Abstract
Obesity is associated with several comorbidities that cause high mortality rates worldwide. Thus, the study of adipose tissue (AT) has become a target of high interest because of its crucial contribution to many metabolic diseases and metabolizing potential. However, many AT-related physiological, pathophysiological, and toxicological mechanisms in humans are still poorly understood, mainly due to the use of non-human animal models. Organ-on-chip (OoC) platform is a promising alternative to animal models. However, the use of adipose-derived human mesenchymal stem cells (hASCs) in these models is still scarce, and more knowledge on the effects properties of culturing hASCs in OoC models is needed. Here, we present the development of an OoC using hASCs to assess adipogenic differentiation. The device capability to increase hASC differentiation levels was confirmed by Nile red staining to verify lipid droplets inside cells after 10 days of culture and fluid flow of 10 µL/h. The Adipo-on-a-chip system increases hASC proliferation and differentiation area compared with the standard culture method under static conditions (96-well plates) verified in hASCs from different donors by image analysis of cells stained with Nile red. The expression of the gene FABP4 is lower in the MPS, which calls attention to different homeostasis and control of lipids in cells in the MPS, compared with the plates. An increase of hASC proliferation in the MPS related to the 96-well plate was verified through protein Ki-67 expression. Cell and nuclei morphology (area, roundness, perimeter, width, length, width to length rate, symmetry, compactness, axial and radial properties to nuclei, and texture) and dominant direction of cells inside the MPS were evaluated to characterize hASCs in the present model. The presented microphysiological system (MPS) provides a promising tool for applications in mechanistic research aiming to investigate adipogenesis in AT and toxicological assessment based on the hASC differentiation potential.
Keywords: Microfluidics, Adipose tissue-on-chip, Microfluidics, Adipose-derived human mesenchymal stem/stromal cells, Lipid droplets, Differentiation potential, Ki-67, hASC morphology
Introduction
Adipose tissue (AT) has historically been considered a simple storage tissue; however, the study of AT recently emerged as a research area of great interest due to its endocrine and metabolic activities and the obesity epidemics. These are associated with other comorbidities, such as type 2 diabetes, cardiovascular disease, and metabolic syndrome—diseases that present high mortality, resulting in ∼ 2.8 million deaths annually [1–3]. Additionally, AT interactions with toxicants suggest that this tissue participate in the kinetics and the toxicity of substances [4]. Thus, there is an increasing demand for the development of AT models, particularly for studies on adipose physiology, pathophysiology, and toxicological approaches. However, the predominant intention behind adipose tissue bioengineering has been the advance of large-scale tissue grafts for regenerative medicine [5]. This is in part explained by several limitations on gaining human-relevant cellular and molecular insights in the currently used models: (i) in vivo animal models allow for more depth of biological level and degree of experimental interventions, but they are ethically questionable, and their predictive value for humans is limited, due to species-specific physiological differences; (ii) ex vivo studies using human AT explants frequently encounter difficulties caused by hypoxia or inflammation; (iii) in vitro studies on primary mature white adipocytes are the best to reflect AT biology, but their culture can be challenging due to their large size, buoyancy, and fragility [6–8]. As an alternative, studies have evaluated in vitro differentiation of human adipose mesenchymal stromal/stem cells (hASCs) [8, 9].
Models using hASCs present the advantages of exploring mechanisms associated with adipogenesis, which can give great insights into obesity and the toxic effects of drugs that may impair differentiation [10–17]. However, the use of 2D cell cultures has recently been questioned because the microenvironment in the cell culture plates is distant from physiological conditions, failing to describe their biological complexity [18].
One of the most promising approaches is to exploit the strength of in vitro microphysiological systems (MPS) to generate biomimetic adipose-on-a-chip models [18]. Organ-on-chip (OoC) models use microfluidics to mimic tissue and organ-level physiology without necessarily replicating organ architecture [19]. This is possible because, in these models, the dynamic flow recreates mechanical forces that can mimic the pressures exerted by vascularization, regulate primary factors such as cellular positioning (cell patterning), and cell–cell and cell–matrix interactions and other parameters crucial for tissue and organ physiology [20]. Although several hASCs on-chip models have been created, these models are still not broadly exploited [9, 21, 22]. Different read-out options and controls to monitor the status of the hASCs during OoC culturing and experimentation are necessary to create powerful methods to investigate adipose tissue–related biology and diseases precisely and consequently contribute to their more widespread use [9]. Here, we present a simple platform that allows hASC attachment, supports their proliferation, and enhances their adipogenic potential under microfluidic conditions. Additionally, we characterize hASCs regarding their morphological features in the presented MPS.
Materials and methods
Stem cells and culture conditions
Human adipose stem cells (hASCs) were obtained from different origins: one commercially purchased (Cat. Num. PT-5006, Lonza®, Walkersville, USA) and four obtained from adult donors undergoing lipoaspiration procedures between November 15, 2014 and September 28, 2023. The donors signed an informed consent form approved by the institutional review board (Ethics Committee—CAAE: 10917112.9.0000.5248). All the donors were from the feminine sex, with an age range of 20–60 years and with no history of chronic diseases. hASCs were cultured in Dulbecco’s modified Eagle medium (DMEM), high glucose, (Cat. Num. 12100046, Gibco Thermo Fischer®, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Cat. Num. 12657029, Gibco Invitrogen®, Carlsbad, CA, USA), and 4 mM L-glutamine (Cat. Num. 21051024, Gibco Invitrogen®, Carlsbad, CA, USA) in a humidified atmosphere with 5.0% CO2 at 37 ºC [13, 14]. Cells were cultivated until they reached 90% confluence with medium changes every 3 or 4 days and plating carried out through enzymatic dissociation with trypsin/EDTA 0.05% (Cat. Num. 25300–062, Gibco Invitrogen®, Carlsbad, CA, USA). Immunophenotypes at passages 5 and 6 of these cells were verified by flow cytometry as required criteria for defining mesenchymal stem cells (MSCs) as determined by the International Society for Cellular Therapy [23]; data not shown. hASCs between passages 4 and 8 were used to perform the assays in this article.
Microphysiological system (MPS)
MPS devices
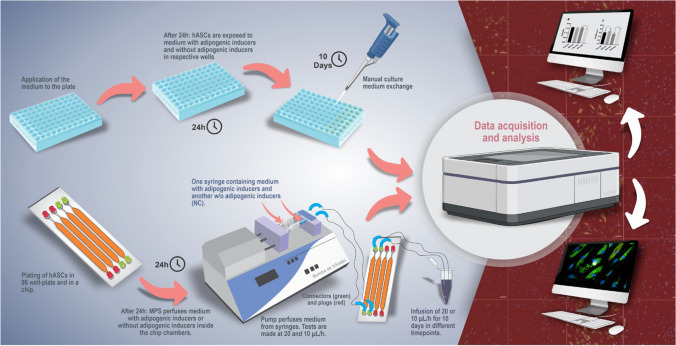
For building up the in vitro MPS, commercial devices Fluidic 221 (Cat. Num. 10000047, ChipShop GmbH, Jena, Germany) with four chambers of 1.59 cm2 each, 100-μL volume, and 600-μm depth were used to culture cells. We selected a device made of zeonor with a thickness of 188 µm, because it offers excellent optical characteristics, very low water absorption, and extremely low permeability. Connectors (Cat. Num. 10000116, ChipShop GmbH, Jena, Germany) and plugs (ChipShop, Cat. Num. 10000054, ChipShop GmbH, Jena, Germany) were used to connect tubes and seal the chambers containing cells on the chips, respectively. Poly-tetra-fluoroethylene (PTFE) tubes with an inner diameter of 0.5 mm and an external diameter of 1 mm were used to generate a laminar flow in the MPS (Cat. Num. 10000032, ChipShop GmbH, Jena, Germany). Luer Lock disposable syringes of 3 mL (Cat. Num. 990174, Luer Lock BD® syringes) were filled with medium. Because of its flexibility and elasticity, silicone tubes with an inner diameter of 0.5 mm and an outer diameter of 2.5 mm (Cat. Num. 10000033, ChipShop GmbH, Jena, Germany) were used in the connections among plugs in the chips, PTFE tubes, and syringes. An infusion pump with two syringes (model EFF-311, Insight Equipment’s Ltda. EPP, Ribeirão Preto, Brazil) was used to infuse the media into the PTFE tubes leading to the chip chambers (inlet) in a laminar flow. MPS (including pumps) was placed in an incubator at 37 °C and in a 5% CO2 atmosphere. Infusion rates of 10 and 20 µL/h (7.11e − 5 and 1.47e − 4 dyn/cm2, respectively) were tested (Fig. 1). To verify if the infusion rates were correct, the medium was collected at different timepoints and weighed on a scale.
Fig. 1.
Design of the MPS and culture in 96-well plates. The image represents an experiment to verify the differentiation of hASCs (10 days in MPS and 96-well plates). The chip is positioned vertically to direct possible bubbles inside the system to the outlet with the fluid flow coming from the inlet. Proliferation tests were performed in 72 h of constant fluid flow at 10 µL/h (7.11e − 5 dyn/cm2) and did not contain medium with adipogenic inducers. hASCs, human adipose mesenchymal stromal/stem cells; MPS, microphysiological system; NC, negative control; PTFE, poly-tetra-fluoroethylene
Culture of hASCs in the MPS
Twenty-four hours before perfusion in the MPS, hASCs (14.55 × 104 cells/chamber) were plated in two chambers of the Fluidic 221 chip in 100 μL of the described culture medium (“Stem cells and culture conditions”), and interfaces were closed with plugs. After 24 h at 37 °C and 5% CO2, cells reached ~ 80 to 90% of confluence, and each chamber was connected to a PTFE tube and a syringe containing culture medium with the specified supplements and antibiotics (100 units/ mL of penicillin and 100 μg/mL of streptomycin—Gibco Invitrogen®, Carlsbad, CA, USA—Cat. Num. 15,140,122). In chambers with cells undergoing adipogenic differentiation, the medium containing adipogenic induction factors was infused (see description in “Adipogenic differentiation”) at rates of 20 and 10 µL/h. As a control for the standard cell culture method, hASCs were plated (3.5 × 103 cells/well) in 96-well plates in the same conditions. Medium changes were performed in the MPS syringes and 96-well plates every 3 or 4 days until day 10 (Fig. 1).
hASC proliferation
To observe if hASC cells normally undergo cell cycle (G1, S, G2, and mitosis) and proliferation within the models after plating, the number of cells marked with Ki-67 protein was assessed after 72 h in culture, according to Reus et al. [24] with modifications. Briefly, cells were plated in the MPS (19.55 × 103 cells/chamber), in the chip w/o fluid flow and 96-well plates (5.25 × 103 cells/chamber) and incubated for 24 h at 37 °C and 5% CO2. After a 24-h incubation period, cells were cultured in a dynamic culture in the MPS and the static condition for 72 h. Cells were then fixed with 4% paraformaldehyde (Cat. Num. 95 30525–89-4, Sigma-Aldrich®, Saint Louis, Missouri, USA) for 10 min. Cells were stained with the primary Ki-67 antibody (clone SP6) 1:300 dilution (Cat Num. MA-514520, Invitrogen) and with the goat anti-Rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 (Cat. Num. A10523, A-11008, Invitrogen) (1:800 dilution) in phosphate-buffered saline (PBS) containing 1% BSA. Nuclei were stained with 0.1% DAPI (Cat num. D9542, Sigma- Aldrich, Saint Louis, Missouri, USA). Nonspecific staining was assessed with the secondary antibody alone. Images were captured at × 20 magnification with Operetta CLS® (PerkinElmer®, Waltham, MA, USA). Proliferation was assessed by defining a mean intensity cut-off between negative and positive groups for Ki-67, using Harmony 4.8® software for Operetta CLS™ high-content screening systems.
Total nuclei count (stained with DAPI) and cell proliferating (Ki-67- positive) were used to calculate the percentage (%) of proliferation by the following formula:
| 1 |
Adipogenic differentiation
After 24 h of incubation, one of the chambers of the Fluidic 221 was connected to the MPS, and a medium containing adipogenic inducers was infused in the chamber for cells undergoing adipogenic differentiation. The medium was prepared according to Abud et al. [14] and patent (BR 102015025791–0 A2) with modifications—routine culture medium containing 100 units/mL of penicillin and 100 μg/mL of streptomycin (Cat. Num. 15140122, Gibco Invitrogen®, Carlsbad, CA, USA), 1 μg/mL insulin (Cat. Num. I6634, Sigma-Aldrich®, St. Louis, MO, USA), 1 μM dexamethasone (Cat. Num. D2915, Sigma-Aldrich®, St. Louis, MO, USA), 200 μM indomethacin (Cat. Num. I7378, Sigma-Aldrich®, St. Louis, MO, USA), and 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) (Cat. Num. I5879, Sigma -Aldrich®, St. Louis, MO, USA). hASCs also underwent differentiation in 96-well plates for later comparison of the methods. Each assay had a negative control (NC) of differentiation (hASCs cultured in DMEM with antibiotics and w/o adipogenic inducers). Additionally, the cells were differentiated in the chip chamber without the fluid flow, as a control for the influence of chip fluidic 221 material (chip condition). After 10 days of differentiation, the cells were fixed with 4% paraformaldehyde (Cat. Num. 95 30525–89-4, Sigma-Aldrich®, Saint Louis, Missouri, USA) for 10 min and stained with Nile red (1000 µg/mL) diluted in PBS 1 × for 30 min (Cat. Num. N3013, Sigma-Aldrich®, St Louis, MO, USA) for detection of lipid droplets, and cell nuclei was stained with DAPI (Cat num. D9542, Sigma-Aldrich, Saint Louis, Missouri, USA) for 10 min. Images were captured at × 20 magnification with Operetta CLS® (PerkinElmer®, Waltham, MA, USA) and analyzed using the Harmony® 4.8 software (Perkin Elmer). Cell differentiation was assessed by defining a mean intensity cut-off between negative and positive groups for the Nile red stain. Total nuclei count (DAPI-positive) and positive cells for adipogenic differentiation (Nile red positive) were used to calculate the percentage of differentiation by the following formula:
| 2 |
The percentage of area occupied with lipid droplets was calculated to assess the efficiency of adipogenic differentiation. The sum of the cell area and the sum of the lipid droplet area in each group were used as parameters. The lipid droplet area was assessed by finding Nile red–positive cells (differentiated cells) and then defining an additional mean intensity cut-off to select regions with lipid droplets within these cells. The following equation then calculated the percent (%) area occupied with lipid droplet
| 3 |
s:
After acquiring data, the level of lipid droplets in MPS or in the chip was calculated by using a fold-change of % of the area with lipid droplets over % of the area with lipid droplets in 96-well plates, with the following formula:
| 4 |
Expression level of FABP4 gene
The expression of FABP4 (FABP4 fatty acid binding protein 4 [Homo sapiens (human)]), a gene which plays a pivotal role in the regulation of lipid storage [25], was quantified in the cells on day 10. Briefly, 14.55 × 104 cells/chamber in the chip and 20 × 103 cells/well in a 24-well plate were grown for 24 h and were treated for 10 days with differentiation medium previously described. RNA was extracted using the Direct-zol™ RNA MiniPrep (Cat. Num. R2053, Zymo Research), following the manufacturer’s instructions. The cDNA was then obtained by ImProm-II™ Reverse Transcription System (Cat. Num. A3800, Promega). The expression of FABP4 was quantified by quantitative reverse transcription polymerase chain reaction (RT-qPCR) using Power SYBR® Green PCR Master Mix (Cat. Num. 4367659; Applied Biosystems).
POLR2A [polymerase (RNA) II (DNA directed) polypeptide A] was used as an endogenous control, and the expression level of FABP4 was normalized relative to the control by the 2 − ΔΔCT method, as previously described [26]. MPS condition was compared with plate condition. The differences related to the plate were considered significant when fold-change > 2 and p < 0.05. The qPCR reaction was performed using the QuantStudio™ 5 Real-Time PCR system (Thermo Fisher, Waltham, MA, USA) and the annealing/extension temperature of 60 °C (60 s). The data was analyzed with the QuantStudio™ Design and Analysis Software version 2.6.0. RT-qPCR was performed in technical and biological replicates (n = 3). The primer sequences are presented in Table 1.
Table 1.
Primer sequences used in the RT-qPCR
| Genes | Direction | Sequence |
|---|---|---|
| POLR2A | F | 5′-TACCACGTCATCTCCTTTGATGGCT-3′ |
| R | 5′-GTGCGGCTGCTTCCATAA-3′ | |
| FABP4 | F | 5'-ACAGCACCCTCCTGAAAACT-3' |
| R | 5'-CAATGCGAACTTCAGTCCAGG-3' |
F forward, R reverse
Cell and nuclei morphology of cells cultured in MPS
After 10 days of differentiation in MPS condition and 96-well plate well condition, the cells were fixed with 4% paraformaldehyde (Cat. Num. 95 30525–89-4, Sigma-Aldrich®, Saint Louis, Missouri, USA), permeabilized 0.5% Triton X-100/PBS for 30 min, and stained with Alexa Fluor™ 488 Phalloidin (Cat. Num. A12379) (1:400) in 1%BSA/PBS for 30 min at R.T. After incubation, the samples were washed thrice for 5 min with 1% BSA/PBS and stained with Nile red (1000 µg/mL) diluted in PBS 1 × for 30 min (Cat. Num. N3013, Sigma-Aldrich®, St Louis, MO, USA) for detection of lipid droplets, and cell nuclei were stained with DAPI (Cat num. D9542, Sigma-Aldrich, Saint Louis, Missouri, USA) for 10 min. Images were captured at × 20 magnification with Operetta CLS® (PerkinElmer®, Waltham, MA, USA) and analyzed using Harmony® 4.8 software (Perkin Elmer). Cell morphology was studied on whole populations of differentiated and undifferentiated hASCs from each donor. Cell and nucleus morphology parameters were analyzed using Harmony 4.8 software, employing a predefined protocol based on the building blocks method. Building blocks included segmentation of the images using “Find nuclei” and “Find Cytoplasm.” After image segmentation, a set of basic intensity and morphological properties (area, roundness, perimeter, width, length, width to length rate, symmetry, threshold compactness, and axial and radial properties of nuclei) were selected. Texture patterns of cytoplasm and nuclei were calculated using “Calculate Intensity Properties,” “Calculate Morphology Properties,” and “SER texture analysis” building blocks, respectively. The SER (Spot, Edge, Ridge) features method includes a set of eight patterns (Spot, Hole, Edge, Ridge, Valley, Saddle, Bright, and Dark), which are sensitive to distinct intensity patterns according to the property geometry designation and identify cytoskeletal and nuclear structure differences. All results are reported as mean ± SD from five independent experiments, each performed in quadruplicate.
Cell direction under dynamic fluid flow
The image regions obtained in the morphological analysis were also analyzed regarding the dominant direction of cells, using the ImageJ (Fiji) software (ImageJ 1.54f (Fiji) Java 1.8.0_322). For that, OrientationJ (OJ) tool was used to manually measure the orientation and coherency in regions of interest (ROI), which generated an ellipse-oriented indicating the dominant direction of the cells. Angle was selected as a unit of measure. A macro was created and applied to all the images. Analysis was performed with three donors, and eight regions were selected for each donor both in MPS and plate condition. Each region presented five subregions. The dominant direction of the five subregions was calculated by OJ and plotted as one result in GraphPad Prism version 8 for Windows (GraphPad Software, San Diego, CA, USA).
High-content imaging analysis
Images of cells from each individual donor were acquired using the High Content Imaging Operetta CLS®, (PerkinElmer®, Waltham, MA, USA), with configuration and channels set according to Abud et al. (2021). Screening was conducted with a × 20 water immersion objective (NA 1.0, working distance 1.7 mm, depth of focus 1.8 mm, effective xy resolution 0.66 mm) and three excitation lasers (DAPI: ex 355–285 nm, em 430–500 nm; Alexa Fluor 488: ex 488 nm, em 500–550 nm; Alexa 546: ex 530–560 nm, em 570–650 nm or other suitable adjustments). Forty different fields of view were imaged per chamber in Fluidic 221 chips using two predetermined Z-focus planes with laser-based autofocusing. For the 96-well plates, 13 images per well in four wells were acquired. The images were analyzed using Harmony® 4.8 software (Perkin Elmer).
Statistical analysis, graphs, and figures
Statistical significance between cells in the MPS culture and standard culture method (96-well plate) was determined by testing the data for normality using the Shapiro–Wilk test and performing unpaired two-tailed Student’s t test, and graphs were made using GraphPad Prism version 8 for Windows (GraphPad Software, San Diego, CA, USA). Differences were considered significant when p < 0.05. Figure boards and illustrations were made using Inkscape 1.3.2 (091e20e, 2023–11-25, custom) and ImageJ 1.54f (Fiji) Java 1.8.0_322 for cell direction analysis.
Results
hASC culture and differentiation in MPS
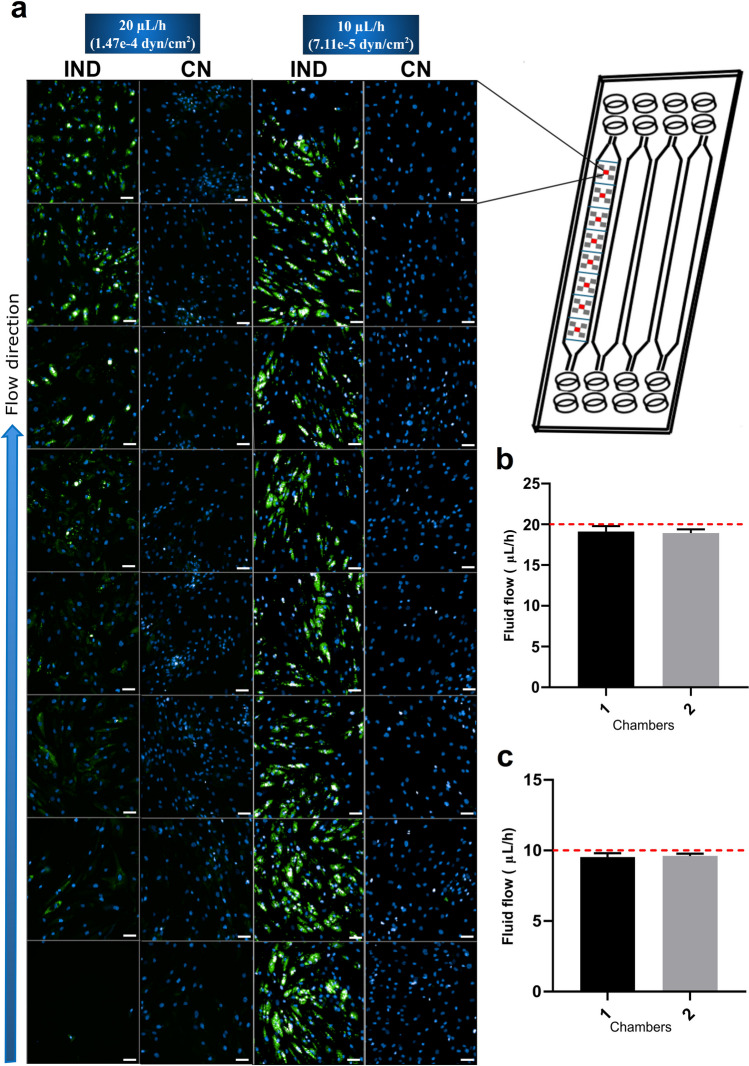
hASC from different donors were seeded and grown in MPS. Cells showed optimal growth when using a medium constant flow of 20 and 10 µL/h. Regarding adipogenic differentiation, setting the flow rate at 20 µL/h generated a gradient of differentiation, where cells in the inlet differentiated less than cells at the end of the chamber. On the other hand, cells show a homogeneous differentiation along the chip chamber when the flow rate is 10 µL/h (Fig. 2). Thus, the subsequent experiments were performed with hASCs cultivated in the MPS at a flow rate of 10 µL/h.
Fig. 2.
hASC differentiation potential is better under fluid flows of 10 µL/h. a Culture of differentiated and undifferentiated hASCs (left) under medium flow (blue arrow) of 20 and 10 µL/h (1.47e − 4 and 7.11e − 5 dyn/cm2, respectively) throughout the chambers of chip Fluidic 221, demonstrating a gradient of differentiation with fluid flow with MPS at 20 µL/h and a homogeneous differentiation at 10 µL/h. For the photos, the chip (represented on the right) had its chambers divided into eight regions, where five photos were captured by Operetta CLS. One of the five photos within all eight regions was selected (red block). The photos are from cells of the same donor (donor 1) in both fluid flows. b MPS using a fluid flow at 20 µL/h shows consistency in medium volume measured with different time points. c MPS using a fluid flow at 10 µL/h shows consistency in medium volume measured with different time points. Fluid rate was verified by measuring media volume weight coming from the outlet, which was collected in microtubes connected to the PTFE tubes. After 10 days, cells were fixed and stained with Nile red (lipid droplets in green) and DAPI (nuclei in blue). NR, Nile red; NC, negative control; Plate, 96-well plate; MPS, microphysiological system; IND, induced with adipogenic differentiation medium. Data are expressed in mean ± SD of 30 measurements (n = 30) (b, c). Scale bars represent 10 µm
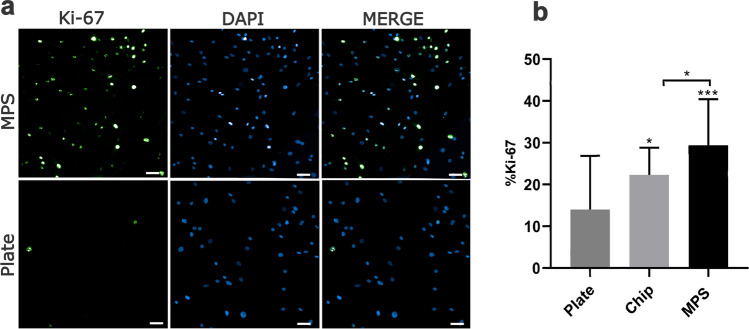
MPS increases hASCs proliferation
We compared hASCs proliferation when cultivated in MPS or 96-well plates for 72 h. Cells were labeled to detect Ki-67 expression. Results show that hASCs in the MPS present statistically significantly higher Ki-67 expression than cells in 96-well plates and in the chip (Fig. 3a, b).
Fig. 3.
Ki-67 expression in hASCs cells is higher in dynamic culture (MPS) than in static conditions (96-well plate). a Qualitative assessment of Ki-67. Ki-67 (green) expression is higher when hASCs are cultivated in MPS compared with plates. b Quantitative assessment of Ki-67 expression in the plate, chip, and MPS. After 72 h, cells were fixed and stained with anti-Ki-67 (nuclei stained, in green) and with DAPI (nuclei in blue). Plate, 96-well plate. MPS, microphysiological system. Data are expressed as mean ± SD and represent three independent experiments (n = 3). Statistical significance between MPS and plate was determined by comparing group means by paired t test with two-tailed p value. ***p > 0.001. Scale bars represent 10 µm
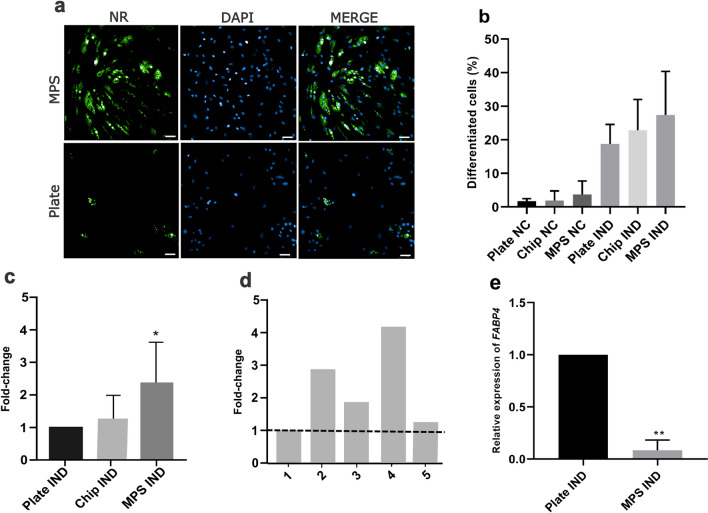
MPS optimizes hASC adipogenic differentiation
hASCs adipogenic differentiation was tested in MPS, the chip and 96-well plates for 10 days after induction. hASCs differentiated in MPS culture under a constant rate flow presented a larger area with lipid droplets relative to the cells differentiated in the chip (w/o fluid flow) and in 96-well plates (Fig. 4a–c), although the number of cells undergoing differentiation on day 10 (positive cells for the presence of lipid droplets) is not statistically significant between the tested conditions (Fig. 4b). These differences were variable among the tested cell donors, but never lower than in 96-well plates (Fig. 4d). To understand the molecular mechanism associated with the increase in lipid droplets in the MPS, gene expression of FABP4, which controls fat mass in adipocytes [25], was evaluated. Additionally, induced cells from the MPS presented a statistically lower expression of FABP4 on day 10 than cell cultured in 96-well plates (Fig. 4e).
Fig. 4.
MPS increases the differentiation potential of hASCs. a Qualitative assessment of differentiated hASCs (with lipid droplets stained in green) in MPS and static condition (96-well plates). The photo is representative of 1 of 13 photos captured in 1 replica of donor 2. b The percentage of differentiated cells indicates that hASC differentiation to adipocytes is not statistically different among dynamic (MPS) and static conditions (96-well plates). c Fold-change of % area of differentiation (lipid droplets) in MPS IND, Chip IND, and Plates IND over % area with lipid droplets in 96-well plates in induced condition. d Fold-change of the area of differentiation (lipid droplets) in cells of different hASCs donors cultured in MPS and compared to their respective differentiation in 96-well plates (dotted line) shows higher differentiation in a dynamic culture. e Gene expression of FABP4 gene in cells on day 10 in the plate and in the MPS. After 10 days, cells were fixed and stained with Nile red (lipid droplets in green) and DAPI (nuclei in blue). The area occupied by cells was calculated through Operetta analysis and converted into SI (a–d). NR, Nile red; NC, negative control; Plate, 96-well plate; MPS, microphysiological system; IND, induced with adipogenic differentiation medium. Fold-change is calculated as % of the area with lipid droplets in MPS over % of the area with lipid droplets in a 96-well plate. Statistical significance between IND Plate and IND MPS was determined by comparing their means by paired T test with two-tailed p value *p < 0.05 (a–d). Significance in RT-qPCR was determined by one-way ANOVA followed by Tukey’s multiple comparisons test using ΔΔCT values of the plate-IND and MPS- cells with *p < 0.05. Data are expressed in mean ± SD and represent five independent experiments (n = 5) (b, c), one representative experiment of each donor (n = 1) (d), and three donors (n = 3) (e). Scale bars represent 10 µm
MPS-cultured hASCs exhibit modifications in cell and nucleus morphology
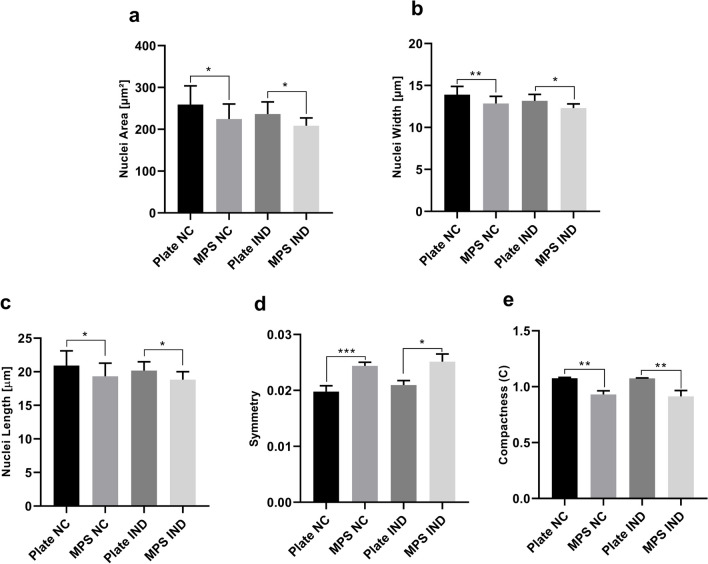
Morphometric characterization of cells differentiated in MPS and 96-well plates was analyzed on day 10. Cell cytoplasm and nucleus morphology were evaluated separately, considering area, roundness, perimeter, with, length, width to length rate, symmetry, compactness, and axial and radial properties of nuclei. In addition, texture parameters—SER analysis, including eight patterns (Spot, Hole, Edge, Ridge, Valley, Saddle, Bright, Dark)—were determined to identify cytoskeletal and nuclear structure differences. Some of the analyzed parameters did not show statistical differences between the two methods of culture (data not shown). Statistically significant differences were observed for cell length, symmetry, radial relative deviation, and compactness (Fig. 5a–d), showing that cells cultured in MPS are less symmetrical, compact, and present lower radial relative deviation; these cells also present higher length than cells cultured in 96-well plates. Additionally, cells in MPS present significantly smaller nuclei with a smaller width, length, and lower compactness (Fig. 6) than hASCs in 96-well plates.
Fig. 5.
hASCs cultivated in MPS and 96-well plates show different cell morphology regardless of being differentiated or not. a Qualitative assessment of differentiated hASCs (with lipid droplets stained in green) in static and MPS conditions. The photo is representative of the differentiation of the same donor in MPS and the 96-well plate (donor 5). The red lines call attention to the characteristic distribution of lipid droplets within cells with significantly higher length (in MPS) and with higher width (in plates). b hASCs differentiated into adipocytes in plates are statistically more symmetrical than in MPS. c Cells in MPS present higher length (µm) than cells cultured in 96-well plates. d hASCs differentiated into adipocytes in MPS present statistically significantly higher radial relative deviation than cells cultured in 96-well plates. e hASC in MPS are statistically more compact than cells cultured in 96-well plates. The parameters were calculated through Operetta analysis after 10 days in 96-well plates and MPS with a medium infusion of 10 µL/h. NC, negative control; Plate, 96-well plate; MPS, microphysiological system; IND, induced with adipogenic differentiation medium. Plate NC was compared with MPS NC and IND Plate and IND MPS. Statistical significance among these data was determined by comparing their means by paired T test with two-tailed P value. *p < 0.05; **p < 0.01; ***p < 0.001. Data are expressed in mean ± SD and represent five independent experiments (n = 5). Scale bars represent 10 µm
Fig. 6.
Nuclei of hASCs cultivated in MPS present different morphology from nuclei of hASCs cultivated in 96-well plates, regardless of whether they go through differentiation to adipocytes or not. a Nuclei of hASCs cultured in MPS are statistically smaller than nuclei of cells cultured in 96-well plates. b hASCs cultured in MPS present nuclei with statistically smaller widths than cells cultured in 96-well plates. c hASCs cultured in MPS present nuclei with statistically smaller lengths than nuclei of cells cultured in 96-well plates. d Nuclei of hASCs in MPS are more symmetric than the nuclei of cells cultured in 96-well plates. e Nuclei of hASC in MPS are statistically less compact than nuclei of cells cultured in 96-well plates. The parameters were calculated through Operetta analysis after 10 days in 96-well plates and in MPS with the medium infusion of 10 µL/h. NC, negative control; Plate, 96-well plate; MPS, microphysiological system; IND, induced with adipogenic differentiation medium. Plate NC was compared with MPS NC and IND Plate and IND MPS. Statistical significance among these data was determined by comparing their means with a paired T test with a two-tailed p value. *p < 0.05; **p < 0.01. Data are expressed in mean ± SD and represent five independent experiments (n = 5)
Additionally, analysis of SER texture parameters was performed to obtain broader information about the morphological variety of hASCs cells among the models. According to statistical analysis, hASC cells cultivated in the MPS present a cytoplasm that is more textured than in hASCs cultivated in 96-well plates, regarding Edge, Valley (significantly more textured in undifferentiated and differentiated cells), and Dark (for differentiated cells) (Fig. 7b, c, respectively). Nuclei from hASC cultivated in MPS also showed statistically significantly higher texture than cells cultured in plates (Fig. 7d, e, respectively).
Fig. 7.
Cytoplasm and nuclei of hASCs cultivated in MPS are statistically significantly more textured than cells cultivated in 96-well plates. a Qualitative assessment of differentiated hASCs (with cytoskeleton in green, lipid droplets in yellow, and nuclei in blue) in MPS and plate condition. The photo is representative of the differentiation of the same donor in MPS and the 96-well plate (donor 1). b Cytoplasm SER texture indexes of NC hASCs cultivated in plates and in MPS. Edge and Valley SER features are significantly higher in cells from MPS than in cells of plates (used as a control of the MPS). c Cytoplasm SER texture indexes of differentiated hASCs cells in plate and MPS. Edge, Valley, and Dark SER features are significantly higher in cells from MPS than in cells of plates. d Nuclei SER texture indexes of undifferentiated NC hASCs cells cultivated in plates and MPS. All SER features of MPS are significantly different from those of plates, except for Hole. e Nuclei SER texture indexes of differentiared hASCs cells cultivated in plates and MPS. All SER features of MPS are significantly different from those of plates. The parameters were calculated through Operetta analysis after 10 days in 96-well plates and MPS with a medium infusion of 10 µL/h. NC, negative control (undifferentiated cells); Plate, 96-well plate; MPS, microphysiological system; IND, induced with adipogenic differentiation medium. Plate NC was compared with MPS NC and IND Plate and IND MPS. Statistical significance among these data was determined by comparing their means with a paired T test with a two-tailed p value. *p < 0.05; **p < 0.01; ***p < 0.001. Data are expressed in mean ± SD and represent five independent experiments (n = 5). Scale bars represent 10 µm
MPS-cultured hASCs direction is not influenced by the applied fluid flow
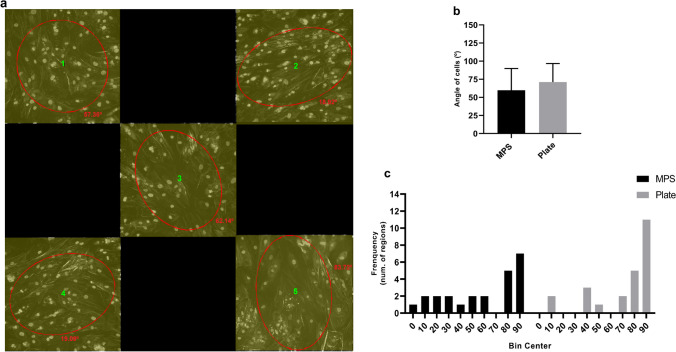
To understand if hASCs could be aligned in the direction of the fluid flow within the MPS, cell direction was also evaluated in both models. Additionally, a perpendicular direction (90º) in the MPS would demonstrate an organization of the cells inside the device that aligned with the direction of infusion of the flow, while angles next to zero represent a more horizontal organization. As demonstrated, cells were organized in variable directions inside the chamber (Fig. 8a). Additionally, no statistically significant differences were observed among MPS and plates, with dominant directions of 60º and 71º, respectively (Fig. 8b). The distribution of these angles within each model can be seen in Fig. 8c.
Fig. 8.
Direction of cells cultivated in MPS and plates. a Variable directions inside regions within the MPS. The image is representative of eight image regions with five subregions analyzed for direction using OrientationJ (OJ) measurement. Each ROI (1–5) is shown in yellow and presents an ellipse oriented in the general direction of cells after analysis. OJ calculated angles of the ellipses, which were added to the image (numbers in red). b Dominant direction of cells in MPS and plates. c Frequency distribution of angles within regions of MPS and plate. The parameters were calculated through ImageJ (Fiji software) after 10 days in 96-well plates and MPS with a medium infusion of 10 µL/h. NC, negative control; Plate, 96-well plate; MPS, microphysiological system. Plate was compared with MPS. Statistical significance among these data was determined by comparing their means with a paired T test with a two-tailed p value. Data are expressed in mean ± SD and represent 24 regions (n = 24) from images of three independent experiments. Each region value is obtained from results of the dominant direction of five subregions
Discussion
Despite its outstanding performance, microfluidic devices still face challenges due to factors like unstable cell viability in the chip and fluid flow injection, which affects cellular behavior [27, 28]. Assessing the impact of different MPS culture conditions on stem cell adipogenesis is crucial for developing more microphysiological models that can be used to study AT. Hence, in this study, we analyzed cell proliferation, adipogenesis, and the morphology features of hASCs in a simple MPS platform compared with the standard culture method in 96-well plates. An organ-on-a-chip platform requires strict environmental control and monitoring. Fluid management and sterility are essential factors in successful cell culture [19, 29]. Thus, the usability of a device is often intersected with flow testing. Before performing the proposed tests, we established a steady flow rate (10 µL/h) applied to hASCs in the MPS, which is essential to maintain the reproducibility of data acquired.
The increase in the proliferation capacity of hASCs in a fluid flow is demonstrated by several studies, as revised by Geraili et al. [30]. Additionally, these cells can respond to extremely low levels of shear flow, for example, using a passive osmosis-driven pump (flow rate down to the 1 μL/h order of magnitude) [31]. The proposed MPS condition in the present study increases the proliferation rate of hASCs, which was shown by a statistically significant increase in Ki-67 expression in hASCs cultured in MPS compared with plates and chips. Different time points were not investigated, because our aim was to determine how hASCs were behaving in regard to cell cycle at a determined timepoint after plating. In our model, hASCs presented a fourfold increase in proliferation after 72 h at a flow rate of 10 µL/h (over plate condition). It is already recognized that shear stress generated by fluid flow can activate cellular signaling involved in cellular proliferation and differentiation in MSCs [32], but the exact mechanism in different models remains to be investigated.
Some described models using fluid flow may increase proliferation rates in culture but when submitted to differentiation medium cells also decrease differentiation capacity, which is negatively correlated [32]. In the present study MPS, chip, and 96-well plates are statistically equal regarding the number of cells undergoing differentiation. However, the MPS had a statistically significant larger area occupied by lipid droplets in the culture after 10 days of differentiation than in 96-well plate conditions. As it is already recognized that several characteristics of materials determine the cell physiology and regulate stem cell differentiation [33], cells in the chip (Fluidic 221 device w/o fluid flow) were also analyzed. It was demonstrated that the chip can increase hASC proliferation compared to 96-well plates, but the proliferation in the MPS is still significantly higher compared with the chip. Although, there is not a statistically significant difference in differentiation area when comparing chips and MPS. This demonstrates that the material properties of the chip may still have influence in cellular behavior, as proliferation is increased independently of fluid flow, but that differentiation in the MPS model might be influenced primarily by the fluid flow rather than by material characteristics.
Previous studies have addressed the mechano-responsiveness of MSCs and have shown that their differentiation into adipocytes may be regulated by several types of physical stimulation, including shear stress, hydrostatic pressure, tension, and microgravity [34]. The effects of a fluidic flow on the adipogenic differentiation and maturation have already been studied in some cell lines, such as in human bone marrow mesenchymal stem cells (hBMSCs) using a peristaltic laminar flow and in 3T3-L1 murine preadipocyte cell line using a cyclic fluid flow [34–36]. A study shows that spheroids of hASCs under perfusion with a flow rate per area of 0.16 mL cm−2 min−1 in a bioreactor system are more driven to adipogenic differentiation than osteogenic and endothelial differentiation [37]. However, to our knowledge, to this date, the influence of a laminar flow has not been tested for hASC adipogenic differentiation.
Given that the MPS exhibited a statistically significant increase in lipid droplet area compared to the 96-well plates, despite having an equal number of differentiated cells in both models, we investigated the expression of the FABP4 gene to understand the mechanism behind the enhanced intracellular lipid mass accumulation in MPS. Fatty acid-binding protein 4 gene (FABP4) expression regulates oleic acid–induced PPARG activity in adipocytes and plays a pivotal role in adipocyte lipid storage, distribution, transportation, decomposition, metabolism, and homeostasis [25, 38]. Additionally, it is generally constant during lipid accumulation and in mature adipocytes. Our results show MPS presented a lower expression of FABP4 than the 96-well plates on day 10. The main function of FABP4 is to regulate the metabolism of fatty acids, and FABP4 has been shown to be released from adipocytes in a non-classical pathway associated with lipolysis, acting as an adipokine and thus reducing the intracellular triglyceride content [38]. The low expression of FABP4 in the MPS is in accordance with higher accumulation of lipid droplets and is an indicator that the MPS might have different homeostasis and control of lipids in the cell, compared with the plates.
Overall, we demonstrate that hASCs in chip chambers increase their proliferation and differentiation potential by increasing the adipocyte area of lipid droplets in a fluid flow of 10 µL/h (7.11e − 5 dyn/cm2). The observable phenotype of adipocytes in MPS suggests that this culture method can create an adequate microenvironment for hASCs adipogenic differentiation. This was observed for several donors, including a commercial cell line (donor 5). This is of particular interest, as hASCs are generally acquired after liposuction surgeries and enable patient-specific experiments, where testing can be performed under multiple experimental and control conditions, providing the possibility of dealing with inter-donor variability [39].
Cell morphology was evaluated by high-content image analysis to understand the effects of fluidic flow on these cells by comparing a set of morphological features of non-differentiated cells in MPS to that of the respective donor in static condition and of differentiated hASCs in MPS to cells in static condition after 10 days of culture.
As observed, hASCs cultivated in MPS are significantly less symmetrical and present higher length, lower radial relative deviation, and lower compactness than cells cultivated in 96-well plates. Additionally, the cytoplasm of these cells is more textured. With more edges and valleys in undifferentiated cells, and with more edges, valleys, and darks in differentiated cells. Kowal et al. [40] have verified the influence of cell area and cell shape in human bone marrow stem cells from different donors and described that smaller cell area is correlated with more proliferative capacity. In contrast, cell area, shape, and cytoplasm texture were not correlated with adipocyte differentiation capacity (also estimated by the lipid droplet area of mature adipocytes) in the same study. Guo et al. [41] described that external physical cues in culture methods may lead to cell water influx/efflux, which cause changes in subcellular macromolecular density (a measure for compactness), which is sufficient to alter their differentiation potential. As some studies reported that changes in MSC morphology in MPS occur to align their structure in a direction perpendicular to the fluid flow [33, 42], the overall direction of cells was calculated. In the analysis, angles next to 90º demonstrate a distribution of cells in the direction of the flow, while the closer to zero, the more horizontal the distribution of cells is. However, cells have similar angles of distribution in the MPS and in plates, showing that fluid flow at 10 µL/h (7.11e − 5 dyn/cm2) does not affect cell arrangement in the present MPS model.
Regarding nuclei morphology, hASCs cultivated in MPS have smaller and more symmetrical nuclei, with smaller width and length and lower compactness than respective hASCs cultivated in 96-well plates. These nuclei are also more textured than the nuclei of hASCs cultivated in 96-well plates. Multivariable analysis identified nucleus geometry as the most stable predictor factor of the differentiation capacity of cells [40]. The cell nucleus has been proposed to function as a mechano-sensor, where cytoskeleton and nucleoskeleton linkers transmit extracellular and cytoplasmic forces that alter the nuclear shape, thus affecting chromatin organization and transcriptional activity as well as cellular differentiation [43–45].
Conclusion
The current study demonstrates the favorable behavior of hASCs in a chip with a simple design, rendering robust adipogenic differentiation with increased lipid accumulation and high levels of cell proliferation. This highlights the applicability of an Adipo-on-a-chip system made of hASCs and how sensitively such a culture method works for hASCs under a fluid flow of 10 µL/h. Additionally, the cell characteristics of these cells inside the fluidic flow were described.
Overall, we presented the development of a reliable and biologically relevant culture in an MPS device for hASCs from different donors that may help to reliably assure their quality regarding proliferation and differentiation for appropriate in vitro studies aiming further investigation of adipose physiological, pathophysiological, and toxicological approaches based on the differentiation potential of these cells.
Data availability
Data will be available on request.
Declarations
Conflict of interest
The authors declare no competing interests.
References
- 1.Auger C, Kajimura S. Adipose tissue remodeling in pathophysiology. Annu Rev Pathol. 2023;18:71–93. 10.1146/annurev-pathol-042220-023633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22. 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2019 Risk Factor Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1223–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustieles V, Arrebola JP. How polluted is your fat? What the study of adipose tissue can contribute to environmental epidemiology. J Epidemiol Community Health. 2020;74(5):401–7. 10.1136/jech-2019-213181. [DOI] [PubMed] [Google Scholar]
- 5.Rogal J, Roosz J, Teufel C, et al. Autologous human immunocompetent white adipose tissue-on-chip. Adv Sci (Weinh). 2022;9(18):e2104451. 10.1002/advs.202104451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lercher A, Baazim H, Bergthaler A. Systemic Immunometabolism: challenges and opportunities. Immunity. 2020;53(3):496–509. 10.1016/j.immuni.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gesta S, Lolmède K, Daviaud D, et al. Culture of human adipose tissue explants leads to profound alteration of adipocyte gene expression. Horm Metab Res. 2003;35(3):158–63. 10.1055/s-2003-39070. [DOI] [PubMed] [Google Scholar]
- 8.Dufau J, Shen JX, Couchet M, et al. In vitro and ex vivo models of adipocytes. Am J Physiol Cell Physiol. 2021;320(5):C822–41. 10.1152/ajpcell.00519.2020. [DOI] [PubMed] [Google Scholar]
- 9.Gibler P, Gimble J, Hamel K, et al. Human adipose-derived stromal/stem cell culture and analysis methods for adipose tissue modeling in vitro: a systematic review. Cells. 2021;10(6):1378. 10.3390/cells10061378. Published 2021 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakab J, Miškić B, Mikšić Š, et al. Adipogenesis as a potential anti-obesity target: a review of pharmacological treatment and natural products. Diabetes Metab Syndr Obes. 2021;14:67–83. 10.2147/DMSO.S281186. Published 2021 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20(4):242–58. 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- 12.Hammarstedt A, Gogg S, Hedjazifar S, Nerstedt A, Smith U. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol Rev. 2018;98(4):1911–41. 10.1152/physrev.00034.2017. [DOI] [PubMed] [Google Scholar]
- 13.Abud APR, Kuligovski C, Corrêa NCR, de Moraes ECP, Caruso RRB, Schuck DC, Brohem CA, Dallagiovanna B, de Aguiar AM. The inhibition of adipogenesis via an in vitro assay can reduce animal use by more precisely estimating the starting dose for the acute toxic class method. Toxicol Lett. 2019;1(311):80–90. 10.1016/j.toxlet.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Abud APR, Paschoal ACC, Kuligovski C, Caruso RRB, Dallagiovanna B, de Aguiar AM. Using inhibition of the adipogenesis of adipose-derived stem cells invitro for toxicity prediction. MethodsX. 2021;8:101515. 10.1016/j.mex.2021.101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikłosz A, Nikitiuk BE, Chabowski A. Using adipose-derived mesenchymal stem cells to fight the metabolic complications of obesity: where do we stand? Obes Rev. 2022;23(5):e13413. 10.1111/obr.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pham DV, Nguyen TK, Park PH. Adipokines at the crossroads of obesity and mesenchymal stem cell therapy. Exp Mol Med. 2023;55(2):313–24. 10.1038/s12276-023-00940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abud AP, Zych J, Reus TL, Kuligovski C, de Moraes E, Dallagiovanna B, de Aguiar AM. The use of human adipose-derived stem cells based cytotoxicity assay for acute toxicity test. Regul Toxicol Pharmacol. 2015;73(3):992–8. 10.1016/j.yrtph.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Cardoso BD, Castanheira EMS, Lanceros-Méndez S, Cardoso VF. Recent advances on cell culture platforms for in vitro drug screening and cell therapies: from conventional to microfluidic strategies. Adv Healthc Mater. 2023;12(18):e2202936. 10.1002/adhm.202202936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung CM, de Haan P, Ronaldson-Bouchard K, et al. A guide to the organ-on-a-chip. Nat Rev Methods Primers. 2022;2:33. 10.1038/s43586-022-00118-6. [Google Scholar]
- 20.Shou Y, Teo XY, Wu KZ, et al. Dynamic stimulations with bioengineered extracellular matrix-mimicking hydrogels for mechano cell reprogramming and therapy. Adv Sci (Weinh). 2023;10(21):e2300670. 10.1002/advs.202300670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy M, Brown T, Alarcon A, et al. Fat-on-a-chip models for research and discovery in obesity and its metabolic co-morbidities. Tissue Eng Part B Rev. 2020;26(6):586–95. 10.1089/ten.TEB.2019.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauschke VM, Hagberg CE. Next-generation human adipose tissue culture methods. Curr Opin Genet Dev. 2023;80:102057. 10.1016/j.gde.2023.102057. [DOI] [PubMed] [Google Scholar]
- 23.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–7. 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 24.Reus TL, Marcon BH, Paschoal ACC, Ribeiro IRS, Cardoso MB, Dallagiovanna B, Aguiar AM. Dose-dependent cell necrosis induced by silica nanoparticles. Toxicol In Vitro. 2020;63:104723. 10.1016/j.tiv.2019.104723. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Hao J, Zeng J, Sauter ER. SnapShot: FABP functions. Cell. 2020;182:1066-e1061. 10.1016/j.cell.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 27.Lee C-S. Grand challenges in microfluidics: a call for biological and engineering action. Front Sens. 2020 Sec. Lab-on-a-Chip Devices Volume 1 - 2020 | 10.3389/fsens.2020.583035.
- 28.Piergiovanni M, Mennecozzi M, Sampani S, Whelan M. Heads on! Designing a qualification framework for organ-on-chip. ALTEX – Altern Animal Exp. 2024;41(2):320–323.10.14573/altex.2401231. [DOI] [PubMed]
- 29.Ahmed T. Organ-on-a-chip microengineering for bio-mimicking disease models and revolutionizing drug discovery. Biosen Bioeletronics:X. 2022;2022(11):100194. 10.1016/j.biosx.2022.100194. [Google Scholar]
- 30.Geraili A, Jafari P, Hassani MS, et al. Controlling differentiation of stem cells for developing personalized organ-on-chip platforms. Adv Healthc Mater. 2018;7(2). 10.1002/adhm.201700426. [DOI] [PubMed]
- 31.Kim S, Ahn K, Park JY. Responses of human adipose-derived stem cells to interstitial level of extremely low shear flows regarding differentiation, morphology, and proliferation. Lab Chip. 2017. 10.1039/C7LC00371D. [DOI] [PubMed] [Google Scholar]
- 32.Kim KM, Choi YJ, Hwang JH, et al. Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PLoS One. 2014;9(3):e92427. 10.1371/journal.pone.0092427. Published 2014 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han S-B, Kim J-K, Lee G, Kim D-H. Mechanical properties of materials for stem cell differentiatioN. Adv Biosystems. 2020. 10.1002/adbi.202000247. [DOI] [PubMed]
- 34.Zhang X, Zhang S, Wang T. How the mechanical microenvironment of stem cell growth affects their differentiation: a review. Stem Cell Res Ther. 2022;13(1):415. 10.1186/s13287-022-03070-0. Published 2022 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi J, Lee SY, Yoo YM, Kim CH. Maturation of adipocytes is suppressed by fluid shear stress. Cell Biochem Biophys. 2017;75(1):87–94. 10.1007/s12013-016-0771-4. [DOI] [PubMed] [Google Scholar]
- 36.Arora S, Srinivasan A, Leung CM, Toh YC. Bio-mimicking shear stress environments for enhancing mesenchymal stem cell differentiation. Curr Stem Cell Res Ther. 2020;15(5):414–27. 10.2174/1574888X15666200408113630. [DOI] [PubMed] [Google Scholar]
- 37.Schneider I, Baumgartner W, Gröninger O, et al. 3D microtissue-derived human stem cells seeded on electrospun nanocomposites under shear stress: modulation of gene expression. J Mech Behav Biomed Mater. 2020;102:103481. 10.1016/j.jmbbm.2019.103481. [DOI] [PubMed] [Google Scholar]
- 38.Berger E, Géloën A. FABP4 controls fat mass expandability (adipocyte size and number) through inhibition of CD36/SR-B2 signalling. Int J Mol Sci. 2023;24(2):1032. 10.3390/ijms24021032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Wu Z, Zhao L, et al. The heterogeneity of mesenchymal stem cells: an important issue to be addressed in cell therapy. Stem Cell Res Ther. 2023;14(1):381. 10.1186/s13287-023-03587-y. Published 2023 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kowal JM, Schmal H, Halekoh U, Hjelmborg JB, Kassem M. Single-cell high-content imaging parameters predict functional phenotype of cultured human bone marrow stromal stem cells. Stem Cells Transl Med. 2020;9(2):189–202. 10.1002/sctm.19-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo M, Pegoraro AF, Mao A, et al. Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc Natl Acad Sci U S A. 2017;114(41):E8618-E8627. 10.1073/pnas.1705179114. [DOI] [PMC free article] [PubMed]
- 42.Dong JD, Gu YQ, Li CM, et al. Response of mesenchymal stem cells to shear stress in tissue-engineered vascular grafts. Acta Pharmacol Sin. 2009;30(5):530–6. 10.1038/aps.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirby TJ, Lammerding J. Emerging views of the nucleus as a cellular mechanosensor. Nat Cell Biol. 2018;20(4):373–81. 10.1038/s41556-018-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pennacchio FA, Nastały P, Poli A, Maiuri P. Tailoring cellular function: the contribution of the nucleus in mechanotransduction. Front Bioeng Biotechnol. 2021;8:596746. 10.3389/fbioe.2020.596746. Published 2021 Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naqvi SM, McNamara LM. Stem cell mechanobiology and the role of biomaterials in governing mechanotransduction and matrix production for tissue regeneration. Front Bioeng Biotechnol. 2020;8:597661. 10.3389/fbioe.2020.597661. Published 2020 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available on request.