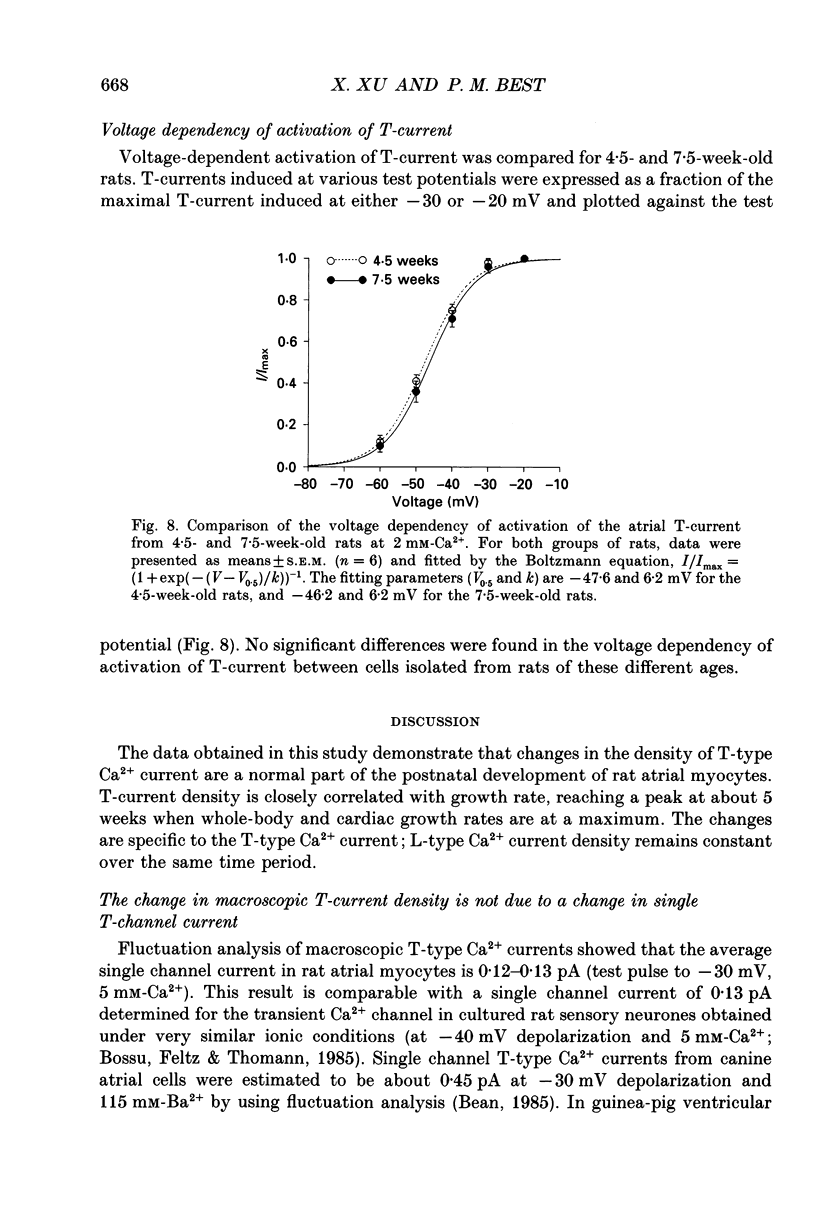
1. Postnatal changes in Ca2+ current were studied in voltage clamped atrial myocytes isolated from Sprague-Dawley rats. T- and L-type Ca2+ currents were identified using standard electrophysiological and pharmacological techniques. Cells were studied from seven groups of male and six groups of female rats ranging in age from 3 to 14 weeks. 2. The density of atrial T-type Ca2+ current showed significant variation during postnatal development, with a maximum density reached at 4.5-5 weeks. At this age, T-current density was 1.44 +/- 0.11 pA/pF (n = 23) for cells isolated from male and 1.25 +/- 0.09 pA/pF (n = 25) for cells isolated from female animals in bathing solutions containing 2 mM-Ca2+. T-current density in atrial cells isolated from younger animals (3.5 weeks postnatal) averaged 1.22 +/- 0.06 (n = 18) and 1.00 +/- 0.05 pA/pF (n = 22) or 85 and 80% of the maximum seen at 4.5-5 weeks for male and female rats, respectively. For rats older than 13 weeks, the average T-current density in atrial cells was 0.50 +/- 0.03 (n = 18) and 0.51 +/- 0.02 pA/pF (n = 35) or 35 and 41% of the maximum seen at 4.5-5 weeks for male and female rats, respectively. 3. In contrast to the T-type current, the density of atrial L-type Ca2+ current remained unchanged in rats from 3 to 14 weeks old. L-type current averaged 8.2 +/- 0.2 (n = 134) in male and 7.9 +/- 0.2 pA/pF (n = 102) in female rats. 4. Fluctuation analysis was used to estimate single T-channel current levels in 4.5- and 7.5-week-old male rats. While the T-current density differed by 70% at these two postnatal ages, no significant difference (P > 0.2) in single channel current was found. Single channel current was 0.12 +/- 0.01 pA (n = 9) for cells from 4.5-week-old and 0.13 +/- 0.01 pA (n = 7) for cells from 7.5-week-old rats. Currents were stimulated by test pulses from -80 to -30 mV at 5 mM-Ca2+. 5. No postnatal changes were seen in either the kinetics of activation or inactivation of macroscopic T-current.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



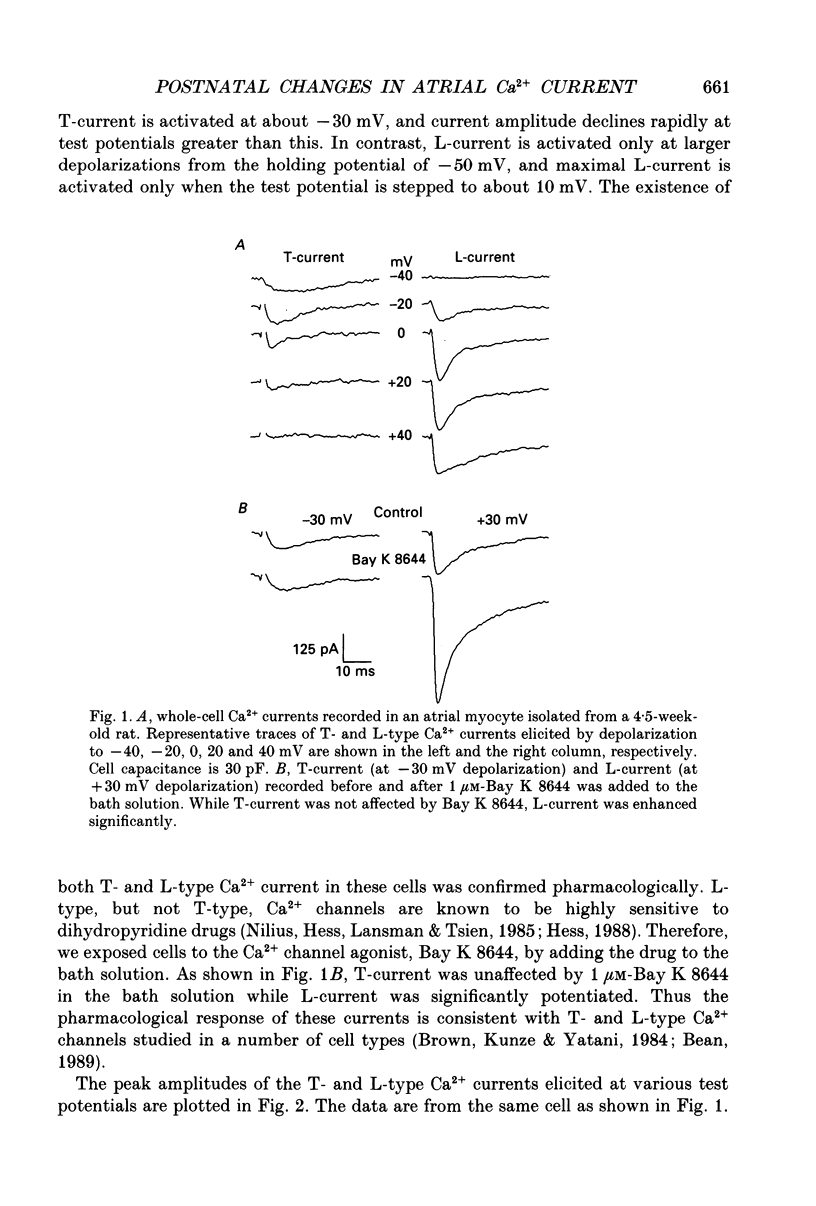
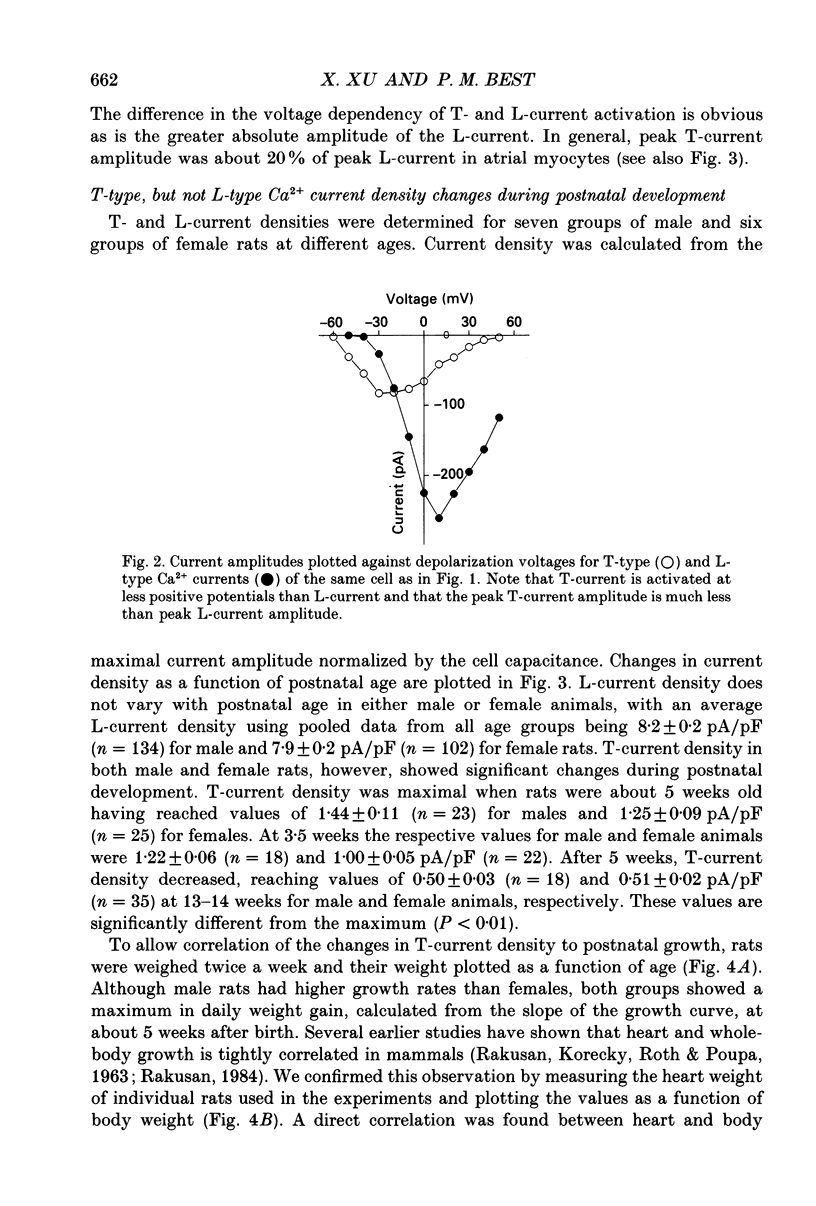
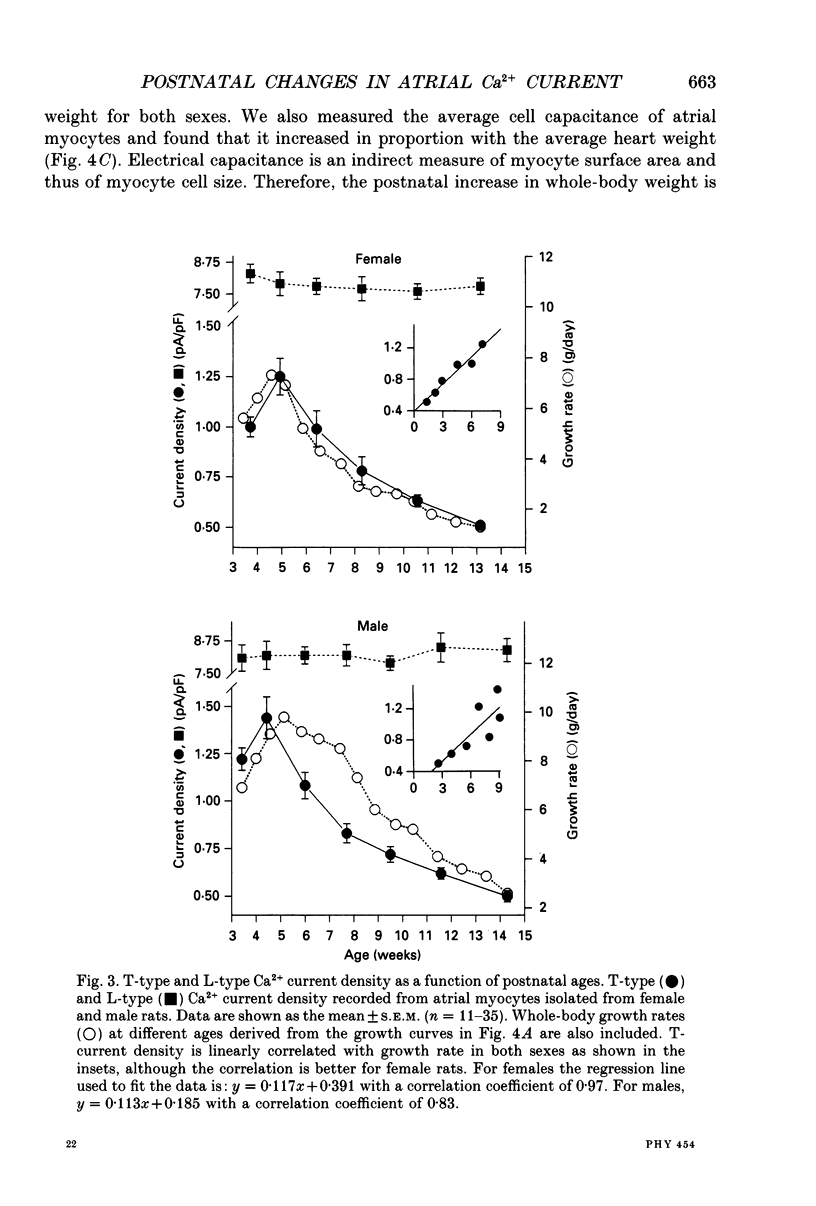
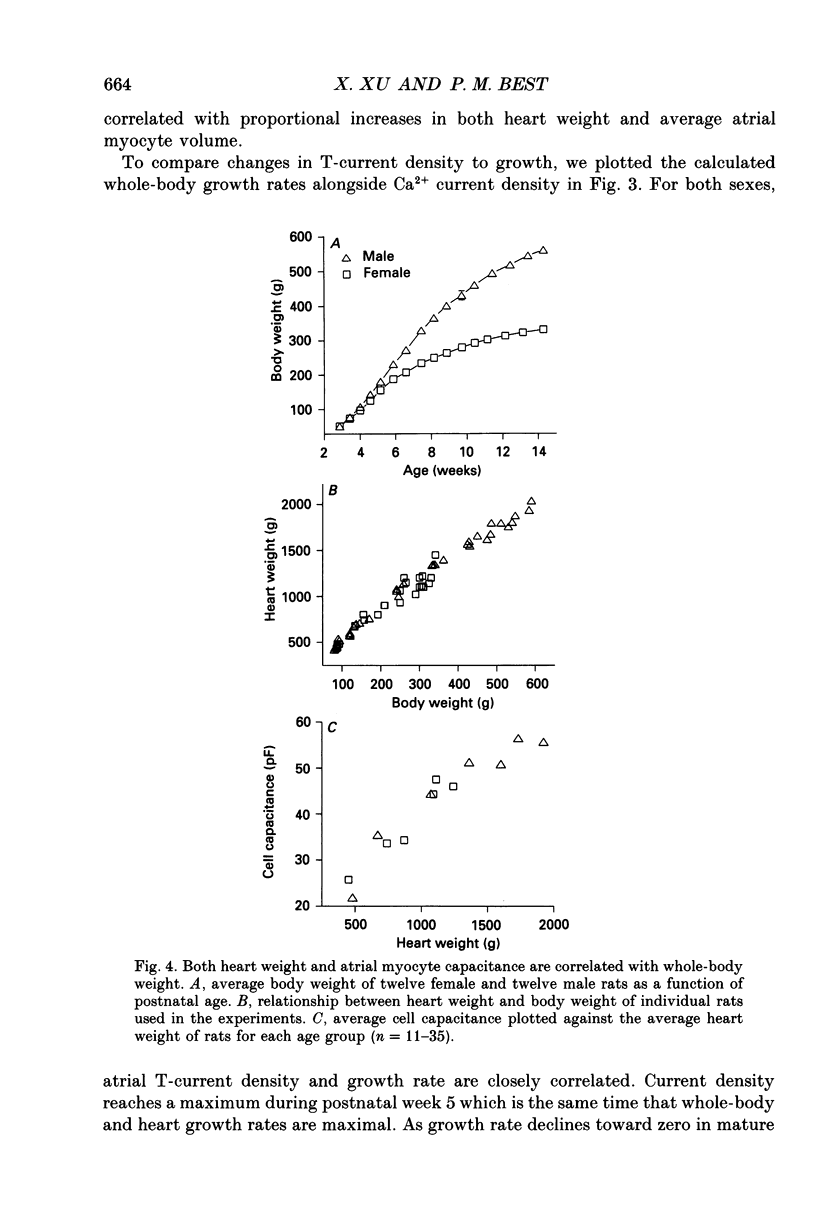




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beam K. G., Knudson C. M. Effect of postnatal development on calcium currents and slow charge movement in mammalian skeletal muscle. J Gen Physiol. 1988 Jun;91(6):799–815. doi: 10.1085/jgp.91.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A., Thomann J. M. Depolarization elicits two distinct calcium currents in vertebrate sensory neurones. Pflugers Arch. 1985 Apr;403(4):360–368. doi: 10.1007/BF00589247. [DOI] [PubMed] [Google Scholar]
- Boyle W. A., Nerbonne J. M. A novel type of depolarization-activated K+ current in isolated adult rat atrial myocytes. Am J Physiol. 1991 Apr;260(4 Pt 2):H1236–H1247. doi: 10.1152/ajpheart.1991.260.4.H1236. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. The agonist effect of dihydropyridines on Ca channels. Nature. 1984 Oct 11;311(5986):570–572. doi: 10.1038/311570a0. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Cohen N. M., Lederer W. J. Changes in the calcium current of rat heart ventricular myocytes during development. J Physiol. 1988 Dec;406:115–146. doi: 10.1113/jphysiol.1988.sp017372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Nilius B. Kinetic properties of the cardiac T-type calcium channel in the guinea-pig. J Physiol. 1989 Dec;419:627–650. doi: 10.1113/jphysiol.1989.sp017890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasscock G. F., Gin K. K., Kim J. D., Hintz R. L., Rosenfeld R. G. Ontogeny of pituitary regulation of growth in the developing rat: comparison of effects of hypophysectomy and hormone replacement on somatic and organ growth, serum insulin-like growth factor-I (IGF-I) and IGF-II levels, and IGF-binding protein levels in the neonatal and juvenile rat. Endocrinology. 1991 Feb;128(2):1036–1047. doi: 10.1210/endo-128-2-1036. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Hasegawa S. Post-natal disappearance of transient calcium channels in mouse skeletal muscle: effects of denervation and culture. J Physiol. 1988 Jul;401:617–637. doi: 10.1113/jphysiol.1988.sp017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P. Elementary properties of cardiac calcium channels: a brief review. Can J Physiol Pharmacol. 1988 Sep;66(9):1218–1223. doi: 10.1139/y88-201. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kawano S., DeHaan R. L. Low-threshold current is major calcium current in chick ventricle cells. Am J Physiol. 1989 May;256(5 Pt 2):H1505–H1508. doi: 10.1152/ajpheart.1989.256.5.H1505. [DOI] [PubMed] [Google Scholar]
- Kilborn M. J., Fedida D. A study of the developmental changes in outward currents of rat ventricular myocytes. J Physiol. 1990 Nov;430:37–60. doi: 10.1113/jphysiol.1990.sp018280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G. Diversity of calcium ion channels in cellular membranes. Neuroscience. 1989;28(2):253–261. doi: 10.1016/0306-4522(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Brady A. J., Tan S. T., Serena D. Correlation of the glycoside response, the force staircase, and the action potential configuration in the neonatal rat heart. Circ Res. 1975 Jun;36(6):744–752. doi: 10.1161/01.res.36.6.744. [DOI] [PubMed] [Google Scholar]
- McCobb D. P., Best P. M., Beam K. G. Development alters the expression of calcium currents in chick limb motoneurons. Neuron. 1989 Jun;2(6):1633–1643. doi: 10.1016/0896-6273(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Moussavi R., Meisami E., Timiras P. S. Compensatory cell proliferation and growth in the rat heart after postnatal hypothyroidism. Am J Physiol. 1985 Mar;248(3 Pt 1):E381–E387. doi: 10.1152/ajpendo.1985.248.3.E381. [DOI] [PubMed] [Google Scholar]
- Nakata K. Quantitative analysis of ultrastructural changes in developing rat cardiac muscle during normal growth and during acute volume load. Jpn Circ J. 1977 Nov;41(11):1237–1250. doi: 10.1253/jcj.41.1237. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Ojeda S. R., Jameson H. E. Developmental patterns of plasma and pituitary growth hormone (GH) in the female rat. Endocrinology. 1977 Mar;100(3):881–889. doi: 10.1210/endo-100-3-881. [DOI] [PubMed] [Google Scholar]
- Pucelík P., Jezek K., Barták F. Postnatal development of electrophysiological manifestations of the working ventricular myocardium of albino rats. Physiol Bohemoslov. 1982;31(3):217–224. [PubMed] [Google Scholar]
- RAKUSAN K., KORECKY B., ROTH Z., POUPA O. DEVELOPMENT OF THE VENTRICULAR WEIGHT OF THE RAT HEART WITH SPECIAL REFERENCE TO THE EARLY PHASES OF POSTNATAL ONTOGENESIS. Physiol Bohemoslov. 1963;12:518–525. [PubMed] [Google Scholar]
- Sasaki R., Watanabe Y., Morishita T., Yamagata S. Determination of deoxyribonucleic acid content of heart muscle and myocardial growth in normal rats. Tohoku J Exp Med. 1968 Jun;95(2):185–192. doi: 10.1620/tjem.95.185. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat J., Nilius B., Carmeliet E. Modulation of the T-type cardiac Ca channel by changes in proton concentration. J Gen Physiol. 1990 Nov;96(5):973–990. doi: 10.1085/jgp.96.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. P., Best P. M. Increase in T-type calcium current in atrial myocytes from adult rats with growth hormone-secreting tumors. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4655–4659. doi: 10.1073/pnas.87.12.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]


