Abstract
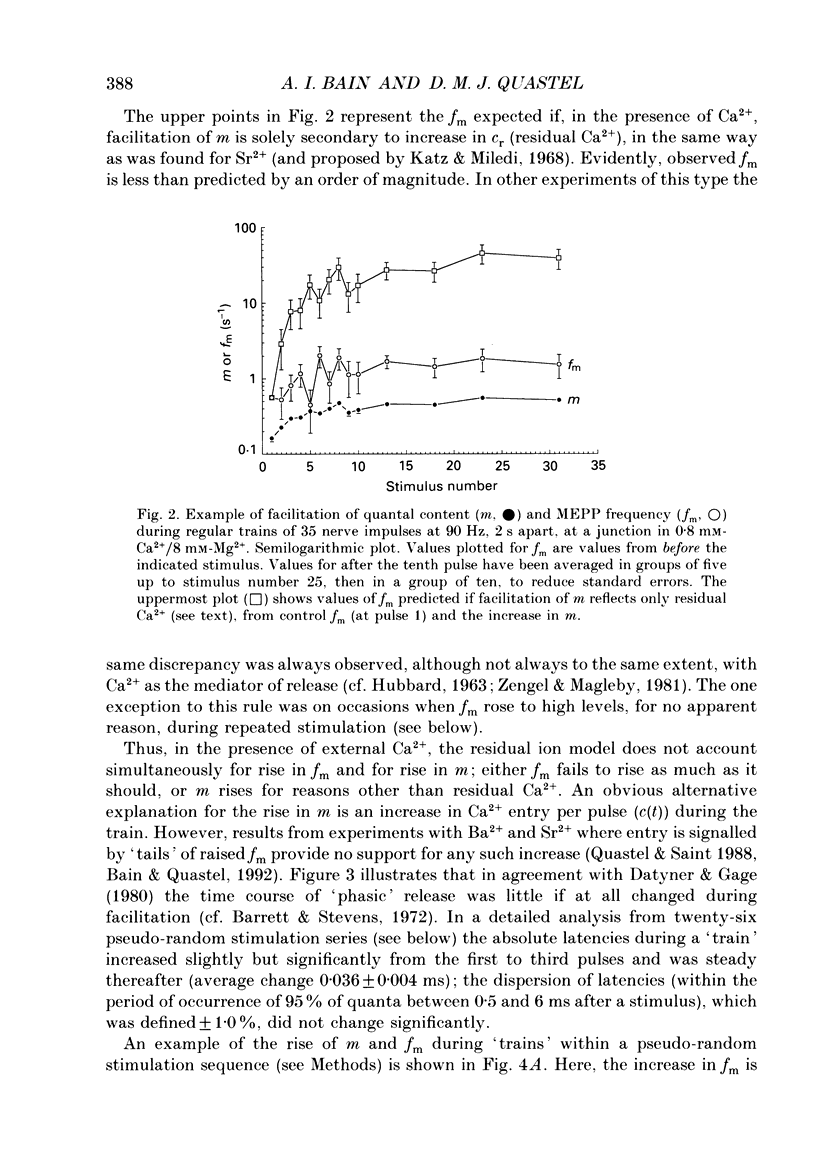
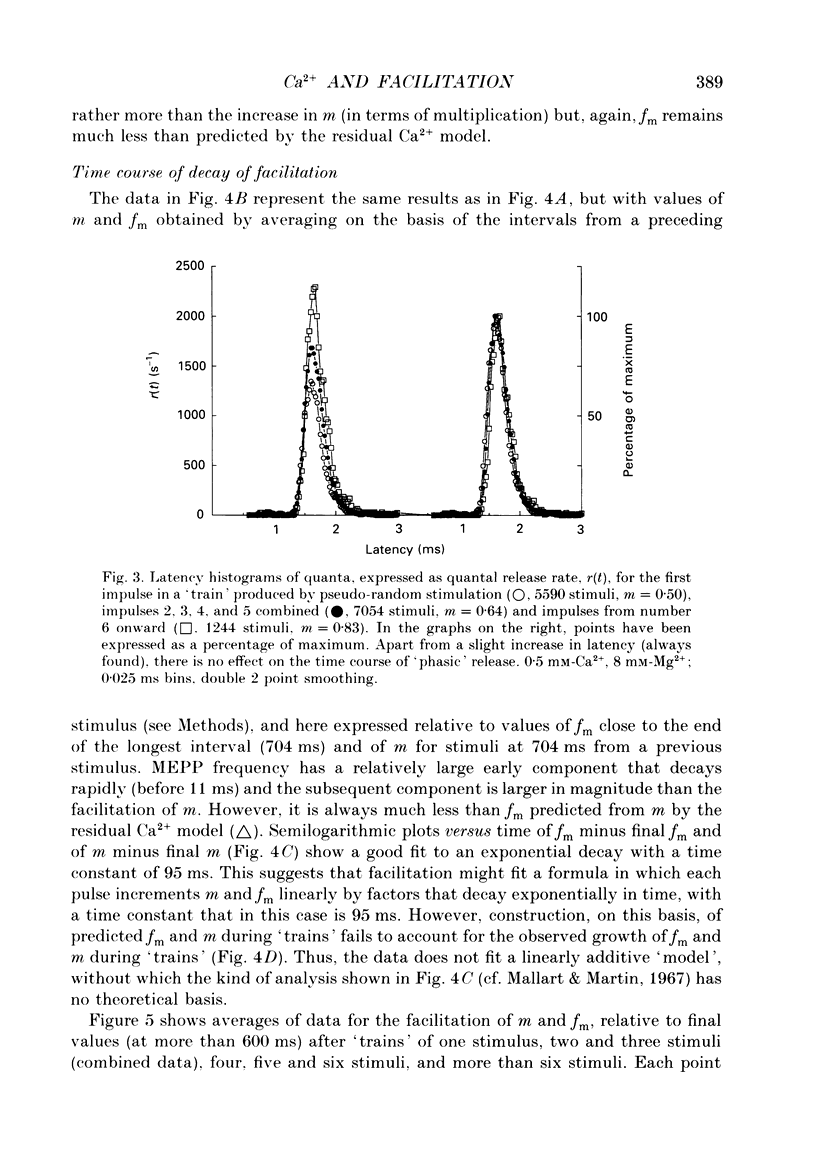
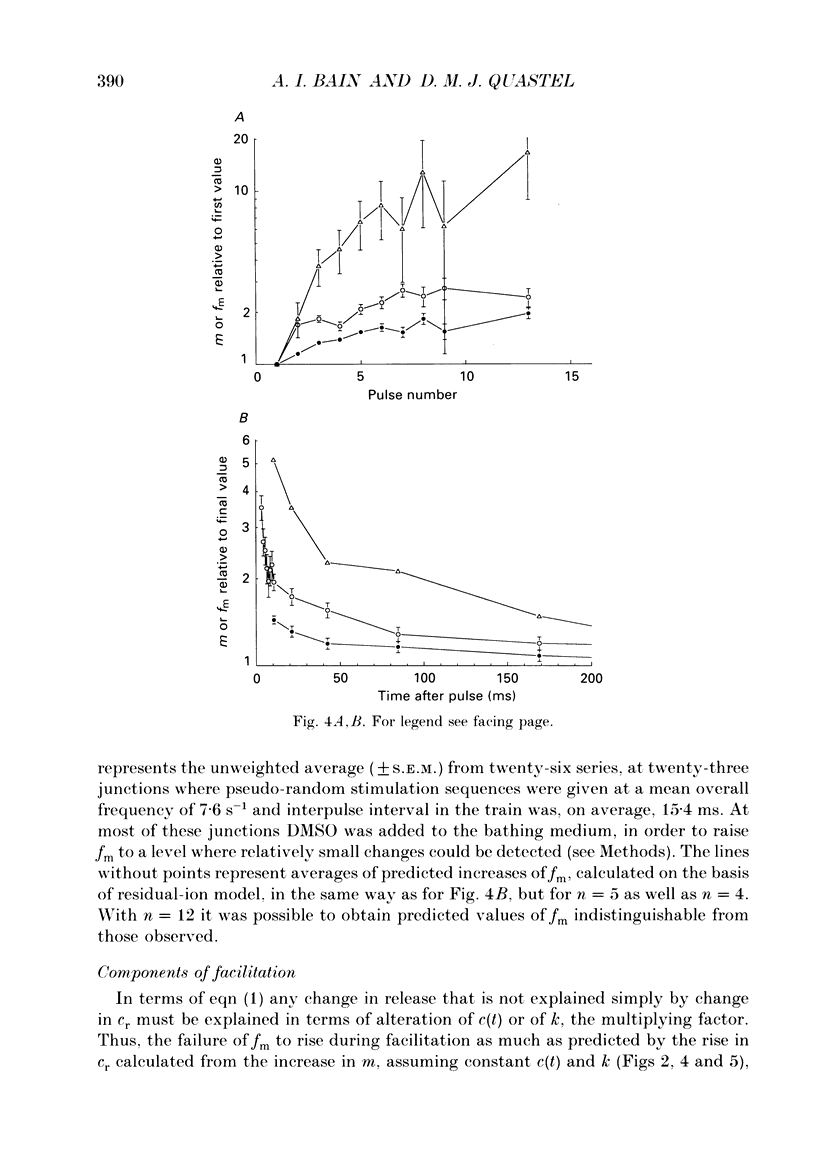
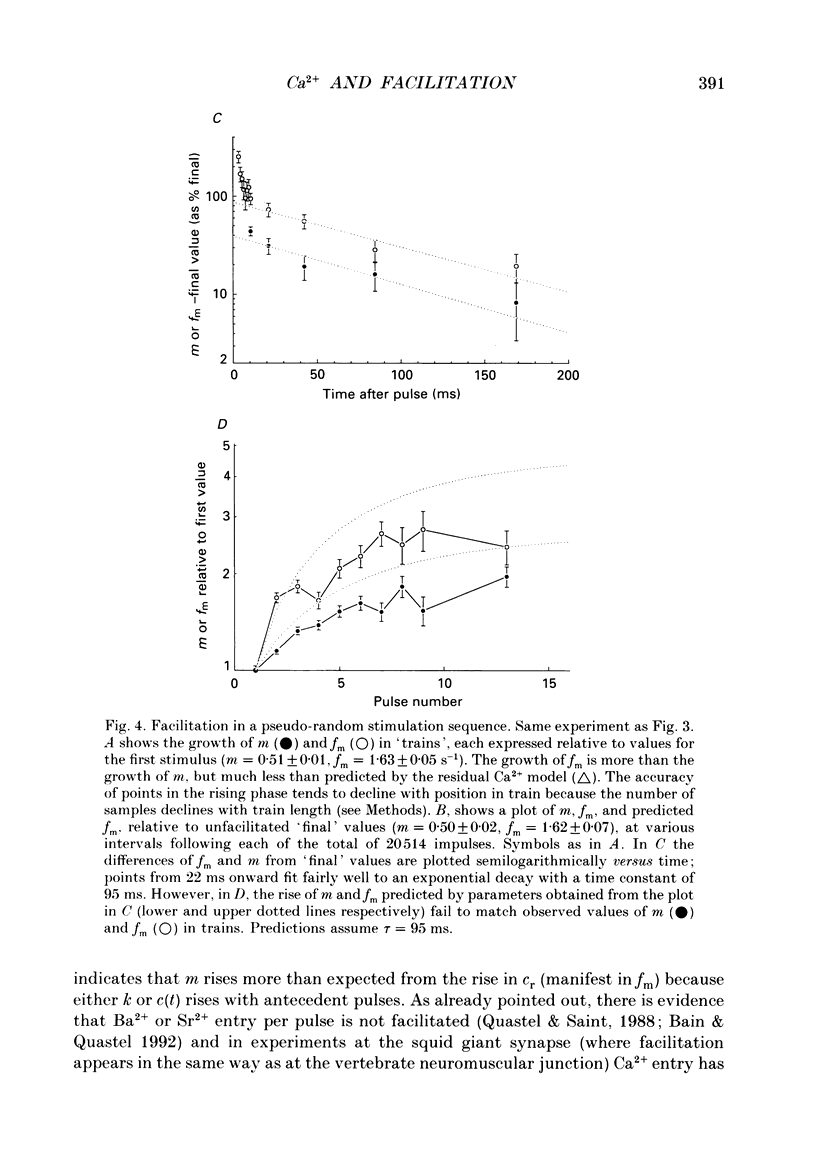
1. Facilitation of endplate potentials (EPPs) and frequency of miniature endplate potentials (MEPPs) were studied, in the presence of low Ca2+/raised Mg2+, in isolated mouse hemidiaphragm, using pseudo-random sequences of nerve stimulation and automated (computer) counting of MEPPs and quantal components of EPPs. 2. The facilitation in quantal content of EPPs (m) produced by one or more antecedent stimuli was accompanied by facilitation of MEPP frequency (fm) that was similar in magnitude and substantially less than expected if facilitation reflects persistent (residual) intraterminal Ca2+. The time course of 'phasic' quantal release, associated with the EPP, was little if at all altered with facilitation. 3. The magnitude and time course of facilitation was consistent with two distinct presynaptic processes, each manifest both in m and fm, (i) an effect to multiply transmitter release, and (ii) residual Ca2+ which adds to Ca2+ brought in by nerve impulses. These have distinct time courses. 4. After loading nerve terminals with bis (O-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA), facilitation of m and fm became very small. 5. At sufficiently low Ca2+/raised Mg2+ facilitation of m and fm became very small although latency histograms showed clear EPPs. However, the multiplicative component of facilitation became maximal at Ca2+/Mg2+ concentrations giving an average m value less than 0.1, corresponding to about 5% of normal Ca2+ entry per pulse. At lower Ca2+, facilitation was restored when EPPs were made larger using 4-aminopyridine. 6. With EPPs elicited by brief 'direct' nerve terminal depolarizations, facilitation was graded with pulse intensity (and m) and could be much less than with EPPs with similar m evoked by nerve stimuli at lower Ca2+ and/or higher Mg2+. 7. It was concluded that fast facilitation is primarily multiplicative and reflects activity within the nerve terminal of a Ca(2+)-sensitive process distinct from that generating Ca(2+)-dependent release.
Full text
PDF







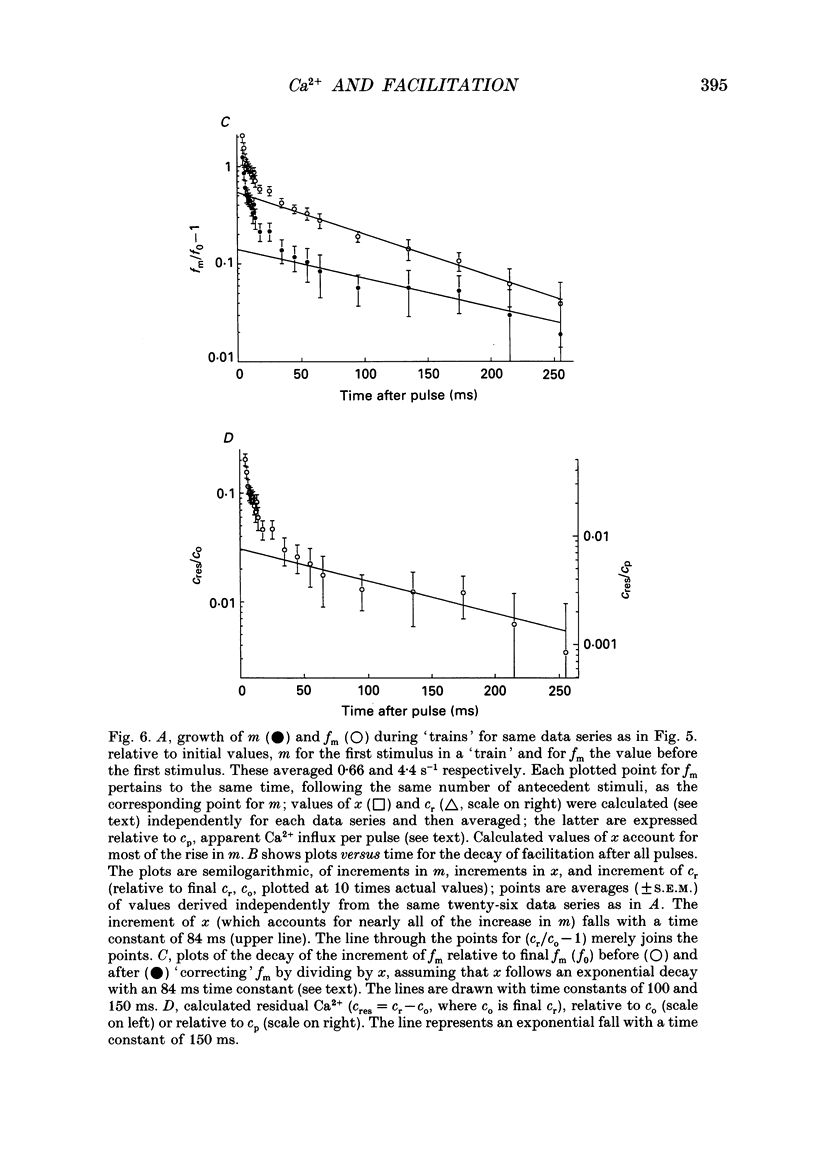


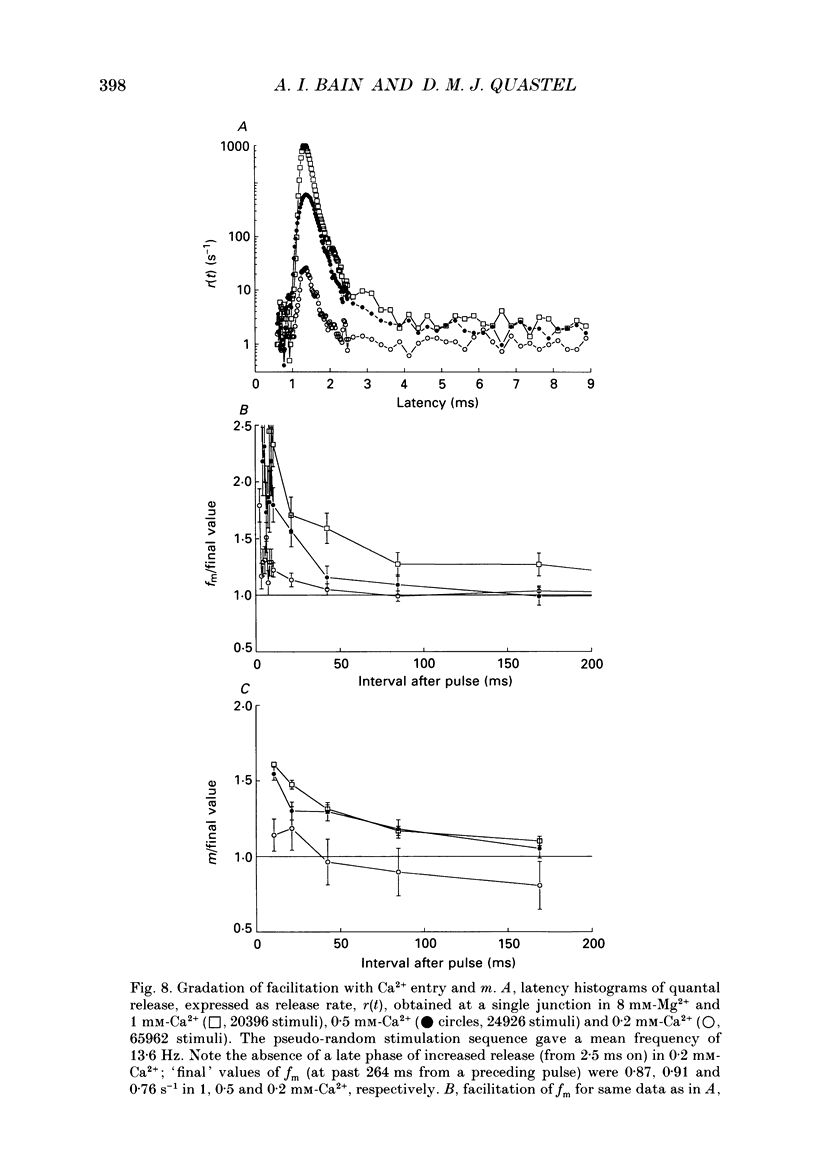

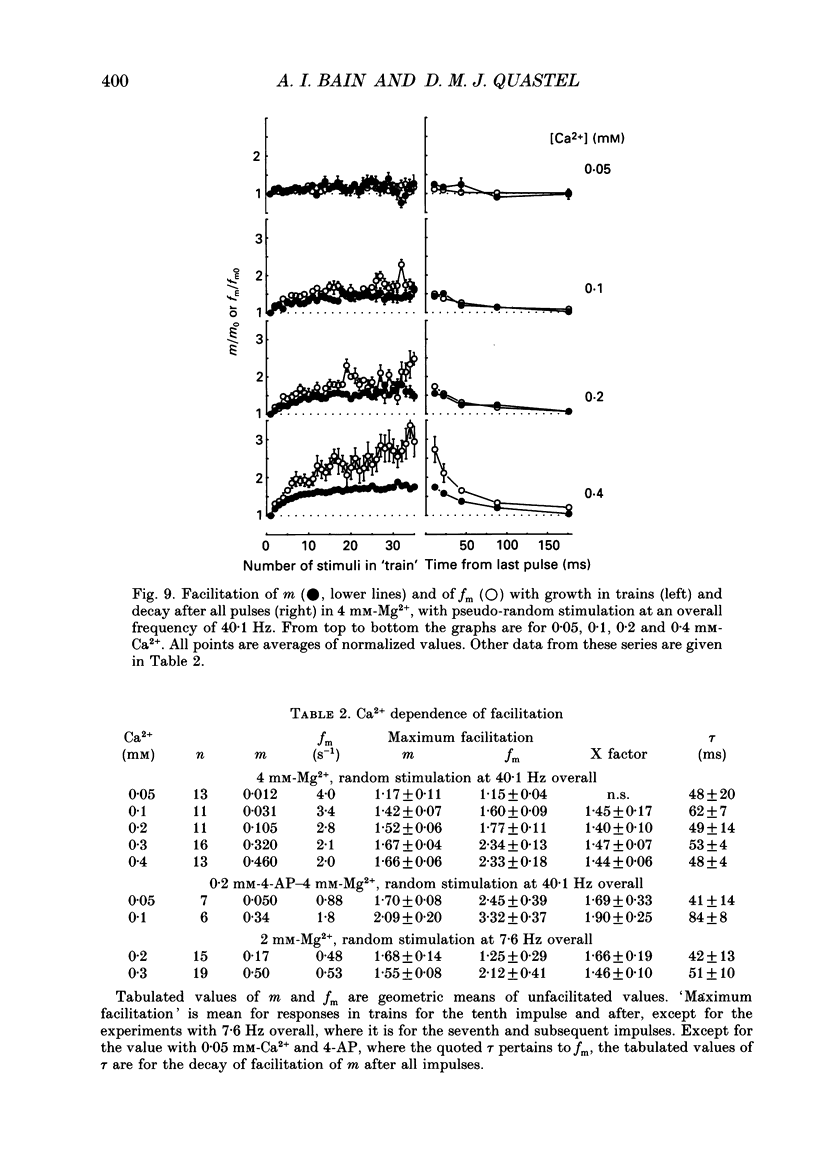
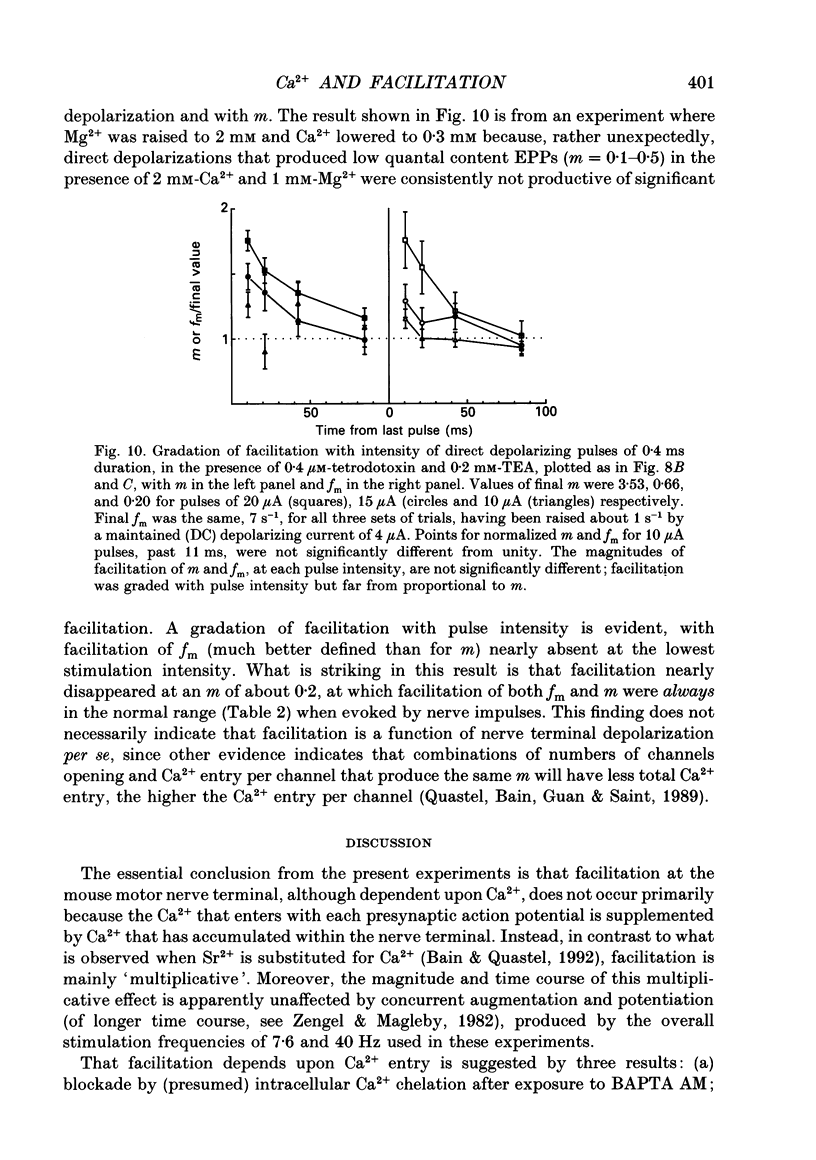




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwood H. L., Swenarchuk L. E., Gruenwald C. R. Long-term synaptic facilitation during sodium accumulation in nerve terminals. Brain Res. 1975 Dec 12;100(1):198–202. doi: 10.1016/0006-8993(75)90260-7. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Bain A. I., Quastel D. M. Quantal transmitter release mediated by strontium at the mouse motor nerve terminal. J Physiol. 1992 May;450:63–87. doi: 10.1113/jphysiol.1992.sp019116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai A., Spanier R., Rahamimoff H. Isolation, purification, and reconstitution of the Na+ gradient-dependent Ca2+ transporter (Na+-Ca2+ exchanger) from brain synaptic plasma membranes. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6521–6525. doi: 10.1073/pnas.81.20.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Atwood H. L. Modulation of transmitter release by intracellular sodium in squid giant synapse. Brain Res. 1977 Oct 7;134(2):367–371. doi: 10.1016/0006-8993(77)91081-2. [DOI] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Biophysical aspects of neuro-muscular transmission. Prog Biophys Biophys Chem. 1956;6:121–170. [PubMed] [Google Scholar]
- Datyner N. B., Gage P. W. Phasic secretion of acetylcholine at a mammalian neuromuscular junction. J Physiol. 1980 Jun;303:299–314. doi: 10.1113/jphysiol.1980.sp013286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S., Tank D. W. Calcium in motor nerve terminals associated with posttetanic potentiation. J Neurosci. 1989 Oct;9(10):3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. Calcium and depolarization dependence of twin-pulse facilitation of synaptic release at nerve terminals of crayfish and frog muscle. Pflugers Arch. 1989 Dec;415(3):304–309. doi: 10.1007/BF00370880. [DOI] [PubMed] [Google Scholar]
- Gage P. W. The effect of methyl, ethyl and n-propyl alcohol on neuromuscular transmission in the rat. J Pharmacol Exp Ther. 1965 Nov;150(2):236–243. [PubMed] [Google Scholar]
- Guan Y. Y., Quastel D. M., Saint D. A. Single Ca2+ entry and transmitter release systems at the neuromuscular synapse. Synapse. 1988;2(5):558–564. doi: 10.1002/syn.890020512. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I. REPETITIVE STIMULATION AT THE MAMMALIAN NEUROMUSCULAR JUNCTION, AND THE MOBILIZATION OF TRANSMITTER. J Physiol. 1963 Dec;169:641–662. doi: 10.1113/jphysiol.1963.sp007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima H., Tanabe N. Calcium-independent increase of transmitter release at frog end-plate by trinitrobenzene sulphonic acid. J Physiol. 1988 Sep;403:135–149. doi: 10.1113/jphysiol.1988.sp017243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan C. Y., Putney J. W., Jr Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells. Dissociation by Sr2+ and Ba2+ of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem. 1990 Jan 15;265(2):678–684. [PubMed] [Google Scholar]
- Linder T. M. Calcium and facilitation at two classes of crustacean neuromuscular synapses. J Gen Physiol. 1973 Jan;61(1):56–73. doi: 10.1085/jgp.61.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L. The effect of tetanic and post-tetanic potentiation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):353–371. doi: 10.1113/jphysiol.1973.sp010349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A quantitative description of stimulation-induced changes in transmitter release at the frog neuromuscular junction. J Gen Physiol. 1982 Oct;80(4):613–638. doi: 10.1085/jgp.80.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarnon J. G., Saint D. A., Quastel D. M. The actions of dimethyl sulfoxide on neuromuscular transmission. Mol Pharmacol. 1986 Dec;30(6):631–638. [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Requena J., Whittembury J. Ca2+ entry in squid axons during voltage-clamp pulses is mainly Na+/Ca2+ exchange. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1847–1851. doi: 10.1073/pnas.82.6.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I., Parnas H. The 'Ca-voltage' hypothesis for neurotransmitter release. Biophys Chem. 1988 Feb;29(1-2):85–93. doi: 10.1016/0301-4622(88)87027-3. [DOI] [PubMed] [Google Scholar]
- Quastel D. M., Hackett J. T., Cooke J. D. Calcium: is it required for transmitter secretion? Science. 1971 Jun 4;172(3987):1034–1036. doi: 10.1126/science.172.3987.1034. [DOI] [PubMed] [Google Scholar]
- Quastel D. M., Saint D. A. Transmitter release at mouse motor nerve terminals mediated by temporary accumulation of intracellular barium. J Physiol. 1988 Dec;406:55–73. doi: 10.1113/jphysiol.1988.sp017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint D. A., Quastel D. M., Guan Y. Y. Multiple potassium conductances at the mammalian motor nerve terminal. Pflugers Arch. 1987 Nov;410(4-5):408–412. doi: 10.1007/BF00586518. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol Rev. 1985 Mar;37(1):81–132. [PubMed] [Google Scholar]
- Tanabe N., Kijima H. Both augmentation and potentiation occur independently of internal Ca2+ at the frog neuromuscular junction. Neurosci Lett. 1989 Apr 24;99(1-2):147–152. doi: 10.1016/0304-3940(89)90280-2. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Differential effects of Ba2+, Sr2+, and Ca2+ on stimulation-induced changes in transmitter release at the frog neuromuscular junction. J Gen Physiol. 1980 Aug;76(2):175–211. doi: 10.1085/jgp.76.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


