Abstract
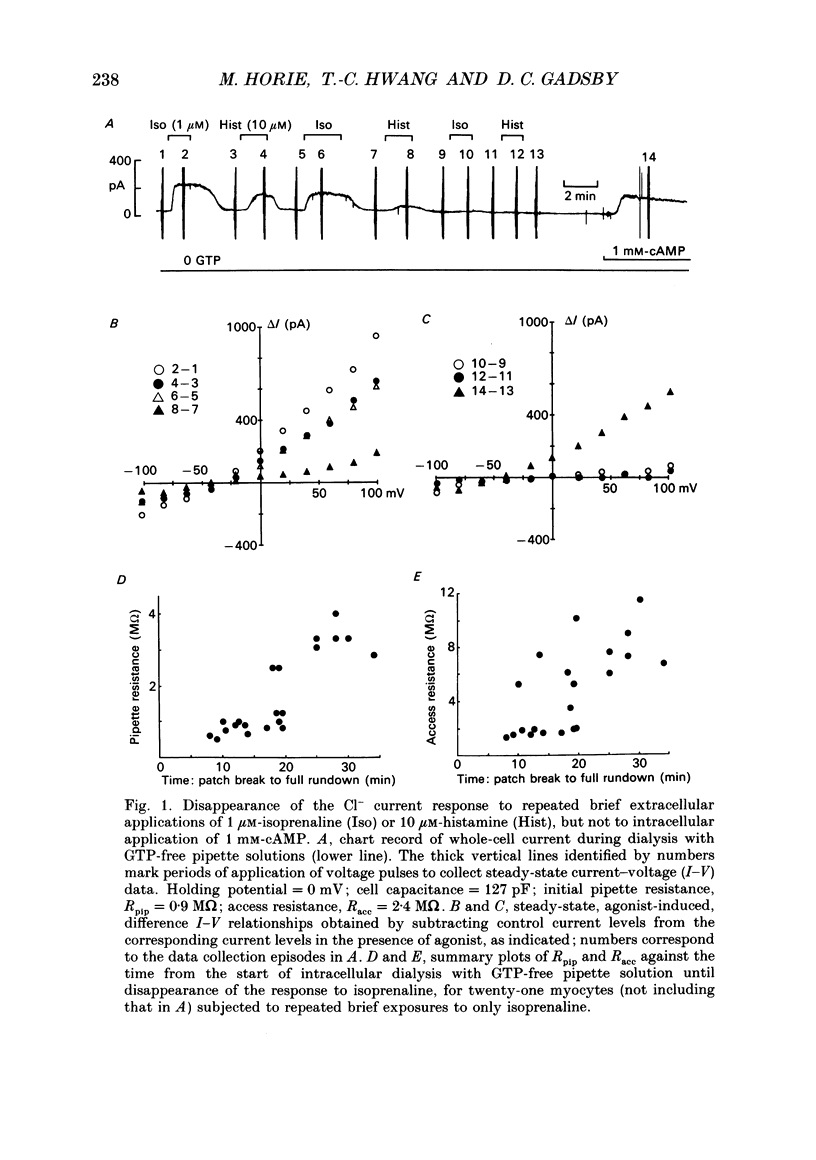
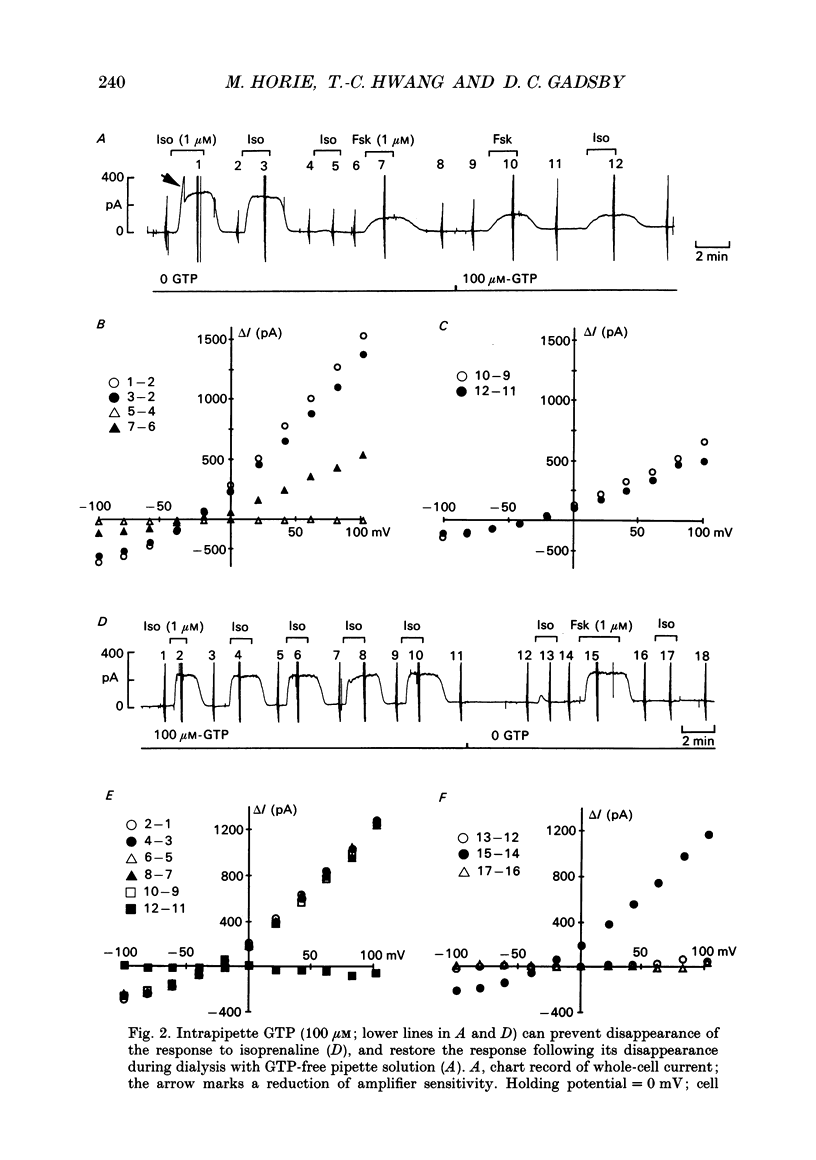
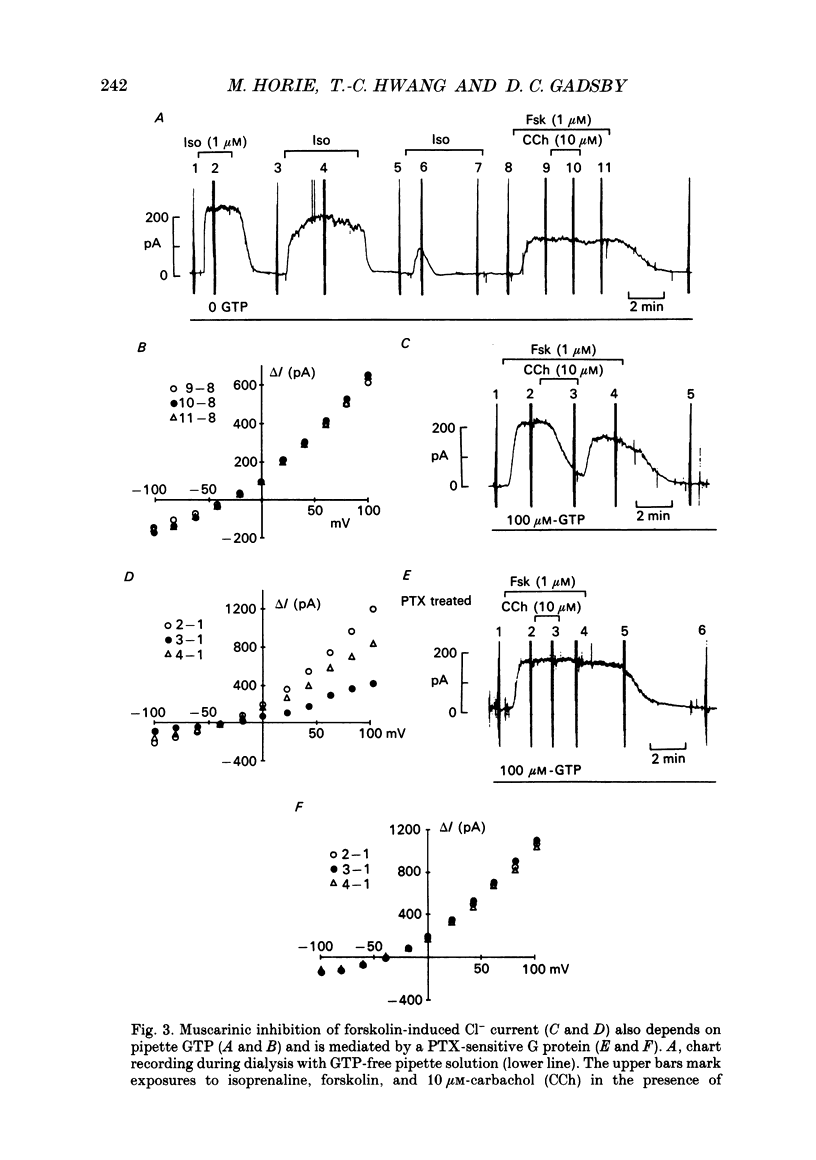
1. Wide-tipped, low-resistance (approximately 1 M omega) pipettes were used to record the whole-cell Cl- current activated by cAMP-dependent protein kinase (PKA) in guinea-pig ventricular myocytes internally dialysed with or without GTP. Without GTP in the pipette, the response to 1 microM-isoprenaline declined with time and eventually disappeared, usually within approximately 20 min of rupturing the membrane and beginning cell dialysis. 2. This rundown of the isoprenaline response occurred more quickly with wider, lower-resistance pipette tips. 3. After complete rundown of the isoprenaline response, histamine (10 microM), another agonist known to elicit the Cl- current, also had no effect, but extracellular forskolin (1 microM) or intrapipette cAMP (1 mM) could still readily elicit the Cl- current. 4. In contrast, with 100 microM-GTP in the pipette, the response to 1 microM-isoprenaline was well maintained for periods greater than 20 min. But, if GTP was then withdrawn from the pipette, a rundown of the isoprenaline response was seen comparable to that in the experiments begun with GTP-free pipette solution. Moreover, in experiments begun without pipette GTP, the addition of 100 microM-GTP to the pipette solution, after the response to isoprenaline had disappeared, was able to restore that Cl- current response. 5. With GTP in the pipette, the forskolin-induced Cl- current could be suppressed by concurrent exposure to carbachol (10 microM). That inhibition was not seen in myocytes pretreated with pertussis toxin. In untreated myocytes dialysed with GTP-free pipette solution, after disappearance of the isoprenaline response, the muscarinic receptor-mediated inhibition was itself abolished. 6. We confirm that both beta-adrenoceptor-mediated activation of the Cl- current by isoprenaline, and muscarinic receptor-mediated inhibition of the forskolin-induced Cl- current, are mediated by G proteins, and conclude that the disappearance of both receptor-mediated responses during whole-cell recording with GTP-free pipette solution reflects the fall of cellular [GTP] below the level required to maintain G protein-dependent signal transduction.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Bielen F. V., Glitsch H. G., Verdonck F. Changes of the subsarcolemmal Na+ concentration in internally perfused cardiac cells. Biochim Biophys Acta. 1991 Jun 18;1065(2):269–271. doi: 10.1016/0005-2736(91)90239-5. [DOI] [PubMed] [Google Scholar]
- Duchatelle-Gourdon I., Lagrutta A. A., Hartzell H. C. Effects of Mg2+ on basal and beta-adrenergic-stimulated delayed rectifier potassium current in frog atrial myocytes. J Physiol. 1991 Apr;435:333–347. doi: 10.1113/jphysiol.1991.sp018513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W. An isoprenaline activated sodium-dependent inward current in ventricular myocytes. Nature. 1987 Aug 13;328(6131):634–637. doi: 10.1038/328634a0. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W., Yamaoka K. On the mechanism of isoprenaline- and forskolin-induced depolarization of single guinea-pig ventricular myocytes. J Physiol. 1988 Jun;400:299–320. doi: 10.1113/jphysiol.1988.sp017121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Kimura J., Noma A. Voltage dependence of Na/K pump current in isolated heart cells. Nature. 1985 May 2;315(6014):63–65. doi: 10.1038/315063a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Jurevicius J. A., Hume J. R. Intracellular Na+ modulates the cAMP-dependent regulation of ion channels in the heart. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6946–6950. doi: 10.1073/pnas.88.16.6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T. C., Horie M., Nairn A. C., Gadsby D. C. Role of GTP-binding proteins in the regulation of mammalian cardiac chloride conductance. J Gen Physiol. 1992 Apr;99(4):465–489. doi: 10.1085/jgp.99.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. Islet-activating protein. A modifier of receptor-mediated regulation of rat islet adenylate cyclase. J Biol Chem. 1981 Aug 25;256(16):8310–8317. [PubMed] [Google Scholar]
- Kimura J., Noma A., Irisawa H. Na-Ca exchange current in mammalian heart cells. Nature. 1986 Feb 13;319(6054):596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- Lucero M. T., Pappone P. A. Membrane responses to norepinephrine in cultured brown fat cells. J Gen Physiol. 1990 Mar;95(3):523–544. doi: 10.1085/jgp.95.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Ehara T., Noma A. Chloride-sensitive nature of the adrenaline-induced current in guinea-pig cardiac myocytes. J Physiol. 1990 Jun;425:579–598. doi: 10.1113/jphysiol.1990.sp018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Wu S., Irisawa H., Giles W. Mechanism of acetylcholine-induced inhibition of Ca current in bullfrog atrial myocytes. J Gen Physiol. 1990 Oct;96(4):865–885. doi: 10.1085/jgp.96.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):539–565. doi: 10.1085/jgp.94.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki K., Yokoyama M. Direct activation of guanine nucleotide binding proteins through a high-energy phosphate-transfer by nucleoside diphosphate-kinase. Biochem Biophys Res Commun. 1987 Oct 14;148(1):300–307. doi: 10.1016/0006-291x(87)91110-7. [DOI] [PubMed] [Google Scholar]
- Oliva C., Cohen I. S., Mathias R. T. Calculation of time constants for intracellular diffusion in whole cell patch clamp configuration. Biophys J. 1988 Nov;54(5):791–799. doi: 10.1016/S0006-3495(88)83017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Tareen F. M., Ono K., Noma A., Ehara T. Beta-adrenergic and muscarinic regulation of the chloride current in guinea-pig ventricular cells. J Physiol. 1991;440:225–241. doi: 10.1113/jphysiol.1991.sp018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. B., Begenisich T. B., Kass R. S. Beta-adrenergic modulation of cardiac ion channels. Differential temperature sensitivity of potassium and calcium currents. J Gen Physiol. 1989 May;93(5):841–854. doi: 10.1085/jgp.93.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990 Feb;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]


