Abstract
The main drawbacks of blood-contacting metallic devices are corrosion and thrombus formation on the surface, so polymeric coatings have been proposed to improve its hemocompatibility. Sulfated chitosan (SC) was obtained from natural chitosan (NC) reaction with chlorosulfonic acid to be used as a coating for metallic surfaces. The sulfated chitosan showed no platelet aggregation, an extended clotting time, and non-toxicity to rat fibroblast L929 cells. In this study, stainless steel (SS) and titanium alloys modified with TiO2 nanotube (NTT) growth received a NC and SC coating. The titanium surface coated with sulfated chitosan presented the lowest percentage of platelet coverage area. Sulfated chitosan proved to be a promising material for use as a coating for metallic surfaces applied for cardiovascular devices.
Keywords: TiO2 nanotubes, Platelet adhesion, Surface functionalization, Platelet aggregation
Introduction
According to the World Health Organization, cardiovascular diseases (CVD) were responsible for 31% of all global deaths in 2020. In this context, blood-contacting surfaces, such as stents that are small, expandable, and mesh-like devices, are inserted into blocked arteries to restore blood flow. Due to their mechanical properties and biocompatibility, these devices are manufactured from materials such as 316L stainless steel and titanium alloys [1, 2]. However, thrombosis and corrosion are the main reasons for blood-implant failure, the degradation products can affect the functions of cells in the vicinity of the biomaterial or even distant cells due to the transport of the products within the body [3], and a blood clot forms on the implant surface, obstructing the blood flow.
Stainless steel (SS) is largely used in medical devices mainly due to its low cost, associated with biocompatibility, malleability, resistance to corrosion, and fatigue [4]. Titanium (Ti) alloys have been established as a material to produce stents, due to their high specific strength-to-weight ratio, improved corrosion, and biocompatibility, compared to SS. Surface modifications on titanium alloys have shown improved cytocompatibility and hemocompatibility [5]. Manivasagam and Popat (2020) modified Ti and Ti-6Al-4 V hydrothermally to obtain nanostructured surfaces and observed that surface morphology (topography and size of the nanostructure) has a direct effect on cell and protein interaction and platelet adhesion [6]. Alves Claro et al. (2018) have demonstrated that the functional oxide layers, such as TiO2, can be oxidized to obtain vertically aligned tubular nanostructures (NTT), allowing improved host-interaction response when inserted into the human body [7].
Another approach to improve the hemocompatibility properties of metallic devices is coating with biopolymers [8] such as chitosan, which is a biopolymer biocompatible and non-toxic with amino (NH2) and hydroxyl (OH) groups in its structure, presenting thrombogenic properties when in contact with blood [9]. Sulfonation reaction on the chitosan structure has been described to reduce its thrombogenicity since this modification gives the chitosan structure a more negative surface charge able to neutralize the positive charges of thrombin amino acid residues preventing the transformation of fibrin to fibrinogen and acting in the coagulation cascade [10, 11], mimicking the heparin effect. Furthermore, the anticoagulant activity of chitosan and its derivatives is influenced by many factors, including the substitution degree [12]. Moraes and collaborators (2019) synthesized 2-N-3,6-O-sulfated chitosan with chlorosulfonic acid, and the hydrophilic material was able to prolong blood clotting time and cause a reduction in protein adhesion when compared to natural chitosan [13].
This work investigates the hemocompatible properties of stainless steel (SS) and titanium alloys modified with TiO2 nanotube (NTT) coated with sulfated chitosan (SC). Different analytical techniques characterized the sulfatation reaction. Platelet adhesion, coagulation time, and cytotoxicity of SC were measured. In addition, the hemocompatibility of coated surfaces was characterized by scanning electron microscopy (SEM) to investigate platelet adhesion.
Materials and methods
Chemical and biological materials
Natural chitosan, N-(3-dimethylaminopropyl)-N′-ethyl carbodiimide (EDAC), poly(ethylene glycol) bis(carboxymethyl) ether (PEGb), and 2-ethane sulfonic acid (MES) were obtained from Sigma-Aldrich; sodium hydroxide (NaOH) and N,N-dimethylformamide (DMF) from Labsynth; and methanol and glutaraldehyde from VETEC Química Fina (Brazil). Chlorosulfonic acid (HClSO3) was obtained from Merck. Coagulation kits were obtained from Bios Diagnóstica.
Chitosan’s sulfonation reaction and film production and characterization
Sulfated chitosan was fabricated as previously reported [13]. Films of natural and sulfated chitosan were cross-linked with different glutaraldehyde concentrations (0.03 or 0.08% v/v). The chitosan films were prepared as described by ourselves in previous works [13]. Fourier transform infrared (FTIR) spectroscopy and thermogravimetric (TG) analysis were performed to investigate if sulfonation reaction occurred. The FTIR measurements were made in attenuated total reflectance (ATR) mode, directly on the films, in a range of 450 to 4500 cm−1 with a resolution of 4 cm−1, using an Agilent Cary 660 model FTIR spectrometer (USA). Thermogravimetric (TG) analysis was carried out from 25 to 700 °C, in a synthetic air atmosphere, with a flow rate of 20 mL/min and heating rate of 10 °C/min, using a simultaneous thermal analyzer equipment STA 409 C/CD from Netzsch (Germany).
Platelet aggregation and coagulation assay
Human blood was collected from healthy donors in 3.6-mL tubes containing 3.2% citrate. The research was approved by the ethics committee of the Federal University of Ceará, with protocol 61,280,822.7.0000.5054 and qualified professionals collected the blood samples. The platelet-rich plasma (PRP) was obtained after centrifugation at 27 °C for 10 min at 1000 rpm. The platelet-poor plasma (PPP) was obtained after centrifugation for 15 min at 3500 rpm and used as a blank in the aggregometer equipment. PRP (450 µL) was incubated at 37 °C with 50 µL of sulfated chitosan (100 µg/mL and 200 µg/mL), natural chitosan (100 µg/mL), or control adenosine diphosphate (ADP) for 5, 10, and 15 min. The Aggrolink 8 (Chrono-log Corp., USA) aggregometer software provided an experimental aggregation curve by the time that gave the area under the curve to calculate the percentage of platelet aggregation.
The anticoagulant activity of the material was obtained by measuring the activated partial thromboplastin time (APTT); 5 µL of the sulfated chitosan (200, 500, and 1000 µg/mL), natural chitosan (200 µg/mL), heparan (5 µg/mL), or saline solution was added to 45 µL of human plasma incubated at 37 °C for 2 min under magnetic stirring. Then 50 µL of the reagent APTT was added to the mixture and incubated for another 3 min. The clotting time in seconds was evaluated after the start of the reaction by adding 50 µL of CaCl2 (25 mmol/L).
Metabolic cell activity assays
The cytotoxicity of chitosan films was evaluated by the indirect method described by Vasconcelos and collaborators (2020) [14]. The sterile samples (1 cm2) were added to 1 mL of Dulbecco’s modified Eagle medium (DMEM) for 24 h at 37 °C while the culture of L929 (mouse fibroblast cells) was seeded in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS) in the 96-well plate at a density of 6 × 103 cells/well and incubated at 37 °C for 24 h (5% CO2 and 95% humidity). After that, the culture medium present in the wells was removed and 100 µL of the supplemented extract was added and incubated at 37 °C for 24 h. After this period, the extract was removed and then 120 µL of the resazurin solution (2.5 mg/mL in DMEM) was added and metabolized for 4 h. Then, 100 μL of the cells’ metabolized solution was transferred to a new 96-well plate and measured in a microplate reader (SpectraMax i3x, Molecular Device, Sunnyvale, USA) in fluorescence mode (λexcitation = 560 nm and λemission = 590 nm). The control cells were exposed to supplemented DMEM and the viability was adjusted (100%) to calculate the mean values and standard deviation (n = 3). The percentage of metabolically active cells was calculated using Eq. 1.
| 1 |
where Fsample represents the fluorescence corresponding to the well where the cells were grown from the sample extract and Fcontrol represents the fluorescence corresponding to the well where the cells were grown in supplemented DMEM.
Functionalization of stainless steel and TiO2nanotubes surfaces
The 316L stainless steel and NTT surfaces were functionalized with natural and sulfated chitosan [15]. Electropolishing was performed only in SS samples in an electrolytic cell system with glycerol, phosphoric acid, and water (50:30:10% v/v) at 60 °C followed by an acidic bath containing water, nitric acid, and hydrochloric acid (88:10:2% v/v) performed at 50 °C. After that, the amination of both the metal surfaces was performed in the dark for 24 h with 2 mL of dopamine hydrochloride (2 mg/mL) in MES buffer solution at pH 8.6 adjusted with NaOH (0.1 mol/L) [16]. Next, the samples were washed several times with ultrapure water. Then, 2 mL of PEGb solution (0.5 g/L) activated with 3 mg of N-(3-dimethylaminopropyl)-N′-ethyl carbodiimide (EDAC) was placed for 3 h on dopamine-coated samples, followed by washing with ultrapure water and dried. Next, the grafting of sulfated or natural chitosan polymer was performed by placing 2 mL of a 2% (w/v) solution to the sample for 3 h. Finally, the samples were washed several times with ultrapure water (see in Fig. 1).
Fig. 1.
Functionalization methodology of SS and NTT surfaces with natural and sulfated chitosan
Platelet adhesion
Blood was collected using the same procedure described in the “Platelet aggregation and coagulation assay” section, so 1.5 mL of PRP was added to each sample and incubated at 37 °C for 1 h. After that, the surface was washed with PBS buffer pH 7.4 to remove unadhered platelets. Next, the adhered cells were fixed with 1% glutaraldehyde for 30 min at 25 °C. Then. The samples were washed with PBS buffer for 10 min followed by drying steps by immersion for 10 min in ethanol solutions (20%, 50%, 90%, and 100% v/v) [17, 18]. The activation of platelets on the surface was characterized by scanning electron microscopy (SEM) conducted at LEO 440i. The samples adhered to stubs with carbon tape and coated with 10 nm gold. Images were obtained from different locations with 5.000 × magnification at 10 kV. The percentage of coverage of the sample by the platelets was calculated in ImageJ software.
Statistical analysis
The statistical significance of experimental data was determined by one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests (p < 0.05) using GraphPad Prism Software (version 8.4.3, San Diego, CA).
Results and discussion
Chitosan sulfation reaction
The FTIR spectra and thermal stability of the natural chitosan surface and the modified sulfated chitosan surfaces are shown in Fig. 2.
Fig. 2.
(A) Fourier transform infrared (FTIR) spectroscopy and (B) thermograms (TG and DTG) of natural chitosan and sulfated chitosan
The characteristic spectra of natural and sulfated chitosan structures can be seen respectively in black and red lines in Fig. 2. The last one presented new peaks around 620 cm−1, 800 cm−1, and 1256 cm−1, corresponding to asymmetric S–O, C-O-S, and S = O, respectively [19]. Xing and collaborators (2004) investigated the structure of natural and sulfated chitosan by FTIR and C NMR and attributed the FTIR vibrational bands at 1222 cm−1 and 806 cm−1 to the sulfated groups S = O and C-O-S [16].
By thermogravimetric analysis, it was possible to identify that the sulfated chitosan had a reduction in stability compared to natural chitosan. Indeed, the decomposition temperature of sulfated chitosan (230 °C) was lower than that of natural chitosan (280 °C) due to the reduction in molecular weight of the modified chitosan caused by the introduction of hydrophilic groups into the chain, so the structure became thermally unstable. Also, the presence of the sulfated groups can contribute to an increase in the free volume between the chains allowing the penetration of a higher energy intensity leading to lower thermal stability [20].
Hemocompatibility of sulfated chitosan (platelet aggregation and coagulation)
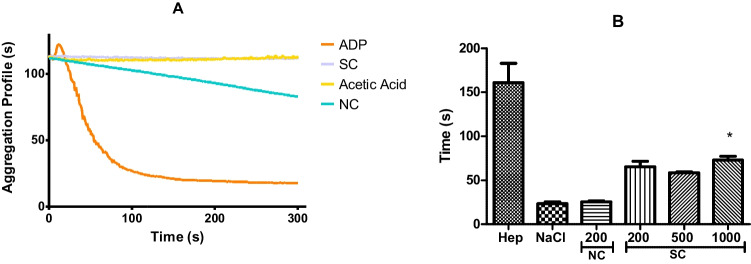
A material with low platelet adhesion and activation indicates hemocompatible surfaces [21]. Activated partial thromboplastin time (APTT) tests were performed for the materials developed to evaluate if sulfated chitosan has heparin mimetic activity in preventing the formation of clots and thrombi. The aggregation of platelets (A) and the coagulation assay (B) are shown in Fig. 3. Table 1 depicts the platelet aggregation percentage caused by sulfated chitosan (SC) and natural chitosan (NC) incubated in platelet-rich plasma (PRP).
Fig. 3.
(A) Aggregation profile provided by the aggregometer equipment. Effect of SC, NC, acetic acid (1%), and ADP control incubated in PRP for 15 min. (B) Activated partial thromboplastin time (APTT) of PRP with heparan 5 µg/mL, saline, NC (200 µg/mL), and SC (200, 500, and 1000 µg/mL). *, p < 0.05, one-way ANOVA with Tukey’s multiple comparison test
Table 1.
Platelet aggregation percentage caused by sulfated chitosan (SC) and natural chitosan (NC) incubated in platelet-rich plasma (PRP)
| Incubation time (min) | Sulfated chitosan, 100 µg/mL | Sulfated chitosan, 200 µg/mL | Natural chitosan, 100 µg/mL |
|---|---|---|---|
| 5 min | 2.0% ± 0.2 | 3.5% ± 0.7 | 13.5% ± 0.7* |
| 10 min | 1.0% ± 0.8 | 2.0% ± 0.0 | 18.0% ± 5.6* |
| 15 min | 2.0% ± 0.9 | 1.5% ± 0.7 | 30.5% ± 2.1* |
The results are expressed in percent of platelet aggregation (mean ± SD). *, p < 0.05, one-way ANOVA with Tukey’s multiple comparison tests; a significant difference was found between natural chitosan and sulfated chitosan. However, no significant difference was found between 100 and 200 µg/mL sulfated chitosan
In Fig. 3(A), it is possible to identify that SC did not induce platelet aggregation (2.0% ± 0.9) due to the negative charge that repels platelets. NC dissolved in 1% (v/v) acetic acid was able to cause PRP aggregation around 30.5% ± 2.12 even though acetic acid (vehicle) aggregated only 3.5% ± 0.2. The platelet aggregation induced by NC is due to the protonated amino groups distributed in the polymer chain. The increase in the incubation time of PRP with NC enhanced the aggregation, because NC presents a positive charge on its surface causing platelet aggregation [22].
In the clotting test that can be seen in Fig. 3(B), the following concentrations of sulfated chitosan —200 µg/mL, 500 µg/mL, and 1000 µg/mL— resulted in the APTT times of 65 s ± 7 s, 58 s ± 2 s, and 72 s ± 5 s, respectively. Furthermore, as can be identified in Fig. 3(B), the coagulation time was prolonged in the plasma that received the sulfated chitosan. A significant difference was found between NaCl control and SC in the concentration of 1000 µg/mL. The result is associated with the presence of sulfated groups according to the literature [13, 23].
Cell metabolic activity
Metabolic activity of cells is an important test that is performed to evaluate the biomaterials that should not release toxic products or lead to adverse reactions. For example, the results obtained for the cell viability test of sulfated and natural chitosan at 24 and 48 h in L929 (mouse fibroblast cells) can be observed in Fig. 4.
Fig. 4.

Viability of L929 cells in extracts from films of natural chitosan, natural chitosan cross-linked with 0.03% glutaraldehyde, natural chitosan 0.08% glutaraldehyde, sulfated chitosan, sulfated chitosan 0.03% glutaraldehyde, and sulfated chitosan 0.08% glutaraldehyde. The results are expressed in percent of cellular viability (mean ± SD). *, p < 0.05, one-way ANOVA with Tukey’s multiple comparison tests; no significant difference was found between sulfated chitosan and negative control
As shown in Fig. 4, the extracts of the developed films can be considered compatible with L929 mouse fibroblast cells (above 70%). The samples with higher glutaraldehyde concentration (0.08%) presented a slight decrease in cell viability with incubation time (48 h) due to the gradual release of toxic residues such as glutaraldehyde. However, at low glutaraldehyde concentrations, the high cell viability for sulfated chitosan cross-linked with 0.03% glutaraldehyde (SC 0.03) suggests a minimal influence of glutaraldehyde content in the cytotoxicity. Indeed, no significant differences were found between negative control and sulfated chitosan samples with 0.03% of glutaraldehyde. This result suggests that the material is harmless to the cells.
Despite being within the margin of error, a slight increase in cell viability (140% and 112%) was observed at 48 h for samples SC and SC 0.03, respectively. This result suggests a tendency observed in some literature studies pointing that sulfated chitosan is safe for cells and can also increase cell growth. Pinto and colleagues (2022) evaluated the cell viability of fibroblasts on natural and sulfated chitosan scaffolds cross-linked with 0.2% glutaraldehyde and concluded that the material is safe for cells, as cell growth was identified after 7 days of incubation [24]. In another study, a PCL polymeric stent modified by the immobilization of sulfated chitosan (2-N, 6-O-sulfated chitosan) showed an increase in cell proliferation by approximately 20% compared to PCL, considered a good candidate for the development of cardiovascular stents [25].
Platelet adhesion on stainless steel and TiO2nanotubes
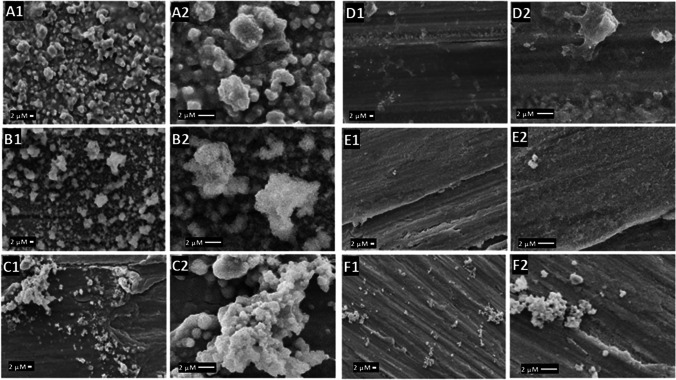
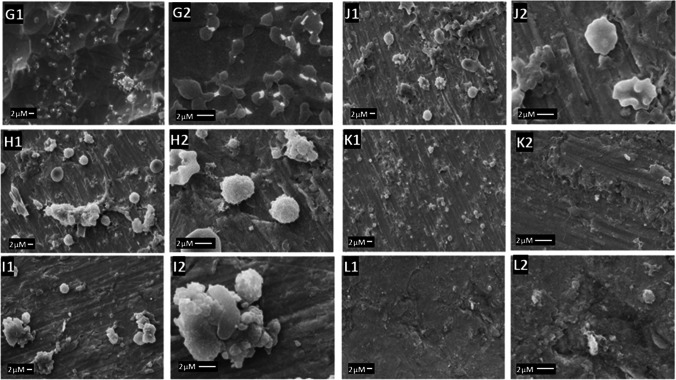
The first step in thrombus formation is the adsorption of proteins to the material’s surface when the medical device is inserted into the body [26]. The platelets are then activated, changing their morphology and forming dendrites that adhere to the surface and other platelets [27]. Thus, the formation of many platelet aggregates rich in dendrite extensions suggests a high propensity for thrombus formation [28]. The platelet’s morphology was observed using SEM imaging in Figs. 5 and 6 for SS and NTT, respectively.
Fig. 5.
SEM images of SS functionalized and subjected to platelet adhesion test. In A SS (A1) fully activated platelets (5000 ×), (A2) dendrite formation. In B SS polished (B1) fully activated platelets (5000 ×), (B2) dendrite formation. In C SS with natural chitosan (C1) activated platelet (5000 ×) (C2) aggregated of platelet. In D SS with natural chitosan and 0.03 glutaraldehyde (D1) activated platelet (5000 ×) (D2) partially activated platelet. In E SS with sulfated chitosan (E1) no activated platelets (5000 ×) (E2) clean surface. In F SS with sulfated chitosan 0.03% glutaraldehyde (F1) few platelets (F2) aggregated of platelet
Fig. 6.
SEM images NTT functionalized and subjected to the platelet adhesion test. In G Ti (G1) activated platelets (5000 ×), (G2) aggregate of fully activated platelets. In H Ti treated (H1) activated platelets (5000 ×), (H2) partially activated platelet. In I Ti with natural chitosan (I1) activated platelets (5000 ×), (I2) aggregate of totally activated platelets. In J Ti with natural chitosan 0.03 glutaraldehyde (J1) activated platelets (5000 ×), (J2) partially activated platelets. In K Ti with sulfated chitosan (K1) no platelets activated (5000 ×), (K2) without activated platelet. In L Ti with sulfated chitosan 0.03 glutaraldehyde (L1) presence of platelets (5000 ×), (L2) non-activated platelet
In Fig. 5, raw and polished SS samples showed platelets fully activated (A1, B1—5.000 × and A2, B2—5000 ×). The SS coated with NC (C1) and glutaraldehyde-cross-linked NC (D1) showed aggregates of fully activated platelets (C2) and partially activated platelets (D2). The SS coated with SC samples (E1) showed no platelets visible on the surface without activated platelets (E2); however, for glutaraldehyde-cross-linked SC (F1), some aggregated platelets (5000 ×) and with high magnification (F2).
In Fig. 6, titanium alloy (G1) showed activated platelets (5000 ×) with aggregate of fully activated platelets. The NTT sample (H1) showed partially activated platelets, demonstrating that titanium modification improves the hemocompatibility [6, 17]. The NTT coated with NC (I1) and glutaraldehyde-cross-linked NC (J1) showed aggregates of fully activated platelets (I2) and partially activated platelets (J2). The NTT coated with SC samples (K1) showed no platelets visible on the surface without activated platelets (K2), and for glutaraldehyde-cross-linked SC (L1), there are some platelets (5000 ×) but non-activated platelets (L2). Platelet adhesion increases with the stiffness of the material, so samples cross-linked with glutaraldehyde showed a higher percentage of platelet coverage compared to samples without cross-linking [29].
For samples coated with NC, partially and fully activated platelets were identified, due to the amino groups that act as a binding site for protein adsorption and clot formation [23, 30]. For the samples coated with SC, no fully activated platelet was found and no dendrites were identified, explained by the reduced availability of amino groups in chitosan structure after the sulfating reaction that are negatively charged and decrease the plasma protein adsorption that is responsible for initiating the coagulation cascade. These results corroborate the APTT results of a previous study presented by Campelo et al. (2017) that evaluated platelet adhesion on 316L stainless steel surfaces coated with natural and sulfated chitosan.
From the images obtained in the SEM, the percentage of platelet coverage was calculated with ImageJ software, as shown in Fig. 7.
Fig. 7.
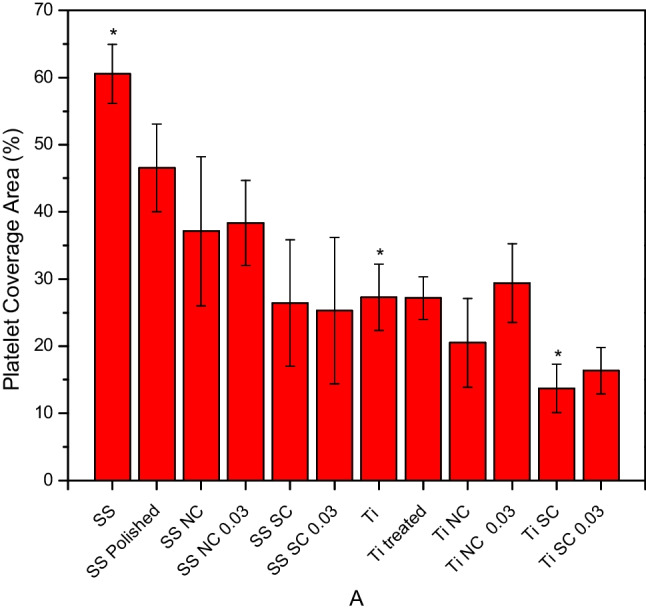
Area of coverage by platelets in SS, SS polished, SS with natural chitosan, SS with natural chitosan 0.03% glutaraldehyde, SS with sulfated chitosan, SS with sulfated chitosan 0.03% glutaraldehyde, NTT, NTT treated, NTT with natural chitosan, NTT with natural chitosan 0.03% glutaraldehyde, NTT with sulfated chitosan, and NTT with sulfated chitosan 0.03% glutaraldehyde. The results are expressed in percentage of platelet coverage area (mean ± SD). *, p < 0.05, one-way ANOVA with Tukey’s multiple comparison tests
NTT samples exhibited a lower platelet adhesion tendency when compared to uncoated and natural chitosan-coated stainless steel samples. Even though there is no significant difference, the results suggest a tendency to decrease platelet adhesion in the NTT samples, which agrees with studies reported in the literature. Huang and colleagues (2015) reported a reduction in platelet adhesion and activation on stainless steel coated with titanium nanotubes. They also observed an improvement in the corrosion resistance of surfaces modified with titanium nanotube growth [27].
Indeed, the greatest reduction in the platelet adhesion was presented by NTT coated with SC, probably due to the presence of negatively charged sulfated groups and the presence of TiO2 nanotubes. This is an important finding for the titanium implant devices compared to SS for clinical applications, associated with the high corrosion resistance, high thermal stability, low solubility, and low toxicity in the physiological environment [31]. Aroussi, Aour, and Bouaziz (2019) compared the performance in biological medium of 316L stainless steel and titanium that proved to be mechanically stronger and exhibited a lower corrosion current which represents an improvement in the hemocompatibility of the titanium alloy material with the lower release of degradation products in the body [32].
Conclusion
The sulfated groups on the natural chitosan structure were confirmed by Fourier transform infrared spectroscopy with an increase of the vibrational band at 800 cm−1 and 1218 cm−1. The sulfated chitosan showed no platelet aggregation, at the concentrations of 200 µg/mL and 100 µg/mL, the prolongation of the coagulation time in the activated partial thromboplastin time test, depicted by the anticoagulant activity in the intrinsic pathway and also non-toxicity to rat fibroblast L929 cells. The TiO2 nanotube coated with sulfated chitosan presented a significant reduction in platelet adhesion with platelet coverage area of 13.7% ± 3.5. Sulfated chitosan proved to be a promising material for use as a coating for metallic surfaces to be used for cardiovascular devices.
Acknowledgements
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES), Cearense Foundation for the Support of Scientific and Technological Development (FUNCAP). The authors also thank the Central Analítica-UFC/CT-INFRA/MCTI-SISANO/Pró-Equipamentos for the SEM/EDS analysis provided.
Funding
This research was supported by the following projects: FUNCAP/PRONEM (PNE-0112–00049.01.00/16), PROCAD/CAPES (88881.068439/2014–01), and Visiting Professor Award for Prof. Vieira from Fulbright.
Data Availability
All data generated or analysed during this study are included in this published article.
Declarations
Conflict of interest
The authors declare no competing interests.
References
- 1.García A, Peña E, Martínez MA. Influence of geometrical parameters on radial force during self-expanding stent deployment. Application for a variable radial stiffness stent. J Mech Behav Biomed Mater. 2012;10:166–75. 10.1016/j.jmbbm.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Hasebe T, Murakami K, Nagashima S, Yoshimoto Y, Ihara A, Otake M, Kasai R, Kasuya S, Kitamura N, Kamijo A, Terada H, Hotta A, Takahashi K, Suzuki T. Design for improved adhesion of fluorine-incorporated hydrogenated amorphous carbon on metallic stent: three-layered structure with controlled surface free energy. Diam Relat Mater. 2011;20:902–6. 10.1016/j.diamond.2011.04.014. [Google Scholar]
- 3.Dee GJ, Rhode O, Wachter R. Chitosan : multi-functional marine polymer. Cosmet Toiletries. 2001;116:39–44. [Google Scholar]
- 4.Bekmurzayeva A, Duncanson WJ, Azevedo HS, Kanayeva D. Surface modification of stainless steel for biomedical applications: revisiting a century-old material. Mater Sci Eng C Mater Biol Appl. 2018;93:1073–89. 10.1016/j.msec.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 5.Cai N, Wong CC, Gong YX, Tan SCW, Chan V, Liao K. Modulating cell adhesion dynamics on carbon nanotube monolayer engineered with extracellular matrix proteins. ACS Appl Mater Interfaces. 2010;2:1038–47. 10.1021/am9008117. [DOI] [PubMed] [Google Scholar]
- 6.Manivasagam VK, Popat KC. In vitro investigation of hemocompatibility of hydrothermally treated titanium and titanium alloy surfaces. ACS Omega. 2020;5:8108–20. 10.1021/acsomega.0c00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alves Claro APR, Konatu RT, do AmaralScada AL, de Souza Nunes MC, Maurer-Morelli CV, Dias-Netipanyj MF, Popat KC, Mantovani D. Incorporation of silver nanoparticles on Ti7.5Mo alloy surface containing TiO2 nanotubes arrays for promoting antibacterial coating – in vitro and in vivo study. Appl Surface Sci. 2018;455:780–8. 10.1016/j.apsusc.2018.05.189. [Google Scholar]
- 8.Mittal H, Ray SS, Kaith BS, Bhatia JK, Sukriti SJ, Alhassan SM. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur Polymer J. 2018;109:402–34. 10.1016/j.eurpolymj.2018.10.013. [Google Scholar]
- 9.Wang B, Wang J, Li D, Ren K, Ji J. Chitosan/poly (vinyl pyrollidone) coatings improve the antibacterial properties of poly(ethylene terephthalate). Appl Surf Sci. 2012;258:7801–8. 10.1016/j.apsusc.2012.03.181. [Google Scholar]
- 10.Negm NA, Hefni HHH, Abd-Elaal AAA, Badr EA, Abou Kana MTH. Advancement on modification of chitosan biopolymer and its potential applications. Int J Biol Macromol. 2020;152:681–702. 10.1016/j.ijbiomac.2020.02.196. [DOI] [PubMed] [Google Scholar]
- 11.Ouerghemmi S, Dimassi S, Tabary N, Leclercq L, Degoutin S, Chai F, Pierlot C, Cazaux F, Ung A, Staelens J-N, Blanchemain N, Martel B. Synthesis and characterization of polyampholytic aryl-sulfonated chitosans and their in vitro anticoagulant activity. Carbohyd Polym. 2018;196:8–17. 10.1016/j.carbpol.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Skorik YA, Kritchenkov AS, Moskalenko YE, Golyshev AA, Raik SV, Whaley AK, Vasina LV, Sonin DL. Synthesis of N-succinyl- and N-glutaryl-chitosan derivatives and their antioxidant, antiplatelet, and anticoagulant activity. Carbohyd Polym. 2017;166:166–72. 10.1016/j.carbpol.2017.02.097. [DOI] [PubMed] [Google Scholar]
- 13.Moraes AF, Filho RNFM, Passos CCO, Cunha AP, Silva LMAe, Freitas LBN, Vasconcelos NF, Ricardo NMPS, Canuto KM, Rosa MF, Leal LKAM, Vieira RS. Hemocompatibility of 2-N-3,6-O-sulfated chitosan films. J Appl Polym Sci. 2019;136:47128. 10.1002/app.47128. [Google Scholar]
- 14.Vasconcelos NF, Andrade FK, Vieira L de AP, Vieira RS, Vaz JM, Chevallier P, Mantovani D, Borges M de F, Rosa M de F. Oxidized bacterial cellulose membrane as support for enzyme immobilization: properties and morphological features. Cellulose. 2020;27:3055–3083. 10.1007/s10570-020-02966-5.
- 15.Souza Campelo C, Chevallier P, Loy C, Silveira Vieira R, Mantovani D. Development, validation, and performance of chitosan-based coatings using catechol coupling. Macromol Biosci. 2019;20:1900253. 10.1002/mabi.201900253. [DOI] [PubMed]
- 16.Xing R, Liu S, Yu H, Zhang Q, Li Z, Li P. Preparation of low-molecular-weight and high-sulfate-content chitosans under microwave radiation and their potential antioxidant activity in vitro. Carbohyd Res. 2004;339:2515–9. 10.1016/j.carres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Montgomerie Z, Popat KC. Improved hemocompatibility and reduced bacterial adhesion on superhydrophobic titania nanoflower surfaces. Mater Sci Eng: C. 2021;119:111503. 10.1016/j.msec.2020.111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocha Neto JBM, Copes F, Chevallier P, Vieira RS, da Silva JVL, Mantovani D, Beppu MM. Polysaccharide-based layer-by-layer nanoarchitectonics with sulfated chitosan for tuning anti-thrombogenic properties. Colloids Surfaces B: Biointerfaces. 2022;213:112359. 10.1016/j.colsurfb.2022.112359. [DOI] [PubMed] [Google Scholar]
- 19.Pavia DL, Lampman GM, Kriz GS, Vyvyan JR. Introduction to spectroscopy. 5th ed. Cengage Learning; 2014.
- 20.Wu D, Fu R, Xu T, Wu L, Yang W. A novel proton-conductive membrane with reduced methanol permeability prepared from Bromomethylated poly(2,6-dimethyl-1,4-phenylene oxide) (BPPO). J Membr Sci. 2008;310:522–530. 10.1016/j.memsci.2007.11.042.
- 21.Salimi E, Ghaee A, Ismail AF, Othman MHD, Sean GP. Current approaches in improving hemocompatibility of polymeric membranes for biomedical application. Macromol Mater Eng. 2016;301:771–800. 10.1002/mame.201600014. [Google Scholar]
- 22.Lan G, Li Q, Lu F, Yu K, Lu B, Bao R, Dai F. Improvement of platelet aggregation and rapid induction of hemostasis in chitosan dressing using silver nanoparticles. Cellulose. 2020;27:385–400. 10.1007/s10570-019-02795-1. [Google Scholar]
- 23.Campelo CS, Chevallier P, Vaz JM, Vieira RS, Mantovani D. Sulfonated chitosan and dopamine based coatings for metallic implants in contact with blood. Mater Sci Eng, C. 2017;72:682–91. 10.1016/j.msec.2016.11.133. [DOI] [PubMed] [Google Scholar]
- 24.Pinto RV, Gomes PS, Fernandes MH, Costa MEV, Almeida MM. Glutaraldehyde-crosslinking chitosan scaffolds reinforced with calcium phosphate spray-dried granules for bone tissue applications. Mater Sci Eng: C. 2020;109:110557. 10.1016/j.msec.2019.110557. [DOI] [PubMed] [Google Scholar]
- 25.Qiu T, Jiang W, Yan P, Jiao L, Wang X. Development of 3D-printed sulfated chitosan modified bioresorbable stents for coronary artery disease. Front Bioeng Biotechnol. 2020;8:462. 10.3389/fbioe.2020.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thromb Haemost. 2015;13:S72–81. 10.1111/jth.12961. [DOI] [PubMed] [Google Scholar]
- 27.Huang Q, Yang Y, Hu R, Lin C, Sun L, Vogler EA. Reduced platelet adhesion and improved corrosion resistance of superhydrophobic TiO2-nanotube-coated 316L stainless steel. Colloids Surf, B. 2015;125:134–41. 10.1016/j.colsurfb.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Bartlet K, Movafaghi S, Dasi LP, Kota AK, Popat KC. Antibacterial activity on superhydrophobic titania nanotube arrays. Colloids Surf, B. 2018;166:179–86. 10.1016/j.colsurfb.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu Y, Brown AC, Myers DR, Sakurai Y, Mannino RG, Tran R, Ahn B, Hardy ET, Kee MF, Kumar S, Bao G, Barker TH, Lam WA. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc Natl Acad Sci USA. 2014;111:14430–5. 10.1073/pnas.1322917111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoven VP, Tangpasuthadol V, Angkitpaiboon Y, Vallapa N, Kiatkamjornwong S. Surface-charged chitosan: preparation and protein adsorption. Carbohyd Polym. 2007;68:44–53. 10.1016/j.carbpol.2006.07.008. [Google Scholar]
- 31.Rosa JL, Nakazato RZ, Scheneider SG, Claro APRA, Rezende MCRA. Wettability behavior of nanotubular TiO2 intended for biomedical applications. Arch Health Invest. 2014;3:43–47. [Google Scholar]
- 32.Aroussi D, Aour B, Bouaziz SA. A comparative study of 316L stainless steel and a titanium alloy in an aggressive biological medium. Eng Technol Appl Sci Res. 2019;9:5093–8. 10.48084/etasr.3208. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.