Abstract
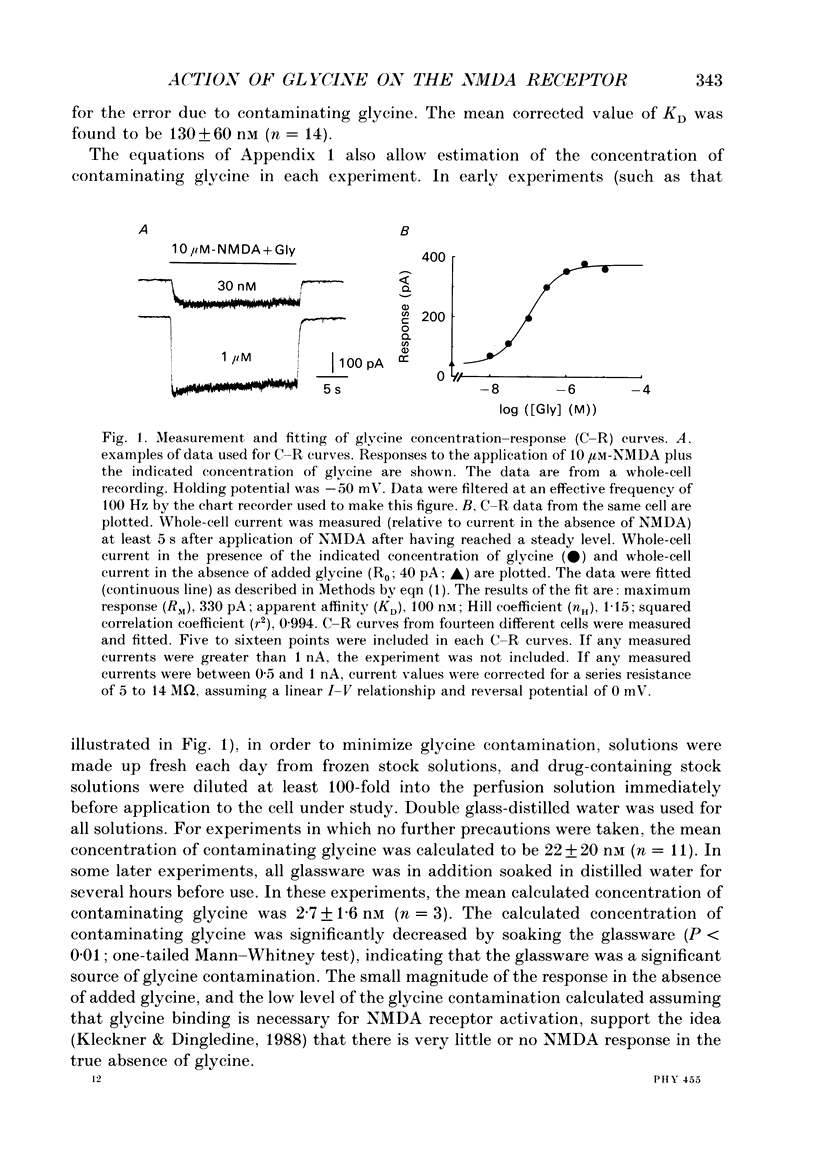
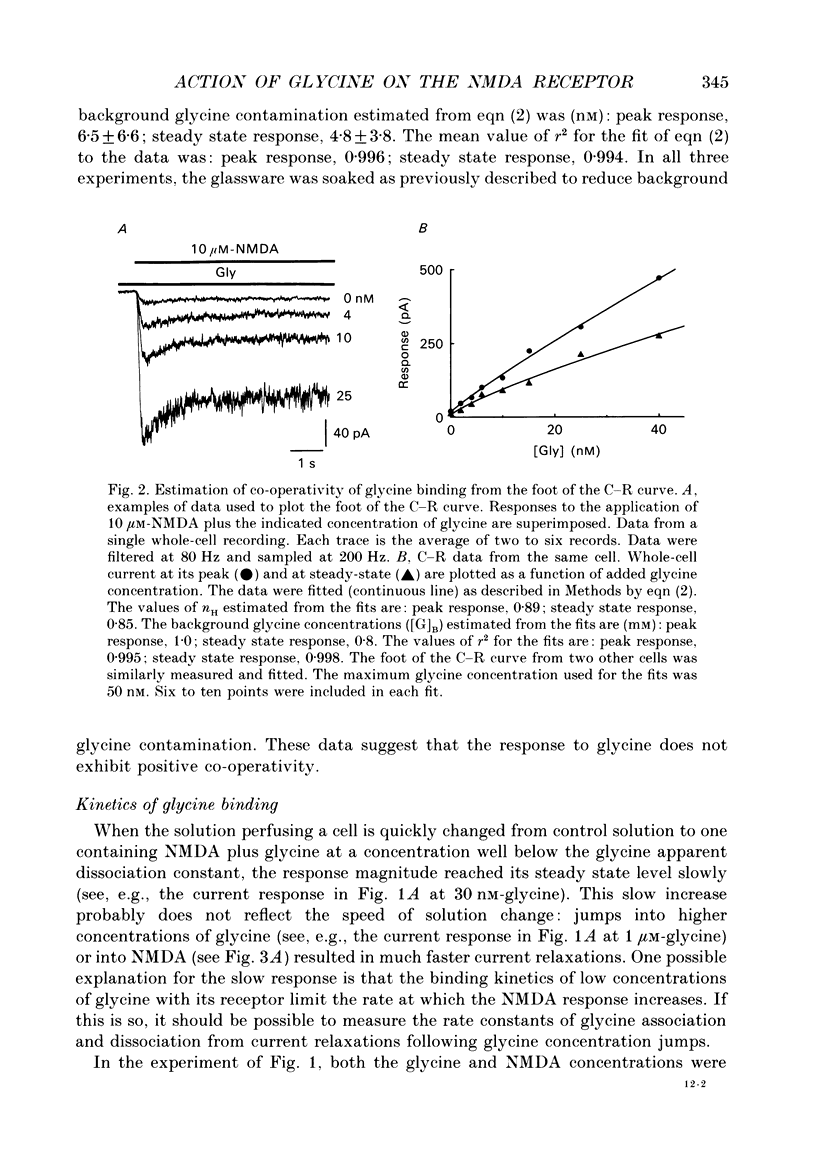
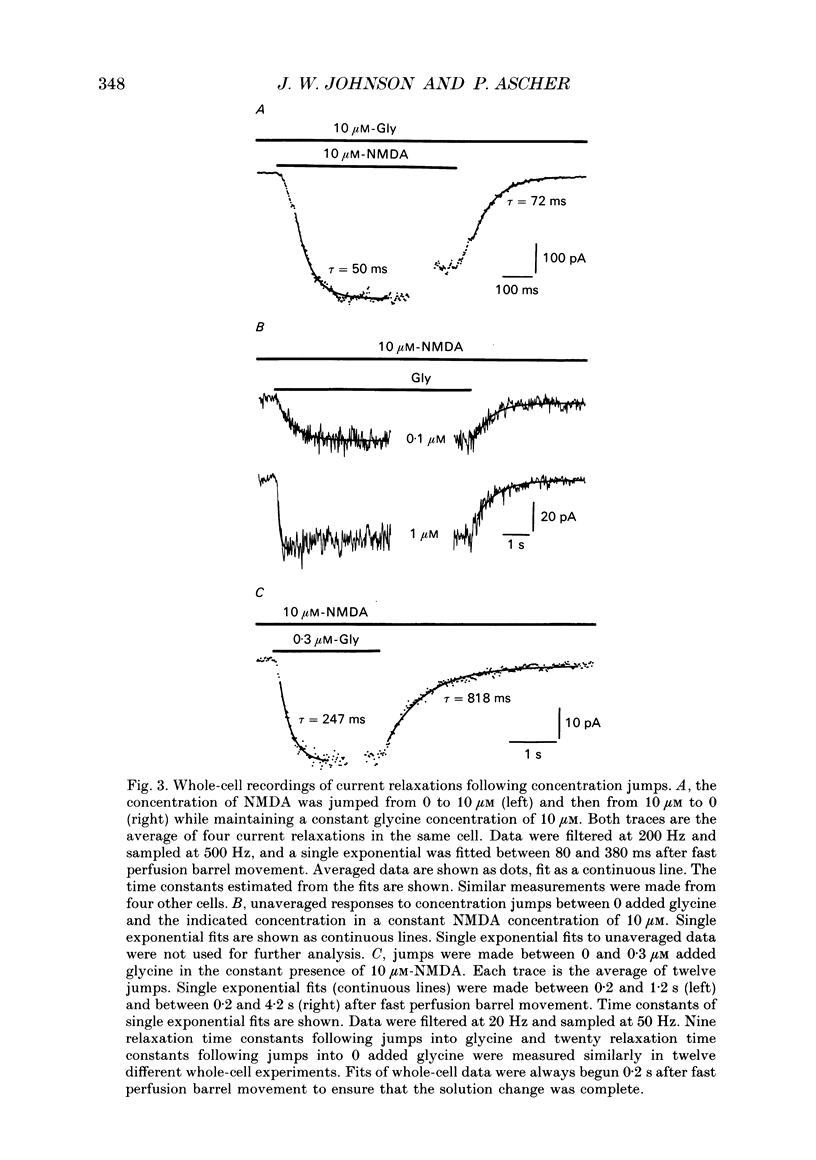
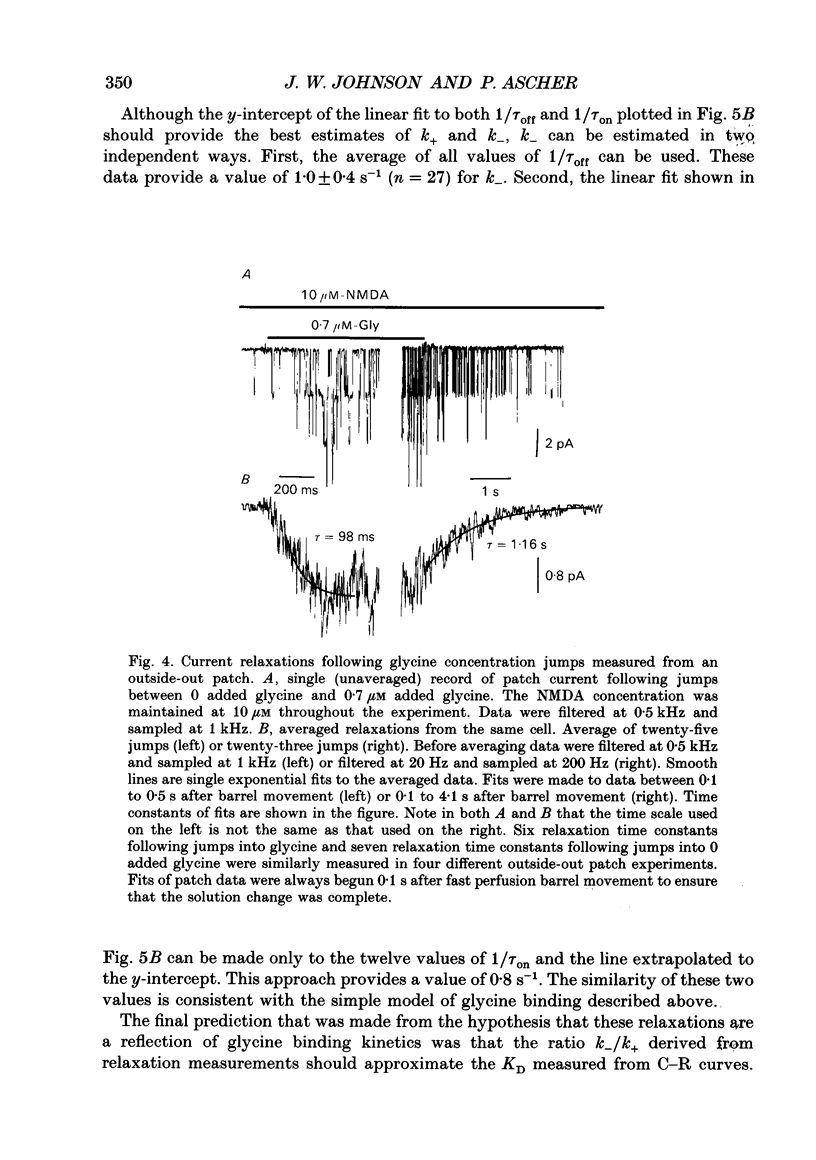
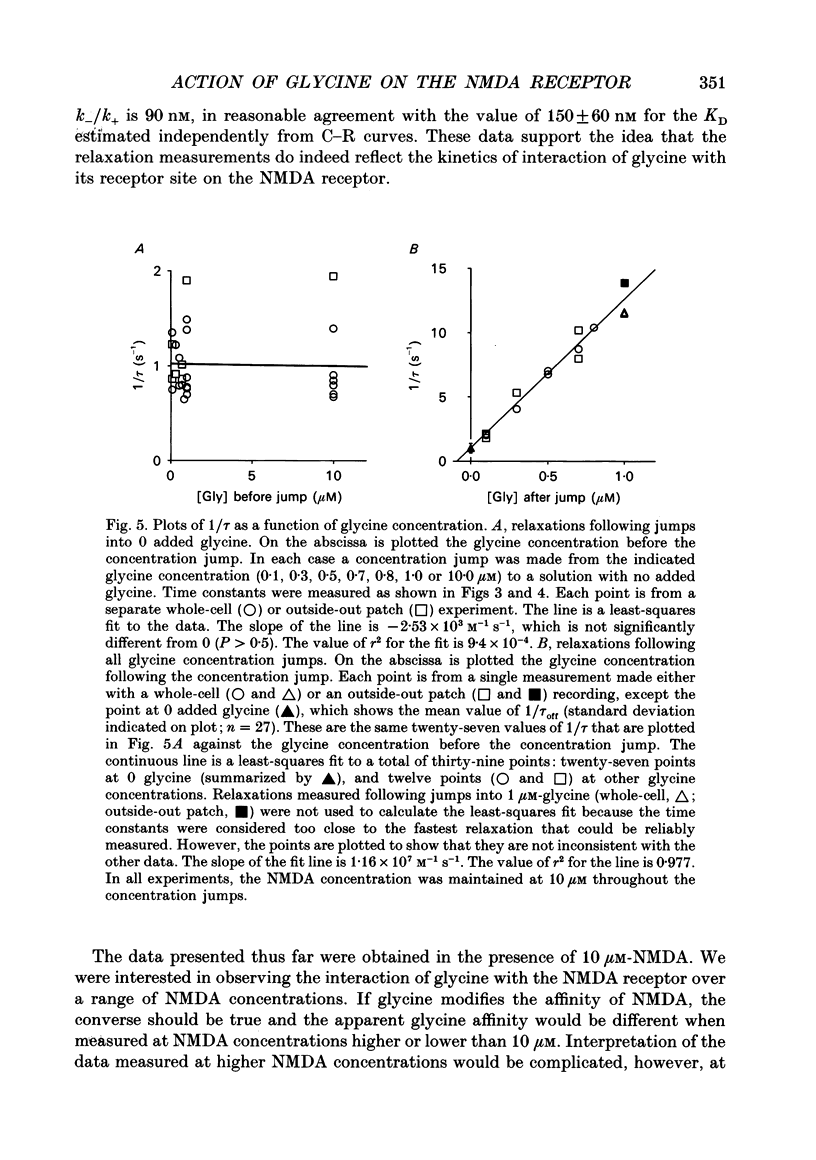
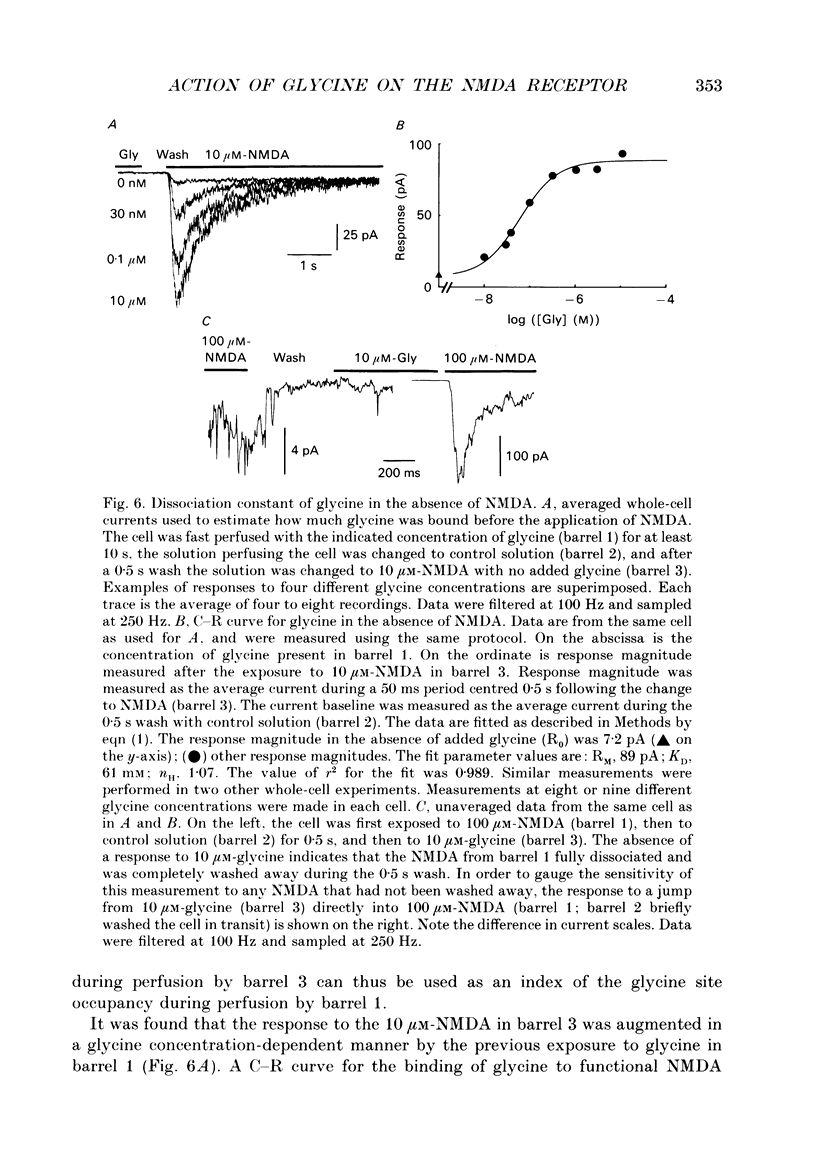
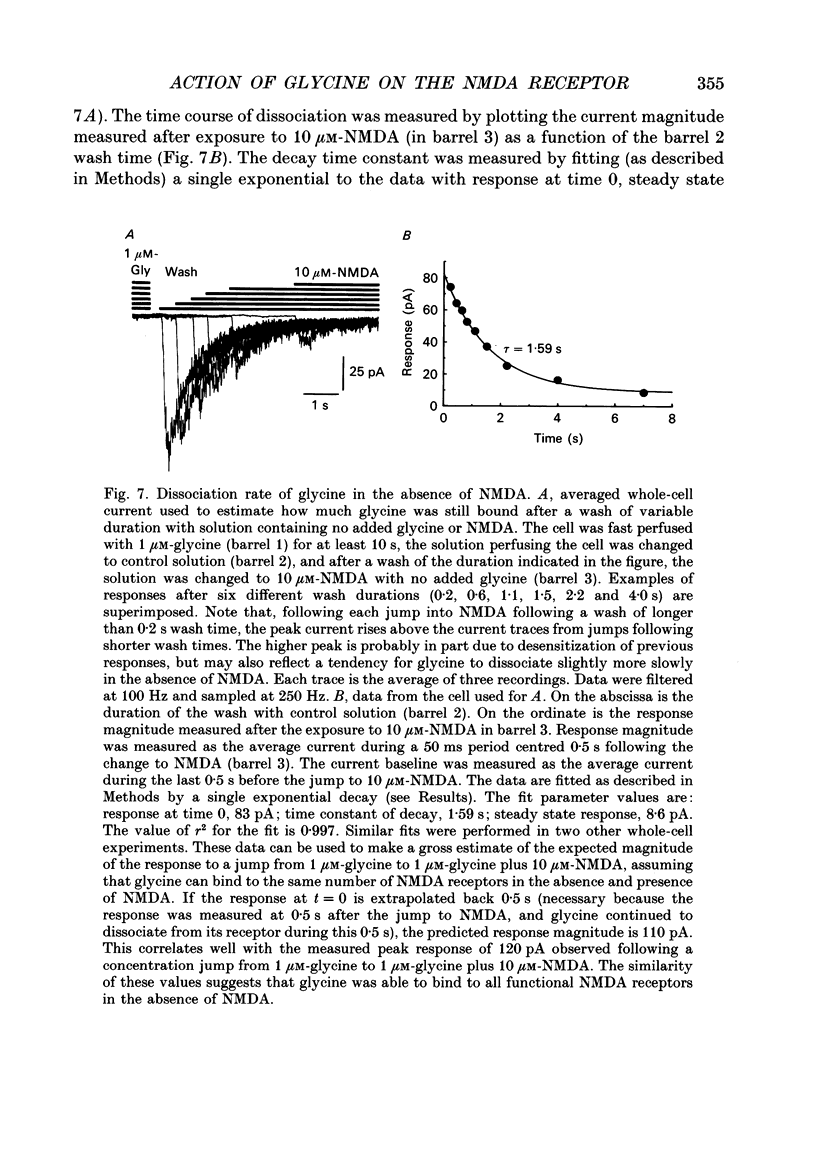
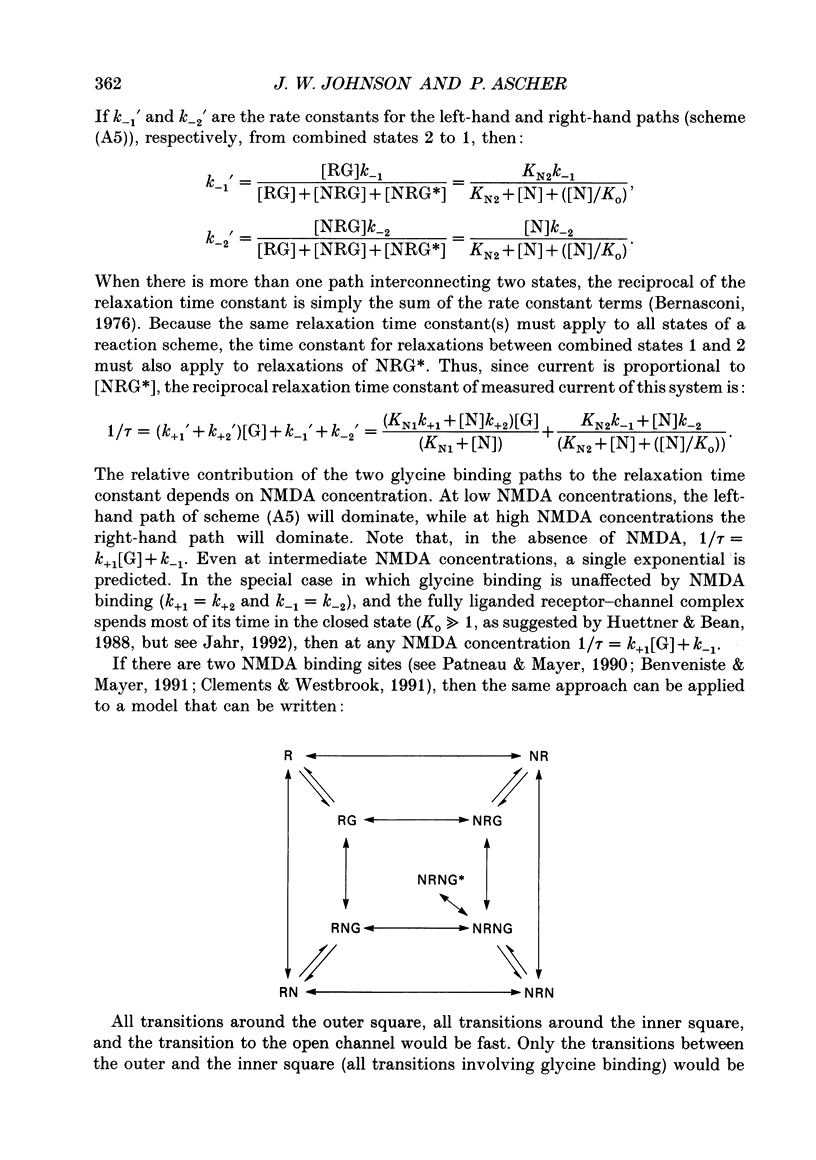
1. The characteristics of the activation of the N-methyl-D-aspartate (NMDA) response by glycine were studied using whole-cell and outside-out patch clamp recording techniques. 2. Glycine concentration-response (C-R) curves were measured in the presence of 10 microM-NMDA and fitted with the Hill equation modified to account for the response to NMDA observed in the absence of added glycine. The mean value of the apparent dissociation constant (KD) was 150 nM, and the mean value of the Hill coefficient (nH) was 1.1. When the KD was corrected for the concentration of contaminating glycine in nominally glycine-free solutions, estimated assuming that there is no response in the absence of glycine, the value was 130 nM. 3. The question of how many glycine binding sites there are on each NMDA receptor-channel complex was addressed by examining the curvature at the foot of the glycine C-R curve. An equation that allowed estimation of both the concentration of contaminating glycine and of the value of nH was fitted to glycine C-R data up to 50 nM. The mean value of nH was found to be 1.0, consistent with the idea that there is one glycine binding site. 4. The kinetics of the interaction of glycine with the NMDA receptor were measured by fitting single exponential curves to the current relaxation following a jump in glycine concentration in the presence of 10 microM-NMDA. The plot of the inverse of the relaxation time constant as a function of glycine concentration after the concentration jump was linear. The association rate constant was estimated from these data as 1.2 x 10(7) M-1 s-1 and the dissociation rate as 1.0 s-1. 5. Experiments were devised to allow the evaluation of the KD and dissociation rates of glycine in the absence of NMDA. They led to a value for KD of 80 nM, slightly but significantly lower than the value of 150 nM estimated in the presence of 10 microM-NMDA. The glycine dissociation rate in the absence of NMDA was found to be 0.7 s-1, not significantly different from that measured in the presence of 10 microM-NMDA. 6. The results are consistent with a model of the NMDA receptor with a single glycine binding site. The characteristics of glycine binding are similar in the absence and the presence of 10 microM-NMDA, although NMDA binding may cause a small increase in the glycine KD.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Clements J., Vyklický L., Jr, Mayer M. L. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Mayer M. L. Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophys J. 1991 Mar;59(3):560–573. doi: 10.1016/S0006-3495(91)82272-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Mienville J. M., Sernagor E., Mayer M. L. Concentration-jump experiments with NMDA antagonists in mouse cultured hippocampal neurons. J Neurophysiol. 1990 Jun;63(6):1373–1384. doi: 10.1152/jn.1990.63.6.1373. [DOI] [PubMed] [Google Scholar]
- Bonhaus D. W., Yeh G. C., Skaryak L., McNamara J. O. Glycine regulation of the N-methyl-D-aspartate receptor-gated ion channel in hippocampal membranes. Mol Pharmacol. 1989 Aug;36(2):273–279. [PubMed] [Google Scholar]
- Boyd N. D., Cohen J. B. Kinetics of binding of [3H]acetylcholine and [3H]carbamoylcholine to Torpedo postsynaptic membranes: slow conformational transitions of the cholinergic receptor. Biochemistry. 1980 Nov 11;19(23):5344–5353. doi: 10.1021/bi00564a031. [DOI] [PubMed] [Google Scholar]
- Bristow D. R., Bowery N. G., Woodruff G. N. Light microscopic autoradiographic localisation of [3H]glycine and [3H]strychnine binding sites in rat brain. Eur J Pharmacol. 1986 Jul 31;126(3):303–307. doi: 10.1016/0014-2999(86)90062-2. [DOI] [PubMed] [Google Scholar]
- Clark G. D., Clifford D. B., Zorumski C. F. The effect of agonist concentration, membrane voltage and calcium on N-methyl-D-aspartate receptor desensitization. Neuroscience. 1990;39(3):787–797. doi: 10.1016/0306-4522(90)90261-2. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeudis F. V., Fando J., Orensanz Muñoz L. M. 'High-affinity' binding sites for glycine in synaptosomal-mitochondrial fractions of rat CNS-regions. Experientia. 1977 Aug 15;33(8):1068–1070. doi: 10.1007/BF01945974. [DOI] [PubMed] [Google Scholar]
- Fadda E., Danysz W., Wroblewski J. T., Costa E. Glycine and D-serine increase the affinity of N-methyl-D-aspartate sensitive glutamate binding sites in rat brain synaptic membranes. Neuropharmacology. 1988 Nov;27(11):1183–1185. doi: 10.1016/0028-3908(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Davidson N., Lester H. A. Properties of two classes of rat brain acidic amino acid receptors induced by distinct mRNA populations in Xenopus oocytes. Synapse. 1988;2(6):657–665. doi: 10.1002/syn.890020613. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Kemp J. A. HA-966 antagonizes N-methyl-D-aspartate receptors through a selective interaction with the glycine modulatory site. J Neurosci. 1989 Jun;9(6):2191–2196. doi: 10.1523/JNEUROSCI.09-06-02191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henderson G., Johnson J. W., Ascher P. Competitive antagonists and partial agonists at the glycine modulatory site of the mouse N-methyl-D-aspartate receptor. J Physiol. 1990 Nov;430:189–212. doi: 10.1113/jphysiol.1990.sp018288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood W. F., Compton R. P., Monahan J. B. N-methyl-D-aspartate recognition site ligands modulate activity at the coupled glycine recognition site. J Neurochem. 1990 Mar;54(3):1040–1046. doi: 10.1111/j.1471-4159.1990.tb02355.x. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Colquhoun D., Cull-Candy S. G. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc R Soc Lond B Biol Sci. 1988 May 23;233(1273):407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Cull-Candy S. G., Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. J Physiol. 1991 Jan;432:143–202. doi: 10.1113/jphysiol.1991.sp018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner J. E. Indole-2-carboxylic acid: a competitive antagonist of potentiation by glycine at the NMDA receptor. Science. 1989 Mar 24;243(4898):1611–1613. doi: 10.1126/science.2467381. [DOI] [PubMed] [Google Scholar]
- Jahr C. E. High probability opening of NMDA receptor channels by L-glutamate. Science. 1992 Jan 24;255(5043):470–472. doi: 10.1126/science.1346477. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Foster A. C., Leeson P. D., Priestley T., Tridgett R., Iversen L. L., Woodruff G. N. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Terramani T., Lynch G., Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989 Apr;52(4):1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto H., Simon J. R., Aprison M. H. Determination of the equilibrium dissociation constants and number of glycine binding sites in several areas of the rat central nervous system, using a sodium-independent system. J Neurochem. 1981 Oct;37(4):1015–1024. doi: 10.1111/j.1471-4159.1981.tb04489.x. [DOI] [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988 Aug 12;241(4867):835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kushner L., Lerma J., Zukin R. S., Bennett M. V. Coexpression of N-methyl-D-aspartate and phencyclidine receptors in Xenopus oocytes injected with rat brain mRNA. Proc Natl Acad Sci U S A. 1988 May;85(9):3250–3254. doi: 10.1073/pnas.85.9.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Kushner L., Zukin R. S., Bennett M. V. N-methyl-D-aspartate activates different channels than do kainate and quisqualate. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2083–2087. doi: 10.1073/pnas.86.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. A., Clements J. D., Westbrook G. L., Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990 Aug 9;346(6284):565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain C. J., Kleckner N. W., Wyrick S., Dingledine R. Structural requirements for activation of the glycine coagonist site of N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Mol Pharmacol. 1989 Oct;36(4):556–565. [PubMed] [Google Scholar]
- Monaghan D. T., Olverman H. J., Nguyen L., Watkins J. C., Cotman C. W. Two classes of N-methyl-D-aspartate recognition sites: differential distribution and differential regulation by glycine. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9836–9840. doi: 10.1073/pnas.85.24.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Patneau D. K., Mayer M. L. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. J Neurosci. 1990 Jul;10(7):2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds I. J., Murphy S. N., Miller R. J. 3H-labeled MK-801 binding to the excitatory amino acid receptor complex from rat brain is enhanced by glycine. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7744–7748. doi: 10.1073/pnas.84.21.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W., Dieudonné S., MacDonald J. F., Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol. 1992 May;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W., Johnson J. W., Henderson G., Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990 May;4(5):725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M., Okamoto K., Sakai Y. Glycine-insensitive NMDA-sensitive receptor expressed in Xenopus oocytes by guinea pig cerebellar mRNA. J Neurosci. 1990 Jul;10(7):2148–2155. doi: 10.1523/JNEUROSCI.10-07-02148.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki T., Nakagawa T., Wakamori M., Tateishi N., Fukuda A., Murase K., Akaike N. Glycine-insensitive desensitization of N-methyl-D-aspartate receptors in acutely isolated mammalian central neurons. Neurosci Lett. 1990 Jan 1;108(1-2):93–98. doi: 10.1016/0304-3940(90)90712-i. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol. 1987 Apr;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell L. D., Morter R. S., Johnson K. M. Structural requirements for activation of the glycine receptor that modulates the N-methyl-D-aspartate operated ion channel. Eur J Pharmacol. 1988 Oct 26;156(1):105–110. doi: 10.1016/0014-2999(88)90152-5. [DOI] [PubMed] [Google Scholar]
- Thedinga K. H., Benedict M. S., Fagg G. E. The N-methyl-D-aspartate (NMDA) receptor complex: a stoichiometric analysis of radioligand binding domains. Neurosci Lett. 1989 Sep 25;104(1-2):217–222. doi: 10.1016/0304-3940(89)90357-1. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. Glycine is a coagonist at the NMDA receptor/channel complex. Prog Neurobiol. 1990;35(1):53–74. doi: 10.1016/0301-0082(90)90040-n. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh G. C., Bonhaus D. W., McNamara J. O. Evidence that zinc inhibits N-methyl-D-aspartate receptor-gated ion channel activation by noncompetitive antagonism of glycine binding. Mol Pharmacol. 1990 Jul;38(1):14–19. [PubMed] [Google Scholar]
- Yeh G. C., Bonhaus D. W., Nadler J. V., McNamara J. O. N-methyl-D-aspartate receptor plasticity in kindling: quantitative and qualitative alterations in the N-methyl-D-aspartate receptor-channel complex. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8157–8160. doi: 10.1073/pnas.86.20.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]


