Abstract
Ischemic stroke (IS) is a complex neurological disorder characterized by the sudden disruption of blood flow to the brain, leading to severe and often irreversible damage. Despite advances in stroke management, the underlying molecular mechanisms and key factors involved in the development and progression of IS remain elusive. In recent years, the integration of high-throughput data analysis techniques has emerged as a powerful approach to unraveling the molecular intricacies of complex diseases. In this study, we comprehensively analyzed gene expression, protein–protein interactions (PPI), and gene regulatory networks to identify IS-associated molecular factors. We utilized publicly available datasets and employed bioinformatics tools to analyze the data. Our analysis revealed many differentially expressed genes (DEGs) in IS, with a predominant down-regulation of genes. Gene ontology (GO) analysis highlighted the involvement of various biological processes, including transcriptional regulation, cell cycle, immune system processes, and cell differentiation. These findings underscore the complexity of stroke pathology, involving dysregulated gene expression and disrupted cellular processes. Constructing PPI networks enabled us to identify specific subnetworks associated with critical biological processes relevant to stroke, such as nucleosome assembly, protein translation, glycosylation, protein folding, and mRNA splicing. These subnetworks provide insights into the dysregulated molecular mechanisms contributing to stroke progression. Furthermore, we focused on identifying differentially expressed transcription factors (DE-TFs) within the gene regulatory network. Several up-regulated DE-TFs, including E2F1, MYB, GFI1B, and NUCKS1, were identified, suggesting their potential involvement in the dysregulation of gene expression in IS.
Keywords: Ischemic stroke, Gene expression, Protein–protein interactions, Gene regulatory networks, Transcription factors
Introduction
Ischemic stroke (IS) is a devastating cerebrovascular disorder characterized by the sudden interruption of blood flow to the brain, leading to neuronal damage, functional impairment, and significant morbidity and mortality worldwide [1, 2]. Despite substantial advancements in stroke management and acute interventions, IS remains a major global health concern, necessitating a deeper understanding of its underlying molecular mechanisms to develop effective therapeutic strategies [3]. The pathogenesis of IS is multifactorial and involves intricate interactions between genetic, environmental, and lifestyle factors. Identifying and characterizing molecular factors associated with IS can provide crucial insights into the disease’s complex etiology, potentially leading to the discovery of novel therapeutic targets and the development of personalized treatment approaches [4, 5]. Ischemic stroke is a complex disease that multiple potential etiologies can cause. Advances in diagnostic technology have allowed us to identify the potential underlying causes of stroke in stroke patients. The fundamental goals of ischemic stroke classification are to make a correct diagnosis, enable prompt treatment, and predict future risks in subgroups of certain discrete features. There are two major approaches to etiologic subclassification of ischemic stroke. The causative approach is based on the underlying pathophysiology of the stroke, while the phenotypic approach is based on the clinical presentation of the stroke. The Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification is a widely used phenotypic classification system that denotes five subtypes of ischemic stroke: large-artery atherosclerosis, cardioembolism, small-vessel occlusion, stroke of other determined etiology, and stroke of undetermined etiology [6, 7]. In recent years, high-throughput technologies such as microarray and next-generation sequencing have revolutionized the field of genomics, enabling the comprehensive analysis of gene expression patterns across different biological conditions [8, 9]. By comparing the transcriptomic profiles of healthy individuals and patients with IS, researchers have sought to identify differentially expressed genes (DEGs) that may serve as potential biomarkers or key players in IS pathology [10, 11]. While identifying DEGs is a significant step toward understanding the molecular basis of IS, it is equally important to decipher the regulatory mechanisms that control their expression [12–14]. Transcription factors (TFs) play a central role in regulating gene expression by binding to specific DNA sequences in gene promoters and activating or repressing transcription [15]. Investigating the TFs that potentially govern the dysregulated gene expression in IS can provide valuable insights into the underlying regulatory networks driving the disease phenotype [16, 17]. Furthermore, it is increasingly recognized that proteins rarely function in isolation but rather interact with each other within complex networks to carry out diverse cellular processes. Protein–protein interactions (PPIs) are critical for maintaining cellular homeostasis and orchestrating complex molecular pathways. Analyzing the PPI networks of the identified DEGs can unravel key hub proteins and pathways that are dysregulated in IS, thereby providing a holistic view of the molecular landscape of the disease [18].
In this study, we aimed to comprehensively analyze and integrate multiple data layers, including DEGs, TFs, and PPI networks, to elucidate the molecular factors associated with IS. We employed a well-defined cohort of control individuals without any history of stroke and patients diagnosed with IS as the treatment group. We sought to identify and predict potential molecular factors underlying IS pathogenesis using cutting-edge bioinformatics tools and machine learning algorithms. This research aims to advance our understanding of the molecular mechanisms driving IS and identify novel targets for therapeutic intervention. By elucidating the molecular landscape of IS, we aim to contribute to developing precision medicine approaches that can improve patient outcomes and reduce the burden of this debilitating neurological condition.
Material and methods
Data preparation and gene expression analysis
To initiate this study, we utilized three datasets with the GEO accession number GSE22255. The treatment group comprised ischemic patients, while the control group comprised healthy individuals. The GEO2R web tool (http://www.ncbi.nlm.nih.gov/geo/geo2r/) was employed to extract and analyze the relevant information from the datasets. Differential expression analysis was performed using the limma package in the R software. Subsequently, differentially expressed genes (DEGs) were generated, removing those with a p-value greater than 0.05 and a log2 fold change (FC) outside the range of ± 0.6. Duplicated DEGs were then eliminated, resulting in final gene lists for subsequent analyses.
Protein–protein interaction networks analysis
Protein–protein interaction (PPI) networks were constructed to identify the proteins encoded by the DEGs. The String database was utilized for this purpose, with the list of DEGs inputted to obtain the PPI network [19]. Cytoscape 3.7.2 [20] and Gephi software v 0.10.1 [21] were utilized for network visualization. Hub genes were identified as the top 10 percent of nodes with the highest degree. Sub-network detection was performed using the ClusterONE 1.0 plugin [22] within Cytoscape, with the following criteria: minimum size (5), density (0.6), and a p-value threshold (< 0.01). Furthermore, common proteins among the three datasets were determined, and the aforementioned steps, except for sub-network detection, were repeated to construct a common PPI network for all datasets.
Transcription factors (TFs) analysis in the list of DEGs
We explored the presence of TFs within the DEG lists and investigated the relationships between genes and TFs to construct a gene regulatory network. The ChEA database [23] was employed to identify TFs within the DEG lists, which contain information on protein-DNA interactions obtained through ChIP-X tests. Only TFs with a p-value less than 0.05 were selected, resulting in differentially expressed TFs (DE-TFs). Based on the pairs of TF-target interactions, networks were constructed, followed by centrality analysis.
Gene ontology
The online DAVID program [24] was used to perform gene ontology (GO) analysis, specifically focusing on biological processes (BP). The analysis considered the top 10 terms with the highest number of members and a p-value below 0.05. By following these methods, we aimed to comprehensively analyze the gene expression profiles, identify protein interactions, investigate TF-gene interactions, and explore the functional annotations of the DEGs. These analyses collectively provide insights into the molecular factors and regulatory networks associated with ischemic stroke.
Results
Gene expression and common DEGs
This study comprehensively analyzed differentially expressed genes (DEGs) to identify genes involved in stroke progression. Our analysis revealed that out of the total DEGs, 377 genes exhibited increased expression, while 622 genes showed decreased expression. Notably, a significant majority of the DEGs were found to be down-regulated genes.
Gene ontology
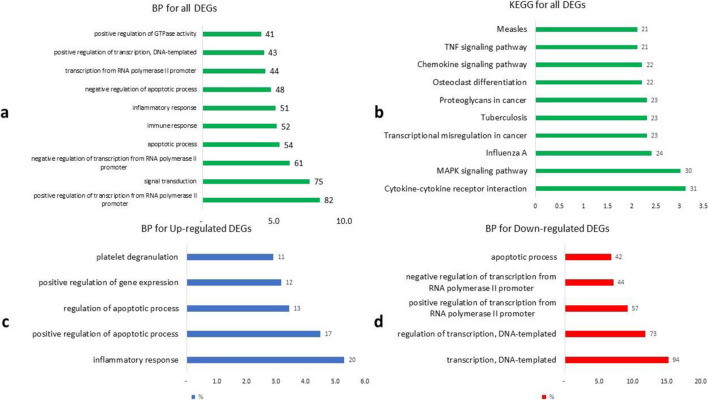
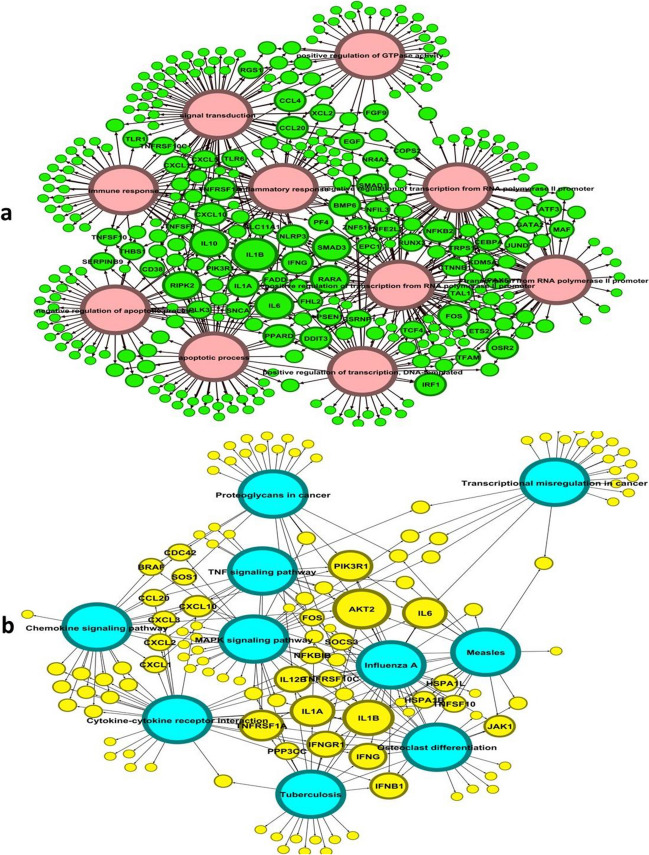
To gain insights into the functional implications of the identified DEGs, we performed gene ontology (GO) analysis. GO analysis allows us to understand how genes are connected to specific biological systems, body parts, or functions. The results of the GO analysis revealed significant changes in various pathways associated with stroke progression. Among the pathways affected by the DEGs, several key processes stood out. These included the inflammatory response, positive regulation of the apoptotic process, transcription, DNA-templated, and regulation of transcription, DNA-templated, among others. These pathways were among the top five pathways identified in the analysis, as illustrated by the bar chart (Fig. 1). These findings highlight the potential involvement of these biological processes in the development and progression of stroke. Also, the common genes involved in the biological process and KEGG are shown in Fig. 2.
Fig. 1.
Gene ontology of the DEGs. The leading biological processes (BP) and KEGG related to a the top ten of BP, b the top ten of KEGG, c the top five of up-regulated DEGs BP, and d the top five of down-regulated DEGs BP are displayed. The number below the diagram indicates the number of genes involved in the process as a percentage of the total number of genes, and the number in front of the column indicates the number of genes involved in the process
Fig. 2.
Gene ontology (GO) and DEGs. a Common genes among different biological processes. b Common genes among different KEGG
Protein–protein interaction network analysis
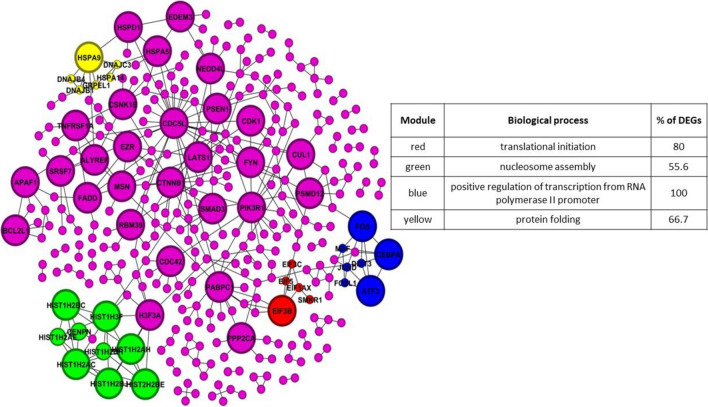
Protein–protein interaction (PPI) networks provide valuable insights into how proteins encoded by genes interact with each other, both physically and functionally. We constructed PPI networks for each cell type to explore the protein interactions associated with stroke using the information obtained from the STRING database. The PPI networks allowed us to identify complex subnetworks and highly interconnected core proteins that play crucial roles in the cellular processes underlying stroke. Our analysis focused on the IS network, which comprised 328 nodes representing proteins and 404 edges representing their interactions (Fig. 3). Furthermore, through network-based analysis, we identified five IS network subnetworks involved in specific biological processes. These subnetworks were associated with positive regulation of transcription from RNA polymerase II promoter, nucleosome assembly, protein folding, and translational initiation. Each subnetwork represented a distinct set of proteins and their interconnections, contributing to understanding the molecular mechanisms underlying stroke (Fig. 3). We gained insights into the underlying biological processes and pathways dysregulated in stroke by examining these subnetworks and their associated gene ontology information. These findings provide a deeper understanding of the molecular interactions and functional implications of the identified proteins within the PPI networks, shedding light on potential targets and pathways for further investigation.
Fig. 3.
Displays protein–protein interaction (PPI) networks with module annotations. The modules, identified through overlapping neighborhood expansion, are represented by colored nodes. The corresponding annotations for the modules are provided in the tables, with modules having p-values less than 0.05 being selected. In the network, modules are indicated by the color of the nodes. Larger-sized nodes indicate a higher degree of connectivity
Transcription factor analysis
To uncover the regulatory relationships between transcription factors (TFs) and the differentially expressed genes (DEGs) within the IS network, we constructed a gene regulatory network (Fig. 4a). The gene regulatory network provides insights into the transcriptional control mechanisms underlying stroke. Several key TFs were identified within the IS network as differentially expressed (DE-TFs). These DE-TFs included GATA2, KLF6, SMAD3, PPARD, CTNNB1, ATF3, JUND, MAF, and NFE2L2. Notably, just GATA2 of these DE-TFs exhibited up-regulation in the context of stroke (Fig. 4b). This suggests that these TFs may play crucial roles in the dysregulation of gene expression and stroke progression. By analyzing the interactions and regulatory connections between these DE-TFs and their target genes within the gene regulatory network, we can better understand the molecular mechanisms by which these TFs contribute to stroke pathology. This information provides valuable insights into the transcriptional regulatory landscape of stroke and highlights potential targets for further investigation and therapeutic intervention.
Fig. 4.
Gene regulatory networks. The node colors in red and blue are used to indicate up- and down-regulation, respectively. a IS TF network. b IS DE-TF network. TFs are transcription factors
Discussion
Ischemic stroke occurs when the blood supply to part of the brain is interrupted or reduced, preventing brain tissue from getting oxygen and nutrients [25]. This can lead to brain cells dying within minutes and can cause severe disability or death [26]. Stroke remains a significant global health concern due to its devastating impact on individuals and society [3]. Lacunar stroke is a subtype of ischemic stroke that accounts for up to 25% of all ischemic strokes. It is caused by the occlusion of a single small perforating artery supplying the subcortical areas of the brain. The pathophysiology, prognosis, and clinical features of lacunar strokes are different from other acute cerebrovascular diseases. Hypertension and diabetes are said to be strongly associated with lacunar ischaemic stroke. However, in studies using risk factor-free ischaemic stroke subtype definitions, there was only a marginal excess of hypertension with lacunar versus non-lacunar infarction and no difference for diabetes. The mechanisms of acute stroke are complex and involve excitotoxicity mechanisms, inflammatory pathways, oxidative damage, ionic imbalances, apoptosis, and more. Understanding the detailed mechanisms underlying acute stroke, mainly in its different subtypes, may lead to the development of effective therapeutic strategies for preventing neuronal death and improving functional recovery from neuronal injury. Regarding the relationship and relevance of identification and prediction of molecular factors associated with lacunar versus non-lacunar ischemic stroke, a study published in the International Journal of Molecular Sciences suggests that lacunar infarcts are small infarcts in the deep cerebral white matter, basal ganglia, or pons, presumed to result from the occlusion of a single small perforating artery supplying the subcortical areas of the brain. The study also suggests that lacunar stroke is a heterogeneous disease with various mechanisms, including most commonly lipohyalinosis and less commonly atheromatous disease and cardioembolism, highlighting the importance of a careful review of brain and neurovascular imaging, a cardiac and systemic evaluation [27, 28]. This study aimed to gain insights into the molecular factors associated with ischemic stroke (IS) by analyzing gene expression patterns, protein–protein interactions (PPI), and gene regulatory networks. Our comprehensive analysis uncovered several key findings that contribute to our understanding of the molecular mechanisms underlying IS and provide potential avenues for therapeutic interventions.
One of the primary outcomes of our study was the identification of differentially expressed genes (DEGs) associated with IS. The analysis revealed a substantial number of DEGs, with a majority exhibiting down-regulation. This observation is consistent with previous studies suggesting that IS is characterized by widespread gene expression changes, particularly down-regulation of genes involved in critical cellular processes. The dysregulation of these genes may contribute to neuronal damage, impaired cellular function, and the progression of stroke pathology. Gene ontology (GO) analysis further elucidated the biological processes and pathways affected by the DEGs. Our analysis highlighted the involvement of various pathways, including the regulation of transcription, cell cycle, immune system processes, and cell differentiation. These findings align with the complex nature of stroke pathology, which encompasses not only neuronal damage but also inflammatory responses, altered cellular proliferation, and disrupted homeostasis. The dysregulation of these biological processes likely contributes to the pathogenesis and progression of stroke. Constructing protein–protein interaction (PPI) networks allowed us to explore the interactions and relationships among the proteins encoded by the DEGs. Through subnetwork analysis, we identified distinct protein modules involved in critical biological processes relevant to stroke. These modules included nucleosome assembly, tRNA aminoacylation for protein translation, protein N-linked glycosylation via asparagine, protein folding, and mRNA splicing via spliceosome. The dysregulation of these processes may disrupt cellular homeostasis, protein synthesis, and post-translational modifications, further contributing to stroke development.
Additionally, our study focused on identifying differentially expressed transcription factors (DE-TFs) within the gene regulatory network. We identified several up-regulated DE-TFs, including E2F1, MYB, GFI1B, and NUCKS1. These DE-TFs have been implicated in various cellular processes and have the potential to drive the dysregulated gene expression observed in IS. Understanding the regulatory roles of these DE-TFs and their interactions with target genes provides valuable insights into the transcriptional control mechanisms underlying stroke. Our study comprehensively analyzes the molecular factors associated with IS, highlighting the dysregulation of gene expression, protein–protein interactions, and gene regulatory networks. These findings contribute to our understanding of the complex pathogenesis of stroke and provide potential targets for future research and therapeutic interventions. By elucidating the molecular landscape of IS, we aim to pave the way for precision medicine approaches that can improve patient outcomes and reduce the burden of this debilitating neurological condition.
GATA2 is a zinc finger transcription factor that regulates endothelial nitric oxide synthase (eNOS) and has been associated with early-onset ischemic stroke in patients with GATA2 deficiency [29]. GATA2 is expressed in vascular tissue and is important in regulating hematopoiesis and inflammation [30]. KLF6 is a member of the zinc finger family of transcription factors and has been implicated in various processes, including organismal development, cell differentiation, function, and apoptosis. KLF6 has been shown to play a role in inflammatory and hypoxic responses and has been associated with oxidative stress and neurological dysfunction following intracerebral hemorrhage [31]. SMAD3 is a key component of the TGF-β1/Smad3 signaling pathway, which has been shown to exert a protective effect on ischemic stroke by reducing apoptosis rates and inflammation in the ischemic region. Overexpression of SMAD3 has been shown to ameliorate cell apoptosis and brain injury after cerebral ischemia/reperfusion [32]. PPARD is a gene encoding peroxisome proliferator-activated receptor delta, which has been associated with ischemic stroke risk in some studies [33]. A meta-analysis has suggested associations between PPAR-γ gene polymorphisms and ischemic stroke development [34]. CTNNB1 is involved in the regulation of cell adhesion and gene transcription. A study found that a circular RNA derived from CTNNB1 (circCTNNB1) ameliorates cerebral ischemia/reperfusion injury by sponging miR-96-5p to up-regulate scavenger receptor class B type 1 (SRB1) expression [35]. ATF3 is a transcription factor that plays a role in cellular stress responses. A study found that the knockdown of ATF3 suppresses the progression of ischemic stroke by inhibiting ferroptosis, a regulated form of cell death involving iron and lipid peroxidation [36]. JUND is a member of the AP-1 transcription factor family. A study found that JunD regulates ischemia/reperfusion brain damage via IL-1β (Interleukin-1β). In vivo, JunD knockdown increased stroke size, reduced neurological function, and increased systemic inflammation [37]. MAF is a transcription factor involved in the regulation of various cellular processes. While there is no direct evidence linking MAF to ischemic stroke in the provided search results, it may still play a role in the complex pathophysiology of ischemic stroke or be involved in related processes, such as inflammation, oxidative stress, and cell survival. NFE2L2, also known as Nrf2, is a transcription factor that regulates the expression of antioxidant proteins. A study found that Nrf2 plays a critical role in experimental ischemic stroke by upregulating antioxidants and inhibiting inflammatory pathways [38].
Our study has contributed significant insights into the molecular landscape of ischemic stroke. There are several promising directions for further research in this field. First, focusing on subtype-specific investigations could unveil the molecular distinctions among different ischemic stroke subtypes, including atherothrombotic, lacunar, cardioembolic, and others, leading to more tailored treatment strategies. Second, exploring the temporal dynamics of stroke is crucial, as it is a dynamic process, and understanding how molecular changes evolve could guide more precise interventions. Additionally, the functional validation of the identified molecular factors through in vitro and in vivo studies is an important step in confirming their roles in stroke pathogenesis. Our findings also pave the way for developing personalized therapies based on individual molecular profiles, marking an exciting avenue for personalized medicine. Integrating a broader range of omics data, including epigenomics, proteomics, and metabolomics, could provide a more holistic understanding of the molecular landscape in ischemic stroke. Leveraging machine learning techniques for predictive modeling and patient stratification is another promising area for further investigation. Moreover, our study opens up possibilities for developing novel therapeutic interventions while emphasizing the importance of translating these molecular findings into clinical practice to improve patient care and outcomes ultimately. These potential research directions collectively hold the promise of advancing our understanding of ischemic stroke and enhancing the quality of care for those affected by this condition.
Our study provides valuable insights into the molecular factors associated with ischemic stroke by integrating gene expression analysis, protein–protein interactions, and gene regulatory networks. The identified DEGs, affected biological processes, and dysregulated transcription factors contribute to our understanding of the pathogenesis of stroke and offer potential targets for further research and therapeutic interventions. Continued efforts in this field hold promise for developing personalized approaches for stroke management and improved patient outcomes.
Conclusion
Our study provides valuable insights into the molecular factors associated with ischemic stroke (IS) through a comprehensive analysis of gene expression, protein–protein interactions (PPI), and gene regulatory networks. By integrating multiple omics data and network-based approaches, we have uncovered key findings that contribute to our understanding of the molecular mechanisms underlying IS and highlight potential avenues for therapeutic interventions. The findings from our study contribute to the growing body of knowledge on the molecular landscape of ischemic stroke and provide insights into potential therapeutic targets. However, it is important to acknowledge the limitations of our study. The results are based on the analysis of publicly available datasets, and further validation in independent cohorts is necessary. Additionally, the complexity of stroke pathology warrants additional research to fully elucidate the underlying molecular mechanisms. We aim to contribute to developing precision medicine approaches that can improve patient outcomes and alleviate the burden of this debilitating neurological condition. Continued efforts in this field will facilitate the translation of these findings into clinical applications, ultimately benefiting individuals affected by ischemic stroke.
Limitation
In our study, we have strived to analyze molecular factors associated with ischemic stroke comprehensively. However, it is important to acknowledge the limitations of our research to provide a balanced perspective on our findings. Several limitations should be considered:
1. Data sources: Our study relies on publicly available datasets, and the quality and comprehensiveness of these datasets are crucial factors. While we have carefully selected datasets with appropriate controls and relevance to our research question, variations in data quality or missing data could impact the results.
2. Sample size: While substantial, the study’s sample size is still limited compared to the complexity of ischemic stroke. This limitation may affect the generalizability of our findings to broader populations.
3. Data integration: Integrating multiple omics data types is a powerful approach, but it also presents challenges, including differences in data formats, experimental methodologies, and normalization procedures. These differences can introduce variability and potential biases in our analysis.
4. Bioinformatics analysis: The bioinformatics tools and algorithms used in this study are based on specific assumptions and parameters. The choice of these parameters can influence the results, and while we have followed best practices, there is a degree of subjectivity in parameter selection.
5. Functional validation: Our study primarily focuses on bioinformatics and computational analyses. While this provides valuable insights, functional validation of the identified molecular factors in experimental models is essential to confirm their roles in ischemic stroke pathogenesis.
6. Clinical heterogeneity: Ischemic stroke is a heterogeneous condition with various etiologies and clinical presentations. Our study did not specifically differentiate between stroke subtypes, and their molecular mechanisms may differ. Future research should investigate the distinctions among subtypes, such as atherothrombotic, lacunar, and cardioembolic strokes.
7. Temporal aspects: Our analysis provides a snapshot of the molecular landscape at a specific time. Stroke is a dynamic process, and the molecular changes may evolve. Longitudinal studies are needed to capture the temporal dynamics.
Author contribution
MR and HF conceived the idea. MR conducted the experiment and prepared the results. HF supervised the procedure and verified the results and discussion. MR wrote the first draft, and HF edited the manuscript. MR and HF prepared the final draft.
Data Availability
Data used in this study is freely available and can be obtained from NCBI using above-mentioned GSE code “GSE22255.”
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
- 1.Peng L, et al. Microglia autophagy in ischemic stroke: a double-edged sword. Front Immunol. 2022;13:6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Y, et al. Neuronal injuries in cerebral infarction and ischemic stroke: from mechanisms to treatment. Int J Mol Med. 2022;49(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell BC, et al. Ischaemic stroke. Nat Rev Dis Prim. 2019;5(1):70. [DOI] [PubMed] [Google Scholar]
- 4.Bevan S, Markus HS. Genetics of common polygenic ischaemic stroke: current understanding and future challenges. Stroke Res Treat. 2011;2011. [DOI] [PMC free article] [PubMed]
- 5.Rutten-Jacobs LC et al. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: cohort study of 306 473 UK Biobank participants. Bmj. 2018;363. [DOI] [PMC free article] [PubMed]
- 6.Adams HP Jr, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. [DOI] [PubMed] [Google Scholar]
- 7.Gasull T, Arboix A. Molecular mechanisms and pathophysiology of acute stroke: emphasis on biomarkers in the different stroke subtypes. 2022;9476 MDPI. [DOI] [PMC free article] [PubMed]
- 8.Zheng K, et al. Single-cell RNA-seq reveals the transcriptional landscape in ischemic stroke. J Cereb Blood Flow Metab. 2022;42(1):56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D-Z, et al. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30(1):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu B, et al. Analysis of expression profiles and bioinformatics suggests that plasma exosomal circular RNAs may be involved in ischemic stroke in the Chinese Han population. Metab Brain Dis. 2022;37(3):665–76. [DOI] [PubMed] [Google Scholar]
- 11.Cho Y-E et al. Circulating immune cell landscape in patients who had mild ischaemic stroke. Stroke Vasc Neurol. 2022;7(4). [DOI] [PMC free article] [PubMed]
- 12.Ri MH et al. Regulatory mechanisms of natural compounds from traditional Chinese herbal medicines on the microglial response in ischemic stroke. Phytomedicine. 2023;154889. [DOI] [PubMed]
- 13.He Q et al. Biological functions and regulatory mechanisms of hypoxia-inducible factor-1α in ischemic stroke. Front Immunol 2021;5377. [DOI] [PMC free article] [PubMed]
- 14.Yang K, et al. The mechanism of ferroptosis regulating oxidative stress in ischemic stroke and the regulation mechanism of natural pharmacological active components. Biomed Pharmacother. 2022;154:113611. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, et al. The protection by octreotide against experimental ischemic stroke: up-regulated transcription factor Nrf2, HO-1 and down-regulated NF-κB expression. Brain Res. 2012;1475:80–7. [DOI] [PubMed] [Google Scholar]
- 16.Waseem A et al. Insight into the transcription factors regulating ischemic stroke and glioma in response to shared stimuli. in Seminars in Cancer Biology. 2023. Elsevier. [DOI] [PubMed]
- 17.Mazur A, et al. Nrf2 as a therapeutic target in ischemic stroke. Expert Opin Ther Targets. 2021;25(3):163–6. [DOI] [PubMed] [Google Scholar]
- 18.Patel S, et al. Pyruvate kinase M2 in chronic inflammations: a potpourri of crucial protein–protein interactions. Cell Biol Toxicol. 2021;37:653–78. [DOI] [PubMed] [Google Scholar]
- 19.Szklarczyk D et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2016;gkw937. [DOI] [PMC free article] [PubMed]
- 20.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks. in Proceedings of the international AAAI conference on web and social media. 2009.
- 22.Nepusz T, Yu H, Paccanaro A. Detecting overlapping protein complexes in protein-protein interaction networks. Nat Methods. 2012;9(5):471–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lachmann A, et al. ChEA: transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics. 2010;26(19):2438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang DW et al. Extracting biological meaning from large gene lists with DAVID. Curr Protoc Bioinforma. 2009;27(1):13.11. 1–13.11. 13. [DOI] [PubMed]
- 25.Sarkar S, et al. Cerebral ischemic stroke: cellular fate and therapeutic opportunities. Front Biosci-Landmark. 2019;24(3):415–30. [DOI] [PubMed] [Google Scholar]
- 26.Jayaraj RL, et al. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudilosso S, et al. The potential impact of neuroimaging and translational research on the clinical management of lacunar stroke. Int J Mol Sci. 2022;23(3):1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regenhardt RW, et al. Advances in understanding the pathophysiology of lacunar stroke: a review. JAMA Neurol. 2018;75(10):1273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bierman-Chow S, et al. Clinical, imaging, and laboratory findings in patients with GATA2 deficiency presenting with early-onset ischemic stroke. Neurology. 2023;100(7):338–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meadows C, Girotra T. An unusual manifestation of ischemic stroke in a patient with rare MonoMAC syndrome caused by N371K GATA2 mutation (2819). 2021;AAN Enterprises.
- 31.Sun J, et al. Krüppel-like factor 6 silencing prevents oxidative stress and neurological dysfunction following intracerebral hemorrhage via sirtuin 5/Nrf2/HO-1 axis. Front Aging Neurosci. 2021;13:646729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song L, et al. Li, Q. Q., Liu Q. Neuroprotective effects of SMADs in a rat model of cerebral ischemia/reperfusion. Neural Regen Res. 2015;10:438. [DOI] [PMC free article] [PubMed]
- 33.Wang Y-Z, et al. Association between PPARG genetic polymorphisms and ischemic stroke risk in a northern Chinese Han population: a case-control study. Neural Regen Res. 2019;14(11):1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng F, et al. Relationship between PPAR-γ gene polymorphisms and ischemic stroke risk: a meta-analysis. Brain Behav. 2021;11(12):e2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C, et al. CircRNA CTNNB1 (circCTNNB1) ameliorates cerebral ischemia/reperfusion injury by sponging miR-96-5p to up-regulate scavenger receptor class B type 1 (SRB1) expression. Bioengineered. 2022;13(4):10258–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz-Cañestro C, et al. AP-1 (activated protein-1) transcription factor JunD regulates ischemia/reperfusion brain damage via IL-1β (interleukin-1β). Stroke. 2019;50(2):469–77. [DOI] [PubMed] [Google Scholar]
- 37.Lian L, et al. Neuroinflammation in ischemic stroke: focus on microRNA-mediated polarization of microglia. Front Mol Neurosci. 2020;13:612439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye J et al. Knockdown of ATF3 suppresses the progression of ischemic stroke through inhibiting ferroptosis. Front Mol Neurosci. 2023;15. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this study is freely available and can be obtained from NCBI using above-mentioned GSE code “GSE22255.”