Abstract
The translational potential of promising anticancer medications and treatments may be enhanced by the creation of 3D in vitro models that can accurately reproduce native tumor microenvironments. Tumor microenvironments for cancer treatment and research can be built in vitro using biomaterials. Three-dimensional in vitro cancer models have provided new insights into the biology of cancer. Cancer researchers are creating artificial three-dimensional tumor models based on functional biomaterials that mimic the microenvironment of the real tumor. Our understanding of tumor stroma activity over the course of cancer has improved because of the use of scaffold and matrix-based three-dimensional systems intended for regenerative medicine. Scientists have created synthetic tumor models thanks to recent developments in materials engineering. These models enable researchers to investigate the biology of cancer and assess the therapeutic effectiveness of available medications. The emergence of biomaterial engineering technologies with the potential to hasten treatment outcomes is highlighted in this review, which also discusses the influence of creating in vitro biomimetic 3D tumor microenvironments utilizing functional biomaterials. Future cancer treatments will rely much more heavily on biomaterials engineering.
Keywords: Tumor microenvironment, 3D in vitro model, Biomaterials, Extracellular matrix
Introduction
Cancer continues to be a major global health concern despite decades of research and advances in cancer diagnosis and treatment methods [1, 2]. Using tumor cells and a matrix or scaffold to recreate the tumor microenvironment (TME) in vitro has helped scientists reduce the margin of error in their assessments of the efficacy of various drugs in recent years [3]. It is possible to conduct mechanistic studies into the biology of cancer as well as therapeutic development and tailored drug testing thanks to the recapitulation of patient tumors in vitro and in vivo as tumor models [4]. The implementation of tissue engineering technologies in cancer biology has been pushed by the significant roles played by the TME in regulating tumor genesis, progression, and metastasis. Recent research has demonstrated that the TME controls the behavior of cancer cells and the development, growth, and metastasis of tumors. It has also been demonstrated that the tumor environment has a significant role in encouraging tumor resistance and recurrence [5, 6]. Tumor cells communicate with their surroundings in a very dynamic and reciprocal manner, suggesting that researching cancer in specially constructed biomaterial scaffolds may more reliably result in the discovery of new therapeutic targets and treatment regimens [7]. Critical insights into the mechanisms of tumor resistance may be revealed when tissue engineering becomes increasingly successful in simulating the natural tumor environment, having an impact on clinical practice, medication development, and biological research [8]. These discoveries have motivated the use of tissue engineering techniques in cancer biology, with the hope that the ability to manipulate the tumor environment will allow for the discovery of therapeutic targets and the creation of novel treatment protocols [9]. Simple two-dimensional (2D) monolayer cultures, more lifelike three-dimensional (3D) spheroid as well as organoid cultures, intricate tumor xenografts, and genetically altered animal models are only a few examples of the models available [7]. Currently, no model systems exist that enable researchers to assess tumor genesis, development, and resistance to therapy in vitro under realistic settings [10].
The extraordinarily intricate microenvironment of the tumor, which includes biophysical signals (interstitial pressure and matrix mechanics) as well as chemical cues (growth factors and cytokines), is crucial to the tumor’s development and spread over time [11, 12]. This microenvironment exhibits a distinct set of significant traits at each stage of the disease (such as cellular and extracellular matrix (ECM) compositions, matrix stiffness, and degree of vascularization) [13, 14]. In order to understand how, why, and when these important factors contribute to the growth and migration of the tumor, it is crucial to break down all the important components that interact with the cancer cells. Additionally, there is a lot of variation not only across different cancer types but also within certain tumors (sometimes referred to as intertumor heterogeneity) (i.e., intratumor heterogeneity) [15–18]. Every patient needs individualized care because of the extreme complexity and diversity that this heterogeneity brings about [19]. In vitro designed human cancer models are greatly wanted in order to completely comprehend the complexity of cancer origins, progression, metastasis, and interactions between the numerous critical players in the tumor microenvironment, as well as the screening of potential anticancer medications. The creation of cancer medicines that work requires this knowledge. This is because the traditional animal-based cancer models, or xenografts, may not always accurately reflect human physiology or the effects that medications have on people [20–22]. A tool for the investigation of individualized anticancer treatments, three-dimensional (3D) cancer models are expected to accurately simulate the in vivo TME in human patients and, as a result, give accurate mechanistic studies. This is achieved by precisely mimicking the qualities and content of the tumor cell/matrix to match the illness type and stage.
Interactions among tumor cells and their surrounding environment influence cancer genesis at all phases of the process. Poor clinical outcomes are caused by significant variables that exacerbate tumor growth and metastasis, including the makeup of the TME and tumor–stromal interactions [6, 23–26]. The active stroma is a key role in cancer cell invasion/extravasation, migrations, angiogenesis, drug resistance, cancer stem cell maintenance, and immunosurveillance evasion, according to mounting evidence that it is a disease-defining factor [8, 27, 28]. Biological and biophysical signals, such as cell–cell and cell–ECM interactions, tissue architecture and dynamics, mass transfer, and tumor growth, among others, govern the connections between cells and the ECM [29]. When developed tumor models are employed in controlled and physiologically appropriate conditions, they provide a perfect platform for examining the effect of a variety of stimuli on tumor malignancy [30, 31]. Although anti-metastatic drugs and other treatments are required, a deeper understanding of tumor genesis, progression, and therapeutic resistance is vital for their successful development [32, 33]. When tumor cells multiply, they infiltrate the surrounding stroma, move via the circulation, and spread to other parts of the body [34]. Due to the focus of research on cancer cells, the genesis of tumors is not well understood. This is true even though the host tissues, the cancer, and the surrounding environment are constantly interacting with each other [35]. This is due to the fact that the molecular pathways behind tumor genesis are still poorly understood [36]. Stephen Paget realized in 1889 that cancer cells, which he termed “bad seeds,” require a favorable microenvironment, or “soil,” to proliferate and spread [8]. Thus, research has shown repeatedly that the TME influences not only metastasis but all stages of tumorigenesis [37, 38]. Research showing the crucial function of the microenvironment in influencing tumorigenesis suggests that cancerous cells can revert to normal when isolated from their environment [39, 40]. To fully understand how cancer develops, it is necessary to look into both the innate features of tumor cells as well as their microenvironment [41, 42].
In vitro models provide the capability to explore mechanistically the unique contributions of various microenvironmental components on tumor cell activity due to their ability to manipulate the biophysical and biomechanical properties. But while assessing them, care must be taken because in vitro models do not completely capture the tumor microenvironment. While it is possible to study the direct effects of interstitial flow on tumor cell invasion in a three-dimensional (3D) culture of tumor cells in a collagen gel with interstitial flow [43–45], this method ignores other factors that are prevalent at an invasive edge of the tumor cells, such as the architecture and composition of the ECM (that is highly heterogeneous), the variability of types of cells present in the tumor stroma and their physiological interactions, as well as gradients of oxygen, pO2, and One can only deduce from such research the direct impact of interstitial flow on equally dispersed tumor cells inside a homogenous collagen ECM because it is uncertain how significant interstitial flow is in contrast to other parameters that were not included in the in vitro model. One should not, however, presume that these results will automatically increase the tumor’s edge in invasiveness. Even as increasingly complicated models are developed, attention must be taken to carefully assess the questions they may be used to answer as well as the ways in which the data they generate can be analyzed. This has been true even for the initial two-dimensional (2D) monocultures [46]. It is certain that advances in TME modeling, an exciting and innovative area of cancer research, will enhance our capacity to discover and assess novel therapy choices [47, 48]. Academics are exerting enormous effort to design models that are applicable to as many situations as feasible, despite the fact that there is currently no ideal model [49, 50].
Tissue engineering continues to develop approaches to simulate various elements of cancer and has played essential roles in gaining a better knowledge of the disease. With the development of new biomaterials and three-dimensional in vitro models that can simulate some of the biological, chemical, and mechanical aspects of the tumor microenvironment.
This review focuses on the use of functional biomaterials to mimic the tumor microenvironment based on the recent advancements in the field of tissue engineering approaches towards modeling tumor microenvironment and tumor matrix engineering. The following section begins with a brief discussion about the 3D TME modeling then it focuses its shift on the engineering approaches of the tumor microenvironment. Next sections give an overview about the ECM models based on the engineered biomaterials and the lab-on-a-chip microfluidic platforms to model the tumor microenvironment as well as the 3D bioprinting techniques to mimic the tumor microenvironment. Finally, the paper addresses the limitations and the future prospects of using functional biomaterials to mimic the tumor microenvironment. Table 1 shows the comparison between the 2D and 3D models. The next section will discuss the three-dimensional modeling aspects of the tumor microenvironment.
Table 1.
A comparison of two-dimensional and three-dimensional models is shown [51]
| SL no | Characteristics | Two dimensional | Three dimensional | References |
|---|---|---|---|---|
| 1 | Tumoral heterogeneity | The most fundamental; all cells receive the same quantity of nutrition. Misrepresentation of the TME during replication | Improved estimate and depiction of the tumor microenvironment; nutrients are not distributed evenly throughout the tumor | [52, 53] |
| 2 | Cell interactions | There are no cell niches formed when cells do not interact with one another or with the ECM | Cell junctions are widespread and enable for cell communication to take place between cells | [54–56] |
| 3 | Response to stimuli | Mechanical and biological inputs are inaccurately represented in the model | Cells develop in a three-dimensional environment and receive stimuli from all directions that are accurate representations of the stimuli they would experience in the environment | [57, 58] |
| 4 | Cost | Large-scale investigations are more affordable | Techniques that are more difficult and expensive | [59–61] |
| 5 | Cell differentiation and proliferation | Poor cell differentiation and unusually fast proliferation are observed | The proliferation of the cells is realistic and dependent on the interactions of the three-dimensional matrix | [54, 62–64] |
| 6 | Reproducibility | Highly repeatable | It is difficult to repeat several of the results | [6, 65] |
| 7 | Analysis and quantification | Results are simple to comprehend, and long-term cultures are preferable | Data analysis is difficult with various cell types or spheroid/organoid structures | [46, 51, 66] |
| 8 | Gene expression | Genes associated with cell adhesion; proliferation and survival are often altered | Representational accuracy of gene expression patterns | [67, 68] |
3D tumor microenvironment modeling
Scientists may use three-dimensional models to conduct controlled research on the interactions of tumor cells, endothelial cells, immune cells, and fibroblasts in a controlled environment [69–71]. The ECM, growth factor proteins (GFP), and environmental variables (EVA) all combine to form a tumor, with each of these cell types altering or being altered on a regular basis [72]. By investigating the connections between various cell types and the TME, the relationship between fibrosis, poor vasculature, immunological control, and treatment complications associated with these disorders may be better understood [73, 74]. The easiest method to replicate the unpredictable behavior of the TME is to use a three-dimensional system. Because two-dimensional cell culture models have a limited surface area available for cell-to-cell contact and communication, reproducing interactions between cells and their microenvironments can be challenging [75]. Among the other indicators are alterations in growth, metabolism, and differentiation. For instance, the company is increasingly utilizing 3D culture technologies, which may be classified as aggregation cultures or encapsulation cultures. Aggregate cultures enable the growth of large amounts of tissue in a small region [76, 77]. Without connection or gravity in the culture environment, it is more difficult for cells in aggregate cultures to adhere to one another. Spheroids may be produced on custom plates, and spinning wall bioreactors can be employed in the laboratory to simulate microgravity [78, 79]. They are well-suited for high-throughput systems that require a high level of cellularity and can assist reduce the time required to configure the system before it can be used in most cases due to its basic structure [80]. When nutrition diffusion to cells inside the cultures is insufficient, the cultures’ very cellular character may also result in very limited control over noncellular mechanical components of the TME [81, 82]. Both outcomes are undesirable, depending on the culture size and cell count. When floating cells in a hydrogel are submerged in water for an extended period of time, they can develop into encapsulation cultures. Collagen, hyaluronic acid, and fibrin are all ECM components that may be employed in cell culture to promote cell proliferation and differentiation [83, 84]. This is a frequently used approach. Encapsulated techniques enable the production of multicellular models with advanced ECM structures that may be used for future study due to their ability to adjust all ECM properties, including self-organization potential [36, 85]. On the other hand, aggregate cultures have a lower throughput and take longer to form in their environment, resulting in a greater throughput. Lower initial cell density causes aggregate cultures to grow more slowly [86]. Traditional three-dimensional models lack the complexity of organoids, which are organotypic, tissue-equivalent models with a diverse array of cell types and the ability to aggregate or cluster cells in a manner comparable to that of an entire organ [65, 87]. To get the required outcomes, a variety of techniques must be applied, including aggregation and encapsulation, because organisms are frequently more complicated than three-dimensional models [88]. As previously demonstrated, progenitor cell fate-specification pathways may be used in a number of ways to govern the formation of organotypic structures [89].
Tissue culture plastic is used to cultivate tumor cell lines for the purpose of researching anticancer medicines. The primary goal is to inhibit tumor cell development or to cause cell death [90]. Dynamic microenvironments provide tissue-specific spatiotemporal modulation of communication between distinct cell types, which is required for tumor cells to form and spread in vivo [91, 92]. Paracrine loops between tumor cells and macrophages in a primary tumor are hypothesized to promote cancer spread by increasing both cells’ motility and preparing them for circulation. As a result of this paracrine loop, breast cancer metastasis is feasible. It has been demonstrated that a heterotypic cell contact pair may be created in vivo and in vitro using a range of 2D and 3D growth platforms employing intravital imaging and a variety of 2D and 3D growth platforms [93]. A significant progress has occurred in the realm of cancer therapy by deliberately targeting heterotypic interactions that occur prior to metastasis. When it comes to quantitative assessments of heterotypic signaling, there are relatively few in vitro systems capable of reproducible tissue morphology that can be used for a variety of other types of studies; this is in contrast to the relatively few in vitro systems capable of reproducible tissue morphology that can be used for drug development [94, 95]. Rather than the conventional 2D petri dish culture, 3D culture gives a more realistic portrayal of tissue and matrix structure [49]. When 3D systems were used instead of 2D systems, in vitro drug screening models were able to identify changes in cell proliferation, morphology, and responsiveness. This was a novel finding when it was made in the field [96, 97].
There is growing interest in the use of microfluidic devices such as cysts and tubules to create three-dimensional microenvironments analogous to the basic building blocks of epithelial tissue in order to enable the creation and maintenance of large-surface-area interfaces between chemically and physiologically distinct domains of tissue [31, 98]. Fibers’ chemical composition and topography may be adjusted throughout the extrusion process, allowing researchers to maintain control over the fibers’ production [99, 100]. This strategy was created lately by a group of academics. Onoe and colleagues have devised a hydrodynamically focused technique for industrial-scale manufacture of cell-encapsulated fibers for the first time [101]. However, because of the limited geometry of single-channel fibers, geometric flexibility and structural control can only be obtained by post-processing processes [102, 103]. Figure 1 illustrates the TME and the engineering aspects for the in vitro modeling of the TME. The following section will elaborate the engineering considerations for the tumor microenvironments.
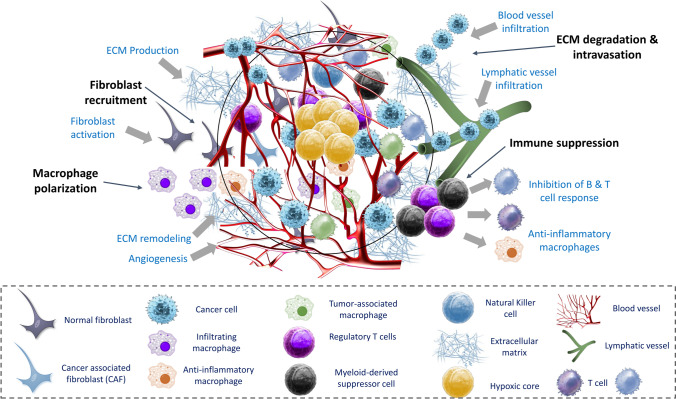
Fig. 1.
Tumor microenvironment (TME) components (reproduced with permission from [28]). The TME is depicted schematically, showing essential mechanisms such as fibroblast recruitment, macrophage polarization, immunological suppression, and ECM breakdown that allow tumor cells to invade and spread
Tumor microenvironment engineering
Using biomimetic approaches to provide cells with attributes of their original microenvironment to recreate a unit of form and function for a given tissue is what has allowed for significant advancements in tissue engineering over the past several years. These advancements have been made possible by employing these strategies. As a result of the fact that researchers view the loss of integration of architecture and function to be the most important hallmark of cancer, it stands to reason that similar methodologies may be utilized in order to get an understanding of the biology of tumors [104].
To this day, the methods that have shown to be the most successful in the field of tissue engineering are those that attempt to replicate the composition, architecture, and chemical presentation of native tissue. For example, inducing the growth of new blood vessels for therapeutic purposes can be accomplished through the sequential release of vascular endothelial growth factor (VEGF) and platelet-derived growth factor, which works to both induce and stabilize the formation of new blood vessels [105]. This method mimics the process that takes place naturally during the physiological process of angiogenesis, which takes place as a result of heterotypic interactions between the stroma and endothelium [106]. Utilizing such biomimetic approaches has already resulted in significant progress made in the study of cancer. Table 2 shows the engineering and design consideration involved in the tumor initiation, progression, and metastasis. Figure 2 illustrates the in vitro tumor models engineered using hydrogels.
Table 2.
Various stages of tumor initiation, progression, and metastasis are represented by models and engineering design considerations [108]
| SL no | Biological phenomenon | Engineering considerations | Models used | References |
|---|---|---|---|---|
| 1 | Tumor cell circulation |
• Platelets, blood cells, clotting factors • Complex geometry (e.g., branching, narrowing capillaries) • Luminal shear stress |
• Microfluidic flow chamber | [91, 97, 109] |
| 2 | Cell invasion and chemotaxis |
• Chemokine gradients • Oxygen gradients/hypoxia • 3D matrix, stiffness, adhesion molecule density • Co-culture with stromal cells • Interstitial flow and ECM stress • Cell contraction and motility |
• Vertical layered assay • Microfluidic devices • 3D cell tracking • Modified Boyden chamber |
[1, 110] |
| 3 | Adhesion and extravasation |
• Chemokines and adhesion molecules • Laminal flow • Co-culture with endothelial cells |
• Parallel plate flow chamber | [111, 112] |
| 4 | Intravasation into blood or lymphatic vessels |
• Luminal flow on endothelial cells • Trans endothelial cells (e.g., into lymphatic vessels) • ECM composition; presence of basement membrane • Interactions between tumor cells and endothelial cells |
• Modified Boyden chamber • Microfluidic devices |
[111, 113, 114] |
| 5 | Growth in ectopic site | • Features of the ectopic site that are tissue-specific, such as chemokines and ECM elements (e.g., lymph nodes, liver, brain, and bone) | • 3D cultures | [115, 116] |
| 6 | Tumor initiation and growth |
• Adhesion molecule density • Compressive stress • ECM composition and stiffness |
• Hanging drop assay • 3D spheroid assay |
[40, 100, 117, 118] |
| 7 | Blood and lymph angiogenesis |
• Role of macrophages, stromal fibroblasts, and other cells • Laminal and interstitial flow • Growth factor gradients • Endothelial sprouting into 3D ECM |
• 3D flow chambers • Microfluidic devices • Tubulogenesis assays • Bead assay |
[53, 61, 119] |
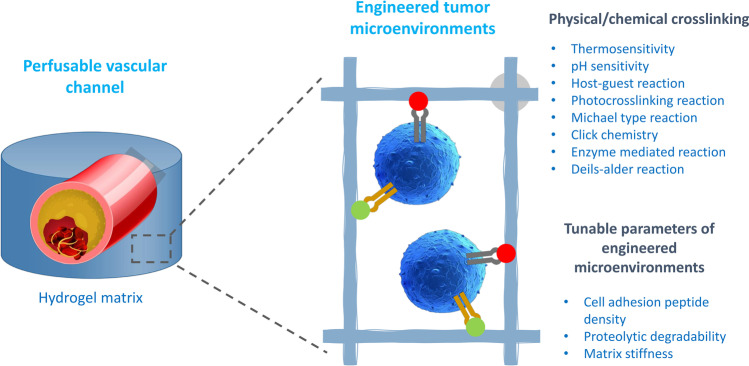
Fig. 2.
In vitro tumor models engineered with natural and synthetic hydrogels (reproduced with permission from [107]). In physiological settings, hydrogel matrices are produced by a series of physical and chemical processes. These hydrophilic networks provide 3D microenvironments favorable to tumor development
Model systems that simulate the intricacy of tumor cells
Conventional methods for researching tumorigenesis in vitro
Preclinical models have been used to predict tumor growth for decades, and animal research continues to be the gold standard for identifying new treatments that are not yet available [120]. It has been challenging to gain a better knowledge of tumor development since animal models are prohibitively expensive and it is difficult to examine the spatiotemporal dynamics of tumor growth [121, 122]. A big advantage of using in vitro systems is that they may increase experimental variable control while maintaining physiologically relevant qualities, which is a substantial advantage. Most often utilized in vitro cancer models in the past were tumor-derived cell lines that had been immortalized histopathologically and grown as adherent 2D cultures [123, 124]. They can also be used to do fast drug testing owing of their broad availability and ease of upkeep. In clinical trials, several medication candidates have failed while displaying promising results in vitro and in vivo and in other research areas [125]. The following are a few examples: One or more of these issues may be to blame for translational research’s lack of predictive capabilities and other performance gaps [126, 127]. The absence of cellular heterogeneity in normal in vitro models contributes significantly to carcinogenesis in the same way that it does in tumors in vivo. Due to the lack of availability to cellular and acellular stimuli in two-dimensional culture, the inherent homogeneity of the cell lines is further exacerbated, which makes the problem even more difficult to deal with [128]. Some intratumor heterogeneity linked with clinical outcome may be reproduced in two-dimensional cell culture and in vivo models together, which may establish a beneficial bridge while avoiding some of the downsides associated with both techniques [129, 130]. The selection of biological components, the selection of appropriate ECM materials, the development of fluid flow, and the generation of biochemical gradients must all be considered when developing physiologically correct 3D models of the TME [131, 132]. Detailed explanations of these design concepts and examples of how they could be used in the future are provided in the following sections [133].
Creating multicellular spheroids
Multicellular aggregates called spheroids can be utilized to mimic the 3D cell–cell adhesions found in the human body. The adhesions between human cells can be mimicked in spheroid cultures. [134]. Inverted tissue culture plates can be used to promote cell–cell adhesion and spheroid growth. This is accomplished by placing a droplet of cell suspension on the plate’s lid and inverting the plate [135]. Rather than that, spherical aggregates form, which must be moved to a new culture tank in order for the organism to continue growing. Growing cells on non-adherent surfaces such as agar, agarose, or commercially supplied ultra-low attachment culture plates with preset well shapes can circumvent both of these constraints (spherical or tapered) [113, 136]. Utilize rotating culture and viscous media to expedite the process (cellulose). Large-scale drug screening requires precise spheroids, which can only be produced by contemporary micropatterning and microfluidic technology, as shown by the most recent investigations [137]. As a result, these systems are more expensive to operate and maintain than traditional ones. Researchers can use spheroid cultures to investigate three-dimensional cell–cell interaction and the creation of physiologically relevant structures such as tumor-like structures in mice and other animals [138]. A deficiency of nutrients and oxygen in the spheroid’s core is caused by the increased cell density and size of the spheroid, which restricts nutrient and oxygen diffusion. Apart from that, it has been demonstrated that the formation of distinct zones in spheres larger than 400–500 microns in diameter is congruent with the pathogenic gradients observed in real patients. In addition to monoculture spheroids, coculturing cancer and stromal cells in the same culture plate can form heterotypic cell–cell interactions [139, 140].
Organoid cultures
Cancer organoids produced from people are being used to further cancer research as a platform capable of imitating the cellular heterogeneity present in human malignancies [141]. A biomimetic matrix can be used to construct tumor organoids from fragmented tissue or circulating tumor cells, allowing the cells to self-assemble and develop into increasingly complex structures [142, 143]. The method most frequently employed to create organoids is matrigel (a bone marrow extract obtained from a mouse Engelbreth-Holm sarcoma that preserves the heterogeneity of cancer cell populations) [144, 145]. Matrigel preserves the heterogeneity of the tumor cell population when it is made from a mouse Engelbreth-Holm tumor, making tumor therapy more successful. The most often utilized method for synthesizing organoid derivatives is matrigel synthesis [146]. It is possible to increase physiological complexity and better comprehend cell–cell interactions in the TME by coculturing tumor organoids with stromal components such cancer-associated fibroblasts (CAFs), immune cells, and vascular networks, in addition to other tumor organoids [147, 148]. If you are seeking for an alternative method for developing organoids, you may use the transwell technique, which includes embedding tissues in collagen and exposing one side of each organoid to the medium while leaving the other open to the air [149, 150]. Thanks to biobanks, cancer organoids may now be employed in scientific study. These biobanks hold samples of malignancies and normal tissue from patients and animals [151]. With these biobanks, it will be easier to use patient samples in larger-scale research, which will benefit both researchers and patients. Due to the use of organoid cultures, ex vivo monitoring and assessment of biomarkers and other morphological changes in organoid cultures can give new insights into the dynamics of biological processes [152]. The capacity to generate matched organoids to assess cellular responses in tumor and normal tissue is crucial in cancer research and drug screening because it enables the construction of tumor and normal tissue matched organoids [35, 153]. This has a significant impact on cancer research and medication screening for individual patients. Additionally, normal tissue organoids produced from pluripotent stem cells may be transformed into tumor organoids, which can be utilized to track and understand the molecular pathways behind tissue morphogenesis and cancer progression, among other applications [33, 154]. Organoid cultures are more difficult to work with due to their great production efficiency and the considerable variety that may be gained by tissue harvesting. Alternatively, organoid proliferation may result in the selective loss or overgrowth of stromal populations, hence obstructing research into tumor-specific heterogeneity [57]. Another issue that may be addressed by the use of matched patient peripheral blood serum is the use of sophisticated media that often contain fetal bovine serum and require the addition of particular chemicals. This can be avoided by using patient-matched peripheral blood serum [155, 156]. For researchers, the addition of fetal bovine serum, a challenging medium, adds another hurdle (FBS). Apart from the fact that glioblastoma tumor organoids may be created from a variety of different types of tumors, the bulk of tumor organoids biobanks are focused on epithelial malignancies [157, 158]. Organoid cultures cannot all be sustained permanently, which is why this discrepancy exists [159]. According to some experts, entire organoid cultures may soon supplant cell line research as the primary method for cancer research [160, 161]. Figure 3 illustrates the development of tumor organoids via investigating the TME.
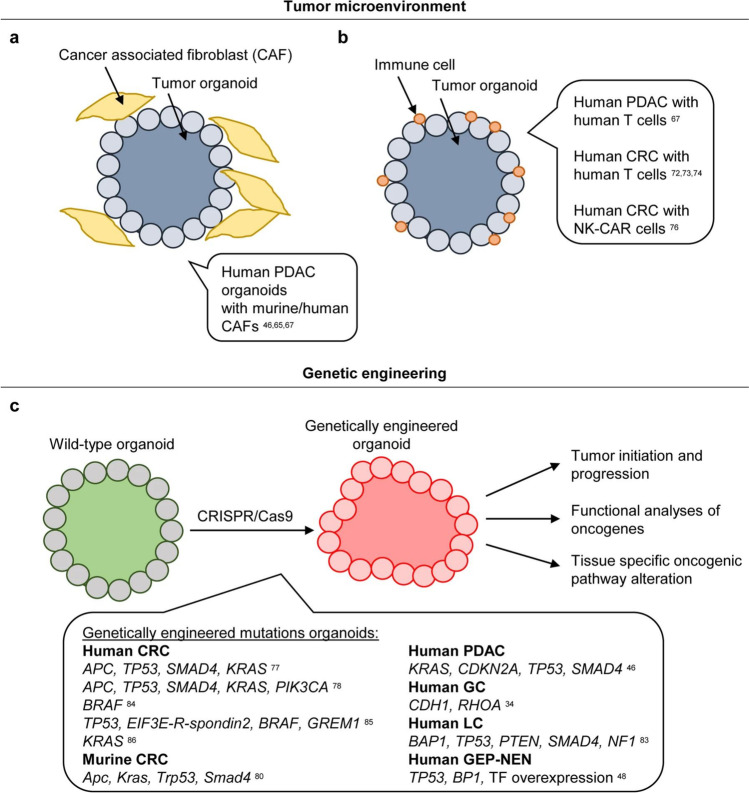
Fig. 3.
Investigating the TME and developing tumor organoids (reprinted from [162]). a Gastrointestinal tumor organoids and cancer-associated fibroblasts (CAFs) were co-cultivated to study the relationship between cancer cells as well as the tumor microenvironment. b Co-cultivating gastrointestinal tumor organoids with immune cells allowed researchers to investigate the interaction among immune cells and malignant cells, explore the interaction, and test immunotherapeutic techniques. c To research tissue-specific oncogenic pathway modifications, to monitor cancer start and progression, and to functionally examine (candidate) oncogenes, tumor organoids can be generated from wild-type organoids using CRISPR/Cas9 by creating tumorigenic mutations
Biomaterials-based ECM models of various types of cancers
Natural biomaterials
Researchers may investigate the functional significance of cancer development in a controlled environment by implanting cells into biomaterials that mimic the ECM found in tumors [163, 164]. Biomaterials, particularly those derived from mammalian tissues, have a broad appeal to a diverse spectrum of people. These materials, which include matrigel and cultrex, have been utilized in research to investigate organotypic tissue development and changed cell penetration into the bone marrow [165]. When oncoproteins such as Papillomavirus E7 and ErbB2 (erythroblastic oncogene B) are activated, cancer cell proliferation and morphogenesis can be accelerated. ErbB2 is an oncoprotein that has been discovered in a number of malignancies ErbB2 [166]. Following intravenous infusion, BM and Matrigel exhibit many of the same biological features, despite significant structural and mechanical differences [109, 167]. Due to the paucity of BM, large-scale examinations in its natural, complete condition are typically not possible (e.g., isolated from rat peritoneum) [168]. Examine the stiffness differential between normal and diseased tissues, as well as the organs most prone to metastasis (for example, breast cancer tissue is ten times stiffer than normal breast tissue: hundreds of pascals in the brain versus gigapascals in bones) [55, 169]. Additionally, commercial BM preparations contain growth factors and cytokines, which regulate cell activity throughout the body. When utilized in conjunction with one another, ECM components such as collagen, fibronectin, and HA can help ease these difficulties ECM. Collagen I, a kind of protein, is frequently employed in the ECM to form hydrogels [170]. Collagen type I hydrogels have aided in our understanding of how the ECM influences cell proliferation and migration, as well as the activity of stromal cells, tumor cells, and their combined activity [60, 171]. According to one study, when cells are transplanted into Matrigel, they develop in an ordered form; nevertheless, when cells are transplanted into collagen gels, they spread out [118, 172]. Additionally, the mechanical and structural properties of collagen hydrogels may be modified by adjusting the collagen content, the gelation conditions (e.g., temperature and pH), or the glycation of the gels [52]. The viscoelastic behavior of the gel varies according to the collagen fibrillar structure. Increased or reduced collagen fibrillar structure enables local strain-strengthening by cells in response to changing gelation circumstances. Numerous techniques exist for altering the collagen fibrillar structure [173, 174]. Electrospinning, magnetic fields, mechanical stretching, and shear flow are only a few examples. Using a strain device for linearized ECM (ECM) synthesis, fiber alignment boosted the directionality of breast cancer cells and increased directionality in a directed pattern [175–177].
On the other hand, the natural ECM is composed of a range of ECM proteins with a diversity of biochemical and physical properties that function in concert. Collagen is one of the ECM proteins [178]. Due to their inherent complexity in terms of composition, mechanical properties, and architectural design, it is challenging to recreate the multiple ECM components that affect cellular activity in more defined ECM cultures [59, 179, 180]. Researchers are examining the impact of diverse stromal cell types’ ECM on carcinogenesis and tumor formation using decellularized cells’ ECM. CAF-derived ECMs appear to promote cancer growth and treatment resistance in vitro [54]. Additionally, changes in the ECM have been demonstrated to contribute in the formation of tumors and the immune system in obese individuals, increasing their cancer risk and prognosis [181]. Regenerative medicine, proteomic mapping of the ECM composition, organoid culture encapsulation, and tumor engineering are only a few applications of this technique that have been successfully implemented [182].
Synthetic and composite biomaterials
Artificial materials are attractive as biomaterial substitutes due to their precision and repeatability, as well as the simplicity with which structural and mechanical modifications may be performed [183, 184]. Due to its potential to demonstrate a wide variety of stiffnesses while retaining a uniform surface chemistry, polyacrylamide (PA) hydrogels have been extensively investigated over the last several decades [185]. PA gels should not be employed in 3D culture due to their cytotoxic properties [186, 187]. To accurately model the interactions between the ECM and the cell, PEG gels must be covalently modified with adhesion ligands such as RGD in fibronectin or GFOGER in collagen, as well as MMP-degradable moieties [188]. It is possible to create adhesion and breakdown sites by cross-linking PEG hydrogels with endogenous proteins such as HA. It is now possible to investigate cell adhesion, motility, and ECM stiffness as a result of these alterations [189, 190]. Despite their adaptability, synthetic ECM models lack the stress-relaxation properties of genuine viscoelastic interstitial ECM. This is because synthetic ECM models lack the stress-relaxation properties of real viscoelastic interstitial ECM. On the other hand, viscoelastic PA gels may be formed by encasing a linear PA solution in an elastic network of PA hydrogels [191]. In other words, a dense network of elastic PA hydrogels isolates the linear PA solution from its surroundings. Reversible hydra zone connections may also be used to crosslink PEG hydrogels, as previously described [192, 193]. Dynamic stress-relaxing crosslinks in hydrogels preserve the benefits of covalently crosslinked hydrogels while allowing for complicated biological activities [194]. In other words, a composite system is one that incorporates both synthetic and natural components. The use of ionically crosslinked alginate hydrogels in conjunction with PEG spacers to develop viscoelastic qualities that were not dependent on the original elastic modulus or breakdown rate is an excellent illustration of this [195]. When heated, this 3D hydrogel displayed increased fibroblast spreading and proliferation, as well as better osteogenic differentiation of mesenchymal stem cells as a result of rapid stress relaxation [196]. Hydrogels may be modified to alter their viscoelastic characteristics, which aids in our understanding of how the ECM supports tumor growth and dissemination. Electrospun synthetic materials such as poly(e-caprolactone), poly(lactide-co-glycolide), polylactic acid, and polylactic acid can be used to build biocompatible and scalable porous scaffolds for a variety of applications (ester urethane) [197, 198]. Tumor-like tissues were generated in experiments using cancer cells placed on porous PLG scaffolds. Due to oxygen and feed supply constraints, tumors grown in this technique were more malignant than tumors produced in 2D culture or 3D Matrigel. According to the researchers, the ECM in bone metastases locations has an inorganic composition similar to that of plastionic acid (PLA), polycaprolactone (PCL), and polyurethane (PUR) scaffolds [62, 104, 199]. By implanting microporous PCL scaffolds into the lungs of mice, researchers can monitor the course of disease and the efficacy of various therapies [200]. While electrospun PCL fibrillar scaffolds resemble aligned ECM substrates, they have the additional benefit of inducing the epithelial-mesenchymal transition (EMT) in breast cancer cells, which is necessary for tumor invasion and proliferation [119, 201]. Another research examined the mechanical characteristics of single dextran methacrylate (DexMA) fibers and a bulk dextran methacrylate (DexMA) matrix. When present, softer fibers can help in the dispersion and multiplication of cells. This was done by recruiting fibers and forming localized adhesions [202]. Previous study with non-fibrous hydrogels suggested that the structure of the biomaterial plays a critical role in influencing cell activity. While synthetic scaffolds may have a number of advantages, they are not repairable by cells, and polymer breakdown can result in the formation of acidic byproducts that inhibit cell function [203, 204]. To get a better understanding of the composition, structure, and mechanical properties of tumor-relevant ECM, which will be utilized to further cancer research, biomaterial scaffolds must be manufactured utilizing carefully selected materials and manufacturing processes [205, 206]. The technique of replicating the cell-surrounding matrix in vitro is one of the most crucial steps in the creation of artificial tissue models. The qualities of the material utilized for the scaffold should correspond to those of the native ECM. ECM is sometimes replaced with hydrogels as discussed above, which are extensively used for 3D modeling. In the spaces between the crosslinked polymer chains, they create a network containing a sizable amount of water [207]. Soft tissues are routinely created in vitro using hydrogels made of polysaccharides [208]. This is mostly due to the resemblance between these hydrogels and natural glycosaminoglycans. The linear microbial exopolysaccharide gellan gum (GG) has many beneficial qualities, including biocompatibility, biodegradability, and a non-toxic composition. Guar gum is yet another name for it. The material’s great transparency, thermoresponsive capabilities, flexible mechanical properties, ease of production and cross-linking, stability under physiological conditions, and reasonable cost are further beneficial traits [208–210]. GG is viewed as a promising material in tissue engineering and regenerative medicine as a result of this. The extensive use of GG in the engineering of spinal cord injury regeneration and the regeneration of cartilage, bone, osteochondral, and other tissues is evidence of this [211–213]. Table 3 shows the use of biomaterials to generate 3D tumor models. The following section will briefly discuss the tumor microenvironment-on-a-chip and will explore the microfluidic design considerations and 3D bioprinting strategies involving TME. Figure 4 illustrates the engineering of ECM to model the TME.
Table 3.
The various categories of biomaterials that can be utilized to produce 3D tumor models, along with the positives and negatives associated with each category [46]
| SL no | Biomaterial | Examples | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| 1 | Synthetic |
• PLG • PLA • PEG • PLGA |
• Wide range tunable material properties • Can be structurally complex or isolate individual microenvironment cues |
• Fabrication methods may be cytotoxic • Limited bioactivity |
[71, 215] |
| 2 | Naturally derived |
• Alginate • Silk • Fibrin • Collagen • Matrigel • Decellularized ECM |
• Recapitulate cell-material interactions present in native tissue • Biomimetic |
• Limited cell adhesion site number • Can only fabricate in limited range of material properties • Batch to batch variability |
[1, 216, 217] |
| 3 | Hybrid and semi synthetic |
• Peptide hydrogels • Chitosan-PCL • Hyaluronic acid |
Combine bioactivity of natural materials with structural complexity and tunable properties of synthetic materials |
• Bioactivity of natural components may be affected • Fabrication methods may be cytotoxic |
[1, 71] |
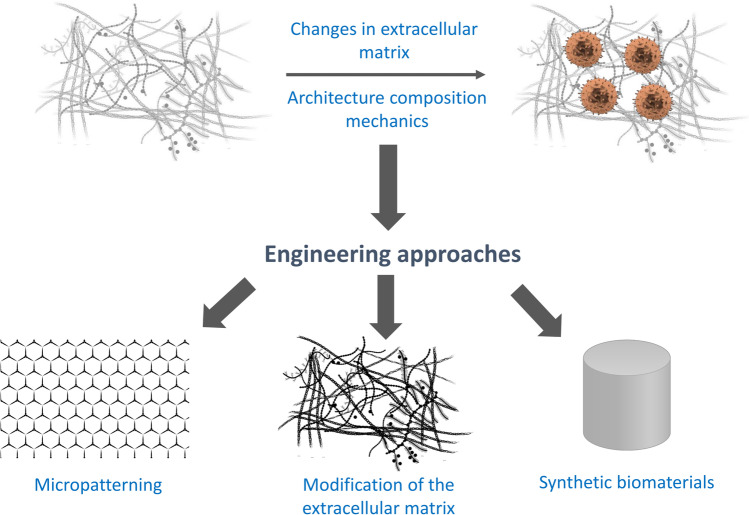
Fig. 4.
Engineering the ECM to model the TME (reproduced with permission from [214]). The cancer cell ECM could be engineered following approaches such as micropatterning, modification of the ECM, synthetic biomaterials, etc.
Modeling tumor-associated ECM changes using biomaterials
Understanding how changes to the ECM affect tumor progression is crucial. To learn more about the role that changes in the composition of the ECM play in the development of cancer, biomaterials-based techniques have been used to create in vitro models of the tumor ECM milieu. The process of creating and exploiting 3D scaffolds made of intact ECM molecules, decellularized ECM molecules, and functional ECM molecules with minimum adhesion sequences is covered in this section. To create a scaffold, these elements are either added to materials that have been changed to allow cross-linking or directly gelled. Gelatin, crosslinker-modified poly(ethylene glycol), and hydroxyapatite are a few examples of such substances (HA).
Matrix in decellularization
It is possible to decellularize animal models, patient samples, and cell cultures to investigate natural matrix elements [218]. Early research relied on decellularized matrix isolated in separate labs, but now commercial vendors supply reconstituted decellularized matrix for use as a surface coating or to make a 3D hydrogel (e.g., Xylyx Bio). These matrices, which can be used to study early tumor formation or metastasis, are produced from healthy tissues. Researchers often need to create their own decellularized matrix to solve biological challenges. Cancer-related disorders may result from ECM changes. Breast cancer cell proliferation was increased by decellularized adipose stromal cells isolated from obese and lean mice [219]. This finding is in line with obese people having an elevated risk of or propensity for breast cancer [220]. Hepatocellular carcinoma cells were driven to undergo EMT by decellularized ECM from healthy or cirrhotic livers in a Smad-dependent manner [221]. These studies highlight the value of applying ECM that is specific to the area of study and show how ECM connects pre-existing issues to cancer risk. The impact of decellularized tumor matrix on the activity of tumor cells has been investigated. ECM that is produced from tumors is typically thought to promote tumor growth. In contrast to collagen gels, glioblastoma cells moved more freely in decellularized tumor matrix [222]. Spheroids of colon cancer that were cultured on ECM from liver metastases expanded more quickly than spheroids of healthy colon [223]. The question of how decellularized matrix from the same tissue distinguishes between tumors from different patients or between normal and malignant states remains unanswered.
Isolated elements of the ECM
Decellularized ECM maintains the complexity of the native ECM environment, although it is challenging due to the scarcity of available tissues and the difficulty in identifying the contributing factors. Scaffolds can be constructed out of ECM components. To investigate the metastasis of breast cancer, collagen I gels were used [224]. Structural scaffolds were created using collagen I and pure ECM. Adding fibronectin to collagen I gels accelerated the migration of breast cancer cells [225]. Increased tumor cell mobility was seen in multicellular models of breast cancer cells and fibroblasts in collagen I or collagen I and fibronectin. The production of extra matrix metalloproteinases (MMPs) by fibroblasts in collagen I plus fibronectin is possible [226]. In an in vitro model of cortical inclusion cysts, the addition of collagen III to collagen I hydrogels boosted fallopian tube epithelial cell invasion [227]. Since full-length ECM proteins play numerous roles, controlling cancer cells is challenging. They are expensive and challenging to employ in scaffolding due to solubility difficulties. Biomaterials can be created from RGD ECM fragments. Glioma cells become chemoresistant due to RGD in HA [228]. To investigate their effect on the size of breast cancer cell clusters, the adhesion peptides RGDS (fibronectin/vitronectin), GFOGER (collagen I), and IKVAV (laminin) were used to make photopolymerizable PEG-based hydrogel systems [229]. (MDA-MB-231) triple-negative and luminal in response to peptide identity, A cells responded differently (T47D). MMP-responsive scaffolds may be required for monitoring cancer cell invasion. PEG hydrogel linkers can be broken down by MMP. Glioblastoma cells expanded and protruded more when cultured in hydrogels that are MMP-degradable [230]. ECM concentrations, not ECM components, frequently fluctuate in malignancies. Many systems function. 3D collagen I gradients show that menaINV enhances breast cancer cell migration to high-fibronectin regions [225]. Breast cancer cells lost their ductal appearance when the density of the collagen I gel was raised and glutamine entry into the TCA cycle increased. This suggests that the ECM plays a role in energy management [231]. Ovarian cancer cells were insensitive to HB-EGF when collagen I density matched omentum values [225, 232]. Ovarian cancer cells multiplied on gels that simulated omentum metastases as a result of HB-EGF [225]. By adjusting the concentration of peptides produced from the ECM, synthetic scaffolds can mimic changes in the ECM’s density. Reducing RGDS or IKVAV density induced an increase in breast cancer cell clustering in the PEG-peptide experiment, indicating a quicker rate of proliferation. GFOGER density is dependent on cell line [229]. Tumors run into new ECM-containing tissues as they spread. To study tumor cell tropism, in vivo replicas of environmental factors were created [233].
Tumor-microenvironment-on-a-chip
Over the past few decades, the cost of medication development has increased significantly due to the inefficiency of preclinical drug screening techniques [215]. Consequently, there is a growing demand for new therapies for people with long-term health conditions [234]. Traditional techniques to drug screening have a number of flaws, the most notable of which are the differences between two-dimensional (2D) in vitro cell culture systems and in vivo models, as well as the evolutionary distance between humans and other animals [235]. Reproducibility has been improved in new 3D cell culture models in addition to delivering more physiologically relevant surroundings and improved prediction capabilities [236]. The fields of microfluidics and cell biology have reached a tipping point with the emergence of “organs on a chip” technologies, which may simulate organ-level in vivo properties in a laboratory environment using a microfluidic substrate that is becoming increasingly popular [237, 238]. These cutting-edge technologies may be used to build better in vitro models for cancer research, expediting the discovery of novel treatments and the transition from laboratory to clinical testing, according to our predictions [239]. It is during the growth of the tumor that the TME is created, which includes both cellular and noncellular components such as blood vessels that encircle the tumor and immune cell populations, cancer stem cells, and ECM [240]. Biological interactions inside the TME are still a significant challenge in cancer treatment research and development because of their complexity [241]. Cancer cannot be examined as a collection of homogenous neoplastic cells, but as a complex multicellular system in order to fully depict the interactions between malignant and nonmalignant cells, according to the most recent study. In particular, this is true as our knowledge of cancer continues to grow [242, 243]. According to cancer biology’s tumor–stroma interaction, this contact between the tumor and the surrounding stroma is crucial to distinguishing the tumor–stroma interaction from other interactions in cancer biology (TMI). Due to the complexity of the TME cancer patients are exposed to, animal models have long been regarded as the gold standard for cancer therapy screening [244, 245]. Even in a laboratory setting, it is nearly impossible to exactly replicate human carcinogenesis. The validity of currently existing in vivo models for the transfer of treatment success data into clinical practice is being questioned as a result of this [38, 246, 247]. Organ-on-a-chip technology has made considerable advances in TME microengineering in recent years. Consequently, it is now possible to create pathophysiologically realistic human cancer models [204, 238].
In the context of tumors, it is vital to keep in mind that they are constantly interacting with their surroundings [195, 197]. Cancer cells and stromal cells (fibroblasts and immune cells) are often found together in a tumor, as are vascular networks in the surrounding tissue that contribute in the tumor’s growth [61, 248]. When developing cancer medicines, it is essential to understand the interplay between the tumor, its stroma, and its circulatory system (blood vessels) [241, 249, 250]. This section focuses on microfluidic devices that are designed to mimic the TME. These systems may be used to investigate the interactions between tumor cells and the stroma, endothelium, and extracellular components that surround them [251]. Table 4 shows the ECM in some common forms of cancer.
Table 4.
ECM deposition in tumors for prevalent kinds of cancer [9]
| SL no | Types of cancer | Tumor-specific ECM deposition | Reference |
|---|---|---|---|
| 1 | Glioblastoma |
• Collagen IV • Procollagen III • Laminin • Fibronectin • Hyaluronic acid • Fibrillar collagens (collagens I, III) |
[240, 252] |
| 2 | Lung cancer |
• Collagen types I and III • Laminin • Fibronectin |
[166, 192] |
| 3 | Pancreatic cancer |
• Collagen I • Hyaluronan collagens I, III, and IV • Laminin • Tenascin • Vitronectin |
[54, 56, 181] |
| 4 | Colon cancer |
• Chondroitin sulfate proteoglycan • Hyaluronic acid • Laminin • Collagen IV Heparan sulfate proteoglycan |
[191, 253, 254] |
| 5 | Hepatocellular carcinoma | • Collagen IV and laminin | [48, 255] |
| 6 | Breast cancer |
• Collagen I • Collagen IV • Collagen V • Fibronectin • Laminins Entacin |
[52, 256–258] |
Microfluidic design considerations
Several key criteria must exist for a chip-based TME to correctly imitate a medically relevant TME [40, 259]. The following conditions apply: TME microfluidic models will be constructed in vitro for drug delivery experiments utilizing data from the study’s in vivo characteristics [249].
Malignancies are hypothesized to stimulate the development of nanoparticles due to their abnormal tumor vasculature and ineffective lymphatic arteries inside the tumor tissue [107, 260]. Their contributions to the EPR phenomenon, which involves the utilization of magnetic fields to transport nanoparticles to solid tumors, are inextricably intertwined [261, 262]. In the laboratory, EPR events have been demonstrated to be hard to adequately replicate using conventional in vitro 2D models. When a solid tumor becomes fibrotic, it develops an increased stiffness, which the illness exacerbates [95, 263].
Collagen and other scaffolding proteins have been connected to the illness’s invasiveness and metastatic potential [103]. Hypoxia and necrosis are more likely to occur in tumors with reduced blood vessel constriction, which decreases oxygen transmission [264, 265]. Because restriction decreases blood flow, it also decreases the effectiveness of therapeutic therapies. As previously stated, it also directly affects cancer cells, triggering death and lowering growth [93, 258, 266]. Long before biomechanical models of tumor formation were developed, mechanical stress was recognized as a factor in tumor growth. To build physiologically appropriate in vitro cancer models, solid tumor stress must be added [267].
Fluid pressure within the tumor cavity increases as a result of the tumor’s highly variable blood flow, both in time and location [268]. Apart from complicating the treatment of malignant tumors, these abnormalities also promote tumor formation. Researchers can employ microfluidic devices to investigate how an aberrant vasculature can be restored to functional normality by increasing pericyte and basement membrane coverage and decreasing vascular permeability [247, 269]. By normalizing the tumor’s vascular system, the tumor’s angiogenic signaling pathways, as well as its abnormal form and function, are restored [270, 271]. When the nanoparticles’ size is raised to compensate for the EPR effect, they become more capable of reaching the tumor site. To provide the most effective and balanced distribution of nanomedicine to a cancer site, it is envisaged that tumor vascular function would need to be normalized [272]. Historically, mutations in tumor vasculature have been explored mostly through computer simulations and mouse models [273, 274]. If researchers employ microfluidic devices, they may get a better understanding of the molecular, cellular, and functional ramifications of dynamic tumor vascular normalization [269, 275].
TME modeling using 3D bioprinting
Extrusion, inkjet printing, stereolithography, and laser-assisted bioprinting are the four primary categories of the currently accessible 3D printing methods that can be employed for 3D in vitro TME modeling. The traditional 3D printing method relies on extrusion, where the material is fed through a nozzle in a continuous stream that can be precisely controlled. A reservoir holds the substance in a specified volume. The extrusion method is a straightforward and reasonably priced procedure. This method’s simplicity allows for any necessary modifications to the printing system, including cross-material printing as well as co-extrusion printing. It is likely that the extrusion process will cause harm and injury to the cells due to the amount of force used. Therefore, the stability and survival of the cells could be jeopardized if the bioprinting parameters, such as the quantity of biomaterial, pressure, and nozzle diameter, are not precisely tuned [276]. In stereolithography bioprinting, bioink is frequently made of light-sensitive polymers, and the fundamental method of this technique is photopolymerization of the bioink. The 3D biomaterial is carefully constructed layer by layer to create the desired pattern. The biomaterial is subjected to light to produce the desired pattern, typically by raster scanning a laser spot or by 2D light projection. Relative to line-by-line raster scanning, 2D light projection allows for speedier printing. It is clear that stereolithography offers superior resolution as compared to bioprinting when extrusion is used. A UV light source is frequently used because the high-energy light required to quickly cure the photopolymer requires it. Stereolithography bioprinters offer good cell viability (> 85%). In contrast to the extrusion procedure, stereolithography bioprinting’s light-induced bioink cross-linking does not subject the cells to shear forces. The third technique, known as inkjet bioprinting, is based on how paper is typically printed in offices. It operates by dropping bioink drops on demand from controlled jets onto a surface. In this trajectory, either heat or piezoelectric actuation controls the bioink’s deposition. The temperature of the bioink increases after the nozzle has been heated, which causes a bubble to form in the thermal actuation. Finally, the force generated by the bubble expansion caused the bioink to be ejected from the nozzle. In piezoelectric actuation, the inkjet printer is under pressure created by a piezoelectric device, which forces the ink out of the nozzle opening. The volume of bioink discharged can be significantly adjusted using the piezoelectric dispensing technique. This technology can provide a greater pattern resolution while employing the least number of cells and biologics since the control can vary from nanoliters to picoliters of bioink. Inkjet bioprinters are thought to be affordable and simple to produce, similar to extrusion bioprinters. As opposed to extrusion bioprinters, which use print heads in sequence, inkjet printers may operate many print heads simultaneously, making this approach speedier and able to deposit multiple cell types quickly. The precise control over the drop-by-drop deposition of bioink is what gives inkjet printers their high resolution (30 mm) [277]. A recent development in tissue engineering is laser-assisted bioprinting. This method’s physical process makes it possible to print liquid materials and cells at the cell level. Laser-assisted bioprinting offers great promise for the production of living tissues with physiologic functionality by offering tissue engineers command over cell density and organization of 3D tissue constructions [278].
Discussion
Although tumor engineering has improved our knowledge of cancer initiation, progression, and therapeutic response, there are still numerous opportunities to advance research and improve treatments. 3-D tumor models must be mechanized and standardized for general use. Hundreds of thousands of spheroids can be produced by bioprinting biomaterials; however, efficiency and size consistency are problems. The size of the spheroid impacts how nutrients diffuse inside the core, which leads to uneven cell growth. In 3-D scaffold-based models, the scaffold material affects medicine absorption and adherence. The choice of the scaffold and the materials should be based on the screening of drugs and their interactions. Even though co-culture of many cell types can simulate the tumor microenvironment in 3-D tumor models, a thorough series of studies must be designed to look at how one cell population affects other cell populations. Different culture media are required for various cell types. In a 3-D in vitro TME model, maintaining distinct growth environments for various cell types may necessitate challenging peripheral component design. In-depth analysis should be done of the ratio of one cell type to other cells in a co-culture setting, and tests should be run to determine not just cell proliferation and invasiveness but also genetic and epigenetic alterations throughout time. Pharma companies may use 3-D TME models that exhibit strong performance in in-depth cross-pharma validations. Imaging and automated readout are required for 3-D tumor models. Limitations of collagenous scaffold autofluorescence 3-D examination of cancer cell images. Cancer cells from 3-D TME models are required for biochemical and cell analyses. It is impossible to gently remove synthetic scaffolding made of polystyrene from growing cancer cells. Enzymatic destruction of PLG-based 3-D scaffolding can liberate cultured cancer cells without hurting the cells. Similar techniques are required for other 3-D TME scaffold models. Despite the fact that they are more automated, scaffold-free 3-D TME models have variable composition and batch-to-batch volatility (basement membrane extract). The composition of the 3-D model can be balanced by allowing stromal cells to deposit extract from the basement membrane before cancer cells are expanded. The development of 3-D tumor models with functional biomaterials using co-cultures of the patient’s cancer, immune, and stromal cells will personalize cancer treatment and establish a new branch of precision medicine. This approach might result in the development of a new class of efficient, specialized drugs that target the cancer cells in the patient. This tactic might also lessen the harmful impacts of drug testing. Finally, by extrapolating observed processes to timelines pertinent to human tumor evolution, computational techniques can support in vitro tumor models. The development, invasion, and treatment outcomes of tumors caused by metabolic heterogeneity can be predicted using computational approaches over timescales of days to years. In silico models can be enhanced by microenvironmental data such as cell deformation, spatial organization, and chemical gradient alterations. Experimentation and computer modeling need to be iteratively integrated to increase relevance. Cancer is a complex disease in which interactions between tumor cells’ microenvironment and their genetic makeup are equally important. Science continues to evolve thanks to in vitro tumor models that focus on the impact of cellular and acellular microenvironmental factors on the development of cancer. Engineered tumor models can offer unique cancer treatment and precision medicine insights that enhance patient outcomes when paired with contemporary imaging, multi-omics, and computational techniques.
Conclusion
Simulating the tumor environment enables the researchers to closely study the tumor progression in relation to its microenvironment. It allows the preclinical testing of new drug candidates for the toxicological and efficacy studies. Using engineered tumor microenvironments based on functional biomaterials will enable researchers to mimic the real TME as closely as possible. Accurate modeling of TME will significantly accelerate the cancer drug development process and will establish a close relation to the results of preclinical studies and the clinical trials since engineered TME contained patients own cells. Therefore, a more personalized form of cancer therapy could be developed and tailored for specific patients. Today’s technological advances have made many aspects of 3D modeling, such as spheroid formation, available to a wider range of researchers and have allowed the creation of multicellular models with increasing complexity. The variety of model systems, cellular sources, and methods for analyzing data may seem daunting, but each has benefits. As a result, the choice of model should be carefully studied in order to meet the goals of the research. Explants give scientists functional units to study TME biology at the sacrifice of tractability, whereas hydrogels give researchers control over the gel’s composition but might produce biomimetic structures that do not exactly replicate the physiology of the host. In the end, 3D modeling is a delicate balance. A balance between usability and model system compatibility must be struck by the ideal cell. Additionally, the 3D system must effectively facilitate the appropriate level of comprehension while accurately displaying the studied biology. With greater access to clinical tissue and improved tools for observing cells in situ and interpreting heterocellular communication, the use of 3D models to understand the TME holds enormous promise for resolving translational research problems across biomedicine.
Abbreviations
- ECM
Extracellular matrix
- TME
Tumor microenvironment
- CAF
Cancer-associated fibroblast
- HOXD10
Homeobox D10
- p53
Tumor suppressor protein
- EMT
Epithelial-mesenchymal transition
- AKT
Protein kinase B
- mTOR
Mammalian/mechanistic target of rapamycin
- MMPs
Matrix metalloproteinases
- AMPK
AMP-activated protein kinase
- LKB1
Liver kinase B1
- ROCK
Rho-associated protein kinase
- HIF-1
Hypoxia-inducible factor-1
- PI3Ks
Phosphoinositide 3-kinases
- MAPKs
Mitogen-activated protein kinases
- TGF
Transforming growth factor
- PGDF
Platelet-derived growth factor
- IGF
Insulin-like growth factor
- IL
Interleukin
- CXCL-8
C-X-C motif chemokine 8
- VEGF
Vascular endothelial growth factor
- IGFBP-3
Insulin-like growth factor-binding protein 3
- ZEB1
Zinc finger E-box binding homeobox 1
Author contribution
Tanvir Ahmed: conceptualization, writing—original draft, and writing—review and editing.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethical approval
Not applicable.
Conflict of interest
The author declares no competing interests.
References
- 1.Do Kim H, Peyton SR. Bio-inspired materials for parsing matrix physicochemical control of cell migration a Review. Integr Biol. 2012;4:37–52. [DOI] [PubMed] [Google Scholar]
- 2.Perrett S, Buell AK, Knowles TPJ (eds). Biological and bio-inspired nanomaterials: properties and assembly mechanisms. Adv Exp Med Biol. 2019;1174. 10.1007/978-981-13-9791-2
- 3.Tomás-Bort E, Kieler M, Sharma S, Candido JB, Loessner D. 3D approaches to model the tumor microenvironment of pancreatic cancer. Theranostics. 2020;10:5074–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernando K, Kwang LG, Lim JTC, Fong ELS. Hydrogels to engineer tumor microenvironments: in vitro. Biomater Sci Royal Soc Chem. 2021;9:2362–83. [DOI] [PubMed] [Google Scholar]
- 5.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–9 (Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asghar W, El Assal R, Shafiee H, Pitteri S, Paulmurugan R, Demirci U. Engineering cancer microenvironments for in vitro 3-D tumor models. Mater Today. 2015;18:539–53 (Elsevier Ltd). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Marion DMS, Domanska UM, Timmer-Bosscha H, Walenkamp AME. Studying cancer metastasis: existing models, challenges and future perspectives. Crit Rev Oncol Hematol. 2016;97:107–17. 10.1016/j.critrevonc.2015.08.009. (Elsevier Ireland Ltd). [DOI] [PubMed] [Google Scholar]
- 8.Maman S, Witz IP. A history of exploring cancer in context. Nat Rev Cancer . 2018;18:359–76. 10.1038/s41568-018-0006-7. (Springer US). [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Vunjak-Novakovic G. Modeling tumor microenvironments using custom-designed biomaterial scaffolds. Curr Opin Chem Eng. 2016;11:94–105. 10.1016/j.coche.2016.01.012. (Elsevier Ltd). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai Y, Li F, Zou Z, Saeed M, Xu Z, Yu H. Bio-inspired amyloid polypeptides: from self-assembly to nanostructure design and biotechnological applications. Appl Mater Today . 2021;22:100966. 10.1016/j.apmt.2021.100966. (Elsevier Ltd). [Google Scholar]
- 11.Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol . 2015;15:669–82. 10.1038/nri3902. (Nature Publishing Group). [DOI] [PubMed] [Google Scholar]
- 12.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Y. Tumor angiogenesis and therapy. Biomed Pharmacother. 2005;59:340–3. [DOI] [PubMed] [Google Scholar]
- 14.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–49. [DOI] [PubMed] [Google Scholar]
- 15.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–34. 10.1038/nrc3261. (Nature Publishing Group). [DOI] [PubMed] [Google Scholar]
- 16.Walter T, Shattuck DW, Baldock R, Bastin ME, Carpenter AE, Duce S, et al. Visualization of image data from cells to organisms. Nat Methods. 2010;7:S26-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein JN, Collisson EA, Mills GB, Shaw KRM, Ozenberger BA, Ellrott K, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet Nature Publishing Group. 2013;45:1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanton C. Intratumor heterogeneity: evolution through space and time. Cancer Res. 2012;72:4875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo DL. Tumor heterogeneity and personalized medicine. N Engl J Med. 2012;366(10):956–7. [DOI] [PubMed]
- 20.Thibaudeau L, Taubenberger AV, Holzapfel BM, Quent VM, Fuehrmann T, Hesami P, et al. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. DMM Dis Model Mech. 2014;7:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi SYC, Lin D, Gout PW, Collins CC, Xu Y, Wang Y. Lessons from patient-derived xenografts for better in vitro modeling of human cancer. Adv Drug Deliv Rev . 2014;79:222–37. 10.1016/j.addr.2014.09.009. (The Authors). [DOI] [PubMed] [Google Scholar]
- 22.Aparicio S, Hidalgo M, Kung AL. Examining the utility of patient-derived xenograft mouse models. Nat Rev Cancer. 2015;15:311–6. 10.1038/nrc3944. (Nature Publishing Group). [DOI] [PubMed] [Google Scholar]
- 23.Mitchell MJ, Jain RK, Langer R. Engineering and physical sciences in oncology: challenges and opportunities. Nat Rev Cancer. 2017;17:659–75. 10.1038/nrc.2017.83. (Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langhans SA. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharmacol. 2018;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avnet S, Lemma S, Cortini M, Di PG, Perut F, Baldini N. Pre-clinical models for studying the interaction between mesenchymal stromal cells and cancer cells and the induction of stemness. Front Oncol. 2019;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodenhizer D, Dean T, D’Arcangelo E, McGuigan AP. The current landscape of 3D in vitro tumor models: what cancer hallmarks are accessible for drug discovery? Adv Healthc Mater. 2018;7:1–36. [DOI] [PubMed] [Google Scholar]
- 27.Mcmillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov . 2013;12:217–28. 10.1038/nrd3870. (Nature Publishing Group). [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues J, Heinrich MA, Teixeira LM, Prakash J. 3D In Vitro Model ( R ) evolution : unveiling tumor – stroma interactions. Trends in Cancer. 2021;7:249–64. 10.1016/j.trecan.2020.10.009. (The Author(s)). [DOI] [PubMed] [Google Scholar]
- 29.Magin CM, Alge DL, Anseth KS. Bio-inspired 3D microenvironments: a new dimension in tissue engineering. Biomed Mater. 2016;11:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egan P, Sinko R, LeDuc PR, Keten S. The role of mechanics in biological and bio-inspired systems. Nat Commun. 2015;6(1):1–12. [DOI] [PubMed]
- 31.Shokooh MK, Emami F, Jeong JH, Yook S. Bio-inspired and smart nanoparticles for triple negative breast cancer microenvironment. Pharmaceutics. 2021;13:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Totti S, Vernardis SI, Meira L, Pérez-Mancera PA, Costello E, Greenhalf W, et al. Designing a bio-inspired biomimetic in vitro system for the optimization of ex vivo studies of pancreatic cancer. Drug Discov Today . 2017;22:690–701. 10.1016/j.drudis.2017.01.012. (Elsevier Ltd). [DOI] [PubMed] [Google Scholar]
- 33.Min LL, Chen SY, Sheng ZZ, Wang HL, Wu F, Wang M, et al. Development and application of bio-inspired and biomimetic microfluidics. Wuli Xuebao/Acta Phys Sin. 2016;65:1–25. [Google Scholar]
- 34.Tu Y, Peng F, Adawy A, Men Y, Abdelmohsen LKEA, Wilson DA. Mimicking the cell: bio-inspired functions of supramolecular assemblies. Chem Rev. 2016;116:2023–78. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Shen G, Yan X. Bio-inspired short peptide self-assembly: from particles to functional materials Particuology. Chinese Soc Particuol. 2022;64:14–34. 10.1016/j.partic.2021.05.007. [Google Scholar]
- 36.Lee DS, Kang J II, Hwang BH, Park KM. Interpenetrating polymer network hydrogels of gelatin and poly(ethylene glycol) as an engineered 3D tumor microenvironment. Macromol Res. 2019;27:205–11. [Google Scholar]
- 37.Zhang L, Liu T, Xie Y, Zeng Z, Chen J. A new classification method of nanotechnology for design integration in biomaterials. Nanotechnol Rev. 2020;9:820–32. [Google Scholar]
- 38.Staymates ME, MacCrehan WA, Staymates JL, Kunz RR, Mendum T, Ong TH, et al. Biomimetic sniffing improves the detection performance of a 3D printed nose of a dog and a commercial trace vapor detector. Sci Rep. 2016;6:1–10 (Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KL, Hubbard LC, Hern S, Yildiz I, Gratzl M, Steinmetz NF. Shape matters: The diffusion rates of TMV rods and CPMV icosahedrons in a spheroid model of extracellular matrix are distinct. Biomater Sci. 2013;1:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X, Farach-Carson MC, Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol Adv . 2014;32:1256–68. 10.1016/j.biotechadv.2014.07.009. (Elsevier B.V). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Shang H, Ding C, Li J. Recent developments and applications of bioinspired dendritic polymers. Polym Chem Royal Soc Chem. 2015;6:668–80. [Google Scholar]
- 42.Barros AS, Costa A, Sarmento B. Building three-dimensional lung models for studying pharmacokinetics of inhaled drugs. Adv Drug Deliv Rev. 2021;170:386–95. [DOI] [PubMed] [Google Scholar]
- 43.Polacheck WJ, Charest JL, Kamm RD. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc Natl Acad Sci U S A. 2011;108:11115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qazi H, Shi ZD, Tarbell JM. Fluid shear stress regulates the invasive potential of glioma cells via modulation of migratory activity and matrix metalloproteinase expression. PloS One. 2011;6(5):e20348. [DOI] [PMC free article] [PubMed]
- 45.Haessler U, Teo JCM, Foretay D, Renaud P, Swartz MA. Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber. Integr Biol. 2012;4:401–9. [DOI] [PubMed] [Google Scholar]
- 46.Wu M, Melody AS. Modeling tumor microenvironments in Vitro. J Biomech Eng. 2014;136:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang YS, Khademhosseini A. Seeking the right context for evaluating nanomedicine: from tissue models in petri dishes to microfluidic organs-on-a-chip. Nanomedicine. 2015;10:685–8. [DOI] [PubMed] [Google Scholar]
- 48.Sükei T, Palma E, Urbani L. Interplay between cellular and non-cellular components of the tumour microenvironment in hepatocellular carcinoma. Cancers (Basel). 2021;13:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park S, Park KM. Engineered polymeric hydrogels for 3D tissue models. Polymers (Basel). 2016;8(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang M, Hou Z, Jin D, Zhou J, Wang M, Wang M, et al. Colorectal tumor microenvironment-activated bio-decomposable and metabolizable Cu2O@CaCO3 nanocomposites for synergistic oncotherapy. Adv Mater. 2020;32:1–11. [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues J, Heinrich MA, Teixeira LM, Prakash J. 3D In vitro model (R)evolution: unveiling tumor–stroma interactions. Trends Cancer. 2021;7:249–64. 10.1016/j.trecan.2020.10.009. (The Author(s)). [DOI] [PubMed] [Google Scholar]
- 52.Stamatelos SK, Bhargava A, Kim E, Popel AS, Pathak AP. Tumor ensemble-based modeling and visualization of emergent angiogenic heterogeneity in breast cancer. Sci Rep. 2019;9:1–14. 10.1038/s41598-019-40888-w. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhat SM, Badiger VA, Vasishta S, Chakraborty J, Prasad S, Ghosh S, et al. 3D tumor angiogenesis models: recent advances and challenges. J Cancer Res Clin Oncol. 2021;147:3477–94. 10.1007/s00432-021-03814-0. (Springer Berlin Heidelberg). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Datta R, Sivanand S, Lau AN, Florek LV, Barbeau AM, Wyckoff J, et al. Interactions with stromal cells promote a more oxidized cancer cell redox state in pancreatic tumors. Sci Adv. 2022;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwak TJ, Lee E. In vitro modeling of solid tumor interactions with perfused blood vessels. Sci Rep. 2020;10:1–9. 10.1038/s41598-020-77180-1. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cave DD, Rizzo R, Sainz B, Gigli G, Del Mercato LL, Lonardo E. The revolutionary roads to study cell–cell interactions in 3d in vitro pancreatic cancer models. Cancers (Basel). 2021;13:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subramaniam S, Joyce P, Thomas N, Prestidge CA. Bioinspired drug delivery strategies for repurposing conventional antibiotics against intracellular infections. Adv Drug Deliv Rev . 2021;177:113948. 10.1016/j.addr.2021.113948. (Elsevier B.V). [DOI] [PubMed] [Google Scholar]
- 58.He Q, Chen J, Yan J, Cai S, Xiong H, Liu Y, et al. Tumor microenvironment responsive drug delivery systems. Asian J Pharm Sci. 2020;15:416–48. 10.1016/j.ajps.2019.08.003. (Elsevier B.V). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clauset A, Behbakht K, Bitler BG. Decoding the dynamic tumor microenvironment. Sci Adv. 2021;7:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liverani C, De Vita A, Minardi S, Kang Y, Mercatali L, Amadori D, et al. A biomimetic 3D model of hypoxia-driven cancer progression. Sci Rep. 2019;9:1–13. 10.1038/s41598-019-48701-4. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soleimani S, Shamsi M, Ghazani MA, Modarres HP, Valente KP, Saghafian M, et al. Translational models of tumor angiogenesis: a nexus of in silico and in vitro models. Biotechnol Adv. 2018;36:880–93. [DOI] [PubMed] [Google Scholar]
- 62.Carey-Ewend AG, Hagler SB, Bomba HN, Goetz MJ, Bago JR, Hingtgen SD. Developing bioinspired three-dimensional models of brain cancer to evaluate tumor-homing neural stem cell therapy. Tissue Eng - Part A. 2021;27:857–66. [DOI] [PubMed] [Google Scholar]
- 63.Bao M, Xie J, Huck WTS. Recent advances in engineering the stem cell microniche in 3D. Adv Sci. 2018;5(8):1800448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tam RY, Smith LJ, Shoichet MS. Engineering cellular microenvironments with photo- and enzymatically responsive hydrogels: toward biomimetic 3D cell culture models. Acc Chem Res. 2017;50:703–13. [DOI] [PubMed] [Google Scholar]
- 65.Rijal G, Li W. Native-mimicking in vitro microenvironment: an elusive and seductive future for tumor modeling and tissue engineering. J Biol Eng. 2018;12:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leight JL, Drain AP, Weaver VM. Extracellular matrix remodeling and stiffening modulate tumor phenotype and treatment response. Annu Rev Cancer Biol. 2017;1:313–34. [Google Scholar]
- 67.Sabu C, Rejo C, Kotta S, Pramod K. Bioinspired and biomimetic systems for advanced drug and gene delivery. J Control Release. 2018. 10.1016/j.jconrel.2018.08.033. (Elsevier B.V). [DOI] [PubMed] [Google Scholar]
- 68.Saglam-Metiner P, Gulce-Iz S, Biray-Avci C. Bioengineering-inspired three-dimensional culture systems: organoids to create tumor microenvironment. Gene. 2019;686:203–12. 10.1016/j.gene.2018.11.058. (Elsevier B.V). [DOI] [PubMed] [Google Scholar]
- 69.Picollet-D’hahan N, Dolega ME, Liguori L, Marquette C, Le Gac S, Gidrol X, et al. A 3D Toolbox to enhance physiological relevance of human tissue models. Trends Biotechnol. 2016;34:757–69. 10.1016/j.tibtech.2016.06.012. (Elsevier Ltd). [DOI] [PubMed] [Google Scholar]
- 70.Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008;18:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adjei I, Blanka S. Modulation of the tumor microenvironment for cancer treatment: a biomaterials approach. J Funct Biomater. 2015;6:81–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hutmacher DW, Loessner D, Rizzi S, Kaplan DL, Mooney DJ, Clements JA. Can tissue engineering concepts advance tumor biology research? Trends Biotechnol. 2010;28:125–33. 10.1016/j.tibtech.2009.12.001. (Elsevier Ltd). [DOI] [PubMed] [Google Scholar]
- 73.Zhang Q, Yang X, Li P, Huang G, Feng S, Shen C, et al. Bioinspired engineering of honeycomb structure - using nature to inspire human innovation. Prog Mater Sci. 2015;74:332–400. 10.1016/j.pmatsci.2015.05.001. (Elsevier Ltd). [Google Scholar]
- 74.Clegg JR, Wagner AM, Shin SR, Hassan S, Khademhosseini A, Peppas NA. Modular fabrication of intelligent material-tissue interfaces for bioinspired and biomimetic devices. Prog Mater Sci. 2019;106:100589. 10.1016/j.pmatsci.2019.100589. (Elsevier Ltd). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu W, Holmes B, Glazer RI, Zhang LG. 3D printed nanocomposite matrix for the study of breast cancer bone metastasis. Nanomedicine Nanotechnology, Biol Med. 2016;12:69–79. 10.1016/j.nano.2015.09.010. (Elsevier Ltd). [DOI] [PubMed] [Google Scholar]
- 76.Sun H, Luo Q, Hou C, Liu J. Nanostructures based on protein self-assembly: from hierarchical construction to bioinspired materials. Nano Today. 2017;14:16–41. 10.1016/j.nantod.2017.04.006. (Elsevier Ltd). [Google Scholar]
- 77.Connors M, Yang T, Hosny A, Deng Z, Yazdandoost F, Massaadi H, et al. Bioinspired design of flexible armor based on chiton scales. Nat Commun. 2019;10:1–13. 10.1038/s41467-019-13215-0. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tekpli X, Lien T, Røssevold AH, Nebdal D, Borgen E, Ohnstad HO, et al. An independent poor-prognosis subtype of breast cancer defined by a distinct tumor immune microenvironment. Nat Commun. 2019;10(1):5499. [DOI] [PMC free article] [PubMed]
- 79.Tan T, Hu H, Wang H, Li J, Wang Z, Wang J, et al. Bioinspired lipoproteins-mediated photothermia remodels tumor stroma to improve cancer cell accessibility of second nanoparticles. Nat Commun . Springer US; 2019;10. 10.1038/s41467-019-11235-4 [DOI] [PMC free article] [PubMed]
- 80.Arina A, Beckett M, Fernandez C, Zheng W, Pitroda S, Chmura SJ, et al. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat Commun. 2019;10(1):1–13. [DOI] [PMC free article] [PubMed]
- 81.Ziai Y, Petronella F, Rinoldi C, Nakielski P, Zakrzewska A, Kowalewski TA, et al. Chameleon-inspired multifunctional plasmonic nanoplatforms for biosensing applications. NPG Asia Mater. 2022;14(1):1–17.
- 82.Wu Q, He Z, Wang X, Zhang Q, Wei Q, Ma S, et al. Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat Commun. 2019;10:1–14. 10.1038/s41467-018-08234-2. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parayath N, Padmakumar S, Nair SV, Menon D, Amiji MM. Strategies for targeting cancer immunotherapy through modulation of the tumor microenvironment. Regen Eng Transl Med. 2020;6:29–49. [Google Scholar]
- 84.Brück O, Blom S, Dufva O, Turkki R, Chheda H, Ribeiro A, et al. Immune cell contexture in the bone marrow tumor microenvironment impacts therapy response in CML. Leukemia. 2018;32:1643–56. 10.1038/s41375-018-0175-0. (Springer US). [DOI] [PubMed] [Google Scholar]
- 85.Shamsi M, Saghafian M, Dejam M, Sanati-Nezhad A. Mathematical modeling of the function of Warburg effect in tumor microenvironment. Sci Rep. 2018;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mertz D, Sentosa J, Luker G, Takayama S. Studying adipose tissue in the breast tumor microenvironment in vitro: progress and opportunities. Tissue Eng Regen Med. 2020;17:773–85. 10.1007/s13770-020-00299-9. (Springer Singapore). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delnero P, Song YH, Fischbach C. Microengineered tumor models: insights & opportunities from a physical sciences-oncology perspective. Biomed Microdevices. 2013;15:583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ingber DE. From cellular mechanotransduction to biologically inspired engineering: 2009 pritzker award lecture, BMES annual meeting October 10, 2009. Ann Biomed Eng. 2010;38:1148–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shieh AC. Biomechanical forces shape the tumor microenvironment. Ann Biomed Eng. 2011;39:1379–89. [DOI] [PubMed] [Google Scholar]
- 90.Lee CH. Engineering bacteria toward tumor targeting for cancer treatment: current state and perspectives. Appl Microbiol Biotechnol. 2012;93:517–23. [DOI] [PubMed] [Google Scholar]
- 91.Zhang WM, Zhang J, Qiao Z, Yin J. Functionally oriented tumor microenvironment responsive polymeric nanoassembly: engineering and applications. Chinese J Polym Sci. 2018;36:273–87 (English Ed). [Google Scholar]
- 92.des Rieux A. Stem cells and their extracellular vesicles as natural and bioinspired carriers for the treatment of neurological disorders. Curr Opin Colloid Interface Sci. 2021;54:101460 (Elsevier Ltd). [Google Scholar]
- 93.Tsai HF, Trubelja A, Shen AQ, Bao G. Tumour-on-a-chip: microfluidic models of tumour morphology, growth and microenvironment. J R Soc Interface. 2017;14(131):20170137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pozzi S, Scomparin A, Israeli Dangoor S, Rodriguez Ajamil D, Ofek P, Neufeld L, et al. Meet me halfway: are in vitro 3D cancer models on the way to replace in vivo models for nanomedicine development. Adv Drug Deliv: Rev; 2021. 10.1016/j.addr.2021.04.001. (Elsevier B.V). [DOI] [PubMed] [Google Scholar]
- 95.Rahmanian M, Seyfoori A, Ghasemi M, Shamsi M, Kolahchi AR, Modarres HP, et al. In-vitro tumor microenvironment models containing physical and biological barriers for modelling multidrug resistance mechanisms and multidrug delivery strategies. J Control Release. 2021;334:164–77. 10.1016/j.jconrel.2021.04.024. [DOI] [PubMed] [Google Scholar]
- 96.Tamo AK, Doench I, Walter L, Montembault A, Sudre G, David L, et al. Development of bioinspired functional chitosan/cellulose nanofiber 3d hydrogel constructs by 3d printing for application in the engineering of mechanically demanding tissues. Polymers (Basel). 2021;13(10):1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fischbach C, Hyun JK, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci U S A. 2009;106:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haidar ZS. Bio-inspired/-functional colloidal core-shell polymeric-based nanosystems: technology promise in tissue engineering, bioimaging and nanomedicine. Polymers (Basel). 2010;2:323–52. [Google Scholar]
- 99.Sorrin AJ, Kemal Ruhi M, Ferlic NA, Karimnia V, Polacheck WJ, Celli JP, et al. Photodynamic therapy and the biophysics of the tumor microenvironment. Photochem Photobiol. 2020;96:232–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dzobo K. Taking a full snapshot of cancer biology: deciphering the tumor microenvironment for effective cancer therapy in the oncology clinic. Omi A J Integr Biol. 2020;24:175–9. [DOI] [PubMed] [Google Scholar]
- 101.Papalazarou V, Salmeron-Sanchez M, Machesky LM. Tissue engineering the cancer microenvironment—challenges and opportunities. Biophys Rev Biophysical Rev. 2018;10:1695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nima ZA, Watanabe F, Jamshidi-Parsian A, Sarimollaoglu M, Nedosekin DA, Han M, et al. Bioinspired magnetic nanoparticles as multimodal photoacoustic, photothermal and photomechanical contrast agents. Sci Rep. 2019;9:1–12. 10.1038/s41598-018-37353-5. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tang Y, Soroush F, Sheffield JB, Wang B, Prabhakarpandian B, Kiani MF. A biomimetic microfluidic tumor microenvironment platform mimicking the EPR effect for rapid screening of drug delivery systems. Sci Rep. 2017;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghajar CM, Bissell MJ. Tumor engineering: the other face of tissue engineering. Tissue Eng - Part A. 2010;16:2153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Richardson TP, Peters MC, Ennett AB, Loomis MDJ. Ilustración creadora Español pdf. Nat Biotech. 2001;19:1029–34. [DOI] [PubMed] [Google Scholar]
- 106.Bevilacqua M, Barrella M, Toscano R, Evangelisti A. Recovery of 0,1 Hz microvascular skin blood flow in dysautonomic diabetic (type 2) neuropathy by using frequency rhythmic electrical modulation system (FREMS). IFMBE Proc. 2007;16:932–5. [DOI] [PubMed] [Google Scholar]
- 107.Song HHG, Park KM, Gerecht S. Hydrogels to model 3D in vitro microenvironment of tumor vascularization. Adv Drug Deliv Rev. 2014;79:19–29. 10.1016/j.addr.2014.06.002. (Elsevier B.V.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gkretsi V, Stylianou A, Papageorgis P, Polydorou C, Stylianopoulos T. Remodeling components of the tumor microenvironment to enhance cancer therapy. Front Oncol. 2015;5:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Machiraju GB, Mallick P, Frieboes HB. Multicompartment modeling of protein shedding kinetics during vascularized tumor growth. Sci Rep. 2020;10:1–16. 10.1038/s41598-020-73866-8. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang H, Cao Z, Zhang Q, Xu J, Yun SLJ, Liang K, et al. Chemotaxis-Driven 2D Nanosheet for directional drug delivery toward the tumor microenvironment. Small. 2020;16:1–8. [DOI] [PubMed] [Google Scholar]
- 111.Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC. In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Front Bioeng Biotechnol. 2016;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saha B, Mathur T, Tronolone JJ, Chokshi M, Lokhande GK, Selahi A, et al. Human tumor microenvironment chip evaluates the consequences of platelet extravasation and combinatorial antitumor-antiplatelet therapy in ovarian cancer. Sci Adv. 2021;7(30):eabg5283. [DOI] [PMC free article] [PubMed]
- 113.Sharma VP, Tang B, Wang Y, Duran CL, Karagiannis GS, Xue EA, et al. Live tumor imaging shows macrophage induction and TMEM-mediated enrichment of cancer stem cells during metastatic dissemination. Nat Commun. 2021;12:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meng F, Meyer CM, Joung D, Vallera DA, McAlpine MC, Panoskaltsis-Mortari A. 3D bioprinted in vitro metastatic models via reconstruction of tumor microenvironments. Adv Mater. 2019;31:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qiu Y, Ren K, Zhao W, Yu Q, Guo R, He J, et al. A “dual-guide” bioinspired drug delivery strategy of a macrophage-based carrier against postoperative triple-negative breast cancer recurrence. J Control Release. 2021;329:191–204. 10.1016/j.jconrel.2020.11.039. (Elsevier B.V). [DOI] [PubMed] [Google Scholar]
- 116.Kato S, Fushimi K, Yabuki Y, Maru Y, Hasegawa S, Matsuura T, et al. Precision modeling of gall bladder cancer patients in mice based on orthotopic implantation of organoid-derived tumor buds. Oncogenesis. 2021;10:1–13. 10.1038/s41389-021-00322-1. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agarwal E, Goldman AR, Tang HY, Kossenkov AV, Ghosh JC, Languino LR, et al. A cancer ubiquitome landscape identifies metabolic reprogramming as target of Parkin tumor suppression. Sci Adv. 2021;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Devarasetty M, Dominijanni A, Herberg S, Shelkey E, Skardal A, Soker S. Simulating the human colorectal cancer microenvironment in 3D tumor-stroma co-cultures in vitro and in vivo. Sci Rep. 2020;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Verbridge SS, Chandler EM, Fischbach C. Tissue-engineered three-dimensional tumor models to study tumor angiogenesis. Tissue Eng - Part A. 2010;16:2147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goh WJ, Zou S, Ong WY, Torta F, Alexandra AF, Schiffelers RM, et al. Bioinspired cell-derived nanovesicles versus exosomes as drug delivery systems: a cost-effective alternative. Sci Rep. 2017;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hussey GS, Dziki JL, Badylak SF. Extracellular matrix-based materials for regenerative medicine. Nat Rev Mater. 2018;3:159–73. 10.1038/s41578-018-0023-x. (Springer US). [Google Scholar]
- 122.Liu D, Schilling B, Liu D, Sucker A, Livingstone E, Jerby-Amon L, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med. 2019;25:1916–27. 10.1038/s41591-019-0654-5. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ouni E, Peaucelle A, Haas KT, Van Kerk O, Dolmans MM, Tuuri T, et al. A blueprint of the topology and mechanics of the human ovary for next-generation bioengineering and diagnosis. Nat Commun. 2021;12(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li G, Xie D, Zhong H, et al. Photo-induced non-volatile VO2 phase transition for neuromorphic ultraviolet sensors. Nat Commun. 2022;13:1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19(5):566–75. [DOI] [PubMed] [Google Scholar]
- 126.Maurer MF, Lewis KE, Kuijper JL, Ardourel D, Gudgeon CJ, Chandrasekaran S, et al. Immunity. US: Springer; 2022. [Google Scholar]
- 127.Kim S, Min S, Choi YS, Jo SH, Jung JH, Han K, et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat Commun. 2022;13(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahmadi S, Sukprasert P, Vegesna R, Sinha S, Schischlik F, Artzi N, et al. The landscape of receptor-mediated precision cancer combination therapy via a single-cell perspective. Nat Commun. 2022;13:1–17 (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Minas TZ, Candia J, Dorsey TH, Baker F, Tang W, Kiely M, et al. Immunity to ancestry and lethal prostate cancer. US: Springer; 2022. p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gatenbee CD, Baker A, Schenck RO, Strobl M, West J, Neves MP, et al. Immunosuppressive niche engineering at the onset of human colorectal cancer. US: Springer; 2022. p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Makam P, Yamijala SS, Bhadram VS, Shimon LJ, Wong BM, Gazit E. Single amino acid bionanozyme for environmental remediation. Nat Commun. 2022;13(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ravi VM, Neidert N, Will P, Joseph K, Maier JP, Kückelhaus J, et al. T-cell dysfunction in the glioblastoma microenvironment is mediated by myeloid cells releasing interleukin-10. Nat Commun. 2022;13(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Prince E, Cruickshank J, Ba-Alawi W, Hodgson K, Haight J, Tobin C, et al. Biomimetic hydrogel supports initiation and growth of patient-derived breast tumor organoids. Nat Commun. 2022;13(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Danaher P, Kim Y, Nelson B, Griswold M, Yang Z, Piazza E, et al. Advances in mixed cell deconvolution enable quantification of cell types in spatial transcriptomic data. Nat Commun. 2022;13:1–13 (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mayse LA, Imran A, Larimi MG, Cosgrove MS, Wolfe AJ, Movileanu L. Disentangling the recognition complexity of a protein hub using a nanopore. Nat Commun. 2022;13:1–11 (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hunter MV, Moncada R, Weiss JM, Yanai I, White RM. Spatially resolved transcriptomics reveals the architecture of the tumor-microenvironment interface. Nat Commun. 2021;12:1–16 (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim MC, Borcherding N, Ahmed KK, Voigt AP, Vishwakarma A, Kolb R, et al. CD177 modulates the function and homeostasis of tumor-infiltrating regulatory T cells. Nat Commun. 2021;12(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qin X, Wu C, Niu D, Qin L, Wang X, Wang Q, et al. Peroxisome inspired hybrid enzyme nanogels for chemodynamic and photodynamic therapy. Nat Commun. 2021;12:1–15. 10.1038/s41467-021-25561-z. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Osuna de la Peña D, Trabulo SMD, Collin E, Liu Y, Sharma S, Tatari M, et al. Bioengineered 3D models of human pancreatic cancer recapitulate in vivo tumour biology. Nat Commun. 2021;12(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Crouchet E, Bandiera S, Fujiwara N, Li S, El Saghire H, Fernández-Vaquero M, et al. A human liver cell-based system modeling a clinical prognostic liver signature for therapeutic discovery. Nat Commun. 2021;12:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tabdanov ED, Rodríguez-Merced NJ, Cartagena-Rivera AX, Puram VV, Callaway MK, Ensminger EA, et al. Engineering T cells to enhance 3D migration through structurally and mechanically complex tumor microenvironments. Nat Commun. 2021;12:1–17. 10.1038/s41467-021-22985-5. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koyuncu B, Melek A, Yilmaz D, Tuzer M, Unlu MB. Chemotherapy response prediction with diffuser elapser network. Sci Rep. 2022;12:1–13. 10.1038/s41598-022-05460-z. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Y, Wang X, Chu C, Zhou Z, Chen B, Pang X, et al. Genetically engineered magnetic nanocages for cancer magneto-catalytic theranostics. Nat Commun. 2020;11:1–11. 10.1038/s41467-020-19061-9. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou Y, Yang D, Yang Q, Lv X, Huang W, Zhou Z, et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat Commun. 2020;11:1–17. 10.1038/s41467-020-20059-6. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Uttam S, Stern AM, Sevinsky CJ, Furman S, Pullara F, Spagnolo D, et al. Spatial domain analysis predicts risk of colorectal cancer recurrence and infers associated tumor microenvironment networks. Nat Commun. 2020;11:1–14. 10.1038/s41467-020-17083-x. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rebecca VW, Somasundaram R, Herlyn M. Pre-clinical modeling of cutaneous melanoma. Nat Commun. 2020;11:1–9. 10.1038/s41467-020-15546-9. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grabovska Y, Mackay A, O’Hare P, Crosier S, Finetti M, Schwalbe EC, et al. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat Commun. 2020;11:1–15. 10.1038/s41467-020-18070-y. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xi X, Xi X, Ye T, Ye T, Wang S, Wang S, et al. Self-healing microcapsules synergetically modulate immunization microenvironments for potent cancer vaccination. Sci Adv. 2020;6:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim K, Hu W, Audenet F, Almassi N, Hanrahan AJ, Murray K, et al. Modeling biological and genetic diversity in upper tract urothelial carcinoma with patient derived xenografts. Nat Commun. 2020;11:1–12. 10.1038/s41467-020-15885-7. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim GB, Nam GH, Hong Y, Woo J, Cho Y, Kwon IC, et al. Xenogenization of tumor cells by fusogenic exosomes in tumor microenvironment ignites and propagates antitumor immunity. Sci Adv. 2020;6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li J, Shi K, Sabet ZF, Fu W, Zhou H, Xu S, et al. New power of self-assembling carbonic anhydrase inhibitor: short peptide–constructed nanofibers inspire hypoxic cancer therapy. Sci Adv. 2019;5:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Korangath P, Barnett JD, Sharma A, Henderson ET, Stewart J, Yu SH, et al. Nanoparticle interactions with immune cells dominate tumor retention and induce T cell–mediated tumor suppression in models of breast cancer. Sci Adv. 2020;6(13):eaay1601. [DOI] [PMC free article] [PubMed]
- 153.Sun Y, You S, Tu H, Spillman Jr DR, Chaney EJ, Marjanovic M, et al. Intraoperative visualization of the tumor microenvironment and quantification of extracellular vesicles by label-free nonlinear imaging. Sci Adv. 2018;4(12):eaau5603. [DOI] [PMC free article] [PubMed]
- 154.Guo X, Bai J, Ge G, Wang Z, Wang Q, Zheng K, et al. Bioinspired peptide adhesion on Ti implants alleviates wear particle-induced inflammation and improves interfacial osteogenesis. J Colloid Interface Sci. 2022;605:410–24. 10.1016/j.jcis.2021.07.079. (Elsevier Inc). [DOI] [PubMed] [Google Scholar]
- 155.Dube S, Rawtani D. Understanding intricacies of bioinspired organic-inorganic hybrid nanoflowers: a quest to achieve enhanced biomolecules immobilization for biocatalytic, biosensing and bioremediation applications. Adv Colloid Interface Sci. 2021;295:102484. 10.1016/j.cis.2021.102484. (Elsevier B.V). [DOI] [PubMed] [Google Scholar]
- 156.Mascheroni P, Savvopoulos S, Alfonso JCL, Meyer-Hermann M, Hatzikirou H. Improving personalized tumor growth predictions using a Bayesian combination of mechanistic modeling and machine learning. Commun Med. 2021;1:1–14 (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tan ML, Ling L, Fischbach C. Engineering strategies to capture the biological and biophysical tumor microenvironment in vitro. Adv Drug Deliv Rev. 2021;176:113852. 10.1016/j.addr.2021.113852. (Elsevier B.V). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Patwa A, Yamashita R, Long J, Risom T, Angelo M, Keren L, et al. Multiplexed imaging analysis of the tumor-immune microenvironment reveals predictors of outcome in triple-negative breast cancer. Commun Biol. 2021;4:1–14. 10.1038/s42003-021-02361-1. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zangooei MH, Margolis R, Hoyt K. Multiscale computational modeling of cancer growth using features derived from microCT images. Sci Rep. 2021;11:1–17. 10.1038/s41598-021-97966-1. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oyelade ON, Ezugwu AE. A bioinspired neural architecture search based convolutional neural network for breast cancer detection using histopathology images. Sci Rep. 2021;11:1–28. 10.1038/s41598-021-98978-7. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Roller DG, Hoang SA, Rawls KD, Owen KA, Simmers MB, Figler RA, et al. Validation of a multicellular tumor microenvironment system for modeling patient tumor biology and drug response. Sci Rep. 2021;11:1–15. 10.1038/s41598-021-84612-z. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seidlitz T, Stange DE. Gastrointestinal cancer organoids—applications in basic and translational cancer research. Exp Mol Med. 2021;53:1459–70 (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Samani ZR, Parker D, Wolf R, Hodges W, Brem S, Verma R. Distinct tumor signatures using deep learning-based characterization of the peritumoral microenvironment in glioblastomas and brain metastases. Sci Rep. 2021;11:1–9. 10.1038/s41598-021-93804-6. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li T, Liu J, Wang Y, Zhou C, Shi Q, Huang S, et al. Liver fibrosis promotes immunity escape but limits the size of liver tumor in a rat orthotopic transplantation model. Sci Rep. 2021;11:1–10. 10.1038/s41598-021-02155-9. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Y, Brodin E, Nishii K, Frieboes HB, Mumenthaler SM, Sparks JL, et al. Impact of tumor-parenchyma biomechanics on liver metastatic progression: a multi-model approach. Sci Rep. 2021;11:1–20. 10.1038/s41598-020-78780-7. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mori V, Bates JHT, Jantz M, Mehta HJ, Kinsey CM. A computational modeling approach for dosing endoscopic intratumoral chemotherapy for advanced non-small cell lung cancer. Sci Rep. 2022;12:1–10. 10.1038/s41598-021-03849-w. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bassi G, Panseri S, Dozio SM, Sandri M, Campodoni E, Dapporto M, et al. Scaffold-based 3D cellular models mimicking the heterogeneity of osteosarcoma stem cell niche. Sci Rep. 2020;10:1–12. 10.1038/s41598-020-79448-y. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pal M, Chen H, Lee BH, Lee JYH, Yip YS, Tan NS, et al. Epithelial-mesenchymal transition of cancer cells using bioengineered hybrid scaffold composed of hydrogel/3D-fibrous framework. Sci Rep. 2019;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bowers HJ, Fannin EE, Thomas A, Weis JA. Characterization of multicellular breast tumor spheroids using image data-driven biophysical mathematical modeling. Sci Rep. 2020;10:1–12. 10.1038/s41598-020-68324-4. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kaduri M, Sela M, Kagan S, Poley M, Abumanhal-Masarweh H, Mora-Raimundo P, et al. Targeting neurons in the tumor microenvironment with bupivacaine nanoparticles reduces breast cancer progression and metastases. Sci Adv. 2021;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vougiouklakis T, Aung PP, Vasudevaraja V, Prieto VG, Torres-Cabala CA, Sulman EP, et al. Correlative study of epigenetic regulation of tumor microenvironment in spindle cell melanomas and cutaneous malignant peripheral nerve sheath tumors. Sci Rep. 2020;10:1–9. 10.1038/s41598-020-69787-1. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fredrich T, Rieger H, Chignola R, Milotti E. Fine-grained simulations of the microenvironment of vascularized tumours. Sci Rep. 2019;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Miedel MT, Gavlock DC, Jia S, Gough A, Taylor DL, Stern AM. Modeling the effect of the metastatic microenvironment on phenotypes conferred by estrogen receptor mutations using a human liver microphysiological system. Sci Rep. 2019;9:1–14. 10.1038/s41598-019-44756-5. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bock N, Kryza T, Shokoohmand A, Röhl J, Ravichandran A, Wille ML, et al. In vitro engineering of a bone metastases model allows for study of the effects of antiandrogen therapies in advanced prostate cancer. Sci Adv. 2021;7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356(6337):eaaf3627. [DOI] [PMC free article] [PubMed]
- 176.Huang Q, Li F, Hu H, Fang Z, Gao Z, Xia G, et al. Loss of TSC1/TSC2 sensitizes immune checkpoint blockade in non–small cell lung cancer. Sci Adv. 2022;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wan Z, Sun R, Liu YW, Li S, Sun J, Li J, et al. Targeting metabotropic glutamate receptor 4 for cancer immunotherapy. Sci Adv. 2021;7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cook DP, Vanderhyden BC. Transcriptional census of epithelial-mesenchymal plasticity in cancer. Sci Adv. 2022;8(1):eabi7640. [DOI] [PMC free article] [PubMed]
- 179.Neufeld L, Yeini E, Reisman N, Shtilerman Y, Ben-Shushan D, Pozzi S, et al. Microengineered perfusable 3D-bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment. Sci Adv. 2021;7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cascio S, Chandler C, Zhang L, Sinno S, Gao B, Onkar S, et al. Cancer-associated MSC drive tumor immune exclusion and resistance to immunotherapy, which can be overcome by Hedgehog inhibition. Sci Adv. 2021;7(46):eabi5790. [DOI] [PMC free article] [PubMed]
- 181.Murphy KJ, Reed DA, Vennin C, Conway JR, Nobis M, Yin JX, et al. Intravital imaging technology guides FAK-mediated priming in pancreatic cancer precision medicine according to Merlin status. Sci Adv. 2021;7(40):eabh0363. [DOI] [PMC free article] [PubMed]
- 182.Opzoomer JW, Anstee JE, Dean I, Hill EJ, Bouybayoune I, Caron J, et al. Macrophages orchestrate the expansion of a proangiogenic perivascular niche during cancer progression. Sci Adv. 2021;7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nikolic A, Singhal D, Ellestad K, Johnston M, Shen Y, Gillmor A, et al. Copy-scAT: deconvoluting single-cell chromatin accessibility of genetic subclones in cancer. Sci Adv. 2021;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee Y, Bogdanoff D, Wang Y, Hartoularos GC, Woo JM, Mowery CT, et al. Xyzeq: Spatially resolved single-cell RNA sequencing reveals expression heterogeneity in the tumor microenvironment. Sci Adv. 2021;7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Whitburn J, Rao SR, Morris EV, Tabata S, Hirayama A, Soga T, et al. Metabolic profiling of prostate cancer in skeletal microenvironments identifies G6PD as a key mediator of growth and survival. Sci Adv. 2022;8(8):eabf9096. [DOI] [PMC free article] [PubMed]
- 186.Chen J, Qiu M, Ye Z, Nyalile T, Li Y, Glass Z, et al. In situ cancer vaccination using lipidoid nanoparticles. Sci Adv. 2021;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Luo H, Xia X, Kim GD, Liu Y, Xue Z, Zhang L, et al. Characterizing dedifferentiation of thyroid cancer by integrated analysis. Sci Adv. 2021;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kilgour MK, MacPherson S, Zacharias LG, Ellis AE, Sheldon RD, Liu EY, et al. 1-Methylnicotinamide is an immune regulatory metabolite in human ovarian cancer. Sci Adv. 2021;7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang S, Zhang L, Zhao J, He M, Huang Y, Zhao S. A tumor microenvironment–induced absorption red-shifted polymer nanoparticle for simultaneously activated photoacoustic imaging and photothermal therapy. Sci Adv. 2021;7(12):eabe3588. [DOI] [PMC free article] [PubMed]
- 190.Kim K, Ryu JH, Koh MY, Yun SP, Kim S, Park JP, et al. Coagulopathy-independent, bioinspired hemostatic materials: a full research story from preclinical models to a human clinical trial. Sci Adv. 2021;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tiwari S, Kajdacsy-Balla A, Whiteley J, Cheng G, Jirstrom K, Birgisson H, et al. INFORM: INFrared-based ORganizational Measurements of tumor and its microenvironment to predict patient survival. Sci Adv. 2021;7(6):eabb8292. [DOI] [PMC free article] [PubMed]
- 192.Guo Y, Lu X, Chen Y, Rendon B, Mitchell RA, Cuatrecasas M, et al. Zeb1 induces immune checkpoints to form an immunosuppressive envelope around invading cancer cells. Sci Adv. 2021;7(21):eabd7455. [DOI] [PMC free article] [PubMed]
- 193.Sun Y, Yang N, Utama FE, Udhane SS, Zhang J, Peck AR, et al. NSG-Pro mouse model for uncovering resistance mechanisms and unique vulnerabilities in human luminal breast cancers. Sci Adv. 2021;7:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.He Y, Hong C, Yan EZ, Fletcher SJ, Zhu G, Yang M, et al. Self-assembled cGAMP-STING∆TM signaling complex as a bioinspired platform for cGAMP delivery. Sci Adv. 2020;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hedegaard CL, Redondo-Gómez C, Tan BY, Ng KW, Loessner D, Mata A. Peptide-protein coassembling matrices as a biomimetic 3d model of ovarian cancer. Sci Adv. 2020;6:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dong X, Pan P, Zheng DW, Bao P, Zeng X, Zhang XZ. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci Adv. 2020;6(20):eaba1590. [DOI] [PMC free article] [PubMed]
- 197.Devkota L, Starosolski Z, Rivas CH, Starosolski Z, Annapragada A, Ghaghada KB, et al. Detection of response to tumor microenvironment targeted cellular immunotherapy using nano-radiomics. Sci Adv. 2020;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang X, Sarvestani SK, Moeinzadeh S, He X, Jabbari E. Three-dimensional-engineered matrix to study cancer stem cells and tumorsphere formation: effect of matrix modulus. Tissue Eng - Part A. 2013;19:669–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Verbridge SS, Choi NW, Zheng Y, Brooks DJ, Stroock AD, Fischbach C. Oxygen-controlled three-dimensional cultures to analyze tumor angiogenesis. Tissue Eng - Part A. 2010;16:2133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng - Part A. 2009;15:205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ingber DE, Mow VC, Butler D, Niklason L, Huard J, Mao J, et al. Tissue engineering and developmental biology: going biomimetic. Tissue Eng. 2006;12:3265–83. [DOI] [PubMed] [Google Scholar]
- 202.Boontheekul T, Hill EE, Kong HJ, Mooney DJ. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007;13:1431–42. [DOI] [PubMed] [Google Scholar]
- 203.Chang R, Nam J, Sun W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng - Part A. 2008;14:41–8. [DOI] [PubMed] [Google Scholar]
- 204.Allison DD, Grande-Allen KJ. Review Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12:2131–40. [DOI] [PubMed] [Google Scholar]
- 205.Garciá-Caballero M, Van De Velde M, Blacher S, Lambert V, Balsat C, Erpicum C, et al. Modeling pre-metastatic lymphvascular niche in the mouse ear sponge assay. Sci Rep. 2017;7:1–16. 10.1038/srep41494. (Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Su Y, Zhang X, Lei L, Liu B, Wu S, Shen J. Tumor microenvironment-activatable cyclic cascade reaction to reinforce multimodal combination therapy by destroying the extracellular matrix. ACS Appl Mater Interfaces. 2021;13:12960–71. [DOI] [PubMed] [Google Scholar]
- 207.Cascone S, Lamberti G. Hydrogel-based commercial products for biomedical applications: a review. Int J Pharm. 2020;573:118803. 10.1016/j.ijpharm.2019.118803. (Elsevier B.V). [DOI] [PubMed] [Google Scholar]
- 208.Bombaldi de Souza RF, Bombaldi de Souza FC, Rodrigues C, Drouin B, Popat KC, Mantovani D, et al. Mechanically-enhanced polysaccharide-based scaffolds for tissue engineering of soft tissues. Mater Sci Eng C. 2019;94:364–75. 10.1016/j.msec.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 209.Albrecht FB, Dolderer V, Nellinger S, Schmidt FF, Kluger PJ. Gellan gum is a suitable biomaterial for manual and bioprinted setup of long-term stable, functional 3D-adipose tissue models. Gels. 2022;8(7):420. 10.3390/gels8070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Morris ER, Nishinari K, Rinaudo M. Gelation of gellan - a review. Food Hydrocoll. 2012;28:373–411. 10.1016/j.foodhyd.2012.01.004. (Elsevier Ltd). [Google Scholar]
- 211.Kim WK, Choi JH, Shin ME, Kim JW, Kim PY, Kim N, et al. Evaluation of cartilage regeneration of chondrocyte encapsulated gellan gum-based hyaluronic acid blended hydrogel. Int J Biol Macromol. 2019;141:51–9. 10.1016/j.ijbiomac.2019.08.176. (Elsevier B.V). [DOI] [PubMed] [Google Scholar]
- 212.Choi JH, Park A, Lee W, Youn J, Rim MA, Kim W, et al. Preparation and characterization of an injectable dexamethasone-cyclodextrin complexes-loaded gellan gum hydrogel for cartilage tissue engineering. J Control Release. 2020;327:747–65. 10.1016/j.jconrel.2020.08.049. (Elsevier). [DOI] [PubMed] [Google Scholar]
- 213.Kim W, Choi JH, Kim P, Youn J, Song JE, Motta A, et al. Preparation and evaluation of gellan gum hydrogel reinforced with silk fibers with enhanced mechanical and biological properties for cartilage tissue engineering. J Tissue Eng Regen Med. 2021;15:936–47. [DOI] [PubMed] [Google Scholar]
- 214.Micek HM, Visetsouk MR, Masters KS, Kreeger PK. iScience ll Engineering the extracellular matrix to model the evolving tumor microenvironment. Iscience. 2020;23:101742. 10.1016/j.isci.2020.101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Thakuri PS, Liu C, Luker GD, Tavana H. Biomaterials-based approaches to tumor spheroid and organoid modeling. Adv Healthc Mater. 2018;7:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Belgodere JA, King CT, Bursavich JB, Burow ME, Martin EC, Jung JP. Engineering breast cancer microenvironments and 3D bioprinting. Front Bioeng Biotechnol. 2018;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Trivedi P, Liu R, Bi H, Xu C, Rosenholm JM, Åkerfelt M. 3D modeling of epithelial tumors—the synergy between materials engineering, 3D bioprinting, high-content imaging, and nanotechnology. Int J Mol Sci. 2021;22(12):6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gratzer PF. Decellularized extracellular matrix. Encycl. Biomed Eng. 2019;1–3:86–96. [Google Scholar]
- 219.Seo BR, Bhardwaj P, Choi S, Gonzalez J, Eguiluz RCA, Wang K, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hefetz-Sela S, Scherer PE. Adipocytes: impact on tumor growth and potential sites for therapeutic intervention. Pharmacol Ther. 2013;138:197–210. 10.1016/j.pharmthera.2013.01.008. (Elsevier Inc). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mazza G, Telese A, Al-Akkad W, Frenguelli L, Levi A, Marrali M, et al. Cirrhotic human liver extracellular matrix 3D scaffolds promote smad-dependent TGF-β1 epithelial mesenchymal transition. Cells. 2019;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Koh I, Cha J, Park J, Choi J, Kang SG, Kim P. The mode and dynamics of glioblastoma cell invasion into a decellularized tissue-derived extracellular matrix-based three-dimensional tumor model. Sci Rep. 2018;8:1–12. 10.1038/s41598-018-22681-3. (Springer US). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Romero-López M, Trinh AL, Sobrino A, Hatch MMS, Keating MT, Fimbres C, et al. Recapitulating the human tumor microenvironment: colon tumor-derived extracellular matrix promotes angiogenesis and tumor cell growth. Biomaterials. 2017;116:118–29. 10.1016/j.biomaterials.2016.11.034. (Elsevier Ltd). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kai FB, Drain AP, Weaver VM. The extracellular matrix modulates the metastatic journey. Dev Cell. 2019;49:332–46. 10.1016/j.devcel.2019.03.026. (Elsevier Inc). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Oudin MJ, Jonas O, Kosciuk T, Broye LC, Guido BC, Wyckoff J, et al. Tumor cell–driven extracellular matrix remodeling drives haptotaxis during metastatic progression. Cancer Discov. 2016;6:516–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lugo-Cintrón KM, Gong MM, Ayuso JM, Tomko LA, Beebe DJ, Virumbrales-Muñoz M, Ponik SM. Breast fibroblasts and ECM components modulate breast cancer cell migration through the secretion of MMPs in a 3D microfluidic co-culture model. Cancers. 2020;12(5):1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fleszar AJ, Walker A, Porubsky V, Flanigan W, James D, Campagnola PJ, et al. The extracellular matrix of ovarian cortical inclusion cysts modulates invasion of fallopian tube epithelial cells. APL Bioeng. 2018;2(3):031902. [DOI] [PMC free article] [PubMed]
- 228.Xiao W, Wang S, Zhang R, Sohrabi A, Yu Q, Liu S, et al. Bioengineered scaffolds for 3D culture demonstrate extracellular matrix-mediated mechanisms of chemotherapy resistance in glioblastoma. Matrix Biol. 2020;85–86:128–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sawicki LA, Ovadia EM, Pradhan L, Cowart JE, Ross KE, Wu CH, Kloxin AM. Tunable synthetic extracellular matrices to investigate breast cancer response to biophysical and biochemical cues. APL Bioeng. 2019;3(1):016101. [DOI] [PMC free article] [PubMed]
- 230.Wang C, Tong X, Jiang X, Yang F. Effect of matrix metalloproteinase-mediated matrix degradation on glioblastoma cell behavior in 3D PEG-based hydrogels. J Biomed Mater Res - Part A. 2017;105:770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Morris BA, Burkel B, Ponik SM, Fan J, Condeelis JS, Aguire-Ghiso JA, et al. Collagen matrix density drives the metabolic shift in breast cancer cells. EBioMedicine. 2016;13:146–56. 10.1016/j.ebiom.2016.10.012. (The Authors). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fogg KC, Renner CM, Christian H, Walker A, Marty-Santos L, Khan A, et al. Ovarian cells have increased proliferation in response to heparin-binding epidermal growth factor as collagen density increases. Tissue Eng - Part A. 2020;26:747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Barney LE, Dandley EC, Jansen LE, Reich NG, Mercurio AM, Peyton SR. A cell-ECM screening method to predict breast cancer metastasis. Integr Biol. 2015;7:198–212. 10.1039/C4IB00218K. ((United Kingdom) . Royal Society of Chemistry). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kumar V, Varghese S. Ex vivo tumor-on-a-chip platforms to study intercellular interactions within the tumor microenvironment. Adv Healthc Mater. 2019;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sontheimer-Phelps A, Hassell BA, Ingber DE. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer. 2019;19:65–81. 10.1038/s41568-018-0104-6. (Springer US). [DOI] [PubMed] [Google Scholar]
- 236.Fan Y, Nguyen DT, Akay Y, Xu F, Akay M. Engineering a brain cancer chip for high-throughput drug screening. Sci Rep. 2016;6:1–12. 10.1038/srep25062. (Nature Publishing Group). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–54. 10.1016/j.tcb.2011.09.005. (Elsevier Ltd). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ahmed T. Biosensors and Bioelectronics : X Organ-on-a-chip microengineering for bio-mimicking disease models and revolutionizing drug discovery. Biosens Bioelectron X. 2022;11:100194. 10.1016/j.biosx.2022.100194. (Elsevier B.V). [Google Scholar]
- 239.Celiku O, Gilbert MR, Lavi O. Computational modeling demonstrates that glioblastoma cells can survive spatial environmental challenges through exploratory adaptation. Nat Commun. 2019;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Akbari H, Kazerooni AF, Ware JB, Mamourian E, Anderson H, Guiry S, et al. Quantification of tumor microenvironment acidity in glioblastoma using principal component analysis of dynamic susceptibility contrast enhanced MR imaging. Sci Rep. 2021;11:1–8. 10.1038/s41598-021-94560-3. (Nature Publishing Group UK). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liu X, Fang J, Huang S, Wu X, Xie X, Wang J, et al. Tumor-on-a-chip: From bioinspired design to biomedical application. Microsyst Nanoeng. 2021;7(1):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shi Y, Van Der MR, Chen X, Lammers T. Theranostics the EPR effect and beyond : strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostic. 2020;10:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ahn J, Sei YJ, Jeon NL, Kim Y. Probing the effect of bioinspired nanomaterials on angiogenic sprouting with a microengineered vascular system. IEEE Trans Nanotechnol. 2017;17(3):393–7. [Google Scholar]
- 244.Shannon AE, Boos CE, Hummon AB. Co-culturing multicellular tumor models: Modeling the tumor microenvironment and analysis techniques. Proteomics. 2021;21(9):2000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Feng M, Lu Y, Yang Y, Zhang M, Xu YJ, Gao HL, et al. Bioinspired greigite magnetic nanocrystals: chemical synthesis and biomedicine applications. Sci Rep. 2013;3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chi Y, Remsik J, Kiseliovas V, Derderian C, Sener U, Alghader M, et al. Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science 80-. 2020;369:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kocal GC, Güven S, Foygel K, Goldman A, Chen P, Sengupta S, et al. Dynamic microenvironment induces phenotypic plasticity of esophageal cancer cells under flow. Sci Rep Nature Publishing Group. 2016;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Aazmi A, Zhou H, Li Y, Yu M, Xu X, Wu Y, et al. Engineered vasculature for organ-on-a-chip systems. Engineering. 2022;9:131–47. [Google Scholar]
- 249.Del Piccolo N, Shirure VS, Bi Y, Goedegebuure SP, Gholami S, Hughes CC, et al. Tumor-on-chip modeling of organ-specific cancer and metastasis. Adv Drug Deliv Rev. 2021;175:113798. [DOI] [PubMed]
- 250.Silvani G, Basirun C, Wu H, Mehner C, Poole K, Bradbury P, et al. A 3D-bioprinted vascularized glioblastoma-on-a-chip for studying the impact of simulated microgravity as a novel pre-clinical approach in brain tumor therapy. Adv Ther. 2021;4:1–14. [Google Scholar]
- 251.Wan L, Neumann CA, Leduc PR. Tumor-on-a-chip for integrating a 3D tumor microenvironment: chemical and mechanical factors. Lab Chip Royal Soc Chem. 2020;20:873–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhang X, Ding K, Wang J, Li X, Zhao P. Chemoresistance caused by the microenvironment of glioblastoma and the corresponding solutions. Biomed Pharmacother. 2019;109:39–46. 10.1016/j.biopha.2018.10.063. [DOI] [PubMed] [Google Scholar]
- 253.Wu H, Zhong D, Zhang Z, Li Y, Zhang X, Li Y, et al. Bioinspired artificial tobacco mosaic virus with combined oncolytic properties to completely destroy multidrug-resistant cancer. Adv Mater. 2020;32:1–10. [DOI] [PubMed] [Google Scholar]
- 254.Evan GI, Hah N, Littlewood TD, Sodir NM, Campos T, Downes M, et al. Re-engineering the pancreas tumor microenvironment: a “regenerative program” hacked. Clin Cancer Res. 2017;23:1647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Özkan A, Stolley DL, Cressman ENK, McMillin M, DeMorrow S, Yankeelov TE, et al. Tumor microenvironment alters chemoresistance of hepatocellular carcinoma through CYP3A4 metabolic activity. Front Oncol. 2021;11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kodack DP, Askoxylakis V, Ferraro GB, Sheng Q, Badeaux M, Goel S, et al. Erratum for The research article: The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci Transl Med. 2019;11(477):aaw6935. [DOI] [PMC free article] [PubMed]
- 257.Premaratne ID, Toyoda Y, Celie KB, Brown KA, Spector JA. Tissue engineering models for the study of breast neoplastic disease and the tumor microenvironment. Tissue Eng Part B Rev. 2020;26(5):423–42. [DOI] [PubMed] [Google Scholar]
- 258.Truong D, Puleo J, Llave A, Mouneimne G, Kamm RD, Nikkhah M. Breast cancer cell invasion into a three dimensional tumor-stroma microenvironment. Sci Rep Nature Publishing Group. 2016;6:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kim HN, Habbit NL, Su CY, Choi N, Ahn EH, Lipke EA, et al. Microphysiological systems as enabling tools for modeling complexity in the tumor microenvironment and accelerating cancer drug development. Adv Funct Mater. 2019;29:1–20. [Google Scholar]
- 260.Jeong SY, Lee JH, Shin Y, Chung S, Kuh HJ. Co-culture of tumor spheroids and fibroblasts in a collagen matrix-incorporated microfluidic chip mimics reciprocal activation in solid tumor microenvironment. PLoS ONE. 2016;11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hassell BA, Goyal G, Lee E, Sontheimer-Phelps A, Levy O, Chen CS, et al. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep. 2017;21:508–16. 10.1016/j.celrep.2017.09.043. (ElsevierCompany). [DOI] [PubMed] [Google Scholar]
- 262.Chung M, Ahn J, Son K, Kim S, Jeon NL. Biomimetic model of tumor microenvironment on microfluidic platform. Adv Healthc Mater. 2017;6:1–7. [DOI] [PubMed] [Google Scholar]
- 263.Truong D, Fiorelli R, Barrientos ES, Melendez EL, Sanai N, Mehta S, et al. A three-dimensional (3D) organotypic microfluidic model for glioma stem cells – vascular interactions. Biomaterials. 2019;198:63–77. 10.1016/j.biomaterials.2018.07.048. (Elsevier Ltd). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Berger Fridman I, Kostas J, Gregus M, Ray S, Sullivan MR, Ivanov AR, et al. High-throughput microfluidic 3D biomimetic model enabling quantitative description of the human breast tumor microenvironment. Acta Biomater. 2021;132:473–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Young EWK. Cells, tissues, and organs on chips: challenges and opportunities for the cancer tumor microenvironment. Integr Biol (United Kingdom). 2013;5:1096–109. [DOI] [PubMed] [Google Scholar]
- 266.Jiménez-Torres JA, Beebe DJ, Sung KE. A microfluidic method to mimic luminal structures in the tumor microenvironment. Methods Mol Biol. 2016;1458:59–69. [DOI] [PubMed] [Google Scholar]
- 267.Tricinci O, De Pasquale D, Marino A, Battaglini M, Pucci C, Ciofani G. A 3D biohybrid real-scale model of the brain cancer microenvironment for advanced in vitro testing. Adv Mater Technol. 2020;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shang M, Soon RH, Lim CT, Khoo BL, Han J. Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab Chip Royal Soc Chem. 2019;19:369–86. [DOI] [PubMed] [Google Scholar]
- 269.Bourn MD, Batchelor DVB, Ingram N, McLaughlan JR, Coletta PL, Evans SD, et al. High-throughput microfluidics for evaluating microbubble enhanced delivery of cancer therapeutics in spheroid cultures. J Control Release. 2020;326:13–24. 10.1016/j.jconrel.2020.06.011. (Elsevier). [DOI] [PubMed] [Google Scholar]
- 270.Sung KE, Beebe DJ. Microfluidic 3D models of cancer. Adv Drug Deliv Rev. 2014;79:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rodrigues T, Kundu B, Silva-Correia J, Kundu SC, Oliveira JM, Reis RL, et al. Emerging tumor spheroids technologies for 3D in vitro cancer modeling. Pharmacol Ther. 2018;184:201–11. 10.1016/j.pharmthera.2017.10.018. (Elsevier Inc). [DOI] [PubMed] [Google Scholar]
- 272.Fisher MF, Rao SS. Three-dimensional culture models to study drug resistance in breast cancer. Biotechnol Bioeng. 2020;117:2262–78. [DOI] [PubMed] [Google Scholar]
- 273.Michna R, Gadde M, Ozkan A, DeWitt M, Rylander M. Vascularized microfluidic platforms to mimic the tumor microenvironment. Biotechnol Bioeng. 2018;115(11):2793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Cox MC, Reese LM, Bickford LR, Verbridge SS. Toward the broad adoption of 3D tumor models in the cancer drug pipeline. ACS Biomater Sci Eng. 2015;1:877–94. [DOI] [PubMed] [Google Scholar]
- 275.Bourn MD, Batchelor DVB, Ingram N, McLaughlan JR, Coletta PL, Evans SD, et al. High-throughput microfluidics for evaluating microbubble enhanced delivery of cancer therapeutics in spheroid cultures. J Control Release. 2020;326:13–24. 10.1016/j.jconrel.2020.06.011. (Elsevier B.V). [DOI] [PubMed] [Google Scholar]
- 276.Oztan YC, Nawafleh N, Zhou Y, Liyanage PY, Hettiarachchi SD, Seven ES, et al. Recent advances on utilization of bioprinting for tumor modeling. Bioprinting. 2020;18:e00079. 10.1016/j.bprint.2020.e00079. (Elsevier B.V). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kim YK, Park JA, Yoon WH, Kim J, Jung S. Drop-on-demand inkjet-based cell printing with 30-μ m nozzle diameter for cell-level accuracy. Biomicrofluidics. 2016;10(6):064110. [DOI] [PMC free article] [PubMed]
- 278.Samadian H, Jafari S, Sepand MR, Alaei L, Sadegh Malvajerd S, Jaymand M, et al. 3D bioprinting technology to mimic the tumor microenvironment: tumor-on-a-chip concept. Mater Today Adv. 2021;12:100160. 10.1016/j.mtadv.2021.100160. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.