Abstract
1. The processes regulating intracellular calcium in the outer segments of salamander rods have been investigated. The main preparation used was the isolated rod loaded with the Ca(2+)-sensitive photoprotein aequorin, from which outer segment membrane current and free [Ca2+]i could be recorded simultaneously. Two other preparations were also used: outer segment membrane current was recorded from intact, isolated rods using a suction pipette, and from detached outer segments using a whole-cell pipette. 2. Measurements of free intracellular [Ca2+] in Ringer solution were obtained from two aequorin-loaded rods. Mean [Ca2+]i in darkness was 0.41 microM, and after a bright flash [Ca2+]i fell to below detectable levels ( < 0.3 microM). No release of intracellular Ca2+ by a bright flash of light could be detected ( < 0.2 microM). 3. Application of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) caused an increase in the size of the light-sensitive current and a rise in [Ca2+]i, but application of IBMX either when the light-sensitive channels had been closed by a bright light or in the absence of external Ca2+ caused no detectable rise in [Ca2+]i. It is concluded that IBMX increases [Ca2+]i by opening light-sensitive channels, and does not release Ca2+ from stores within the outer segment. 4. Removal of external Na+ caused a rise in [Ca2+]i to around 2 microM and completely suppressed the light-sensitive current. 5. The Na(+)-Ca2+, K+ exchange current in aequorin-loaded rods was activated in first-order manner by internal free calcium, with a mean Michaelis constant, KCa, of 1.6 microM. 6. The KCa of the Na(+)-Ca2+, K+ exchange was increased by elevating internal [Na+]. 7. The Michaelis relation between [Ca2+]i and the activity of the Na(+)-Ca2+, K+ exchange was used to calculate the change in [Ca2+]i occurring during the response to a bright light. In aequorin-loaded rods in Ringer solution the mean change in free [Ca2+]i after a bright flash was 0.34 microM. In these rods 10% of the dark current was carried by Ca2+. 8. Most of the calcium entering the outer segment was taken up rapidly and reversibly by buffer systems. The time constant of equilibration between free and rapidly bound Ca2+ was less than 20 ms. No slow component of calcium uptake was detected. 9. Two components of calcium buffering could be distinguished in the outer segments of aequorin-loaded rods.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


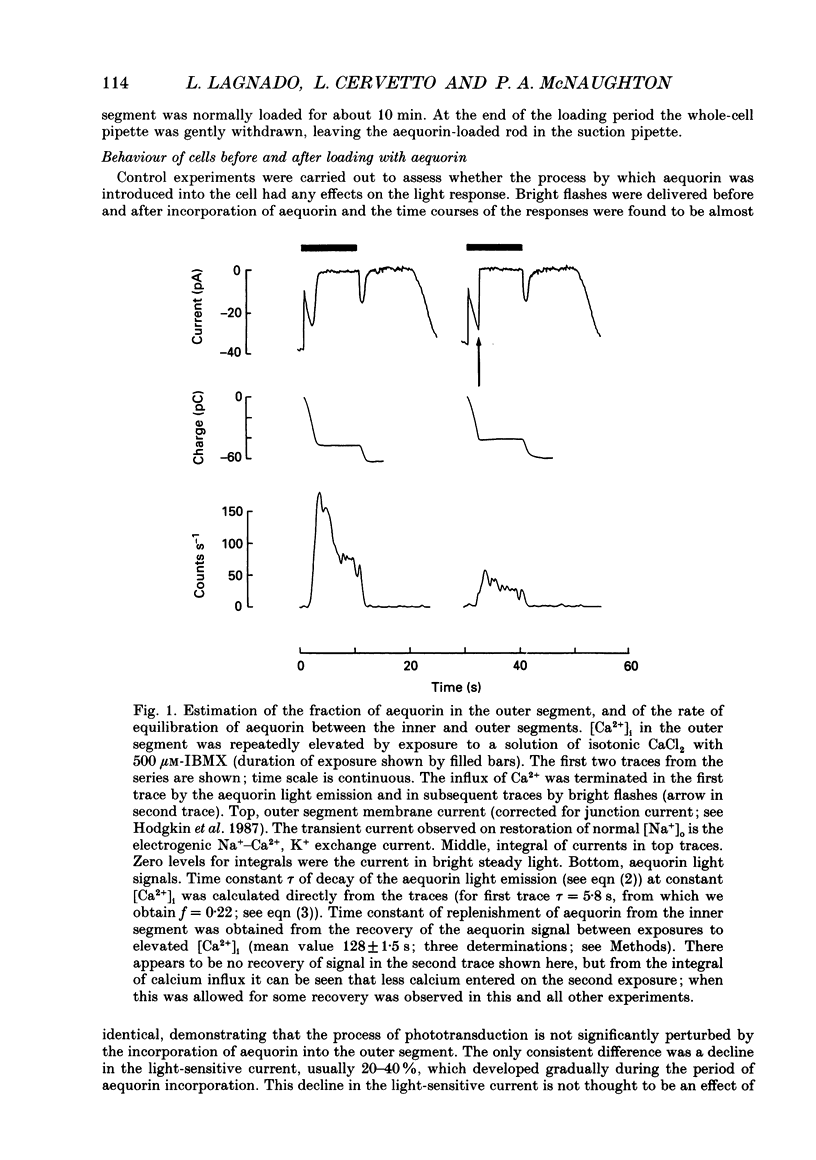



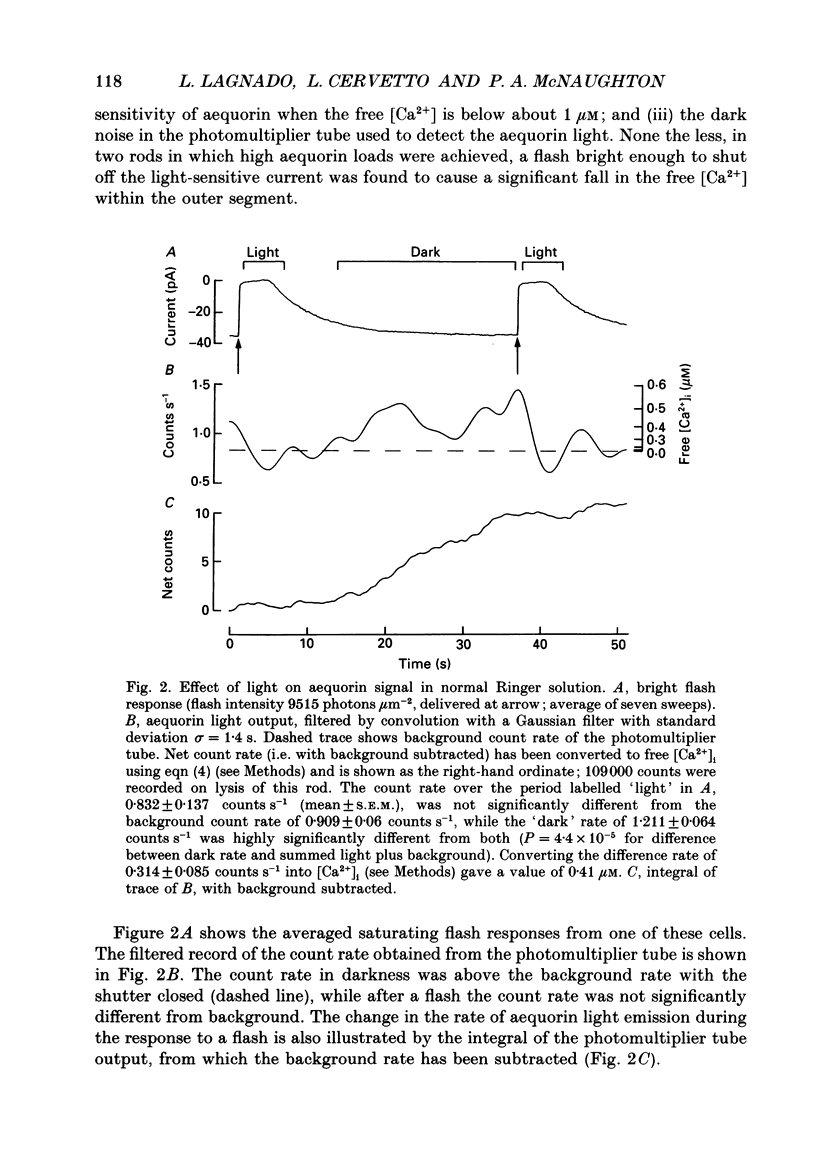

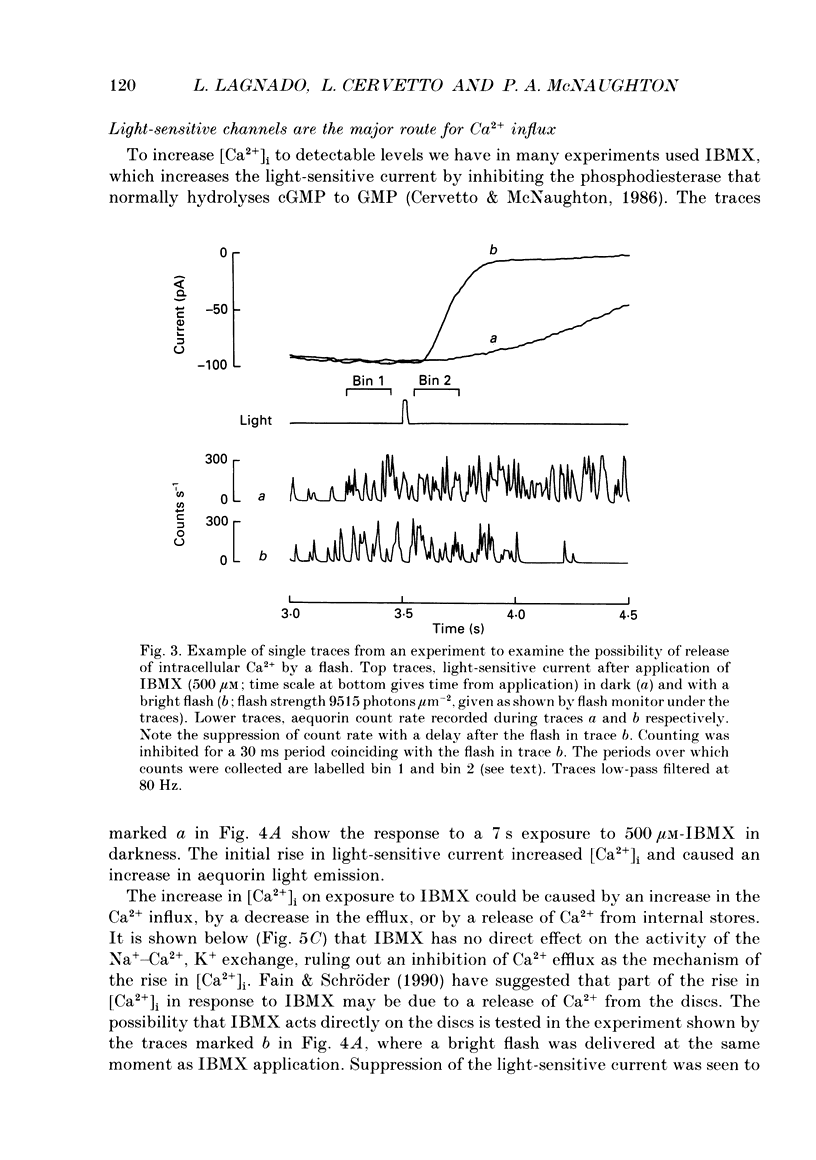
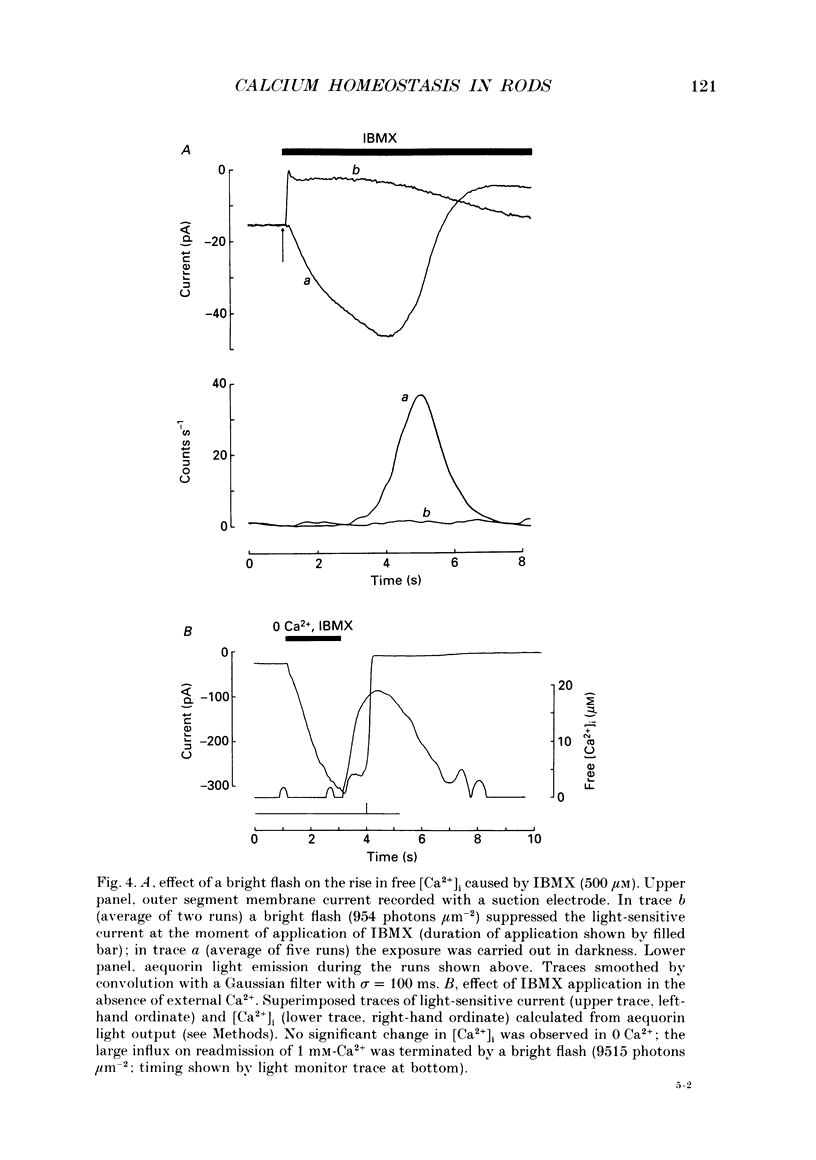












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Nunn B. J. Location and function of voltage-sensitive conductances in retinal rods of the salamander, Ambystoma tigrinum. J Physiol. 1984 Sep;354:203–223. doi: 10.1113/jphysiol.1984.sp015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Coles J. A., Pinto L. H. Effects of injections of calcium and EGTA into the outer segments of retinal rods of Bufo marinus. J Physiol. 1977 Aug;269(3):707–722. doi: 10.1113/jphysiol.1977.sp011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Lagnado L., Perry R. J., Robinson D. W., McNaughton P. A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989 Feb 23;337(6209):740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Cervetto L., McNaughton P. A. The effects of phosphodiesterase inhibitors and lanthanum ions on the light-sensitive current of toad retinal rods. J Physiol. 1986 Jan;370:91–109. doi: 10.1113/jphysiol.1986.sp015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Torre V., Rispoli G., Marroni P. Mechanisms of light adaptation in toad rods. Exp Biol. 1985;44(3):147–157. [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. In squid axons, ATP modulates Na+-Ca2+ exchange by a Ca2+i-dependent phosphorylation. Biochim Biophys Acta. 1987 Mar 12;897(3):347–354. doi: 10.1016/0005-2736(87)90432-9. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Schröder W. H. Calcium content and calcium exchange in dark-adapted toad rods. J Physiol. 1985 Nov;368:641–665. doi: 10.1113/jphysiol.1985.sp015881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Schröder W. H. Calcium in dark-adapted toad rods: evidence for pooling and cyclic-guanosine-3'-5'-monophosphate-dependent release. J Physiol. 1987 Aug;389:361–384. doi: 10.1113/jphysiol.1987.sp016661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Schröder W. H. Light-induced calcium release and re-uptake in toad rods. J Neurosci. 1990 Jul;10(7):2238–2249. doi: 10.1523/JNEUROSCI.10-07-02238.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. Measurement of sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:347–370. doi: 10.1113/jphysiol.1987.sp016742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. Control of light-sensitive current in salamander rods. J Physiol. 1988 Sep;403:439–471. doi: 10.1113/jphysiol.1988.sp017258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S., Murakami M. Calcium-dependent regulation of cyclic GMP phosphodiesterase by a protein from frog retinal rods. Nature. 1991 Jan 31;349(6308):420–423. doi: 10.1038/349420a0. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Cervetto L., McNaughton P. A. Ion transport by the Na-Ca exchange in isolated rod outer segments. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4548–4552. doi: 10.1073/pnas.85.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado L., McNaughton P. A. Net charge transport during sodium-dependent calcium extrusion in isolated salamander rod outer segments. J Gen Physiol. 1991 Sep;98(3):479–495. doi: 10.1085/jgp.98.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolley R. N., Racz E. Calcium modulation of cyclic GMP synthesis in rat visual cells. Vision Res. 1982;22(12):1481–1486. doi: 10.1016/0042-6989(82)90213-9. [DOI] [PubMed] [Google Scholar]
- Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988 Jul 7;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Brown J. Diffusion of calcium ions in retinal rods. A theoretical calculation. J Gen Physiol. 1981 Apr;77(4):475–487. doi: 10.1085/jgp.77.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton P. A. Light response of vertebrate photoreceptors. Physiol Rev. 1990 Jul;70(3):847–883. doi: 10.1152/physrev.1990.70.3.847. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and light adaptation in retinal rods and cones. Nature. 1988 Jul 7;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988 Jan;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. J., McNaughton P. A. Response properties of cones from the retina of the tiger salamander. J Physiol. 1991 Feb;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett K. L., Goldin S. M. Guanosine 3',5'-cyclic monophosphate stimulates release of actively accumulated calcium in purified disks from rod outer segments of bovine retina. Biochemistry. 1986 Apr 8;25(7):1739–1746. doi: 10.1021/bi00355a044. [DOI] [PubMed] [Google Scholar]
- Ratto G. M., Payne R., Owen W. G., Tsien R. Y. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1988 Sep;8(9):3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder W. H., Fain G. L. Light-dependent calcium release from photoreceptors measured by laser micro-mass analysis. Nature. 1984 May 17;309(5965):268–270. doi: 10.1038/309268a0. [DOI] [PubMed] [Google Scholar]
- Torre V. The contribution of the electrogenic sodium-potassium pump to the electrical activity of toad rods. J Physiol. 1982 Dec;333:315–341. doi: 10.1113/jphysiol.1982.sp014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984 May 24;309(5966):352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]


