Abstract
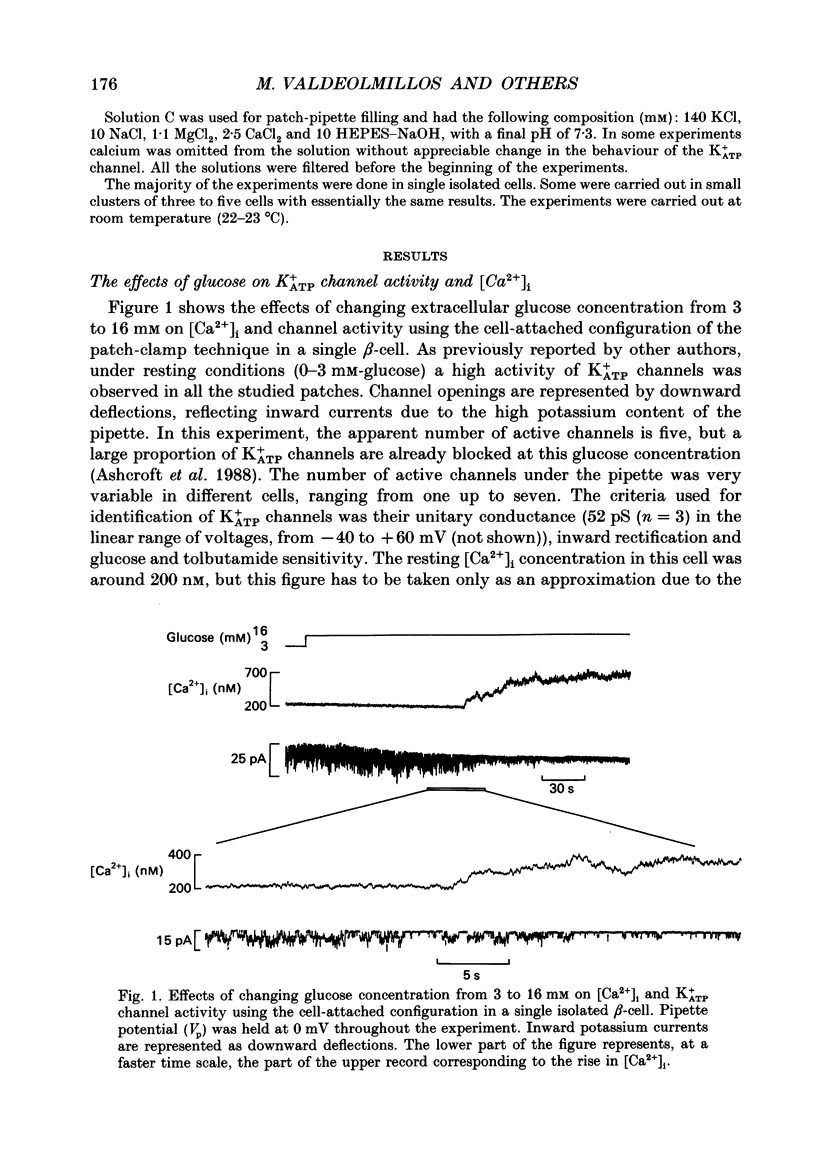
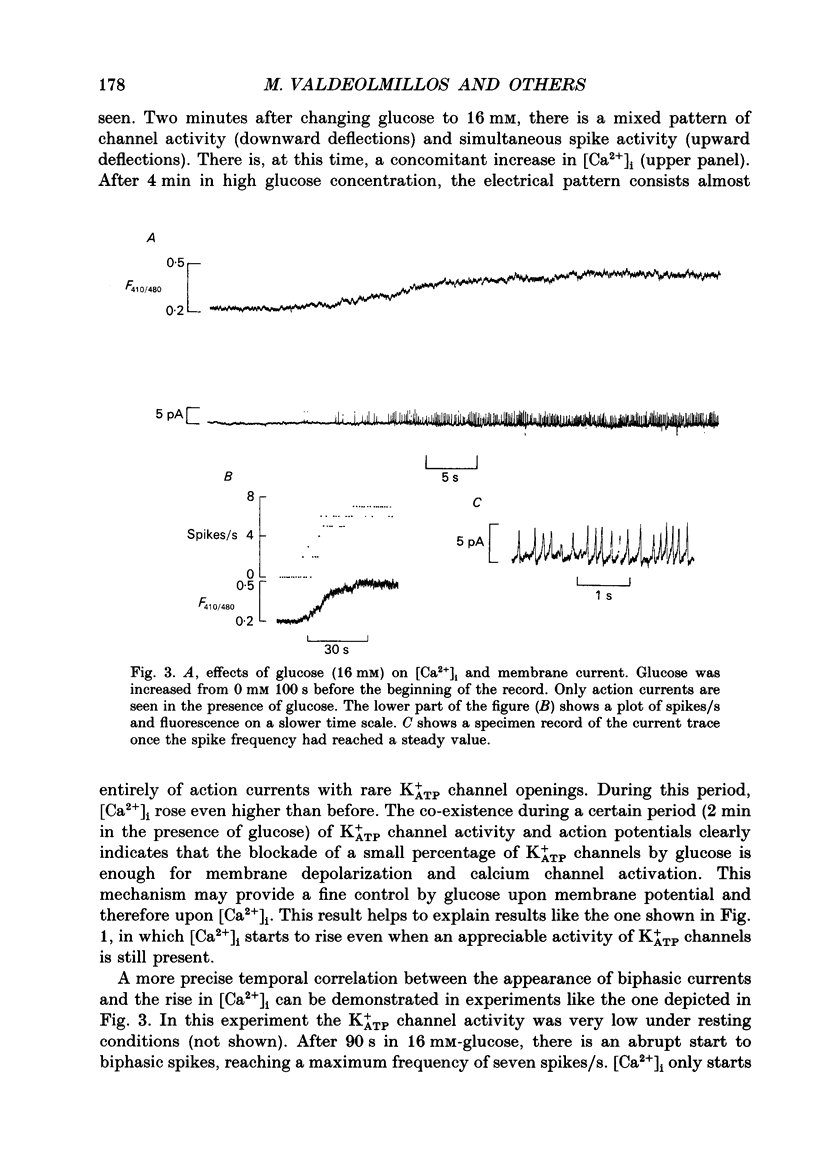
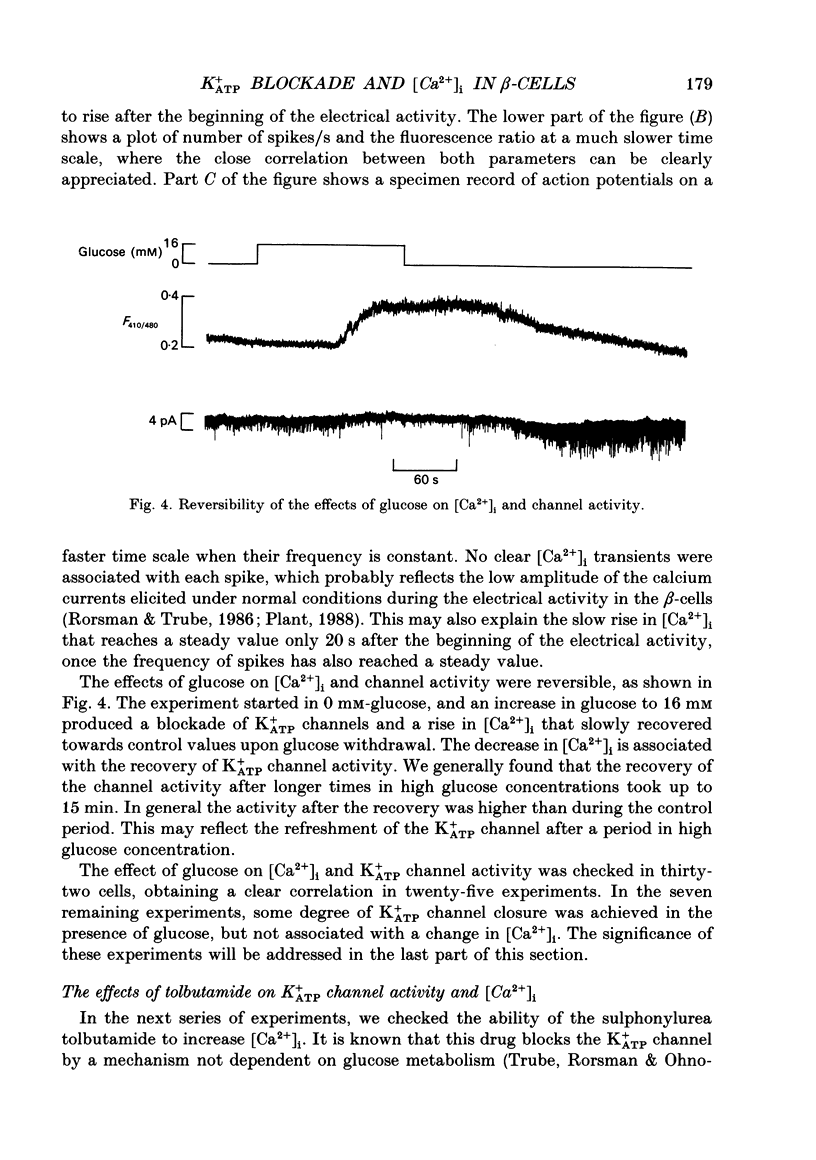
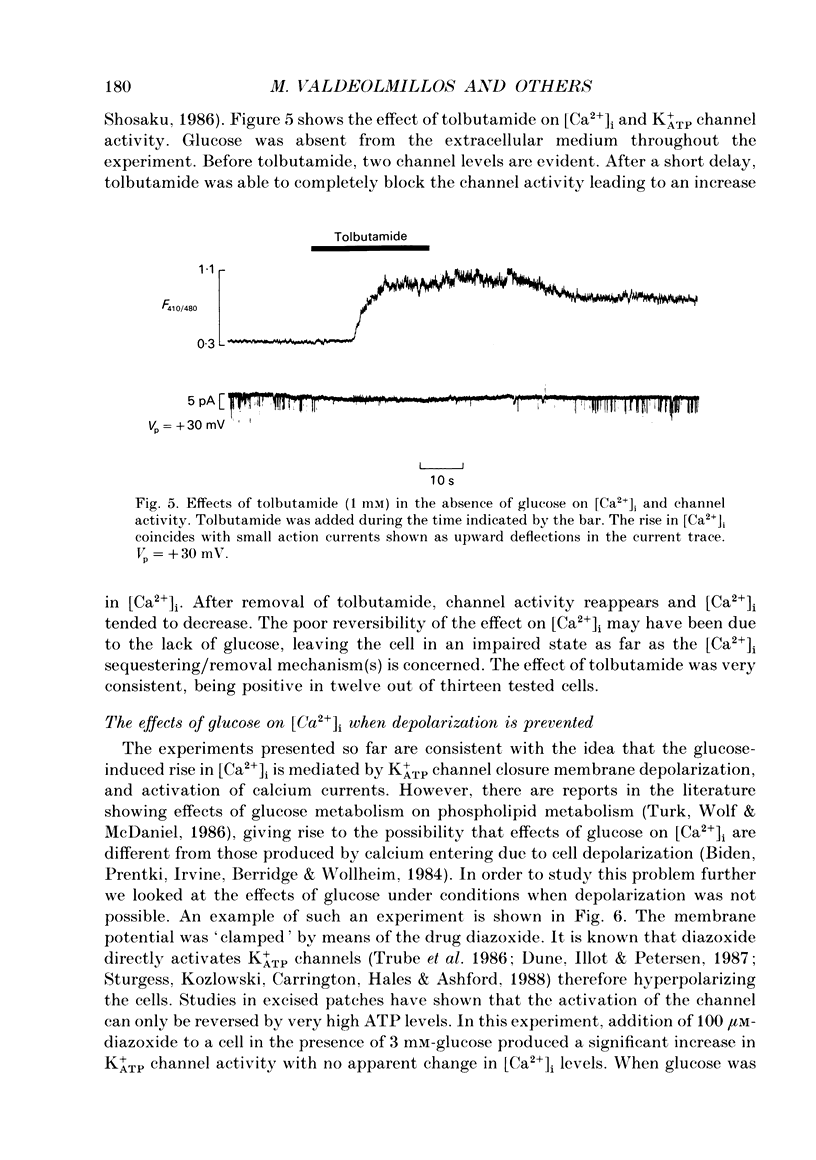
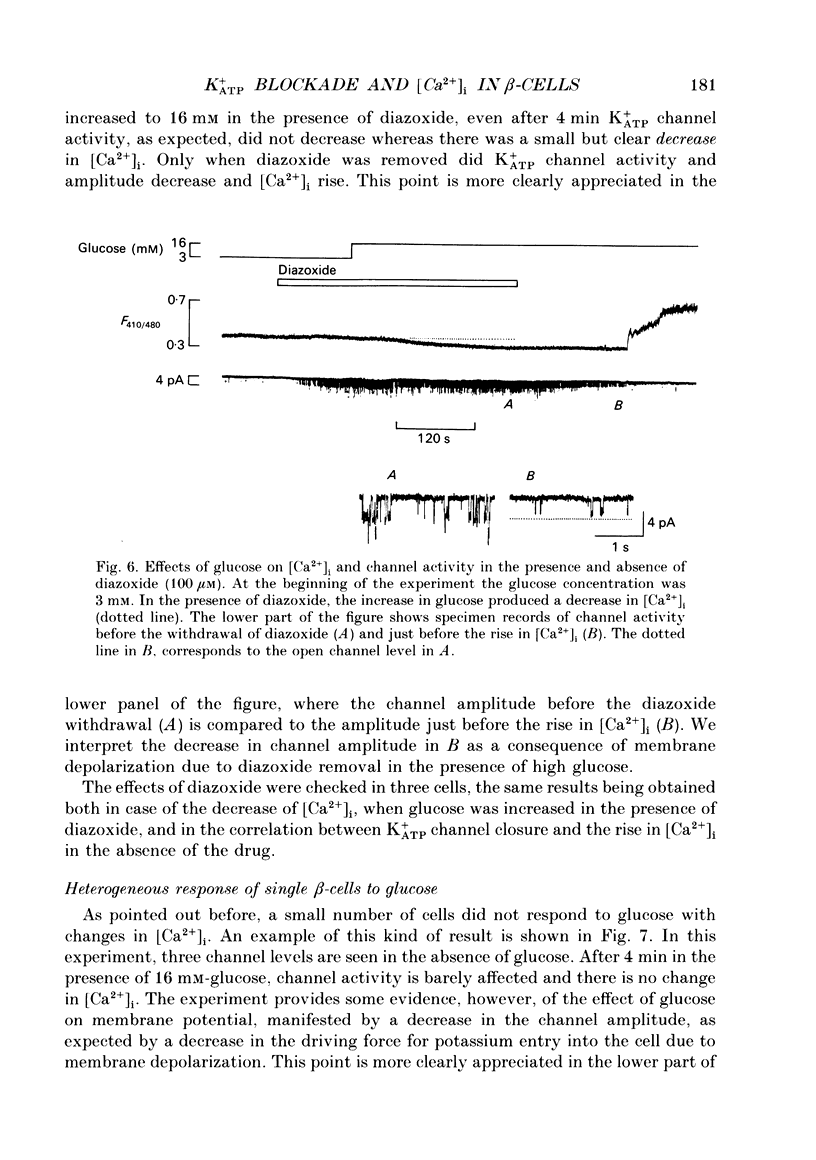
1. Intracellular calcium [Ca2+]i and channel activity were simultaneously recorded in single, dissociated mouse beta-cells kept in culture for 1-3 days. [Ca2+]i was estimated from microfluorometric ratio methods using Indo-1. Channel activity was measured using the cell-attached configuration of the patch-clamp technique. 2. At low glucose concentrations (0.3 mM), resting K+ATP channel activity was prevalent. Increasing glucose up to 16 mM, produced a gradual decrease in K+ATP channel activity over a time course of 90-120 s (temperature = 23 degrees C) and an increase in [Ca2+]i. 3. In the majority of experiments, glucose elicited biphasic action currents (action potentials) which preceded the rise in [Ca2+]i. There was a close correlation between spike frequency and the levels of [Ca2+]i. 4. The sulphonylurea tolbutamide (1 mM) blocked K+ATP channels in 10-20 s. K+ATP channel blockade was associated with a quick rise in [Ca2+]i. 5. When K+ATP channel activity was stimulated in the presence of diazoxide (100 microM), increasing the glucose concentration from 3 to 16 mM produced a decrease in [Ca2+]i. Only when diazoxide was removed did glucose produce an increase in [Ca2+]i. 6. In a small population of cells, glucose (16 mM) produced a small decrease in K+ATP channel activity but not an increase in [Ca2+]i. In such cells, tolbutamide blocked K+ATP channels and produced an increase in [Ca2+]i. 7. These results demonstrate a close correlation between K+ATP channel activity and [Ca2+]i in beta-cells. The findings are consistent with the model in which glucose metabolism produces a rise in [Ca2+]i through the blockade of K+ATP channels, membrane depolarization and calcium current activation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
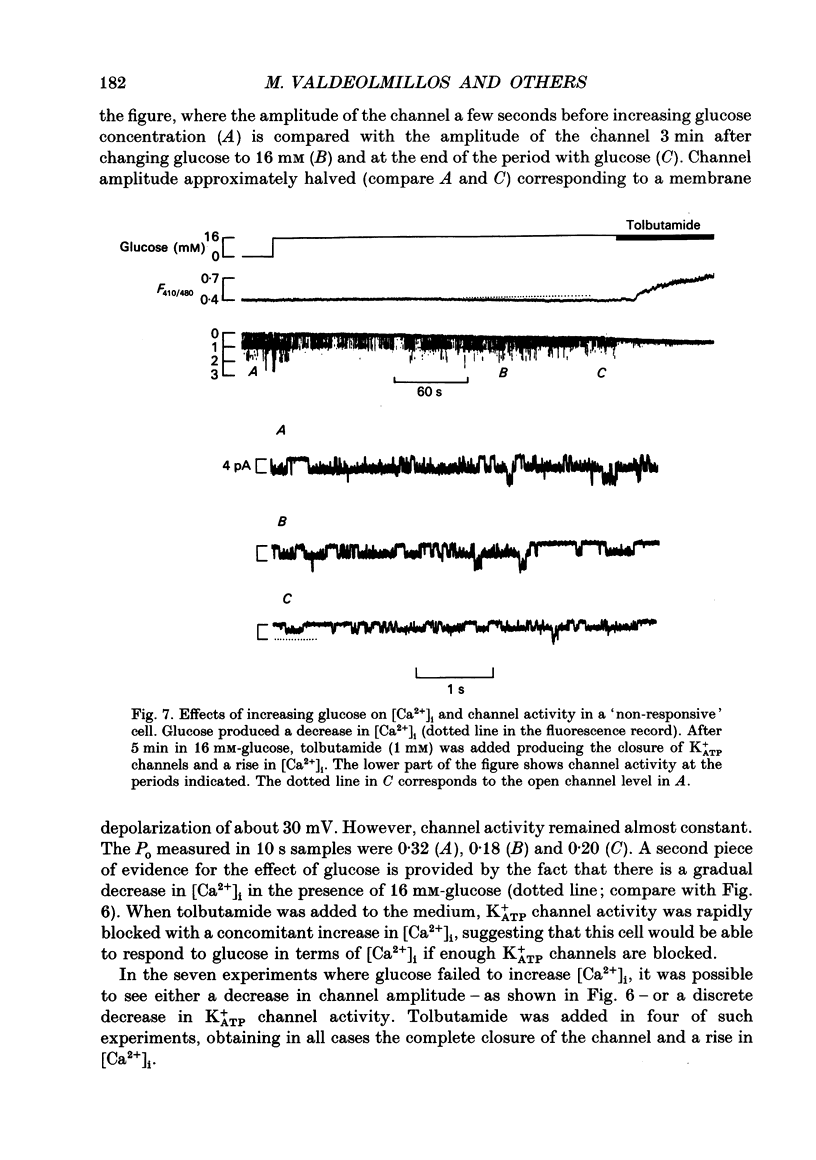
- Ashcroft F. M., Ashcroft S. J., Harrison D. E. Properties of single potassium channels modulated by glucose in rat pancreatic beta-cells. J Physiol. 1988 Jun;400:501–527. doi: 10.1113/jphysiol.1988.sp017134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Atwater I., Ribalet B., Rojas E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J Physiol. 1978 May;278:117–139. doi: 10.1113/jphysiol.1978.sp012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Prentki M., Irvine R. F., Berridge M. J., Wollheim C. B. Inositol 1,4,5-trisphosphate mobilizes intracellular Ca2+ from permeabilized insulin-secreting cells. Biochem J. 1984 Oct 15;223(2):467–473. doi: 10.1042/bj2230467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells. Nature. 1968 Jul 27;219(5152):389–390. doi: 10.1038/219389a0. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Illot M. C., Peterson O. H. Interaction of diazoxide, tolbutamide and ATP4- on nucleotide-dependent K+ channels in an insulin-secreting cell line. J Membr Biol. 1987;99(3):215–224. doi: 10.1007/BF01995702. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser R. N., Vaughan-Jones R. D. Mechanism of potassium efflux and action potential shortening during ischaemia in isolated mammalian cardiac muscle. J Physiol. 1990 Dec;431:713–741. doi: 10.1113/jphysiol.1990.sp018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapengiesser E., Gylfe E., Hellman B. Dual effect of glucose on cytoplasmic Ca2+ in single pancreatic beta-cells. Biochem Biophys Res Commun. 1988 Jan 15;150(1):419–425. doi: 10.1016/0006-291x(88)90537-2. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gylfe E. Glucose-induced early changes in cytoplasmic calcium of pancreatic beta-cells studied with time-sharing dual-wavelength fluorometry. J Biol Chem. 1988 Apr 15;263(11):5044–5048. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hellman B., Gylfe E., Grapengiesser E., Panten U., Schwanstecher C., Heipel C. Glucose induces temperature-dependent oscillations of cytoplasmic Ca2+ in single pancreatic beta-cells related to their electrical activity. Cell Calcium. 1990 Jun-Jul;11(6):413–418. doi: 10.1016/0143-4160(90)90053-w. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G., Smith G. L. The mechanism of early contractile failure of isolated rat ventricular myocytes subjected to complete metabolic inhibition. J Physiol. 1989 Jun;413:329–349. doi: 10.1113/jphysiol.1989.sp017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974 Oct;10(5):431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- Meda P., Kohen E., Kohen C., Rabinovitch A., Orci L. Direct communication of homologous and heterologous endocrine islet cells in culture. J Cell Biol. 1982 Jan;92(1):221–226. doi: 10.1083/jcb.92.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner H. P. Electrophysiological evidence for coupling between beta cells of pancreatic islets. Nature. 1976 Aug 5;262(5568):502–504. doi: 10.1038/262502a0. [DOI] [PubMed] [Google Scholar]
- Mourre C., Ben Ari Y., Bernardi H., Fosset M., Lazdunski M. Antidiabetic sulfonylureas: localization of binding sites in the brain and effects on the hyperpolarization induced by anoxia in hippocampal slices. Brain Res. 1989 May 1;486(1):159–164. doi: 10.1016/0006-8993(89)91288-2. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. D. Properties and calcium-dependent inactivation of calcium currents in cultured mouse pancreatic B-cells. J Physiol. 1988 Oct;404:731–747. doi: 10.1113/jphysiol.1988.sp017316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong W. F., Bartley C., Wollheim C. B. Single islet beta-cell stimulation by nutrients: relationship between pyridine nucleotides, cytosolic Ca2+ and secretion. EMBO J. 1990 Jan;9(1):53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Pérez-Armendariz M., Roy C., Spray D. C., Bennett M. V. Biophysical properties of gap junctions between freshly dispersed pairs of mouse pancreatic beta cells. Biophys J. 1991 Jan;59(1):76–92. doi: 10.1016/S0006-3495(91)82200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D., Meda P. Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp Cell Res. 1986 Feb;162(2):507–520. doi: 10.1016/0014-4827(86)90354-x. [DOI] [PubMed] [Google Scholar]
- Santos R. M., Rosario L. M., Nadal A., Garcia-Sancho J., Soria B., Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflugers Arch. 1991 May;418(4):417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Ashcroft F. M., Rorsman P. Simultaneous recordings of glucose dependent electrical activity and ATP-regulated K(+)-currents in isolated mouse pancreatic beta-cells. FEBS Lett. 1990 Feb 12;261(1):187–190. doi: 10.1016/0014-5793(90)80667-8. [DOI] [PubMed] [Google Scholar]
- Soria B., Chanson M., Giordano E., Bosco D., Meda P. Ion channels of glucose-responsive and -unresponsive beta-cells. Diabetes. 1991 Aug;40(8):1069–1078. doi: 10.2337/diab.40.8.1069. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Kozlowski R. Z., Carrington C. A., Hales C. N., Ashford M. L. Effects of sulphonylureas and diazoxide on insulin secretion and nucleotide-sensitive channels in an insulin-secreting cell line. Br J Pharmacol. 1988 Sep;95(1):83–94. doi: 10.1111/j.1476-5381.1988.tb16551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Turk J., Wolf B. A., McDaniel M. L. Glucose-induced accumulation of inositol trisphosphates in isolated pancreatic islets. Predominance of the 1,3,4-isomer. Biochem J. 1986 Jul 1;237(1):259–263. doi: 10.1042/bj2370259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdeolmillos M., Santos R. M., Contreras D., Soria B., Rosario L. M. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of Langerhans. FEBS Lett. 1989 Dec 18;259(1):19–23. doi: 10.1016/0014-5793(89)81484-x. [DOI] [PubMed] [Google Scholar]


