Osteoarthritis (OA), also known as degenerative joint disease, is the most common form of arthritis. It involves gradual and progressive wearing of the articular cartilage, synovial membrane inflammation, and subchondral bone remodelling that generate osteochondral (OC) defects characterized by an unbalanced regeneration of articular cartilage and bone, where the intrinsic repair mechanisms are insufficient. BAMOS project particularly addresses the challenges in OA treatment by providing novel cost-effective OC scaffold technology for early intervention of OA. While microfracture (MF) marrow stimulation and commercially available tissue-engineered scaffolds [1] are currently used for the treatment of small defects, none of these methods promotes a satisfactory durable regeneration of large OA defects. Thus, BAMOS aims to give clinicians a viable treatment option in situations where OA has progressed beyond a small defect, but where a full joint replacement could still be avoided. The main objectives of the project have been [2] the following: (a) define clinical specifications for OC scaffolds, (b) develop new OC scaffolds biomaterials, (c) develop innovative additive manufacturing (AM) techniques to produce patient-tailored OC scaffolds, (d) assess the OC scaffold in both in vitro and in vivo, and (e) train early-stage researchers in the context of collaborative research.
More than 60 secondments have taken place in the context of BAMOS, involving 41 staff members to complete a total stay time over 124 months. Apart from conducting research, the secondees have participated in many training activities for acquiring knowledge especially relevant for early-stage researchers, including cell culture protocols and procedures, mechanical characterization and imaging of the proposed scaffolds, joint replacement operations and clinical procedures for cartilage repair, molecular biology techniques, etc.
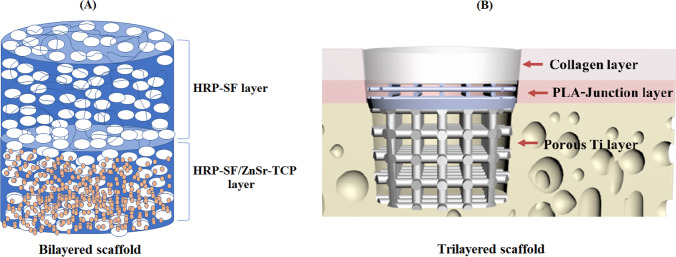
Different biomaterials have been evaluated for OC scaffold manufacturing in BAMOS project, including biodegradable polymers, ceramic materials, titanium alloys, and naturally derived hydrogels. Multi-layered and multi-material 3D structures with optimized designs for in vitro 3D models and OC tissue regeneration have been developed. One promising approach involved the use of horseradish peroxidase cross-linked silk fibroin as base material for the development of hierarchical OC scaffolds [3]. While the cartilage layer of these novel biofunctional hierarchical scaffolds was solely composed of the enzymatically cross-linked silk fibroin (HRP-SF), the bone-like layer also contained a 20% (w/w) of zinc (Zn) and strontium (Sr)-doped tricalcium phosphate (TCP) (Fig. 1A). The proposed 3D structures have shown to be able to support combined compression-shear loadings for OC tissue, possess a controllable porosity and memory-shape properties, and prevent bacterial biofilm formation. In vitro evaluation of the bilayered structures was carried out by using a co-culture system of human osteoblasts (hOBs) and human articular chondrocytes (hACs) [4]. Good adhesion, proliferation, and extracellular matrix (ECM) formation were observed for both types of cells, with no significant differences between scaffolds with and without ion incorporation. Not only osteoblasts produced a mineralized ECM in the bone layer and chondrocytes showed glycosaminoglycan (GAG) deposition in the cartilage layer, but also the formation and adequate integration of an interface region were confirmed. The structural characteristics and mechanical properties of these bilayered scaffolds, which closely mimic cartilage and subchondral bone properties, as well as their adequate biological performance, make them potential candidates for applications in OC tissue regeneration and as artificial extracellular matrices for in vitro OC models. More recently [5], we explored the properties of silk fibroin, decellularized extracellular matrix, and carbon nanotubes resulting in elastic and bioactive scaffolds for bone tissue engineering. Such promising carbon nanotube-reinforced cell-derived matrix-silk fibroin hierarchical scaffolds stimulated the expression of osteogenic-related markers such as ALP, Runx-2, Col Iα, and OPN. In brief, the developed 3D carbon nanotube-reinforced cell-derived matrix-silk fibroin hierarchical scaffolds showed great potential for applications in bone tissue engineering.
Fig. 1.
Main osteochondral scaffolds developed in BAMOS: (A) Horseradish peroxidase cross-linked silk fibroin-based bilayered scaffold; (B) Trilayered scaffold composed of titanium, PLA and collagen/PLGA layers
Following a different design strategy, tri-layered OC scaffolds were also developed by integrating various AM technologies and biomaterials (Fig. 1B) [6]. Thus, a porous titanium matrix obtained by powder bed fusion of metal (DMLS) was used as bone layer. On the other hand, a composite layer composed of collagen and poly(lactic-co-glycolic acid) (PLGA), produced by casting and freeze-drying methods, was intended for cartilage regeneration. These two components were joined by a two-part junction polylactic acid (PLA) layer manufactured by material extrusion, commonly referred to as fused deposition modelling (FDM). This junction layer served as calcified cartilage of the engineered OC scaffold, providing a graded structure in terms of morphological, mechanical, and compositional features. The biocompatibility of the multilayer scaffold was evaluated through cell viability and proliferation tests on the scaffold layers using sheep bone marrow mesenchymal stem cells (sBMMSCs) [7]. Results of the Live/Dead assay confirmed the viability of cells on the titanium, PLA, and collagen/PLGA layers throughout 14 days of culture, with sBMMSCs evenly distributed in the samples. Finally, the proliferation analysis using the AlamarBlue™ (Thermo Fisher, UK) reagent revealed a continuous increase of viable cells on the samples on days 1, 7, and 14.
Polymeric-based scaffolds obtained by 3D printing were also developed in BAMOS project to serve as support structures for the regeneration of subchondral bone in OC defects. Specifically, polycaprolactone (PCL) and PLA were used as matrices, to which natural or ceramic additives were incorporated to enhance their biofunctionality. Calcium carbonate and β‐TCP particles were used as additives in the case of PLA-based scaffolds [8]. Apart from an increase of the porosity, hydrophilicity, and surface roughness, composite scaffolds showed a very highly statistically significant (p < 0.001) improvement of the metabolic activity of human osteoblastic osteosarcoma cells (SaOS‐2) after 7 days of culture. Improved mechanical and biological properties were also obtained by introducing microcrystalline cellulose (MCC) as an additive to the PCL matrix when manufacturing 3D printed bone scaffolds [9]. The results of the proliferation assay of sBMMSCs cells after 1, 3, and 8 days of culture showed a significantly higher (p < 0.05) value for the composite PCL-based scaffolds containing 2% MCC.
Another strategy explored in the context of BAMOS to improve the biological performance of PLA and PCL bone scaffolds involved the application of surface treatments to the 3D structures. In this sense, a novel surface treatment method has been developed, comprising the use of oxygen plasma and the subsequent application of an Aloe vera bioactive coating. In the first published work regarding this method [10], Aloe vera-coated PLA scaffolds showed an improvement in terms of cell metabolic activity of human fetal osteoblastic (hFOB) cells cultured in vitro for 10 days. Surface-modified PLA- and PCL-based scaffolds have shown improved biofunctionality and, thus, great potential to be applied for bone tissue regeneration or as a bone component of an OC scaffold.
Interestingly, different bioreactors for the in vitro and ex vivo culture of tissue-engineered constructs, human OC tissue, and other tissue interfaces (gradients) are being developed. These are of great interest for applications involving 3D dynamic cell culture, tissue engineering strategies, tissue modelling, and personalized medicine. The scaffolds developed in BAMOS, and presented in this manuscript, are also being tested using ex vivo sheep condyle OC defect and human tissue explant models, as well as in vivo clinical OC defect animal models. The scaffolds will be ready at the end of the project for clinical trial.
Acknowledgements
Not applicable
Author contribution
Mario Monzón: resources, writing—original draft, project administration, funding acquisition. Ricardo Donate: writing—original draft. Chaozong Liu: resources, writing—review and editing. Maryam Tamaddon: visualization. J. Miguel Oliveira: resources, writing—review and editing, visualization.
Funding
The authors would like to thank H2020‐MSCA‐RISE program, as this work is part of the developments carried out in BAMOS project, funded from the European Unions Horizon 2020 research and innovation programme under grant agreement no. 734156.
Data availability
The data presented in this study are available on reasonable request from the authors.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
- 1.Bicho D, Pina S, Reis RL, Oliveira JM. Commercial products for osteochondral tissue repair and regeneration. Adv Exp Med Biol 1058, Springer New York LLC; 2018; 415–28. 10.1007/978-3-319-76711-6_19 [DOI] [PubMed]
- 2.Monzón M. Biomaterials and additive manufacturing: osteochondral scaffold innovation applied to osteoarthritis (BAMOS project). J Zhejiang Univ A. 2018;19:329–30. 10.1631/JZUS.A18NW001. [Google Scholar]
- 3.Ribeiro VP, Pina S, Gheduzzi S, Araújo AC, Reis RL, Oliveira JM. Hierarchical HRP-crosslinked silk fibroin/ZnSr-TCP scaffolds for osteochondral tissue regeneration: assessment of the mechanical and antibacterial properties. Front Mater. 2020;7:1–12. 10.3389/fmats.2020.00049. [Google Scholar]
- 4.Ribeiro VP, Pina S, Costa JB, Cengiz IF, García-Fernández L, Fernández-Gutiérrez MDM, et al. Enzymatically cross-linked silk fibroin-based hierarchical scaffolds for osteochondral regeneration. ACS Appl Mater Interfaces. 2019;11:3781–99. 10.1021/acsami.8b21259. [DOI] [PubMed] [Google Scholar]
- 5.Lemos R, Maia FR, Ribeiro VP, Costa JB, Coutinho P, Reis RL, Oliveira JM. Carbon nanotubes-reinforced cell-derived matrix-silk fibroin hierarchical scaffolds for bone tissue engineering applications. J Mater Chem B. 2021;9:9561–74. 10.1039/D1TB01972D. [DOI] [PubMed] [Google Scholar]
- 6.Blunn G, Liu C, Tamaddon M. Improved bone and cartilage regeneration in a rapid-manufactured functionally-graded osteochondral. Hangzhou International Conference on Biomaterials, Bio-Design and Manufacturing (BDMC2018). Aug. 26 - 28, 2018; Hangzhou (China). https://2020.aconf.org/conf_156776/abstract/77.html
- 7.Tamaddon M, Blunn G, Tan R, Yang P, Sun X, Chen SM, Luo J, Liu Z, Xu W, Lu X, Donate R, Monzón M, Liu C. In vivo evaluation of additively manufactured multi-layered scaffold for the repair of large osteochondral defects. Bio-Design Manuf 202210.1007/s42242-021-00177-w [DOI] [PMC free article] [PubMed]
- 8.Donate R, Monzón M, Ortega Z, Wang L, Ribeiro V, Pestana D, et al. Comparison between calcium carbonate and β-tricalcium phosphate as additives of 3D printed scaffolds with polylactic acid matrix. J Tissue Eng Regen Med. 2020;14:272–83. 10.1002/term.2990. [DOI] [PubMed] [Google Scholar]
- 9.Alemán-Domínguez ME, Giusto E, Ortega Z, Tamaddon M, Benítez AN, Liu C. Three-dimensional printed polycaprolactone-microcrystalline cellulose scaffolds. J Biomed Mater Res Part B Appl Biomater. 2019;107:521–8. 10.1002/JBM.B.34142. [DOI] [PubMed] [Google Scholar]
- 10.Donate R, Alemán-Domínguez ME, Monzón M, Yu J, Rodríguez-Esparragón F, Liu C. Evaluation of Aloe vera coated polylactic acid scaffolds for bone tissue engineering. Appl Sci. 2020;10:2576. 10.3390/APP10072576. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on reasonable request from the authors.