Abstract
Objectives:
Investigate the immediate resonance magnetic image changes undergone by the lumbar canal after indirect decompression and compare them at one-year post-intervention. We also investigate the clinical outcome of indirect decompression at one-year follow-up.
Methods:
Imaging changes in patients who underwent indirect lumbar decompression and percutaneous posterior fixation were analyzed with one-year follow-up. Radiographic measurements were performed preoperatively and postoperatively (at one year), and the area of lumbar canal occupation and yellow ligament by nuclear magnetic resonance was compared preoperatively, at 48 hours post-surgery, and at one year. Radiographic measurements included disc height, foraminal height, total lumbar lordosis, and segmental lordosis. The VAS lumbar and lower limb scales and the Oswestry Disability Index (ODI) were used to assess clinical outcomes.
Results:
A total of 21 male and 23 female patients underwent indirect decompression at 64 lumbar levels. A significant improvement was observed in the clinical evaluation of all patients’ post-surgery (p < 0.001) in all radiographic parameters. There was an immediate increase in the lumbar canal at 48 hours (p < 0.001), which continued to increase at one year post-intervention (p < 0.05). The yellow ligament occupation area decreased at 48 hours (p < 0.001) and continued to decrease until one year (p < 0.01). Four complications were recorded, one of which was a posterior tract infection requiring open decompression.
Conclusion:
Indirect decompression for degenerative lumbar disease provided successful clinical outcomes, including indirect expansion of the dural sac at 48 hours post-procedure, with progressive increase in the lumbar canal area at one-year follow-up.
Key Words: Degenerative spine disorders, Ligamentotaxis, Lumbar region, Magnetic resonance imaging, Minimally invasive
Introduction
Degenerative spine pathology leads to clinical deterioration that negatively affects the quality of life of individuals.1 in recent years, there has been an increasing demand from patients to return to their daily activities as soon as possible, and different techniques offering a faster recovery period have been developed.2
Degenerative facet hypertrophy as a cause of lumbar canal stenosis was first described in 1954.3 When disc height is reduced due to degenerative processes or poor alignment, such as spondylolisthesis, the interlaminar space and intervertebral foramina are also reduced .4,5
The primary indication and goal of surgery in patients with symptomatic degenerative lumbar stenosis is neurological decompression. Traditionally, this was achieved through direct posterior resection of bone and/or soft tissue.6 In addition to canal decompression, instrumentation and fusion may be required in some situations due to the resection of elements responsible for the natural stability of the spine.7
Recently, lateral interbody fusion techniques (XLIF) and oblique (OLIF) are increasingly being used for the treatment of degenerative diseases. Fusion techniques offer advantages, such as restoration of disc height, correction of spinal sagittal balance, and decompression of both the spinal canal and neural foramina through ligamentotaxis, mainly of the yellow ligament.8,9 Another advantage of this approach is avoiding aggressive muscle dissection by preserving the musculature, which plays a crucial role in postoperative recovery.10,11
Recent studies report that even in the presence of severe stenosis, clinical outcomes after indirect decompression are highly favorable, and lumbar canal expansion by magnetic resonance imaging (MRI) continues even after two years postoperatively.12,13 The primary objective of this study was to investigate the immediate changes undergone by the lumbar canal after indirect decompression and compare them at one-year post-intervention.
Materials and Methods
Design
A retrospective observational cohort study of patients who underwent indirect decompression and percutaneous posterior arthrodesis between January 2020 and December 2022. The indirect decompression techniques used were OLIF (Oblique Lumbar Interbody Fusion) for lumbar levels L2 to L5 and ALIF (Anterior Lumbar Interbody Fusion) for the L5-S1 level.
This study was authorized by the institutional review committee of our Hospital.
Study Population
Adults aged 18 years and older diagnosed with degenerative lumbar stenosis, degenerative spondylolisthesis, and lumbosacral instability, who underwent all their imaging follow-ups at our center, were included. Patients with previous lumbar spine surgeries, a history of oncological pathologies and fractures, and those with less than 1 year of follow-up were excluded from our study.
Variables Recorded
Age of patients at the time of surgery, sex, BMI (body mass index), duration of symptoms, time to surgery, instrumented lumbar level, type of anterior fusion approach, length of hospital stay, months taken to return to work and sports activities. Postoperative complications were also recorded.
Definitions
Lumbar canal stenosis is defined as the structural narrowing of the lumbar canal, lateral recesses, or neural foramina in the lumbar region.14 The diagnosis of canal stenosis was made through clinical evaluation and imaging. Instability was defined as significant mechanical low back pain (VAS > 7) (pain that increases with load).15,16
Clinical Variables
The Oswestry Disability Index (ODI) questionnaire was administered to assess the extent to which pain disrupts the daily activities of each patient.17 The Visual Analog Scale (VAS) for low back and lower limb pain, which consists of a subjective pain scale from 1 to 10 according to the patient, was also used. These questionnaires were conducted before surgery and at one-year follow-up.
Imaging Variables
At the radiographic level, the following measurements were calculated (preoperative and immediate postoperative):
Total lumbar lordosis (L1-S1) (degrees), measured from the superior endplate of S1 to the superior endplate of L1.
Caudal segmental lordosis L4-S1 (degrees), measured from the superior endplate of S1 to the inferior endplate of L4.
Disc height of the fused segment (millimeters), on a lateral lumbosacral radiograph, from the inferior edge of the superior endplate to the superior edge of the inferior endplate. This was performed at the anterior and posterior edges of each segment, and the final result was the average of both measurements.
Foraminal height of the fused segment (millimeters), from the superior edge of the foramen of the lower vertebra to the inferior edge of the foramen of the upper vertebra.
In the magnetic resonance imaging (MRI), axial cuts at the instrumented level were used to measure the occupation area of the spinal canal and the area of occupation of the yellow ligament using the ROI tool of Synapse (Synapse PACS Version 5.7 | Fujifilm Medical Informatics) [Figure 1 A-B]. Both measurements were conducted using an integrated digital area measurement setup. The region of interest, delineated using a graphic cursor around the area of the lumbar canal and the yellow ligament, was calculated to obtain both results.
Figure 1.

In an axial MRI image, measurement of the lumbar canal area (A) and yellow ligament occupation (B) is conducted. Both images display the outlined contour of the areas using the ROI tool of Synapse, along with the measurement results
Additionally, in a profile cut, the anteroposterior diameter of the intervertebral disc occupation was measured [Figure 2]. The Schizas classification was used as the morphological classification of lumbar spinal stenosis.14 all these parameters from the MRI were measured both preoperatively, at 48 hours post-surgery, and at one year postoperatively.
Figure 2.
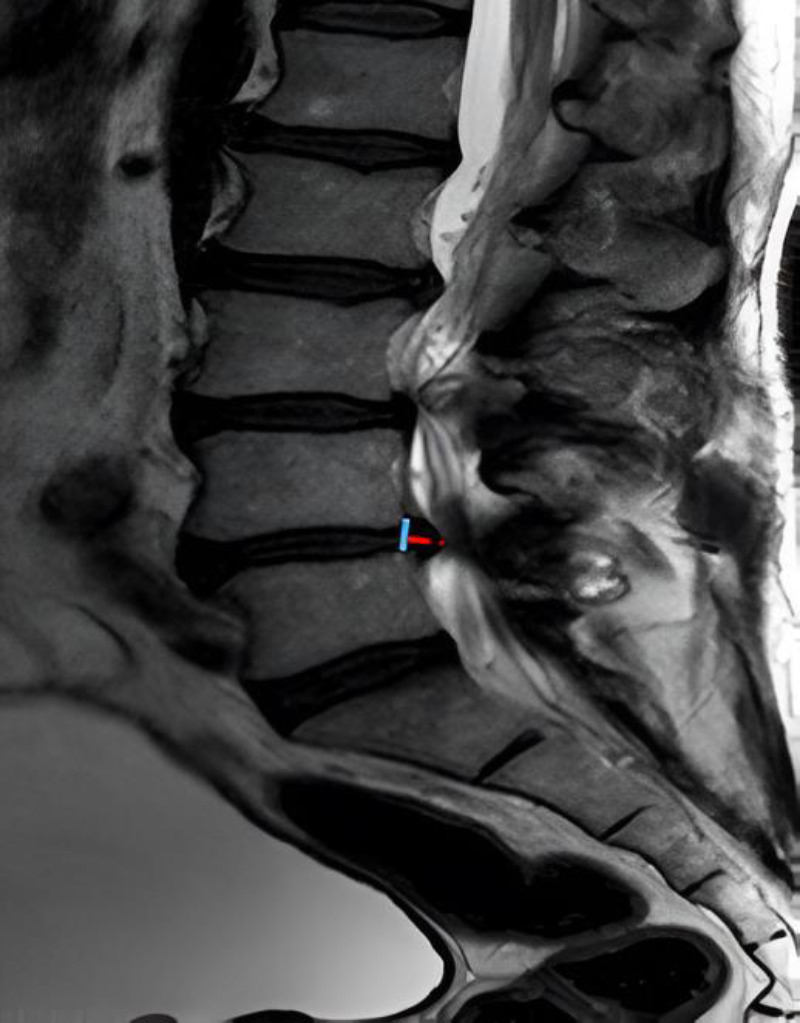
In a lateral MRI image, measurement of the herniated disc diameter is conducted from the posterior edge of the posterior longitudinal ligament
All patients underwent percutaneous posterior arthrodesis in the same surgical procedure. While the stand-alone technique has been described with good results in certain pathologies,18 based on our experience and the literature, we believe that the addition of posterior column arthrodesis provides greater long-term stability and a lower risk subsidence.19 Therefore, the clinical and radiological measurements were performed after the posterior approach.
Technique description:
OLIF (oblique lateral interbody fusion)
The patient is positioned in the right lateral decubitus. A 7-8 cm incision is made, positioned two finger widths anterior to the disc space to be treated, in a longitudinal direction. Once the transversalis fascia is opened, we identified the anterior border of the psoas muscle and the working corridor in the intervertebral disc. A guide needle is placed to confirm the level using fluoroscopy. Then sequential dilators are placed, followed by the tubular retractor. The discectomy is performed in the standard manner.
The so-called orthogonal maneuver refers to the change in the direction of the instruments during the preparation of the disc space and the placement of the instrumentation. The size of the implant is determined during surgery based on the space obtained after disc cleaning, using trial implants previously, determined by individual anatomy. Subcutaneous tissue is closed and the skin is sutured intradermally.
ALIF (anterior lumbar interbody fusión)
A transverse incision is made, with prior marking using fluoroscopy. A longitudinal incision is made from the anterior rectus sheath to the transversalis fascia. Then the peritoneum is separated and the left and right iliac vessels are retracted to their respective sides to expose the L5-S1 space.
A Steinmann pin is placed under fluoroscopic control to confirm the correct. A total discectomy is performed in the conventional manner. When distraction forceps is placed, the first spacer is inserted into the center of the distractor and parallel to the endplates, and the T-handle is rotated 90 degrees, applying constant force with the forceps. The height of the spacers is gradually increased until the desired distraction is achieved. The size of the implant is determined during surgery based on the space obtained after disc cleaning, using trial implants previously, determined by individual anatomy.
Posterior approach
The patient is positioned in the prone position. The superolateral quadrant of the pedicles of the vertebrae to be instrumented was located using fluoroscopy, and incisions of 15 to 20 mm were made. A 14-gauge Jamshidi needle was introduced, with its tip positioned at the lateral margin of the pedicle oval. Guided by fluoroscopy, the needle was advanced through the cortical bone until its tip was located at the junction of the middle and anterior thirds of the vertebral body.
A guidewire was then placed through the needle, which was subsequently removed. A pedicle screw was inserted into the prepared hole using the guidewire for orientation. Once the screws were in place, a tunnel was created beneath the fascia connecting the heads of the ipsilateral screws, and a rod was placed. A locking cap was then applied to the head of each screw through the same incision.
Statistical Analysis
The population was described using mean and standard deviation or median and interquartile range, depending on the distribution. Patients were grouped by instrumented levels, and two-tailed analysis of variance (ANOVA) with Tukey's multiple comparisons test was performed to compare changes over time in radiographic outcomes (for dural sac, yellow ligament, and disc bulge) and ODI results. A P-value of < 0.05 was considered statistically significant. All analyses were conducted using GraphPad Prism version 8.0.1 for Windows, GraphPad Software, La Jolla, California, USA.
Results
Demographic data is presented in [Table 1]. During the analyzed period, a total of 55 patients with lumbar canal stenosis underwent indirect decompression and posterior percutaneous arthrodesis. Two patients had preoperative studies conducted at another center, and 9 patients were lost to follow-up postoperatively, resulting in an analysis group of 44 patients. Of these, 30 patients underwent surgery at a single level (unilevel), and the remaining 14 underwent surgery at more than one level (multilevel).
Table 1.
Demographic Data of Patients
| N | 44 |
|---|---|
| Follow-up (months) | 21.4 (+/- 10) |
| Age | 65 (+/- 13.43) |
| Sex (M/F) | 21/23 |
| Body mass index (BMI) | 27 (+/- 8.9) |
| Diabetes (DBT) | 11 |
| Smoker | 15 |
| Return to work (months) | 3 (+/- 1.42) |
| Return to sport (months) | 3 (+/- 1.5) |
| Length of hospital stay | 3 (+/- 3.5) |
| Preoperative diagnosis | |
| Lumbar Spinal Stenosis (LSS) | 21 |
| Spondylolisthesis | 15 |
| Degenerative Disc Disease with Instability | 8 |
| Total surgery levels | 64 |
| L2-L3 | 3 |
| L3-L4 | 15 |
| L4-L5 | 38 |
| L5-S1 | 8 |
From the total number of patients, 8 underwent both anterior and posterior approaches, while the rest underwent lateral and posterior approaches.
The average postoperative follow-up was 21.4 months (12 - 33 months). The average age of patients at the time of surgery was 65 years (25 -75 years). The average body mass index was 27 (21 -35). There were 21 males (47.72%) and 23 females (52.73%). Both return to work and return to sports activities occurred on average at 3 months after surgery.
Clinical Parameters
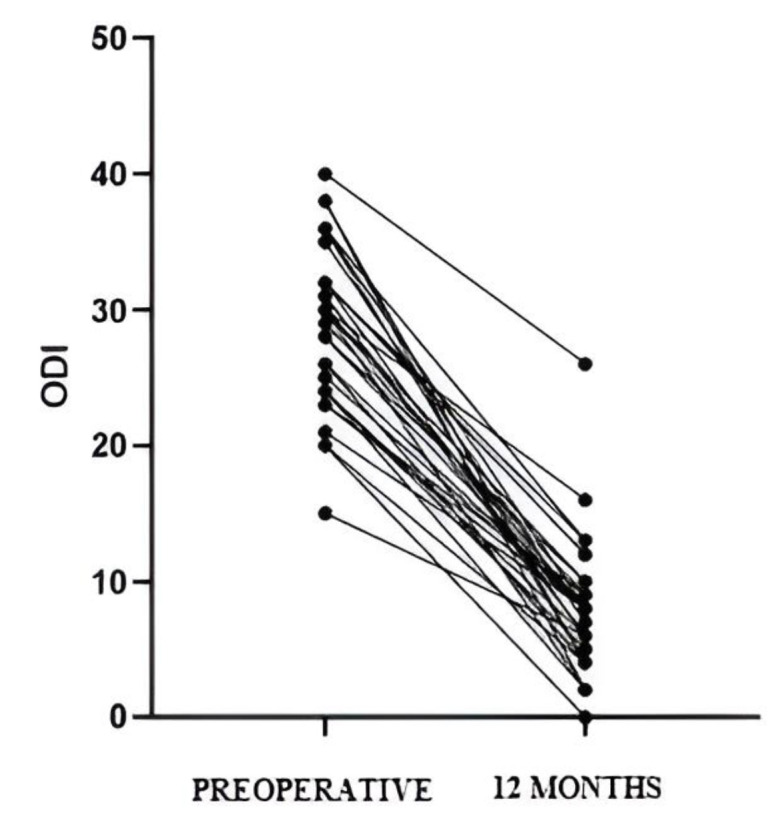
The preoperative ODI was 28 +/- 6.02 (15-40), while at 12 months it significantly improved to 6 +/- 4.71 (0-26) (p < 0.0001) [Figure 3]. The lumbar VAS was 8.5 +/- 1.13 preoperatively (5-10), and at 12 months it was 2 +/- 1.80 (0-7) (p < 0.0001). The lower limb VAS was 7.8 +/- 1.54 (3-9) preoperatively, while at 12 months it was 1 +/- 2.4 (0-7) (p < 0.0001).
Figure 3.
Significant improvement (p<0.0001) in ODI calculated at one year post-intervention is observed. ODI: Oswetry disability index
Radiological Parameters
The radiological parameters are shown in [Table 2].
Table 2.
Analysis of Radiographic Results at One-Year Follow-Up
| Preoperative | Postoperative | p | |
|---|---|---|---|
| Disc height | |||
| L2-L3 | 8.32 (+/- 2.4) | 10.45 (+/- 1.65) | <0.001 |
| L3-L4 | 5.81 (+/-3.63) | 11.23 (+/- 4.19) | <0.0001 |
| L4-L5 | 4.89 (+/- 4.32) | 12.19 (+/- 3.11) | <0.0001 |
| L5-S1 | 5.76 (+/- 2.22) | 10.76 (+/- 2.43) | <0.001 |
| Foraminal height | |||
| L2-L3 | 9.23 (+/-2.43) | 10.43 (+/- 1.23) | |
| L3-L4 | 8.78 (+/- 3.58) | 12.76 (+/- 4.78) | <0.001 |
| L4-L5 | 6.41 (+/- 4.5) | 15.32 (+/- 6.76) | <0.0001 |
| L5-S1 | 7.1 (+/- 1.76) | 9.43 (+/- 2.98) | <0.001 |
| Total lumbar lordosis | 54.12 (+/- 22.45) | 56.35 (+/- 24.67) | |
| Lordosis L1-L4 | 23.68 (+/- 13.45) | 24.57 (+/- 14.76) | |
| Lordosis L4-S1 | 31.6 (+/- 15.43) | 32.17 (+/- 13.21) |
Imaging Changes on MRI
The preoperative cross-sectional area of the canal (overall) was 84 +/- 32, and it improved to 121 +/- 28 at 48 hours post-surgery (p < 0.001). The area continued to increase during the follow-up period, reaching 134 +/- 24 at one year postoperatively (p < 0.05). We show an example case [Figure 4]. When subdividing patients into two groups (single level and multilevel), both showed significant improvements in both immediate postoperative and one-year outcomes [Figure 5]. The morphology of the dural sac was significantly improved at 48 hours post-surgery (p < 0.001).
Figure 4.
Example case with OLIF technique. (A, B, C) Preoperative sagittal and axial MRI scans showing severe central stenosis (Schizas grade C) at L3/L4 and L4/5. (D, E, F) Sagittal and axial MRI scans at immediate postoperative (48 hours) demonstrating indirect expansion of the thecal sac. (G, H, I) MRI scans at one-year postoperative showing continued increase in the area of the spinal canal
Figure 5.
Comparison of the spinal canal area (mm2) at preoperative, 48 hours, and one year postoperative levels; subdivided into single-level (A) and multilevel (B)
We conducted an analysis comparing the canal area and classification in patients operated at a single level [Figure 6]. This revealed an average area value in group A of 111.5 (+/-20.52), in group B 84 (+/-9.39), group C 62 (+/- 10.78), and group D of 36 (+/-10.78).
Figure 6.

We compare the average area of the instrumented spinal canal with its corresponding morphological classification (Schizas). A(normal); B (moderate); C (severe), D (extreme severe)
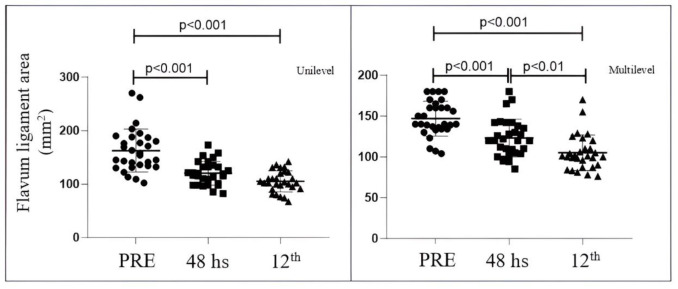
The cross-sectional area of yellow ligament occupation significantly reduced after surgery (p < 0.001). The preoperative area (overall) was 152 mm2 +/- 38.1 mm2; it reduced to 117.2 mm2 +/- 20.18 in the immediate postoperative period. The area continued to decrease until one year postoperatively (101 mm2 +/- 15.7) (p < 0.01). When subdividing the total group into single level and multilevel, both groups showed significant improvements [Figure 7]. In the single-level group, the preoperative cross-sectional area was 156 +/- 40.17; it reduced to 119 +/- 22.18 immediately postoperatively and was 103 +/- 19.70 at one year. In the multilevel group, the preoperative cross-sectional area was 140 +/- 21.4, at 48 hours it was 120 +/- 22.94, and at one year postoperatively, it was 101 +/- 21.60.
Figure 7.
Comparison of the area of occupation of the yellow ligament (mm2) at the preoperative level with respect to 48 hours and one year; subdivided into single-level (A) and multilevel (B)
The anteroposterior diameter of the disc herniation preoperatively was 5.32 +/- 1.99, and it showed improvement to 2.3 +/- 1.4 at 48 hours post-surgery (p < 0.001). The diameter continued to decrease during the follow-up period, reaching 1.52 +/- 1.04 at one year postoperatively (p < 0.05) [Figure 8].
Figure 8.

Global comparison of the anteroposterior measurement of lumbar disc occupation
Regarding postoperative complications, a total of 4 were recorded: a fracture in the vertebral body of L5 was recorded after placing an interbody cage via OLIF; a cage revision was performed at 48 hours due to displacement, and a screw replacement was done at one month due to misplacement. The remaining complication was an acute infection in the posterior approach requiring a direct decompression one-week post-surgery and subsequent debridement at 13 days, with isolation of Enterococcus faecalis. The patient received intravenous antibiotic treatment for 4 weeks without the need for revision of materials from the original surgery.
Discussion
Our study demonstrates an immediate increase in the diameter of the lumbar canal following indirect decompression. The literature supports the use of fusion for indirect decompression in patients with spinal pathology presenting neurological symptoms, mainly with instability and lumbar canal stenosis.20
Lateral (XLIF), oblique (OLIF), and anterior (ALIF) decompression techniques provide sufficient indirect decompression for disc bulge, collapsed disc with foraminal height loss, and/or invasion of soft tissue into the canal. The degree of disc height is directly related to the increase in foraminal height and ligamentotaxis, indirectly decompressing neural elements. In our study, both disc height and foraminal height significantly increased at all instrumented lumbar levels [see Table 2]. Yingsakmongkol et al. analyzed success criteria for lumbar indirect decompression in 119 patients.21 One parameter they found to influence treatment failure was postoperative disc height less than 10mm. This is similar to what was described by Park et al., who set a cutoff for failure at 9.4 mm of disc height.12 in our series, all lumbar levels showed a disc height exceeding 10 mm, with significant improvements compared to preoperative values.
As Gabel, we defined clinical symptoms of instability as pain improvement of more than 50% in the supine position compared to standing or walking.22 Pain improvement occurs because dynamic disc distraction and the effects of ligamentotaxis after indirect decompression increase the interlaminar space and decrease the diameter of yellow ligament occupation. Our results showed an average preoperative yellow ligament occupation area of 152 mm2, which improved to 117.2 mm2 at 48 hours and continued to improve during follow-up to 101 +/- 15 mm2. Nakashima et al. followed patients who underwent indirect decompression for 2 years.13 they obtained an average yellow ligament occupation area of 150.9 +/- 44.2, which improved at 2 weeks to 132.6 +/- 44.3 mm2. The area continued to decrease over the 2 years, supporting the constant remodeling of the ligament postoperatively. However, the reasons why the thickness of the ligamentum flavum continues to decrease over time are inconclusive. Further histochemical investigation in cases of patients with lumbar spinal stenosis is essential to clarify the mechanism of ligament remodeling after indirect decompression.
On the other hand, we also included patients with severe lumbar canal stenosis. Historically, it was said that indirect decompression was indicated in patients with Schizas B or C. Shimizu analyzed indirect decompression in patients with Schizas C and D.23 The average preoperative lumbar canal area in that study was 54.5 +/- mm2, which is expectedly lower than our result (84 +/- 32 mm2), as we also analyzed patients with Schizas A and B. In accordance with Shimizu, the values increased postoperatively, although Shimizu performed the first imaging follow-up at 3 weeks, whereas we did it at 48 hours post-intervention. This ongoing improvement of the thecal sac is believed to result from the continuous reduction in the cross-sectional area of the ligamentum flavum and the diameter of disc bulging, as it is an anatomical fact that the size of the thecal sac is influenced by changes in the size of the ligamentum flavum and the intervertebral disc due to its location between these two structures.
The ODI is one of the most commonly used scales in the evaluation of patients with lumbar pathology.24,25 It is a survey that gathers different parameters of the patient's life such as pain, ability to perform hygiene tasks, lifting weights, sleeping, having sexual intercourse, social life, among others. All our patients showed clinical improvement at one year of follow-up, transitioning from an average of 28 +/- 6, representing severe disability, to obtaining an average of 6 +/- 4 at one year, representing mild disability. Our clinical results are significantly better compared to other similar studies published in the literature. Tseng et al. analyzed changes in ODI in two groups of patients with direct and indirect decompression, respectively.26 the indirect decompression group achieved an average score of 11.1 at one year postoperatively. While we know that indirect decompression increases the cross-sectional area of the thecal sac and thereby relieves nerve roots, it is difficult to determine how much clinical improvement in the patient is attributable to this and how much is due to posterior fixation. Cohort studies comparing both surgical techniques will be necessary to provide a more accurate answer.
Important perioperative complications related to the lateral approach include injuries to the ureter, vascular structures, and the lumbosacral plexus, especially the genitofemoral nerve and lateral femoral cutaneous nerve.27 In our series, none of the complications were related to the lateral approach; instead, they were due to inadequate screw placement, insufficient foraminal decompression, vertebral body fracture due to cage subsidence, and a percutaneous posterior pathway infection.
Some limitations of this study deserve consideration. First, the single-center, retrospective design with relatively short follow-up. The small number of patients may be insufficient to be representative of the general population. Third, more cases and long-term follow-up data will support our understanding of the efficacy of indirect decompression, especially to determine the reasons why some patients may require an open decompression in the long term. Finally, fluoroscopy time and radiation dose were not analyzed, which are essential for the surgical technique used in our series.
Conclusion
Immediately after indirect decompression and percutaneous posterior fixation, expansion of the thecal sac begins and continues to progress steadily during the follow-up period. The thickness of the yellow ligament and disc bulging consistently decreased over time following fixation. These findings suggest that indirect decompression is an effective form of lumbar decompression for patients with canal stenosis and degenerative spondylolisthesis.
Acknowledgment
N/A
Authors Contribution:
Matias Leonardo Cullari: Investigation, manuscript writing, and proofreading.
Juan Pablo Taleb: Investigation, manuscript writing, and
Proofreading.
Lucio Gutierrez: Investigation and manuscript writing
Facundo Martín Aguirre: Investigation and manuscript writing.
Santiago Alejandro Aguer: Conceptualization, Supervision
Ruy Lloyd: Conceptualization, supervision.
Glenda Ernst: Statistical analysis.
Declaration of Conflict of Interest:
The author(s) do NOT have any potential conflicts of interest for this manuscript.
Declaration of Funding:
The author(s) received NO
financial support for the preparation, research, authorship, and publication of this manuscript.
Declaration of Ethical Approval for Study:
The study was approved by the Research Ethics Committee of British Hospital of Buenos Aires, Argentina (approval number: 8476 Resolution 2476/MSGC/2019 and was conducted in accordance with the ethical standards in the 1964 Declaration of Helsinki).
Declaration of Informed Consent:
There is no information (names, initials, hospital identification numbers, or photographs) in the submitted manuscript that can be used to identify patients.
References
- 1.Schwab F, Patel A, Ungar B, Farcy JP, Lafage V. Adult spinal deformity-postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976) 2010;35(25):2224–31. doi: 10.1097/BRS.0b013e3181ee6bd4. [DOI] [PubMed] [Google Scholar]
- 2.Allain J, Dufour T. Anterior lumbar fusion techniques: ALIF, OLIF, DLIF, LLIF, IXLIF. Orthop Traumatol Surg Res. 2020;106(1S):S149–S157. doi: 10.1016/j.otsr.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36-B(2):230–7. doi: 10.1302/0301-620X.36B2.230. [DOI] [PubMed] [Google Scholar]
- 4.Costa F, Sassi M, Cardia A, et al. Degenerative lumbar spinal stenosis: analysis of results in a series of 374 patients treated with unilateral laminotomy for bilateral microdecompression. J Neurosurg Spine. 2007;7(6):579–86. doi: 10.3171/SPI-07/12/579. [DOI] [PubMed] [Google Scholar]
- 5.Weber C, Giannadakis C, Rao V, et al. Is There an Association Between Radiological Severity of Lumbar Spinal Stenosis and Disability, Pain, or Surgical Outcome? A Multicenter Observational Study. Spine (Phila Pa 1976) 2016;41(2):E78–83. doi: 10.1097/BRS.0000000000001166. [DOI] [PubMed] [Google Scholar]
- 6.Airaksinen O, Herno A, Turunen V, Saari T, Suomlainen O. Surgical outcome of 438 patients treated surgically for lumbar spinal stenosis. Spine (Phila Pa 1976) 1997;22(19):2278–82. doi: 10.1097/00007632-199710010-00016. [DOI] [PubMed] [Google Scholar]
- 7.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis Attempted meta-analysis of the literature. Spine (Phila Pa 1976) 1992;17(1):1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Cho JH, Joo YS, Lim C, Hwang CJ, Lee DH, Lee CS. Effect of one- or two-level posterior lumbar interbody fusion on global sagittal balance. Spine J. 2017;17(12):1794–1802. doi: 10.1016/j.spinee.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 9.Ghandhari H, Ameri Mahabadi M, Nikouei F, et al. The Role of Spinopelvic Parameters in Clinical Outcomes of Spinal Osteotomies in Patients with Sagittal Imbalance. Arch Bone Jt Surg. 2018;6(4):324–330. [PMC free article] [PubMed] [Google Scholar]
- 10.Limthongkul W, Tanasansomboon T, Yingsakmongkol W, Tanaviriyachai T, Radcliff K, Singhatanadgige W. Indirect Decompression Effect to Central Canal and Ligamentum Flavum After Extreme Lateral Lumbar Interbody Fusion and Oblique Lumbar Interbody Fusion. Spine (Phila Pa 1976) 2020;45(17):E1077–E1084. doi: 10.1097/BRS.0000000000003521. [DOI] [PubMed] [Google Scholar]
- 11.Ebrahimi Takamjani I, Ezzati K, Khani S, Sarrafzadeh J, Tabatabaiee A. Reliability of Ultrasound Findings in Patients with Lumbar Multifidus Myofascial Pain Syndrome. Arch Bone Jt Surg. 2023;11(4):248–255. doi: 10.22038/ABJS.2022.63591.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park D, Mummaneni PV, Mehra R, et al. Predictors of the need for laminectomy after indirect decompression via initial anterior or lateral lumbar interbody fusion. J Neurosurg Spine. 2020;32(6):781–787. doi: 10.3171/2019.11.SPINE19314. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima H, Kanemura T, Satake K, et al. Indirect Decompression on MRI Chronologically Progresses After Immediate Postlateral Lumbar Interbody Fusion: The Results From a Minimum of 2 Years Follow-Up. Spine (Phila Pa 1976) 2019;44(24):E1411–E1418. doi: 10.1097/BRS.0000000000003180. [DOI] [PubMed] [Google Scholar]
- 14.Arnoldi CC, Brodsky AE, Cauchoix J, et al. Lumbar spinal stenosis and nerve root entrapment syndromes Definition and classification. Clin Orthop Relat Res. 1976(115):4–5. [PubMed] [Google Scholar]
- 15.Anderson DG, Limthongkul W, Sayadipour A, et al. A radiographic analysis of degenerative spondylolisthesis at the L4-5 level. J Neurosurg Spine. 2012;16(2):130–4. doi: 10.3171/2011.10.SPINE11140. [DOI] [PubMed] [Google Scholar]
- 16.Boden SD, Wiesel SW. Lumbosacral segmental motion in normal individuals Have we been measuring instability properly? Spine (Phila Pa 1976) 1990;15(6):571–6. doi: 10.1097/00007632-199006000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25(22):2940–52; discussion 2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 18.Calvo-Echenique A, Cegoñino J, Perez Del Palomar A. Is there any advantage of using stand-alone cages? A numerical approach. Biomed Eng Online. 2019;18(1):63 . doi: 10.1186/s12938-019-0684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JD, Poffyn B, Sys G, Uyttendaele D. Are stand-alone cages sufficient for anterior lumbar interbody fusion? Orthop Surg. 2012;4(1):11–4. doi: 10.1111/j.1757-7861.2011.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He W, He D, Sun Y, et al. Standalone oblique lateral interbody fusion vs combined with percutaneous pedicle screw in spondylolisthesis. BMC Musculoskelet Disord. 2020;21(1):184 . doi: 10.1186/s12891-020-03192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yingsakmongkol W, Jitpakdee K, Kerr S, Limthongkul W, Kotheeranurak V, Singhatanadgige W. Successful Criteria for Indirect Decompression With Lateral Lumbar Interbody Fusion. Neurospine. 2022;19(3):805–815. doi: 10.14245/ns.2244058.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabel BC, Hoshide R, Taylor W. An Algorithm to Predict Success of Indirect Decompression Using the Extreme Lateral Lumbar Interbody Fusion Procedure. Cureus. 2015;7(9):e317. doi: 10.7759/cureus.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu T, Fujibayashi S, Otsuki B, Murata K, Matsuda S. Indirect decompression via oblique lateral interbody fusion for severe degenerative lumbar spinal stenosis: a comparative study with direct decompression transforaminal/posterior lumbar interbody fusion. Spine J. 2021;21(6):963–971. doi: 10.1016/j.spinee.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Derman PB, Ohnmeiss DD, Lauderback A, Guyer RD. Indirect Decompression for the Treatment of Degenerative Lumbar Stenosis. Int J Spine Surg. 2021;15(6):1066–1071. doi: 10.14444/8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagliardi MJ, Guiroy AJ, Camino-Willhuber G, et al. Is Indirect Decompression and Fusion More Effective than Direct Decompression and Fusion for Treating Degenerative Lumbar Spinal Stenosis With Instability? A Systematic Review and meta-Analysis. Global Spine J. 2023;13(2):499–511. doi: 10.1177/21925682221098362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng SC, Lin YH, Wu YC, et al. Indirect decompression via oblique lumbar interbody fusion is sufficient for treatment of lumbar foraminal stenosis. Front Surg. 2022;9:911514. doi: 10.3389/fsurg.2022.911514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujibayashi S, Kawakami N, Asazuma T, et al. Complications Associated With Lateral Interbody Fusion: Nationwide Survey of 2998 Cases During the First 2 Years of Its Use in Japan. Spine (Phila Pa 1976) 2017;42(19):1478–1484. doi: 10.1097/BRS.0000000000002139. [DOI] [PubMed] [Google Scholar]