Abstract
The importance of the macrophage in innate immunity is underscored by its secretion of an array of powerful immunoregulatory and effector molecules. We report herein that macrophage migration inhibitory factor (MIF), a product of activated macrophages, sustains macrophage survival and function by suppressing activation-induced, p53-dependent apoptosis. Endotoxin administration to MIF−/− mice results in decreased macrophage viability, decreased proinflammatory function, and increased apoptosis when compared with wild-type controls. Moreover, inhibition of p53 in endotoxin-treated, MIF-deficient macrophages suppresses enhanced apoptosis and restores proinflammatory function. MIF inhibits p53 activity in macrophages via an autocrine regulatory pathway, resulting in a decrease in cellular p53 accumulation and subsequent function. Inhibition of p53 by MIF coincides with the induction of arachidonic acid metabolism and cyclooxygenase-2 (Cox-2) expression, which is required for MIF regulation of p53. MIF's effect on macrophage viability and survival provides a previously unrecognized mechanism to explain its critical proinflammatory action in conditions such as sepsis, and suggests new approaches for the modulation of innate immune responses.
Keywords: apoptosis‖arachidonic acid‖Cox-2‖sepsis
The macrophage is a pivotal mediator of innate immunity and a vanguard of the host response to tissue invasion (1). Once activated, macrophages produce an assortment of microbicidal effectors and immunoregulatory cytokines that act to eliminate the invasive agent and influence the course of the ensuing, cognate immune response. An overly robust macrophage response, however, can lead to pathological sequelae that contribute significantly to septic shock, autoimmune diseases, and various granulomatous disorders (2).
Innate immune responses are kept in check by specialized counterregulatory mechanisms (3). One such proposed mechanism involves apoptosis or programmed cell death, which is induced in macrophages by stimulation with bacterial toxins such as lipopolysaccharide (LPS; refs. 4 and 5).
Apoptosis has been suggested to limit the half-life of activated macrophages and to restrict the expression of their inflammatory products to the site of tissue invasion (6–8). The LPS-induced apoptotic response in macrophages has been shown to require the production of nitric oxide (NO), the intracellular accumulation of the tumor suppressor gene product p53, and activation of a caspase-dependent cytolytic pathway (4–6, 9).
Historically, activated T cells were considered to be the source of macrophage migration inhibitory factor (MIF) and monocyte/macrophage populations to be the target of its migration inhibitory effects. More recent studies have established that monocytes/macrophages, in fact, are the primary site of MIF production after exposure of the host to bacterial endo- and exotoxins (LPS, toxic shock syndrome toxin-1, and streptococcal pyrogenic exotoxin), malaria pigment (hemozoin), or cytokines (TNF-α and INF-γ; refs. 10–12). Once released, MIF modulates the expression of proinflammatory mediators by macrophages, and is an important component of T cell activation (10, 13). The expression of MIF also has been tightly linked to the lethality associated with Gram-negative and Gram-positive septic shock in various experimental models (14–16). Deletion of the MIF gene (15) or immunoneutralization of MIF (14, 16) confers protection against these conditions, even in mice with a TNF-α-deficient background (16), verifying an intrinsic role of MIF in the innate immune response and the pathogenesis of sepsis.
MIF−/− mice are resistant to endotoxic shock (15), but the molecular mechanism that underlies this resistance is unknown. The recent observation that MIF can regulate p53 activity in vitro (17) suggested to us a potential means to explain MIF's global proinflammatory properties in sepsis and other inflammatory conditions (14, 15, 18). Programmed cell death is a potentially important pathway in the regulation of the host inflammatory response (19–21), and we hypothesized that deficits in the regulation of p53-dependent apoptotic pathways in macrophages could both explain the sensitivity of MIF−/− mice to endotoxemia and suggest new approaches for therapeutic intervention.
Materials and Methods
Apoptosis and Cell Viability.
Male 129/Bl6 F3 MIF+/+ and MIF−/− mice were injected intraperitoneally (i.p.) with either vehicle or LPS (Escherichia coli 0111:B4, 15 mg/kg) in 0.4 ml sterile PBS. After 18 h, peritoneal macrophages were collected by lavage and immediately assessed for apoptosis by an ELISA that detects cytoplasmic oligonucleosomes (Roche Diagnostics). Resting primary mouse macrophage apoptosis [10 μg/ml LPS and 100 units/ml IFN-γ (R & D Systems)] and RAW 264.7 macrophage apoptosis [1 mM sodium nitroprusside (Calbiochem)] also was quantified by ELISA. Results are expressed as OD405 and represent relative DNA fragmentation. Annexin-V-FLUOS Staining Kit (Roche Diagnostics) was performed as described by the manufacturer. Apoptotic cells were visualized by fluorescence microscopy and expressed as a percentage of apoptotic cells. Cell viability of peritoneal macrophages was analyzed by trypan blue exclusion. Viability was calculated as the percentage of dead vs. total cells. Light microscopy analysis of morphologic features of apoptosis was additionally quantified by scoring cells displaying characteristic cell shrinkage and apoptotic bodies (data not shown).
p53 Luciferase Assay.
RAW 264.7 macrophages were transiently transfected (16 h) by using Fugene 6 transfection reagent (Roche Molecular Biochemicals) and 2 μg of the multimeric, p53-responsive luciferase plasmid, p53-Luc (Stratagene, La Jolla, CA) and 0.1 μg of Renilla pRL-TK vector (Promega). Cells then were treated with recombinant MIF (rMIF; refs. 22 and 23) or vehicle (PBS) for an additional 8 h, followed by 1 mM sodium nitroprusside (SNP) overnight. Luciferase and Renilla luciferase activities were measured by the Dual Luciferase Reporter Assay System (Promega) on a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). Results are expressed as fold-increase over control after normalizing ratios of luciferase/Renilla luciferase and averaging quadruplicate samples.
MIF Plasmid Construct and Transfection.
The MIF coding region was amplified by PCR by using the following primers: 5′-CACCATGCCGATGTTCATCGTAAAC-3′ and 5′-GGCGAAGGTGGAGTTGTTCC-3′. The MIF ORF was verified by sequencing and cloned into TOPO TA Eukaryotic Expression Vector pcDNA 3.1 GS (Invitrogen). Transfection of pcDNA GS/MIF into RAW 264.7 cells was by the use of the liposome reagent, Fugene 6 (Roche Molecular Biochemicals). Transfection efficiencies generally ranged between 10–15% of cells being transfected as assessed by β-galactosidase expression and substrate staining as described (24). Optimal transfection consistently resulted in a 4- to 5-fold increase in extracellular MIF levels (3–6 ng/ml in vector control transfectants vs. 20–30 ng/ml MIF in supernatants from cells expressing pcDNA GS/MIF) as assessed by ELISA (25) and Western blotting of MIF of cell supernatants. In addition to MIF Western blotting, transfected MIF expression levels were routinely analyzed by immunoblotting of cell supernatants with anti-V5 epitope antibodies, a C-terminal fusion protein generated by cloning into the pcDNA 3.1 GS vector (data not shown). For MIF neutralization experiments, isotype control mAb or a neutralizing anti-MIF monoclonal antibody (25) were added at 20 μg/ml just before transfection. Of note, the anti-MIF antibody is specific for MIF-dependent processes and does not interfere with cyclooxygenase-2 (Cox-2) induction in response to other stimuli such as IL-1β or TNF-α (unpublished observations).
Western Blotting and ELISA.
p53 (mAb Pab 122; PharMingen), phospho-specific Ser15p53 (Cell Signaling Technology; Beverly, MA), and Cox-2 (Santa Cruz Biotechnology) antibodies were used for immunoblotting, which was performed as previously described (25). For TAT-fusion protein experiments, transduced levels were analyzed by Western blotting of nontransduced and transduced parallel cultures of MIF+/+ and MIF−/− macrophages plated at 0.5 × 106 cells per milliliter. Briefly, lysates (40 μg total protein) were immunoblotted with an anti-hemagglutinin (anti-HA; Roche Molecular Biochemicals) antibody that recognizes an epitope on the TAT-fusion protein (26). Male 129/Bl6 F3 MIF+/+ and MIF−/− mice were injected i.p. with either vehicle or LPS (E. coli 0111:B4, 15 mg/kg) in 0.4 ml sterile PBS. After 18 h, peritoneal macrophages were collected by lavage and plated at 0.5 × 106 viable cells per milliliter for an additional 24 h, at which time supernatants were collected for determination of TNF-α, IL-1β, and prostaglandin E2 (PGE2) concentrations by ELISA (R & D Systems). Nitric oxide was measured by Griess's assay as described (15).
TAT-Fusion Proteins and Purification.
TAT-fusion protein constructs were the kind gift of S. Dowdy (Washington University, St. Louis) and have been previously described (26, 27). Fractions containing the TAT-fusion proteins underwent buffer exchange on PD-10 columns (Amersham Pharmacia) into TE (10 mM Tris/1 mM EDTA, pH 7.5); TAT-p53DD, TAT-p73DD) or PBS (TAT-GFP). TAT-fusion proteins were added to primary macrophages (0.5 × 106 cells per milliliter) 15 min before challenge with 10 μg/ml LPS and 100 units/ml IFN-γ and incubated for 24 h. Apoptosis was analyzed by Annexin-V-FLUOS Staining Kit (Roche Molecular Biochemicals) followed by fluorescence microscopy.
Results
Potentiation of Macrophage Apoptosis in MIF−/− Mice.
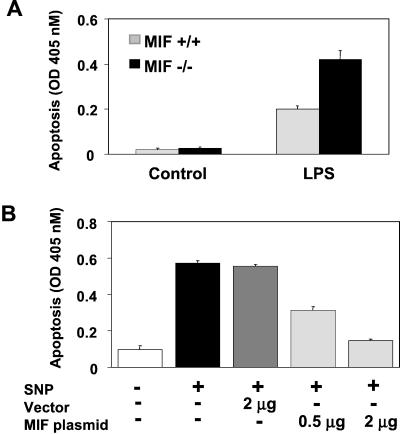
We isolated peritoneal macrophages from MIF−/− and MIF+/+ mice primed in vivo with endotoxin (LPS) and observed that a significantly lower proportion of recovered MIF−/− macrophages were viable (MIF−/− mice, 58% ± 1.6% viability, vs. MIF+/+ mice, 79.1% ± 4% viability, P < 0.02). Decreased macrophage viability was accompanied by a substantial increase in macrophage apoptosis, as assessed by DNA fragmentation and by characteristic morphologic changes visible under light microscopy (Fig. 1A and data not shown).
Figure 1.
MIF modulates activation-induced macrophage apoptosis. (A) MIF-deficient mice are sensitized to LPS-induced peritoneal macrophage apoptosis. MIF+/+ and MIF−/− mice were injected i.p. with either vehicle or LPS (E. coli 0111: B4, 15 mg/kg) in 0.4 ml of sterile PBS. After 18 h, peritoneal macrophages were collected by lavage and immediately assessed for apoptosis by an ELISA that detects cytoplasmic oligonucleosomes. (B) MIF rescues RAW 264.7 macrophages from nitric oxide-induced apoptosis. RAW 264.7 macrophages were transfected with an MIF-expressing plasmid, pcDNA GS/MIF, or an empty pcDNA GS control plasmid for 20 h. The NO donor, SNP, was added at 1 mM for 16 h, and DNA fragmentation was assessed by ELISA. Data are mean ± SD of three experiments.
Macrophages obtained from the endotoxin-treated MIF−/− mice showed significantly less production of the proinflammatory mediators TNF-α, IL-1β, and PGE2, whereas the levels of NO released were comparable between the MIF+/+ and MIF−/− macrophages (Table 1 and ref. 15). The decreased level of TNF-α production in the MIF-deficient macrophages argues against a dominant role for TNF-α-induced death in these cells (15, 28) and supports the inference, in accordance with prior studies (4), that NO is the primary mediator of apoptosis in this model.
Table 1.
MIF is required for production of proinflammatory mediators
| LPS stimulated | MIF+/+ | MIF−/− | ||
|---|---|---|---|---|
| TNF-α, pg/ml | 250 ± 7 | 130 ± 7* | ||
| IL-1β, pg/ml | 760 ± 90 | 370 ± 40* | ||
| PGE2, pg/ml | 5,800 ± 400 | 400 ± 25* | ||
| NO, ng/ml | 44 ± 4 | 42 ± 5 |
MIF+/+ and MIF−/− mice were injected i.p. with either vehicle or LPS. Peritoneal macrophages were cultured ex vivo for an additional 24 h, at which time supernatants were collected for determination of TNF-α, IL-1β, and PGE2 concentrations by ELISA. Nitric oxide was measured by Griess's assay as described (15). Results shown are from two mice per group and represent the mean ± SD of triplicate samples from each.
, P < 0.01 by Student's t test.
Exogenous MIF Suppresses NO-Induced Macrophage Apoptosis.
Recent results obtained from a cell-based genetic screen have identified MIF to inhibit p53-dependent cell cycle arrest, extend fibroblast life span, and decrease NO-induced apoptosis in RAW 264.7 macrophages (17). Despite these findings, however, no mechanism of action for p53 inhibition by MIF has been proposed. We sought to confirm the finding that MIF could effectively inhibit macrophage apoptosis in vitro as an explanation for the sensitization of MIF−/− macrophages to endotoxin-induced apoptosis observed in vivo (Fig. 1A). It was recently suggested that MIF may be posttranslationally modified and this unspecified modification could be important for its bioactivity (29). To ensure complete bioactivity of MIF, eliminate any possibility of contamination of recombinant proteins, and closely mimic a setting of increased MIF expression by macrophages, we performed transient transfection experiments with an MIF-expressing plasmid construct. Transient transfection of the MIF-expressing plasmid into RAW 264.7 macrophages inhibited macrophage apoptosis induced by the NO donor, SNP, as assessed by DNA fragmentation (Fig. 1B) and fluorescent annexin staining [vector control (2 μg) + SNP = 44% ± 3.5% apoptotic cells vs. MIF plasmid (2 μg) + SNP = 11.6% ± 1.5% apoptotic cells, P < 0.01). Of note, transfection with the MIF plasmid (2 μg transfection condition) consistently resulted in a 4- to 5-fold increase in extracellular MIF compared with empty vector as determined by both ELISA and Western blotting (see Materials and Methods). It is also important to note that, whereas only ≈10% of cells contain the MIF-expressing plasmid after transfection (2 μg transfection condition), MIF's autocrine action (25) will be exerted on the entire population of cultured cells.
Exogenous MIF Suppresses NO-Induced p53 Accumulation.
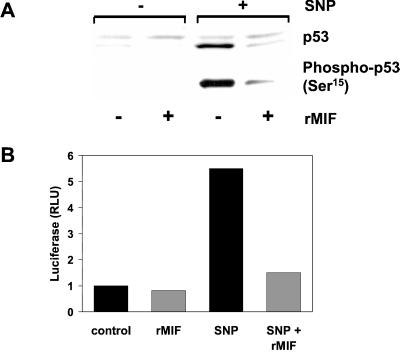
Treatment of RAW 264.7 macrophages with NO leads to an accumulation of p53 protein in cells (30), and both rMIF and ectopically expressed MIF significantly attenuated the NO-stimulated increase in intracellular p53 protein levels (Fig. 2A and data not shown). NO treatment was associated with a coordinate increase in the phosphorylation of p53 on Ser15, and immunoblotting for phosphorylated p53 was a sensitive means to detect the influence of MIF on intracellular p53 content.
Figure 2.
MIF inhibits p53 accumulation and function. (A) RAW 264.7 macrophages were pretreated overnight with 50 ng/ml rMIF. The NO donor, SNP, then was added at 1 mM for 4 h. Lysates were assessed for total p53 and p53 Ser15 phosphorylation by Western blotting. (B) RAW 264.7 macrophages were transiently cotransfected with the multimeric p53 responsive luciferase plasmid, p53-Luc, and the Renilla pRL-TK vector for 16 h. rMIF or vehicle then were added to cells for 8 h, followed by the addition of 1 mM SNP, as indicated, for 16 h. Results are expressed as fold increase over control after normalizing ratios of luciferase/Renilla luciferase from quadruplicate samples.
We next sought to determine whether there was a corresponding decrease in functional p53 activity in MIF-treated macrophages vs. controls. A p53-sensitive promoter assay was used to investigate the relative transactivation potential of p53 in cells challenged with NO in the presence or absence of rMIF. NO treatment of RAW 264.7 cells transfected with a p53-luciferase construct strongly induced p53-dependent transcription in cells that had not been pretreated with rMIF. By contrast, cells exposed to either rMIF or transfected with an MIF-expressing plasmid and then challenged with NO displayed p53-dependent transactivation only slightly above control (Fig. 2B and data not shown). Taken together, these data indicate that MIF acts to inhibit macrophage apoptosis by decreasing the intracellular accumulation and subsequent function of the tumor suppressor protein p53.
MIF Induces Arachidonic Acid Metabolism in Macrophages.
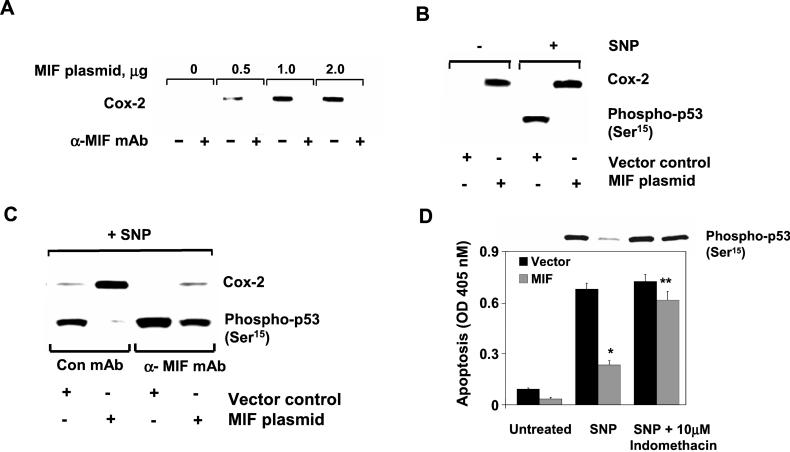
It has been suggested that arachidonic acid metabolism and the expression of Cox-2 negatively regulates NO-mediated p53 accumulation and apoptosis in macrophages (31). We previously observed that MIF induces cytoplasmic phospholipase A2 activity in fibroblasts, leading to an increase in arachidonic acid production by these cells (25). Because the expression of enzymes mediating arachidonic acid metabolism are frequently coupled (32), we examined whether Cox-2 activity in macrophages was regulated by MIF and whether it was mechanistically linked to the suppression of NO-dependent p53 accumulation by MIF. Transfection of an MIF-expressing plasmid into macrophages induced the expression of Cox-2 mRNA and protein when compared with vector controls, and this effect was attenuated by the addition of a neutralizing anti-MIF mAb (Fig. 3A). After challenge with NO, Cox-2 expression and p53 accumulation were inversely related in MIF-treated macrophages, suggesting a potential role for Cox-2 in MIF-mediated suppression of p53 (Fig. 3B). Moreover, when p53 accumulation was examined in cells exposed to MIF in the presence of a neutralizing anti-MIF mAb, there was a nearly complete inhibition of Cox-2 induction and a comparable restoration of p53 accumulation that was inversely related to the expression of Cox-2 (Fig. 3C). As expected, macrophages exposed to both MIF and anti-MIF antibody showed an increase in the level of NO-mediated apoptosis corresponding to the restored level of p53 as shown in (Fig. 3C and data not shown).
Figure 3.
MIF induction of Cox-2 inhibits p53. (A) MIF induces Cox-2 expression in RAW 264.7 macrophages. Increasing concentrations of pcDNA GS/MIF plasmid or pcDNA GS vector control (lanes 1 and 2) were transfected into macrophages, and Cox-2 expression was examined. (B) Inverse relationship between MIF-induced Cox-2 and p53 expression. RAW 264.7 macrophages were transfected with either pcDNA GS vector control or pcDNA GS/MIF plasmid. SNP (1 mM) was added to cells as indicated for an additional 4 h. (C) Suppression of MIF-induced Cox-2 and p53 restoration by anti-MIF. Same as B but in the presence of anti-MIF or isotype control. (D) Inhibition of MIF-induced arachidonate metabolism restores p53 accumulation and function. Indomethacin was added at the time of transfection to inhibit the activity of cyclooxygenases. Data are the mean ± SD of three determinations and are representative of three independent experiments (*, P < 0.01, and **, P < 0.05 for MIF vs. vector).
Inhibition of p53 Accumulation by MIF Requires Cyclooxygenase Activity.
To further elucidate the role of MIF-mediated Cox-2 expression in suppressing p53-dependent apoptosis, we added indomethacin, a potent inhibitor of cyclooxygenases, to block the formation of arachidonate metabolites in macrophages (33). Macrophage apoptosis induced by NO was almost completely suppressed by overexpression of MIF, and cells exposed to indomethacin were resistant to the anti-apoptotic effects of MIF (Fig. 3D). Of importance, the inhibition of Cox activity by indomethacin was associated with a restoration of p53 to levels observed in the absence of MIF expression (Fig. 3D). These data indicate that the induction of Cox-2 activity by MIF is required for the MIF-mediated suppression of p53 accumulation and macrophage apoptosis in response to NO challenge.
MIF−/− Macrophages Are Resistant to Activation-Induced Cox-2 Expression and Activity.
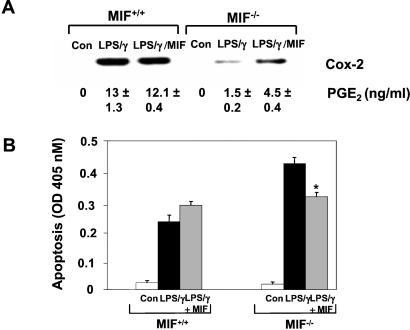
We next sought to examine the relationship between MIF-induced Cox-2 expression and the previously observed susceptibility of MIF−/− macrophages to apoptosis in vivo (Fig. 1A). Resting peritoneal macrophages from MIF−/− and MIF+/+ mice were isolated and treated in vitro with LPS together with IFN-γ, a potent and well-characterized stimulus for activation-induced macrophage apoptosis (30). The level of Cox-2 expression and PGE2 release was almost 10-fold less in the MIF-deficient vs. the wild-type macrophages (Fig. 4A). Treatment with LPS/IFN-γ revealed an inverse relationship between the level of MIF-modulated Cox-2 activity and the extent of apoptosis in LPS/IFN-γ-challenged MIF−/− and MIF+/+ macrophages (Fig. 4B). Of importance, the addition of rMIF to the MIF−/− macrophages partially restored the defect in LPS/IFN-γ-mediated Cox-2 expression, PGE2 production, and suppression of apoptosis.
Figure 4.
MIF is required for LPS-induced Cox-2 expression, activity and suppression of apoptosis in murine peritoneal macrophages. (A) Resting peritoneal macrophages were stimulated for 18 h with 10 μg/ml LPS and 100 units/ml IFN-γ together with or without mouse rMIF. Results shown are the mean ± SD of duplicate samples (PGE2) and are representative of two independent experiments. (B) Restoration of Cox-2 expression and activity by rMIF rescues MIF−/− macrophages from augmented activation-induced apoptosis. Cells were treated as in A and assessed for apoptosis by ELISA of cytosolic oligonucleosomes as shown. Results shown are the mean ± SD of duplicate samples and are representative of three independent experiments. *, P < 0.04 for LPS/IFN-γ/MIF vs. LPS/IFN-γ treatment alone.
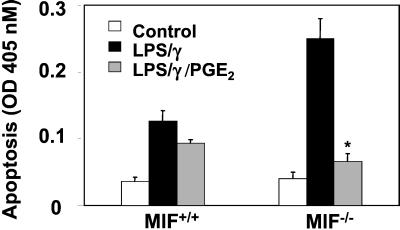
PGE2 Inhibits Enhanced Activation-Induced Apoptosis in MIF−/− Macrophages.
These data suggest that the conversion of arachidonic acid into PGE2 is a critical component of MIF-mediated inhibition of p53-dependent macrophage apoptosis. Because MIF−/− macrophages are resistant to LPS-induced Cox-2 expression and activity, but highly sensitive to LPS-mediated apoptosis, we then performed add-back experiments to verify a direct role for PGE2 in MIF's capacity to inhibit macrophage apoptosis. As shown in Fig. 5, peritoneal macrophages obtained from MIF−/− mice and treated with PGE2 were strongly protected from the augmented apoptosis associated with treatment of MIF−/− macrophages with LPS/IFN-γ. MIF+/+ macrophages by contrast were only minimally protected by PGE2 addition.
Figure 5.
The Cox-2 product, PGE2, rescues activation-induced apoptosis in MIF−/− macrophages. Resting peritoneal macrophages from MIF+/+ and MIF−/− mice were stimulated with 10 μg/ml LPS and 100 units/ml IFN-γ in the absence or presence of exogenously added PGE2. PGE2 was added to cultures 4 h after the addition of LPS/IFN-γ to mimic relative time of PGE2 accumulation in MIF+/+ macrophages. Data shown are the mean ± SD of duplicate samples and are representative of two independent experiments. *, P < 0.03 for LPS/IFN-γ/PGE2 vs. LPS/IFN-γ alone.
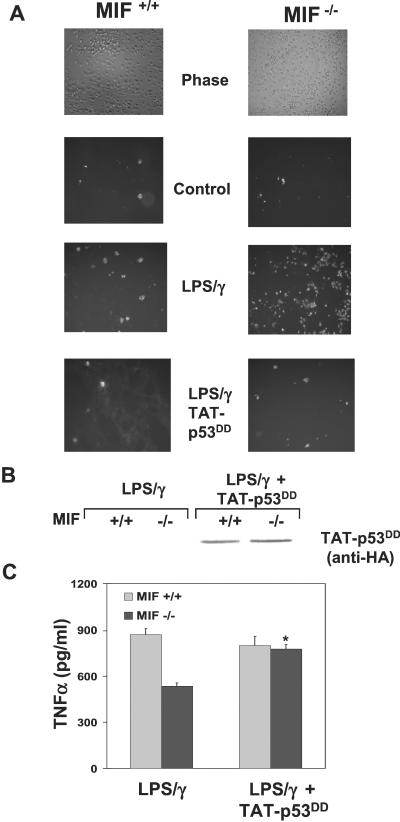
Dominant-Negative p53 Inhibits Enhanced Activation-Induced Apoptosis in MIF−/− Macrophages and Restores Function.
The inhibition of activation-induced apoptosis in MIF−/− macrophages by PGE2 suggested to us that direct inhibition of p53 could similarly suppress this enhanced apoptosis and potentially restore the proinflammatory function of these cells. To examine the precise role of p53 in primary mouse macrophage apoptosis, we used the TAT transducible protein system (26, 27), which has been shown to transduce greater than 99% of any given cell population (26). A well characterized p53 tetramerization dominant-negative domain was generated as a fusion protein with TAT and expressed as a recombinant protein (27). Enhanced LPS-mediated macrophage apoptosis associated with the lack of endogenous MIF was completely abolished in the presence of dominant negative p53 (TAT-p53DD) (Fig. 6A). When macrophages were treated with a dominant-negative mutant of the p53 family member p73 (TAT-p73DD) or green fluorescent protein (TAT-GFP), no significant decrease in activation-induced apoptosis was observed (data not shown). To determine whether inhibition of cellular p53 by TAT-p53DD could restore proinflammatory function to the MIF−/− macrophages, we analyzed the levels of TNF-α released from LPS/IFN-γ-treated cells. As shown in Fig. 6C, MIF-deficient macrophages produced significantly lower amounts of TNF-α than did the MIF-containing cells, which is consistent with earlier findings (Table 1 and ref. 15). However, when apoptosis was inhibited by dominant-negative p53, the levels of TNF-α production were restored to levels seen in the MIF+/+ macrophages. These results suggest that the enhanced apoptosis observed in MIF−/− macrophages depends on p53 and that an increase in cellular p53 and subsequent apoptosis confers decreased proinflammatory function to MIF-deficient cells.
Figure 6.
Dominant-negative p53 inhibits enhanced activation-induced apoptosis in MIF−/− macrophages and restores function. Peritoneal macrophages from MIF+/+ and MIF−/− mice were treated with or without 250 nM TAT-p53DD for 15 min before stimulation with 10 μg/ml LPS and 100 units/ml IFN-γ for 24 h. (A) Activation induced apoptosis was assessed by fluorescent-annexin staining and fluorescence microscopy. (B) Anti-hemagglutinin (HA) immunoblot analysis of primary macrophages from MIF+/+ and MIF−/− mice transduced with TAT-p53DD, demonstrating equivalent transduction efficiencies. (C) Supernatants from indicated samples were assessed for TNF-α production by ELISA. Data shown are the mean ± SD of duplicate samples and are representative of two independent experiments. *, P < 0.05 for LPS/IFN-γ/TAT-p53DD vs. LPS/IFN-γ alone.
Discussion
We conclude that MIF regulates the activation pathway leading to LPS-stimulated macrophage apoptosis by functionally inactivating p53 activity. This finding is manifest by an increase in activation-induced arachidonic acid metabolism and a decrease in NO-induced p53 accumulation. Conversely, activated macrophages deficient in MIF display decreased arachidonic acid metabolism that results in decreased macrophage survival, increased apoptosis, and a corresponding decrease in proinflammatory function. The ability of an inhibitory p53 to completely suppress the enhanced activation-induced macrophage apoptosis inherent in MIF-deficient cells strongly implies that p53 is responsible for this phenomenon. Moreover, the restoration of macrophage function to MIF−/− macrophages by inhibition of p53 further implies that increased apoptosis is at least partially responsible for the decrease in proinflammatory cytokine production in these cells. The precise role of macrophage apoptosis in macrophage function in the presence of MIF is less easy to predict. Our results suggest that, whereas there is a moderate amount of activation-induced macrophage apoptosis in MIF+/+ cells, inhibition of p53 suppresses macrophage apoptosis but confers no additional proinflammatory function as assessed by TNF-α production. We conclude that MIF is required for the maintenance of activation-induced p53 induction and macrophage apoptosis and that, when MIF is lost, cell survival and function are compromised.
The ability of MIF to sustain macrophage function by regulating p53-dependent apoptosis provides the first mechanism to explain the global hyporesponsiveness of MIF−/− macrophages to LPS, and the resistance of MIF−/− mice to endotoxic shock (14, 15). Whereas various immune effector cells have been known for some time to undergo apoptosis as a consequence of activation (20, 34), the impact of this phenomenon on the evolution of the innate immune response has only recently become an area of high interest (19). Immune cell dysfunction late in sepsis is an important complication of the host response to bacterial products, and programmed cell death appears to be a significant contributor to the depression of late phase host responses (7, 19, 34). Inhibition of the cytokine mediators that affect sepsis progression thus far has proven disappointing in the clinic—in part because of the complexity of their regulatory actions (19). Exploration of the apoptotic pathways that contribute to sepsis lethality may be instructive both for revealing important regulatory features of the late-phase of the sepsis response, and for suggesting new targets for potential therapeutic intervention (34). Potential intracellular effectors of MIF action that have been described to date include components of the mitogen-activated protein (MAP) kinase pathway (25), the c-Jun coactivator JAB1 (35), and the proliferation-associated gene product PAG (36).
Our findings additionally support an emerging body of data that the Cox-2 isozyme is of critical importance in the tissue pathology associated with septic shock (37, 38). Regulation of p53 function via MIF induction of Cox-2 therefore is likely to be an important mechanism for the overall proinflammatory spectrum of action of MIF. Whether functional inactivation of p53 by MIF modulation of Cox-2 activity extends beyond the macrophage and the innate immune response is also of interest. MIF and the target of nonsteroidal anti-inflammatory drug (NSAID) therapy, Cox-2, are highly expressed in colon carcinoma (39, 40), and MIF has been implicated to be a proliferative and/or survival factor for these cancer cells (40). It is noteworthy that prophylaxis with NSAIDs has been reported to reduce the development of adenocarcinoma of the colon (41). Inhibition of MIF-induced Cox-2 activity by NSAIDs may underlie the protective effect of these drugs in oncogenesis, and supports a potentially fundamental link between MIF expression and p53 function in a variety of cell systems.
Acknowledgments
We are grateful to Jian Wang for excellent technical assistance and Kirk Manogue, Marc Symons, and Maria Ruggieri for their comments and input on the manuscript. These studies were supported by National Institutes of Health Grant 1 R01 AI 42310-03 (to R.B.).
Abbreviations
- Cox-2
cyclooxygenase-2
- LPS
lipopolysaccharide
- MIF
macrophage migration inhibitory factor
- PGE2
prostaglandin E2
- SNP
sodium nitroprusside
- rMIF
recombinant MIF
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hoffmann J A, Kafatos F C, Janeway C A, Ezekowitz R A. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello C A. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 3.Ohmori Y, Hamilton T A. Pharmacol Ther. 1994;63:235–264. doi: 10.1016/0163-7258(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 4.Albina J E, Cui S, Mateo R B, Reichner J S. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- 5.Sarih M, Souvannavong V, Adam A. Biochem Biophys Res Commun. 1993;191:503–508. doi: 10.1006/bbrc.1993.1246. [DOI] [PubMed] [Google Scholar]
- 6.Williams T E, Ayala A, Chaudry I H. J Surg Res. 1997;70:113–118. doi: 10.1006/jsre.1997.5117. [DOI] [PubMed] [Google Scholar]
- 7.Ayala A, Urbanich M A, Herdon C D, Chaudry I H. J Trauma. 1996;40:568–573. doi: 10.1097/00005373-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 8.von Knethen A, Callsen D, Brune B. J Immunol. 1999;163:2858–2866. [PubMed] [Google Scholar]
- 9.Messmer U K, Reimer D M, Reed J C, Brune B. FEBS Lett. 1996;384:162–166. doi: 10.1016/0014-5793(96)00311-0. [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Bernhagen J, Mitchell R A, Bucala R. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calandra T, Spiegel L A, Metz C N, Bucala R. Proc Natl Acad Sci USA. 1998;95:11383–11388. doi: 10.1073/pnas.95.19.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martiney J A, Sherry B, Metz C N, Espinoza M, Ferrer A S, Calandra T, Broxmeyer H E, Bucala R. Infect Immun. 2000;68:2259–2267. doi: 10.1128/iai.68.4.2259-2267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacher M, Metz C N, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. Proc Natl Acad Sci USA. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernhagen J, Calandra T, Mitchell R A, Martin S B, Tracey K J, Voelter W, Manogue K R, Cerami A, Bucala R. Nature (London) 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 15.Bozza M, Satoskar A R, Lin G, Lu B, Humbles A A, Gerard C, David J R. J Exp Med. 1999;189:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calandra T, Echtenacher B, Roy D L, Pugin J, Metz C N, Hultner L, Heumann D, Mannel D, Bucala R, Glauser M P. Nat Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 17.Hudson J D, Shoaibi M A, Maestro R, Carnero A, Hannon G J, Beach D H. J Exp Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leech M, Metz C, Hall P, Hutchinson P, Gianis K, Smith M, Weedon H, Holdsworth S R, Bucala R, Morand E F. Arthritis Rheum. 1999;42:1601–1608. doi: 10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 19.Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer L L. FASEB J. 2001;15:879–892. doi: 10.1096/fj.00-058rev. [DOI] [PubMed] [Google Scholar]
- 20.Hotchkiss R S, Chang K C, Swanson P E, Tinsley K W, Hui J J, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, et al. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 21.Grobmyer S R, Armstrong R C, Nicholson S C, Gabay C, Arend W P, Potter S H, Melchior M, Fritz L C, Nathan C F. Mol Med. 1999;5:585–594. [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson L N, Noble M E, Owen D J. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- 23.Bernhagen J, Mitchell R A, Calandra T, Voelter W, Cerami A, Bucala R. Biochemistry. 1994;33:14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- 24.Lim K, Chae C B. Biotechniques. 1989;7:576–579. [PubMed] [Google Scholar]
- 25.Mitchell R A, Metz C N, Peng T, Bucala R. J Biol Chem. 1999;274:18100–18106. doi: 10.1074/jbc.274.25.18100. [DOI] [PubMed] [Google Scholar]
- 26.Nagahara H, Vocero-Akbani A M, Snyder E L, Ho A, Latham D G, Lissy N A, Becker-Hapak M, Ezhevsky S A, Dowdy S F. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 27.Irwin M, Marin M C, Phillips A C, Seelan R S, Smith D I, Liu W, Flores E R, Tsai K Y, Jacks T, Vousden K H, Kaelin W G J. Nature (London) 2000;407:645–648. doi: 10.1038/35036614. [DOI] [PubMed] [Google Scholar]
- 28.Xaus J, Comalada M, Valledor A F, Lloberas J, Lopez-Soriano F, Argiles J M, Bogdan C, Celada A. Blood. 2000;95:3823–3831. [PubMed] [Google Scholar]
- 29.Watarai H, Nozawa R, Tokunaga A, Yuyama N, Tomas M, Hinohara A, Ishizaka K, Ishii Y. Proc Natl Acad Sci USA. 2000;97:13251–13256. doi: 10.1073/pnas.230445397. . (First Published November 7, 2000; 10.1073/pnas.230445397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messmer U K, Brune B. Arch Biochem Biophys. 1996;327:1–10. doi: 10.1006/abbi.1996.0085. [DOI] [PubMed] [Google Scholar]
- 31.von Knethen A, Brune B1. FASEB J. 1997;11:887–895. [PubMed] [Google Scholar]
- 32.Heasley L E, Thaler S, Nicks M, Price B, Skorecki K, Nemenoff R A. J Biol Chem. 1997;272:14501–14504. doi: 10.1074/jbc.272.23.14501. [DOI] [PubMed] [Google Scholar]
- 33.von Knethen A, Lotero A, Brune B. Oncogene. 1998;17:387–394. doi: 10.1038/sj.onc.1201926. [DOI] [PubMed] [Google Scholar]
- 34.Mahidhara R, Billiar T R. Crit Care Med. 2000;28:N105–N113. doi: 10.1097/00003246-200004001-00013. [DOI] [PubMed] [Google Scholar]
- 35.Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, Johannes F J, Roger T, Calandra T, Kapurniotu A, et al. Nature (London) 2000;408:211–216. doi: 10.1038/35041591. [DOI] [PubMed] [Google Scholar]
- 36.Jung H, Kim T, Chae H Z, Kim K T, Ha H. J Biol Chem. 2001;276:155504–155510. doi: 10.1074/jbc.M009620200. [DOI] [PubMed] [Google Scholar]
- 37.McAdam B F, Mardini I A, Habib A, Burke A, Lawson J A, Kapoor S, FitzGerald G A. J Clin Invest. 2000;105:1473–1482. doi: 10.1172/JCI9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinchuk J E, Car B D, Focht R J, Johnston J J, Jaffee B D, Covington M B, Contel N R, Eng V M, Collins R J, Czerniak P M. Nature (London) 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 39.Williams C S, Goldman A P, Sheng H, Morrow J D, DuBois R N. Neoplasia. 1999;1:170–176. doi: 10.1038/sj.neo.7900024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi N, Nishihira J, Sato Y, Kondo M, Ogawa H, Ohshima T, Une Y, Todo S. Mol Med. 1998;4:707–714. [PMC free article] [PubMed] [Google Scholar]
- 41.Williams C, Shattuck-Brandt R L, DuBois R N. Ann NY Acad Sci. 1999;889:72–83. doi: 10.1111/j.1749-6632.1999.tb08725.x. [DOI] [PubMed] [Google Scholar]