Abstract
Background:
The combination of bazedoxifene 20 mg (BZA) and conjugated estrogens 0.45 mg (CE) marketed as Duavee® is approved for vasomotor symptom relief and osteoporosis prevention. Our pilot study suggested it had potential breast cancer risk reduction, and we proposed a multisite Phase IIB primary prevention trial assessing change in breast imaging and tissue risk biomarkers. However, by the time funding was aquired in February 2021, Duavee® was unavailable with an uncertain return date. A redesign was needed to salvage the study.
Methods:
The basic trial design was minimally altered. Women age 45–64 at elevated risk for breast cancer with vasomotor symptoms and no menses for at least 2 months have mammography, phlebotomy, and benign breast tissue sampling before and after 6 months of intervention. However, instead of Duavee® (single pill) vs placebo, women are randomized to 6 months of BZA+CE vs Waitlist. Those initially randomized to Waitlist can receive BZA+CE after 6 months. The primary endpoint is between arm difference in change in a fully automated measure of mammographic density with blood and tissue-based secondary endpoints.
Outcomes:
Accrual initiation was delayed due to contractual difficulties surrounding BZA importation during COVID-19 and deploying a fully automated method (Volpara®) to assess the primary endpoint. To accommodate this delay, a mid-grant no cost extension along with amended eligibility requirements were employed. 61/120 participants needed were entered in the initial 27 months of accrual and 37 months of award. Despite a late start, accrual is likely to be completed within the funding period.
Keywords: breast cancer prevention, Duavee, Fibroglandular volume, random periareolar fine needle aspiration
1.0. Background
1.1. Lack of Acceptable Agents for Breast Cancer Risk Reduction in Women with Menopausal Symptoms
Vasomotor symptoms occur in 70% of women in menopause transition and last an average of 7 years. [1,2]. Because standard antiestrogen therapy increases vasomotor symptoms, it is unlikely to be adopted for primary prevention by symptomatic women whose uptake of these agents is already low [3–10]. Low-dose tamoxifen is an acceptable option for postmenopausal women with fewer side effects. However, as it can still worsen vasomotor symptoms it is not likely to be adopted for women already seeking relief from hotflashes and night sweats [11,12].
Systemic administration of estrogen with or without a progestin is the most effective treatment of vasomotor symptoms [2]. Five years of estrogen without a progestin is unlikely to be associated with a significant increase in risk [13–16]. Indeed, in the Women’s Health Initiative, conjugated estrogens alone, compared to placebo, was associated with a 22% reduction in risk for breast cancer and 40% reduction in breast cancer-related mortality [16]. Conjugated Estrogens (CE) differ from 17-B estradiol in that the preferred estrogen receptor (ER) binding partner is ERβ rather than ERα [17] with less of a proliferative effect on the terminal lobular duct units [18]. However, for women without a prior hysterectomy, the progestin added to CE to protect the uterus increases the risk for breast cancer [13,14,16]. Progestins exhibit a pro-proliferative effect on differentiated luminal epithelial cells and via RANK ligand, progenitor cells as well [19–21].
1.2. Bazedoxifene and Conjugated Estrogen for Safe Relief of Menopausal Symptoms and Breast Cancer Risk Reduction
Bazedoxifene is a selective estrogen receptor modulator (SERM) with both breast and uterine antiestrogenic properties, permitting the omission of a progestin when used with estrogen [22,23]. In clinical trials, BZA given with CE given for up to 2 years relieved hot flashes, improved vaginal dryness, and decreased osteoporotic fracture with no increase in the incidence of endometrial hyperplasia or breast cancer [24–27]. Duavee®, the co-formulation of CE 0.45 mg and BZA 20 mg, received FDA approval in 2013 for relief of menopausal symptoms and prevention of osteoporosis [24]. BZA+CE reduced normal mammary tissue proliferation and estrogen response gene expression in non-human primates [28] and growth of cancer xenografts in mice [29,30]. These observations along with long-term human safety data [31] suggested a potential role for BZA+CE in breast cancer risk reduction.
As an initial step, we performed a pilot study assessing the effects of 6 months of Duavee® on risk biomarkers for breast cancer in peri and postmenopausal women at high risk for development of breast cancer [32]. In addition to a dramatic reduction in hot-flash score, favorable modulation was observed for the mammographic density measure fibroglandular volume (FGV), the breast epithelial proliferation indicator (Ki-67), as well as serum progesterone, sex hormone binding globulin (SHBG) and insulin growth factor-1 (IGF-1) [32]. Higher levels of mammographic density, epithelial proliferation, serum progesterone, and IGF-1 and lower levels of SHBG are positively associated with risk for breast cancer [33–41]. In postmenopausal women insulin resistance may also be associated with risk for breast cancer [42]. Despite promising pre-clinical studies, the effect of Duavee® on visceral fat and serum markers of insulin resistance remain underexplored [43,44].
1.3. Rationale for Study Design
We originally proposed a multisite trial of 6 months of Duavee® vs Placebo in high-risk women with vasomotor symptoms using the same biomarker panel as the pilot. However, at the time of the Notice of Award (NCI grant 1R01CA249437) Duavee® had been temporarily removed from the market due to a problem with internal foil packaging with an uncertain date of return. BZA 20 mg could be paired with commercially available CE (Premarin®) 0.45mg, but BZA as a single agent was not approved by the FDA for use in the US, although it was available in Europe and Japan. With the appropriate IND we were able to import BZA from Japan with direct shipment to the sites. Provision of a placebo under these circumstances was not possible, necessitating the use of randomization to a Waitlist control instead of placebo. The final design offered women randomized to Waitlist 6 months of BZA+CE at the end of the randomized period, which was thought likely to aid recruitment and retention.
To offset the lack of an assessment blind to treatment assignment, we used a fully automated, FDA-approved method (Volpara®) for volumetric density measures and will assess the difference between the two arms in absolute mammographic fibroglandular volume (FGV) as our primary endpoint. Fibroglandular volume is a risk biomarker for breast cancer [41,45] and fully automated measures improve reliability by eliminating interpretive variance [46,47]. A decrease in mammographic density, including FGV, is associated with favorable response to tamoxifen [48–51]. Minimizing variance is particularly important in postmenopausal women where both baseline density and magnitude of change in response to preventive treatment may be lower than for their premenopausal counterparts [51,52]. Using change in FGV instead of change in percent dense area or volume reduces variance between exams due to differences in breast compression [53]. However, a change in FGV is more susceptible to variance than percent dense volume (FGV/Total Breast Volume) if there is variation in the volume of breast captured [53].
Decrease in mammary epithelial cell proliferation as measured by Ki-67 is associated with favorable response to preventive endocrine therapy in premenopausal women and is not highly correlated with density [54,55]. However, since proliferation is low in postmenopausal women and was <1% in 46% of women in our pilot study, we utilized change in Ki-67 as a secondary endpoint [32]. To assure a similar distribution of women with high and low FGV and Ki67 in both arms, women are stratified by these variables at randomization (Figure 1).
Figure 1:
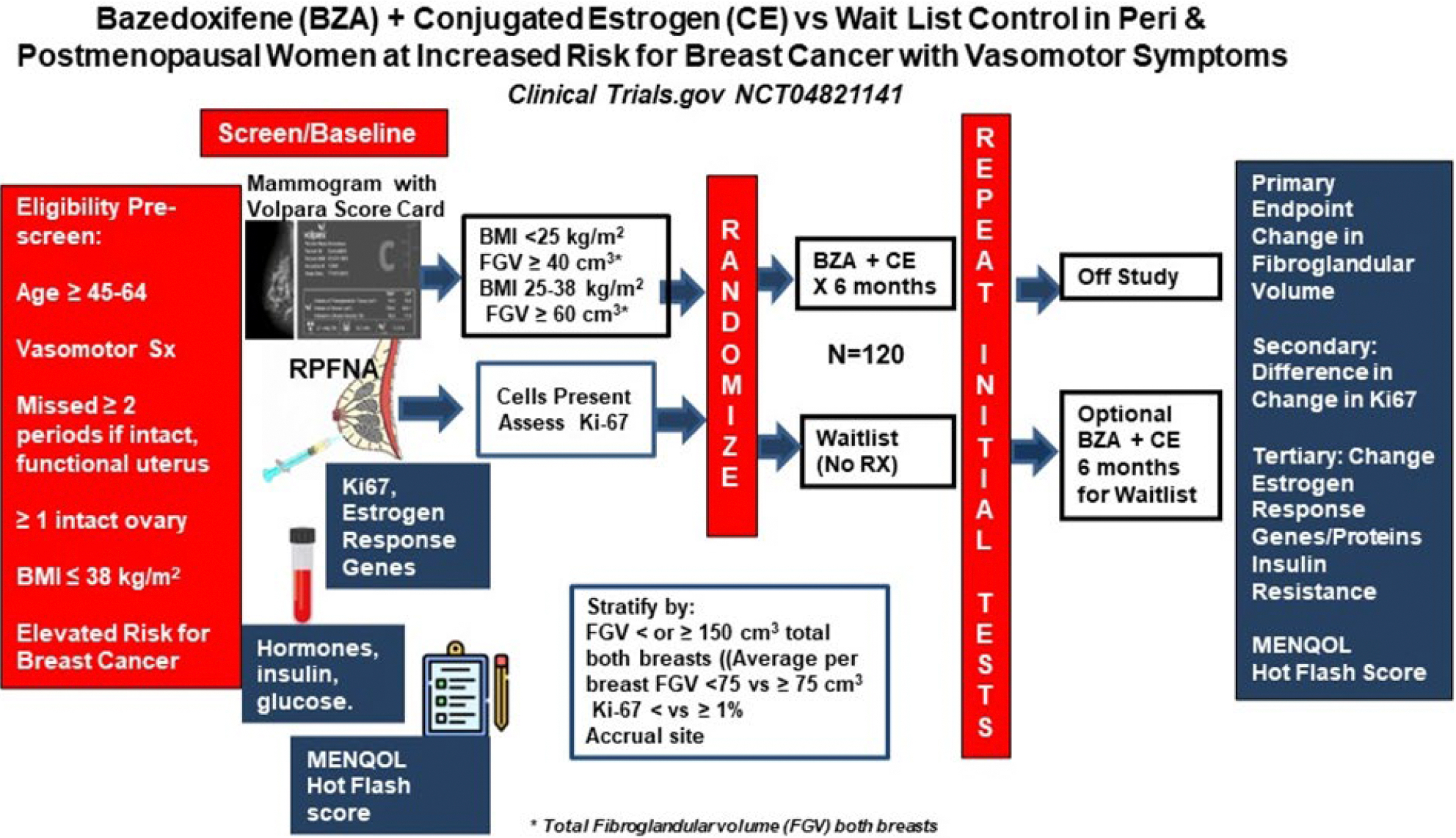
Schema of study design
2.0. Objectives
2.1 Our primary objective is to determine if women randomized to 6 months of BZA+CE have a significant decrease in mammographic FGV compared to those randomized to waitlist control.
2.2 Our secondary objective is to determine if 6 months of BZA+CE modifies epithelial cell Ki-67 expression. Specifically, do women with baseline Ki-67 ≥1% randomized to BZA+CE show a decrease relative to controls? Is there evidence of significant increase (i.e., post-intervention Ki-67 ≥2% for those with baseline ≤ 1% or doubling in those with higher baseline Ki-67).
2.3 Exploratory objectives include change in:
Menopause Quality of Life (MENQOL) and Hot Flash Score
Benign breast tissue estrogen response gene expression (TFF1, PGR, GREB1, ESR1/ESR2, AREG, CCND1, CCNB1, AGR2, FASN, CXCL12, and CYP19A1) and correlation with change in bioavailable hormones.
Benign breast epithelial cell Estrogen Receptor Alpha (ERα), Progesterone Receptor (PR), and Anterior Gradient Receptor 2 (AGR2) protein.
Variables impacting insulin resistance including visceral adipose, adiponectin, omentin, leptin, HOMA-IR, HOMA-%B, and HOMA-%S, and IGF-1.
2.4 Explanatory objectives include:
Modifying effect of baseline FSH, Inhibin-B, and 6-month BZA levels on breast tissue biomarker changes.
Modifying effect of systemic hormones and SHBG on mammographic density and tissue biomarkers
3. Methods
The study protocol was approved by the Institutional Review Board at the University of Kansas Medical Center, which served as the single IRB for all sites per NIH requirements. The trial was registered at ClinicaTrials.gov: NCT04821141
3.1. Study eligibility
Women from specialized clinics designed for women at elevated risk at the University of Kansas Medical Center, Northwestern University, Dana Farber Cancer Institute, University of California San Francisco, and City of Hope are prescreened for eligibility and exclusion criteria. However, prior to randomization women must demonstrate both a minimum fibroglandular volume (FGV) plus epithelial cellularity in a benign breast tissue specimen obtained by random periareolar fine needle aspiration (RPFNA) [32]. See Table 1 Inclusion and Exclusion Criteria
Table 1.
Inclusion and Exclusion Criteria
| Inclusion and Exclusion Criteria |
|---|
| Inclusion |
| Age 45–64 Current Vasomotor symptoms |
| No menstrual periods for 2 months or more Elevated risk for Breast Cancer by any one or more of following factors Positive family history High to moderate penetrance germline gene mutation carrier Prior breast biopsy showing atypical hyperplasia, usual hyperplasia, LCIS, Prior treated ER-PR- or low risk ER+ DCIS Multiple breast biopsies regardless of histology 10-year IBIS 8 or BCSC model risk ≥2 x that for age group BMI ≤ 38 kg/m2 At least 1 intact non-irradiated breast Reasonably normal liver and renal functions No systemic hormones ≥ 8 weeks before biomarker screen. FGV ≥ 60 cm3 (total both breasts) if BMI ≥ 25 kg/m2 or ≥ 40 cm3 if BMI <25 kg/m2 RPFNA specimen contains epithelial cells |
|
|
|
Exclusion Prior Thromboembolic event Prior bilateral oophorectomy Prior High- Risk ER+ and or PR+ DCIS Prior pleomorphic LCIS Prior invasive breast, ovarian or uterine cancer Current significant renal or liver disease Receiving anti-coagulants Prior tamoxifen, raloxifene, aromatase inhibitor within 6 months Known hypoparathyroidism or triglycerides > 300 mg/dl |
3.2. Consent and Study Registration
A consent is signed before research testing and the participant is registered in the Comprehensive Research Information System (CRIS) using an institution-specific screening ID. CRIS is managed by the Department of Biostatistics and the University of Kansas Cancer Center’s Biostatistics and Informatics Shared Resource.
3.3. Specimen Acquisition and Research Biomarker Testing
3.3.1. Mammographic Volumetric Measures.
The baseline mammogram is performed at accruing sites. Raw data Digital Imaging and Communications in Medicine (DICOM) files are sent to an institutional research server where Volpara® software (Volpara® Solutions™, Wellington, New Zealand) has been installed. A Volpara® score card reporting FGV, total breast volume, and % volumetric density for each breast is automatically generated. A copy is securely transmitted to KUMC via a REDCAP application before randomization.
3.3.2. Benign Breast Tissue is Obtained by Random Periareolar Fine Needle Aspiration (RPFNA)
Aspirations are performed on the inner and outer quadrants of both breasts under local anesthesia. RPFNA is processed to both fixed (4/5) and frozen (1/5) aliquots for all KUMC participants, but frozen specimen processing is optional for other sites.
3.3.2.1 Immunostaining for Ki-67 is performed with MIB-1 monoclonal antibody using a DAKO autostainer on ThinPrep™ processed slides [32]. Only participants with ≥ 500 epithelial cells on baseline slides assessed by two laboratory technicians for Ki-67 are considered evaluable for this endpoint.
The remainder of the fixed RPFNA specimen is transferred to a paraffin cell block via a Cellient™ processor and stored until the baseline and 6-month specimen can be processed and assessed in the same batch. Sections are cut, processed to slides, and stained for ERα, PR, and AGR2 in that order of priority. (See Supplemental Table S1 for antibodies).
3.3.2.2 Gene expression from frozen tissue is performed by RT-qPCR after RNA extraction, purification, amplification, and reverse transcription to cDNA. Baseline and postintervention specimens are assessed together in batches from ten or more participants who have completed the randomized period. Primer and probe sequences are provided in Supplementary Table S2 and details regarding testing have been previously published [32].
3.3.3 Research Serum and Plasma is obtained at the time of the RPFNA, processed to serum and plasma, and stored at −80°C until shipment to KUMC on dry ice. Assays for hormones, SHBG, and adipocytokines are performed at the KUMC Breast Cancer Prevention Research Laboratory with baseline and 6-month samples run together. (See supplemental file for assay methods). Plasma for bazedoxifene and major metabolites is obtained at 6 months only and frozen until batch analysis by tandem mass spectrometry in the Clinical Pharmacology Shared Resource at KUMC [56] Bioavailable estradiol and testosterone are calculated according to standard formulae [57]. Fasting insulin, glucose, and lipid profile for KUMC only participants are assayed in the KUMC clinical lab the same day as drawn.
3.3.4. Body Composition Assessment is performed at KUMC only. Women undergo dual x-ray absorptiometry (GE Lunar iDXA) at baseline and 6 months. iDXA coefficient of variation (CV) is < 1% for total mass and 17% for visceral fat [58].
3.3.5. Hot Flash Score and Menopause Specific Quality of Life
The hot flash scoring system developed at Mayo Clinic [59] is the average number of hot-flashes + night sweats/day x the average intensity (1 for mild, 2 for moderate, 3 for severe). The hot-flash score and a 29 Item validated Menopause Quality of Life Questionnaire is utilized at baseline and 6-months [60]. They are also completed at 12 months for those participants originally randomized to Waitlist and subsequently taking drug months 7–12.
3.4. Data Entry
Clinical, iDXA, Volpara® score card and Ki-67 data, are entered into CRIS by site study coordinators. Data from Menopause Specific Quality of life questionnaires are entered directly into REDCAP by participants. Data generated from the Breast Cancer Prevention Research Laboratory obtained from blood and tissue biosamples are entered into an Excel spread sheet by laboratory personnel. After check for completeness and internal consistency lab datat is merged into the central CRIS data base. Central calculation and entry of Breast Cancer Surveillance Consortium 10-year risk version 3 [63] is performed by KUMC research coordinators.
3.5. Stratification and Randomization
Women are stratified by average FGV per breast <75 cm3 vs ≥ 75 cm3; Ki-67 <1% vs ≥ 1%; and enrollment site. Once pre-study data has been entered into CRIS by study coordinators, randomization assignment is automatically performed with generation of a permanent study ID number.
3.6. Intervention:
Women randomized to BZA+CE receive a 7-month supply of bazedoxifene 20 mg and conjugated estrogen 0.45 mg to be taken daily. The intended duration of the intervention is 6 months, but an extra month’s supply is dispensed to provide for any delays in repeat biomarker testing.
3.7. Adverse Events
NCI CTCAE, version 5.0 is used to code adverse events. There is no dose reduction for adverse events. Participants who experience grade 3–4 adverse events have both drugs suspended until the adverse event resolves. Both drugs may be re-instituted after resolution of the adverse event if the investigator is relatively certain the event is not due to BZA+CE.
3.8. Data Collection and Data Sources
Source documents consist of clinical/electronic medical record reports as well as forms created specifically for this study. All CRFs and questionnaires are only identified by participant initials and study ID number. Source documents are kept in institutional participant-specific study binders and are available for monitoring.
3.9. Retention Strategies
The primary retention strategy is the potential for relief of vasomotor symptoms immediately or for those randomized to wait list control the opportunity to take drug at the conclusion of the randomized period.
3.10. Safety Surveillance and Monitoring
Surveillance is detailed in Table 2. This study is monitored by the University of Kansas Cancer Center Data Safety and Monitoring Committee. There is a quarterly review of recruitment, retention, and adverse events.
Table 2.
Evaluation During Study
| Evaluation/ Procedure | Pre-Study/ Screening | Baseline | Mo 1 | Mo 6 | Mo 7* | Mo 12* | 2 weeks after participant’s last study visit |
|---|---|---|---|---|---|---|---|
| Pre-screen for eligibility and conditions of ineligibility | X | ||||||
| Informed consent | X | ||||||
| Liver and Renal Functions | X | ||||||
| Mammogram with Volpara® Score Card | X | X | X** | ||||
| Medical history, PE, and breast exam | X | ||||||
| Vital signs, height, weight, and waist circumference | X | X | X* | ||||
| RPFNA for tissue collection | X | X | |||||
| KUMC Only: iDXA scan for body composition | X | X | X* | ||||
|
KUMC only: Fasting blood Other sites: Blood fasting or non-fasting |
X | X | X* | ||||
| Hot Flash Score assessment; Menopause Quality of Life, other symptoms | X | X | X* | ||||
| Concomitant medications | X | X | X* | ||||
| Final Eligibility Assessed | X | ||||||
| Negative pregnancy test if <55 and uterus intact | X | X* | |||||
| Dispense study agent | X | X** | |||||
| Adverse events | X | X | X* | X | |||
| Collect study agent | X | X* |
4.0. Analytic approach
Our analytic approach is described in a previous publication [61]. Randomization (1:1) of 120 women presuming 10% premature dropout will provide 80% power to detect a mean 20.1 cm3 reduction in FGV for the treatment group compared to 2.5 cm3 increase in the control group. A standard deviation of 51.4 cm3 is assumed for FGV change in the treatment group and 22 cm3 for controls with a Type I error rate of 5% (one-sided test). An interim analysis will be conducted when 60 women complete their 6-month visit.
The change in FGV and Ki-67 from baseline to 6 months will be modeled using Bayesian two-sample normal distributions with weakly informative priors, meaning the majority of analyses will rely on data from the trial rather than prior assumptions. These distributions will be estimated using Markov Chain Monte Carlo at both the interim analysis and the final analysis. Although final success is based only on FGV, the interim analysis consists of stopping criteria (for early success or early futility) based on both FGV and Ki-67. Operating characteristics (e.g., power and sample size) were estimated using trial simulations under various scenarios. Posterior cut-points for both analyses were chosen to maintain a one-sided Type I error rate of 5% and a futility probability of 25%. This two-stage adaptive design with the use of the endpoints in an early stopping decision ensures that adequate information is obtained for Ki-67 but avoids inefficiencies that can arise from using two endpoints in the final analysis.
5.0. Outcomes
5.1. Overcoming Challenges to Start- up and Accrual
5.1.1. Drug Supply
Pfizer voluntarily withdrew Duavee® from the commercial market in May of 2020 due to FDA-identified problems with inner foil inner packaging. At the time of the notice of award (February 2021), Duavee® was still unavailable with an uncertain return to market date. Conjugated Estrogens (CE) as Premarin™ is commercially available in the US but bazedoxifene as a single agent is not. We eventually determined that we would be able to import bazedoxifene from Japan as Viviant® where it is approved for use as a single agent. Consequently, we redesigned the protocol and submitted an IND for bazedoxifene 20 mg to be given with conjugated estrogen. We received the first drug shipment October 2021 and the first participant entered at KUMC on December 14, 2021. We were able to demonstrate to the FDA that bazedoxifene blood levels achieved with our imported BZA+ CE as two pills were in the range of those previously reported after Duavee® [62].
5.1.2. Generation of Research Volumetric Assessments for the Primary Endpoint.
In the clinical setting, raw data DICOM files are sent from the mammography gantries to Picture Archiving and Communication Systems (PACS). Installed Volpara® software automatically generates volumetric measures from the raw data files which are then summarized on a Volpara® “Score Card”. In most institutions, only the processed DICOM files are saved on PACS after Volpara assessment while the raw data files are deleted from the gantries after a few days to weeks. Use of Volpara software for research although attractive because of full automation is complicated by 1) Score cards with FGV cannot be accurately generated after removal of clinical identifiers and 2) clinical Volpara® algorithms are periodically updated.
To ensure participant confidentiality and that the same analysis program was used at baseline and 6 months research, the same version of Volpara research software was installed on institution-specific research servers. These servers were configured to receive the raw data DICOM files, and generate the score card. Following score card generation clinical identifiers could be be replaced with a study ID number prior to secure transfer to a KUMC research server via REDCAP. Concerns regarding transfer of raw data DICOM files from a hospital system to an academic research server caused substantial contractual delays. Multiple meetings between hospital systems, University/Cancer Center Information Technology, legal teams, radiology departments, and site PIs eventually resolved these issues but caused serious accrual delays for sites outside of KUMC.
5.1.3. Early Accrual Lag Due to Delayed Start from Problems with Drug Supply and Institutional Volpara® Research Installation
KUMC randomized 48 participants (the institution’s 4 year goal) within the first 3 years. Overall accrual was still behind as the other sites did not begin accrual until 27 months after the notice of award.
5.1.4. Measures to Increase the Accrual Rate
Several amendments designed to broaden eligibility were approved by the IRB and FDA (see 5.2).
Systematic prescreen of electronic medical records of breast cancer prevention clinics (Year 2) and surgical benign breast disease clinics (Year 3) was initiated to alert providers to potential eligible candidates prior to clinic arrival. This process was not as successful as we would have anticipated as these individuals are generally seen only 1–2x yearly and medications, vasomotor symptoms and menopause status can change during this time. Data from Year 2 when this was initiated suggested that out of 646 women identified by coordinator pre-screen from the high-risk clinics by age, risk, and BMI, only 15 (2.3%) women were eventually enrolled into study. The two main reasons for non-enrollment were menses within prior 2 months (33%) or no concerning hot flashes (36%). Eight percent of women declined, 6% had provider-identified health issues. Since their last appointment, 5% of women had been started on an antiestrogen, 3% had been started on hormones, and 3% had a BMI outside the acceptable range. In the same time period, 12 women not identified in the prescreen were identified by providers and enrolled.
We also continued with more traditional means of institution-specific, IRB-approved, high quality fliers in radiology, surgery, and high-risk clinics.
The consent form has been translated into Spanish and we meet with community representatives to increase protocol exposure and help promote diversity. We meet with individual site PIs and coordinators monthly.
5.1.5. Current Accrual and Projected Completion Date
As of March 2024, 64 women have undergone biomarker screening post consent. One was ineligible because of no epithelial cells in the RPFNA specimen, two because their mammographic fibroglandular volume was too low, and one because she resumed periods. 61 women have been enrolled as of March 21, 2024, 37 months from NOA and 28 months from initiation of accrual (48 from KUMC and 13 from the four other sites). A tool developed by our statistical unit updates current accrual (Figure 2) and anticipated accrual completion (by 2026) based on performance to date on a weekly basis.
Figure 2:

Accrual from December 2021 to March 2024. Date of individual site first enrollment indicated.
5.2. Study Eligibility Amendments to Improve Accrual
We have relaxed certain eligibility criteria that would not impact our main outcomes. Amendments include increasing the maximum age to 64 for women with higher breast density and reducing the minimum FGV for women with a BMI of < 25 kg/m2 to 40 cm3. Three of our sites are headed by breast surgeons. Based on favorable prognostic and safety data, we have amended the study to allow women with prior treated ER negative DCIS to participate.
5.3. Baseline Characteristics in Initial Entrants
Of the 61 women entered, median age is 54, BMI 26 kg/m2, and FGV 129 cm3. 67% of women had an affected first-degree relative and 34% a prior biopsy showing atypical hyperplasia or LCIS. No one has yet been entered with DCIS. Median calculated 10-year risk from Version 3 of the Breast Cancer Surveillance Consortium [63] is 5.74% which is ~ 2x that of women in their age group. Race distribution is 56 Caucasian, 2 Black, 2 Asian, 1 other; 6 identify as Hispanic. This distribution is consistent with race and ethnicity distribution at KUMC, the major contributor to date. Hormones are assayed when both pre-study and 6-month specimens can be analyzed together in the same run so that baseline values are not yet available for all 61 individuals entered. For 34 women with baseline values, 6 (18%) showed a progesterone ≥ 0.5 ng/ml and 6 (18%) an FSH <50 mIU/ml, consistent with premenopausal status. However, only two women had this in common, demonstrating the difficulty of describing an individual as pre or postmenopausal based on a single type of hormone value.
5.4. AEs and Early Discontinuations
As of March 21, 2024, 40 women randomized have completed or would have completed the 6-month initial period. AEs have been mild and generally not thought to be related to drug. Of the 40, four did not have an end of study RPFNA and 2 did not have a 6-month mammogram. One randomized to BZA+CE with increasing depression after 3 months wanted to change to bioidentical hormones and withdrew from study. One on Waitlist stopped having hot flashes and wanted to withdraw. Two, one randomized to waitlist and another randomized to BZA+CE, did not want a repeat RPFNA.
Discussion
We eventually overcame our major barriers of drug supply and multisite generation of research Volpara® volumetric assessments, but this placed us behind in accrual. A 6-month no cost extension to the grant in year 3 will allow the trial to extend into a 6th year to help overcome this delay. Several adjustments have been made in the eligibility criteria which will not impact study outcome but will hopefully increase accrual. We are also in the process of adding an additional site.
Our statistical considerations were based on our pilot. To date baseline characteristics of the current trial participants are very similar to those in the pilot study (median age 53, BMI 26 kg/m2 and FGV 133 cm3) [33]. Of the 40 entrants who should have completed the randomized portion, “6 month” mammograms for FGV the primary endpoint were performed in 95% and RPFNAs for tissue endpoints in 90%. However only 43% of our trial entrants to date have a Ki-67 of ≥1% compared to 54% of the pilot. This may impact our ability to detect significant change in Ki-67 between BZA/CE and placebo. Our exploratory endpoint of change in estrogen responsive gene expression, particularly a gene index we have developed for SERMs of the the average log2(FoldChange) for TFF1, PGR, GREB1, and the ratio of ESR1:ESR2, may be more sensitive to tissue changes as a result of BZA+CE exposure.
The only other Phase II trial assessing effects on risk for breast cancer is a so called window of opportunity trial (NCT02694809) in which women with newly diagnosed ER+ DCIS are randomized between BZA+CE (as Duavee®) and placebo for the 4–6 weeks between initial biopsy and re-excision. This trial funded in 2016 with planned accrual of 160 is likely to complete in 2024. This trial does not assess change in mammographic density but does assess change in Ki-67 and several estrogen response genes.
Supplementary Material
Table S1: Antibodies for ImmunoHistoChemistry
Table S2: RT-qPCR primers and transcripts.
Table 3.
Projected and actual accrual
| Accrual Originally Proposed by Year | ||||||
|---|---|---|---|---|---|---|
| Location | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
| KUMC | 10 | 16 | 16 | 6 | 48 | |
| Other Sites | 10 | 28 | 28 | 6 | 72 | |
| Total | 20 | 44 | 44 | 12 | 120 | |
| Actual Accrual to Date | ||||||
| Location | Year 1 | Year 2 | Year 3 | Year 4* | Year 5 | Total @ 3 years.* |
| KUMC | 3 | 23 | 20 | 2 | 48 | |
| Other Sites | 0 | 0 | 12 | 1 | 13 | |
| Total | 3 | 23 | 29 | 3 | 61 | |
Note that this is 37 months from notice of funding award; 27 months from initiation of accrual at KUMC and 10 months from first accrual at sites other than KUMC.
Table 4.
Baseline Characteristics
| Variable | Number | Median | Range |
|---|---|---|---|
| Age | 61 | 54 years | 45–60 years |
| BCSC 10 -year Risk | 61 | 5.74 (3.1 for age) | 2.22 – 15.43 |
| FSH mIU/ml | 34 | 83 | 3 – 165 |
| Estradiol pg/ml | 34 | 22 | UD - 1287 |
| Progesterone ng/ml | 34 | 0.2 | UD - 15.3 |
| SHBG, nM | 34 | 52 | 20 – 98 |
| BMI, kg/m2 | 59 | 26.1 | 19– 37.6 |
| BIRADs Density | 55 | C (heterogenous) | A - D |
| Absolute FGV, cm3 | 61 | 128.8 | 40.7 – 370.1 |
| Cell number category | 61 | 500–999 | <100 to 5000 |
| Cytology Score | 61 | 13 | 10 – 16 |
| Ki-67* | 56 | 0.8% | 0 – 6.0 % |
| Ki-67 ≥1% | 26 | 1.4 | 1 – 6% |
| Ki-67 0.2–0.8% | 22 | 0.6 | |
| Ki-67 = 0 | 8 |
Baseline Ki-67 not assessed in 5/61 because of < 500 cells on ThinPrep slides
Funding
This work (and specifically the clincal trial) are supported by NIH grant 5R01CA249437, Breast Cancer Research Foundation grant BCRF-22–049, and NIH/NCI Cancer Center Support Grant P30CA168524. The funders had no involvement in the design, conduct, or interpretation of results from the trial.
Footnotes
Declaration of Competing Interest
Accomplished via a form for each author. If none for any author, then single entry on one form will suffice –.
References
- 1.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR, Duration of menopausal hot flushes and associated risk factors, Obstet. Gynecol. 117 (2011) 1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crandall CJ, Mehta JM, Manson ME, Management of menopausal symptoms: A review, JAMA. 329 (5) (2023) 405–420. [DOI] [PubMed] [Google Scholar]
- 3.Crew KD, What factors influence decision-making about breast cancer chemoprevention among high-risk women? Cancer Prev. Res. 10 (2017) 609–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hackett J, Thorneloe R, Side L, Wolf M, Horne R, Cuzick, et al. , Uptake of breast cancer preventive therapy in the UK: results from a multicentre prospective survey and qualitative interviews, Breast Cancer Res. Treat. 170 (2018) 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuzick J J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, et al. , Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data, Lancet 381 (2013) 1827–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goss PE PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende j., et al. , Exemestane for breast-cancer prevention in postmenopausal women, N. Engl. J. Med. 364 (2011) 2381–2391. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. , Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial, Lancet 383 (2014) 1041–1048. [DOI] [PubMed] [Google Scholar]
- 8.Ropka ME, Keim J, Philbrick JT, Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis, J. Clin. Oncol. 28 (2010) 3090–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Port ER, Montgomery LL, Heerdt AS, Borgen PI, Patient reluctance toward tamoxifen use for breast cancer primary prevention, Ann. Surg. Oncol 8 (2001) 580–585. [DOI] [PubMed] [Google Scholar]
- 10.Smith SG, Sestak I, Forster A, Partridge A, Side L, Wolf MS, et al. , Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis, Ann. Oncol. 27 (2016) 575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeCensi A, Puntoni M, Guerrieri-Gonzaga A, Caviglia S, Avino F, Cortesi L, et al. , Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia, J. Clin. Oncol. 37 (19) (2019) 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, DeCensi A, et al. , Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update, J. Clin. Oncol. 37 (33) (2019) 3152–3165. [DOI] [PubMed] [Google Scholar]
- 13. .Chew F, Wu X, Sources of information influencing the state-of-the-science gap in hormone replacement therapy usage, PLoS One 12 (2) (2017) e0171189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collaborative Group on Hormonal Factors in Breast Cancer, Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence, Lancet 394 (10204) (2019) 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manson JE, Aragaki AK, Bassuk SS, Chlebowski RT, Anderson GL, Rossouw JE, et al. , Menopausal estrogen-alone therapy and health outcomes in women with and without bilateral oophorectomy: A randomized trial, Ann. Intern. Med. Sep 10 (2019) doi: 10.7326/M19-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chlebowski RT, Anderson GL, Aragaki AK, Manson JE, Stefanick ML, Pan K, et al. , Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials, JAMA. 324 (4) (2020) 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhavnani BR, Stanczyk FZ, Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action, J. Steroid Biochem. Mol. Biol. 142 (2014) 16–29. [DOI] [PubMed] [Google Scholar]
- 18.Berrodin TJ, Chang KC, Komm BS, Freedman LP, Nagpal S, Differential biochemical and cellular actions of Premarin estrogens: distinct pharmacology of bazedoxifene-conjugated estrogens combination, Mol. Endocrinol. 23 (1) (2009) 74–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ, Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast, J.. Clin Endocrinol. Metab. 84 (1999) 4559–4565. [DOI] [PubMed] [Google Scholar]
- 20.Hilton HN, Clarke CL, Impact of progesterone on stem/progenitor cells in the human breast. J. Mammary Gland Biol. Neoplasia 20 (1–2) (2015) 27–37. [DOI] [PubMed] [Google Scholar]
- 21.Cenciarini ME, Proietti CJ, Molecular mechanisms underlying progesterone receptor action in breast cancer: Insights into cell proliferation and stem cell regulation, Steroids. 152 (2019) 108503. [DOI] [PubMed] [Google Scholar]
- 22.Chang KC, Wang Y, Bodine PV, Nagpal S, Komm BS, Gene expression profiling studies of three SERMs and their conjugated estrogen combinations in human breast cancer cells: insights into the unique antagonistic effects of bazedoxifene on conjugated estrogens, J. Steroid Biochem. Mol. Biol. 118 (1–2) (2010) 171–1124 [DOI] [PubMed] [Google Scholar]
- 23.Han SJ, Begum K, Foulds CE, Hamilton RA, Bailey S, Malovannaya A, et al. , The dual estrogen receptor α inhibitory effects of the tissue-selective estrogen complex for endometrial and breast safety, Mol. Pharmacol. 89 (2016) 14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komm BS, Mirkin S, Jenkins SN, Development of conjugated estrogens/bazedoxifene, the first tissue selective estrogen complex (TSEC) for management of menopausal hot flashes and postmenopausal bone loss, Steroids 90 (2014) 71–81. [DOI] [PubMed] [Google Scholar]
- 25.Pickar JH, Boucher M, Morgenstern D, Tissue selective estrogen complex (TSEC): a review, Menopause 25 (2018) 1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinkerton JV, Pickar JH, Racketa J, Mirkin S, Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention, Climacteric 15 (5) (2012) 411–418. [DOI] [PubMed] [Google Scholar]
- 27.Kagan R, Komm BS, Ryan KA, Lavenberg J, Yu CR, Pinkerton JV, Timing and persistence of effect of conjugated estrogens/bazedoxifene in postmenopausal women, Menopause 23 (2016) 1204–1213. [DOI] [PubMed] [Google Scholar]
- 28.Ethun KF, Wood CE, Register TC, Cline JM, Appt SE, Clarkson TB, Effects of bazedoxifene acetate with and without conjugated equine estrogens on the breast of postmenopausal monkeys, Menopause 19 (2012) 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y, Santen RJ, Wang JP, Yue W, Effects of the conjugated equine estrogen/bazedoxifene tissues elective estrogen complex (TSEC) on mammary gland and breast cancer in mice, Endocrinology 153 (2012) 5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue W, Wang J, Atkins KA, Bottalico L, Mesaros C, Blair IA, et al. , Santen RJ. Effect of a tissue selective estrogen complex on breast cancer: Role of unique properties of conjugated equine estrogen, Int. J. Cancer 143 (2018) 1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman SR, Governor S, Daniels K, Seals RM, Ziyadeh NJ, Wang FT, et al. , Comparative safety of conjugated estrogens/bazedoxifene versus estrogen/progestin combination hormone therapy among women in the United States: a multidatabase cohort study, Menopause 30 (8) (2023) 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabian CJ, Nye L, Powers KR, Nydegger JL, Kreutzjans AL, Phillips TA, et al. , Effect of bazedoxifene and conjugated estrogen (Duavee) on breast cancer risk biomarkers in high-risk women: A pilot study, Cancer Prev. Res. 12 (2019) 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brand JS, Czene K, Shepherd JA, Leifland K, Heddson B, Sundbom A, et al. , Automated measurement of volumetric mammographic density: a tool for widespread breast cancer risk assessment, Cancer Epidemiol. Biomarkers Prev. 23 (2014) 1764–1772. [DOI] [PubMed] [Google Scholar]
- 34.Cheddad A, Czene K, Eriksson M, Li J, Easton D, Hall P, et al. , Area and volumetric density estimation in processed full-field digital mammograms for risk assessment of breast cancer, PLoS One 9 (10) (2014) e110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huh SJ, Oh H, Peterson MA, Almendro V, Hu R, Bowden M, et al. , The proliferative activity of mammary epithelial cells in normal tissue predicts breast cancer risk in premenopausal women, Cancer Res. 76 (2016) 1926–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaaban AM, Sloane JP, West CR, Foster CS, Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor-alpha and Ki-67 expression, Am. J. Pathol. 160 (2002) 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Missmer SA, Eliassen H, Barbier RL, Hankinson SE, Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women, J. Natl. Cancer Inst. 96 (2004) 1856–1865. [DOI] [PubMed] [Google Scholar]
- 38.Trabert B, Bauer DC, Buist DSM, Cauley JA, Falk RT, Geczik AM, et al. , Association of circulating progesterone with breast cancer risk among postmenopausal women, JAMA Netw. Open. 3 (4) (2020) e203645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He XY, Liao YD, Yu S, Zhang Y, Wang R, Sex hormone binding globulin and risk of breast cancer in postmenopausal women: a meta-analysis of prospective studies, Horm. Metab. Res. 47 (7) (2015) 485–490. [DOI] [PubMed] [Google Scholar]
- 40.Endogenous Hormones Breast Cancer Collaborative Group, Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, et al. , Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies, Lancet Oncol. 11 (2010) 530–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shepherd JA, Kerlikowske K, Ma L, Duewer F, Fan B, Wang J, Malkov S, et al. , Volume of mammographic density and risk of breast cancer, Cancer Epidemiol. Biomarkers Prev. 20 (7) (2011) 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez AV, Guarnizo M, Miranda Y, Pasupuleti V, Deshpande A, Paico S, et al. , Association between insulin resistance and breast carcinoma: a systematic review and meta-analysis, PLoS One 9 (2014) e99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JH, Meyers MS, Khuder SS, Abdallah SL, Muturi HT, Russo L, et al. , Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice, Mo.l Metab. 3 (2014) 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marlatt KL, Lovre D, Beyl RA, Tate CR, Hayes EK, Burant CF, et al. , Effect of conjugated estrogens and bazedoxifene on glucose, energy and lipid metabolism in obese postmenopausal women, Eur. J. Endocrinol. 183 (4) (2020) 439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeffers AM, Sieh W, Lipson JA, Rothstein JH, McGuire V, Whittemore AS, et al. , Breast cancer risk and mammographic density assessed with semiautomated and fully automated methods and BI-RADS, Radiology 282 (2) (2017) 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Astley SM, Harkness EF, Sergeant JC, Warwick J, Stavrinos P, Warren R, et al. , A comparison of five methods of measuring mammographic density: a case-control study, Breast Cancer Res. 20 (1) (2018) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anlonzo-Proulx O, Mawdsley GE, Patrie JT, Yaffe MJ, Harvey JA, Reliability of automated breast density measurements, Radiology 275 (2) (2015) 366–376. [DOI] [PubMed] [Google Scholar]
- 48.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. , FTamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study, J. Natl. Cancer Inst. 103 (9) (2011) 744–752. [DOI] [PubMed] [Google Scholar]
- 49.Eriksson M, Eklund M, Borgquist S, Hellgren R, Margolin S, Thoren L, et al. , Low-dose tamoxifen for mammographic density reduction: a randomized controlled trial, J. Clin. Oncol. 39 (17) (2021) 1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bäcklund M, Eriksson M, Hammarström M, Thoren L, Bergqvist J, Margolin S, et al. , Time to mammographic density decrease after exposure to tamoxifen, Oncologist 27 (7) (2022) e601–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engmann NJ, Scott CG, Jensen MR, Ma L, Brandt KR, Mahmoudzadeh AP, et al. , Longitudinal changes in volumetric breast density with tamoxifen and aromatase inhibitors, Cancer Epidemiol. Biomarkers Prev. 26 (6) (2017) 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harvey JA, Pinkerton JV, Baracat EC, Shi H, Chines AA, Mirkin S, Breast density changes in a randomized controlled trial evaluating bazedoxifene/conjugated estrogens, Menopause 20 (2013) 138–145. [DOI] [PubMed] [Google Scholar]
- 53.Lau S, Ng KH, Abdul Aziz YF, Volumetric breast density measurement: sensitivity analysis of a relative physics approach, Br. J. Radiol. 89 (1066) (2016) 20160258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan QJ, Kimler BF, O’Dea AP, Zalles CM, Sharma P, Fabian CJ, Mammographic density does not correlate with Ki-67 expression or cytomorphology in benign breast cells obtained by random periareolar fine needle aspiration from women at high risk for breast cancer, Breast Cancer Res. 9 (3) (2007) R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Decensi A, Robertson C, Viale G, Pigatto F, Johansson H, Kisanga ER, et al. , A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers, J. Natl. Cancer Inst. 95 (2003) 779–790. [DOI] [PubMed] [Google Scholar]
- 56.Shen L, Ahmad S, Park S, DeMaio W, Oganesian A, Hultin T, et al. , In vitro metabolism, permeability, and efflux of bazedoxifene in humans, Drug Metab. Dispos. 38 (9) (2010) 1471–1479. [DOI] [PubMed] [Google Scholar]
- 57.Endogenous Hormones and Breast Cancer Collaborative Group, Free estradiol and breast cancer risk in postmenopausal women: comparison of measured and calculated values, Cancer Epidemiol. Biomarkers Prev. 12 (2003) 1457–1461. [PubMed] [Google Scholar]
- 58.Mellis MG, Oldroyd B, Hind K, In vivo precision of the GE Lunar iDXA for the measurement of visceral adipose tissue in adults: the influence of body mass index, Eur. J. Clin. Nutr. 68 (12) (2014) 1365–1367. [DOI] [PubMed] [Google Scholar]
- 59.Loprinzi L, Barton DL, Sloan JA, Zahasky KM, Smith DA, Pruthi S, et al. , Pilot evaluation of gabapentin for treating hot flashes, Mayo Clin. Proc. 77 (2002) 1159–1163. [DOI] [PubMed] [Google Scholar]
- 60.Lewis JE, Hilditch JR, Wong CJ, Further psychometric property development of the Menopause-Specific Quality of Life questionnaire and development of a modified version, MENQOL-Intervention questionnaire, Maturitas 50 (3) (2005) 209–221. [DOI] [PubMed] [Google Scholar]
- 61.Gajewski BJ, Kimler BF, Koestler DC, Mudaranthakam DP, Young K, Fabian CJ, A novel Bayesian adaptive design incorporating both primary and secondary endpoints for randomized IIB chemoprevention study of women at increased risk for breast cancer, Trials 23 (1) (2022) 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cada DJ, Baker DE, Conjugated estrogens and bazedoxifene, Hosp. Pharm. 49 (3) (2014) 273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breast Cancer Surveillance Consortium Risk Calculator Version 3.0. [accessed 4-19-2024]. https://tools.bcsc-scc.ucdavis.edu/AdvBC6yearRisk/#/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Antibodies for ImmunoHistoChemistry
Table S2: RT-qPCR primers and transcripts.


