Abstract
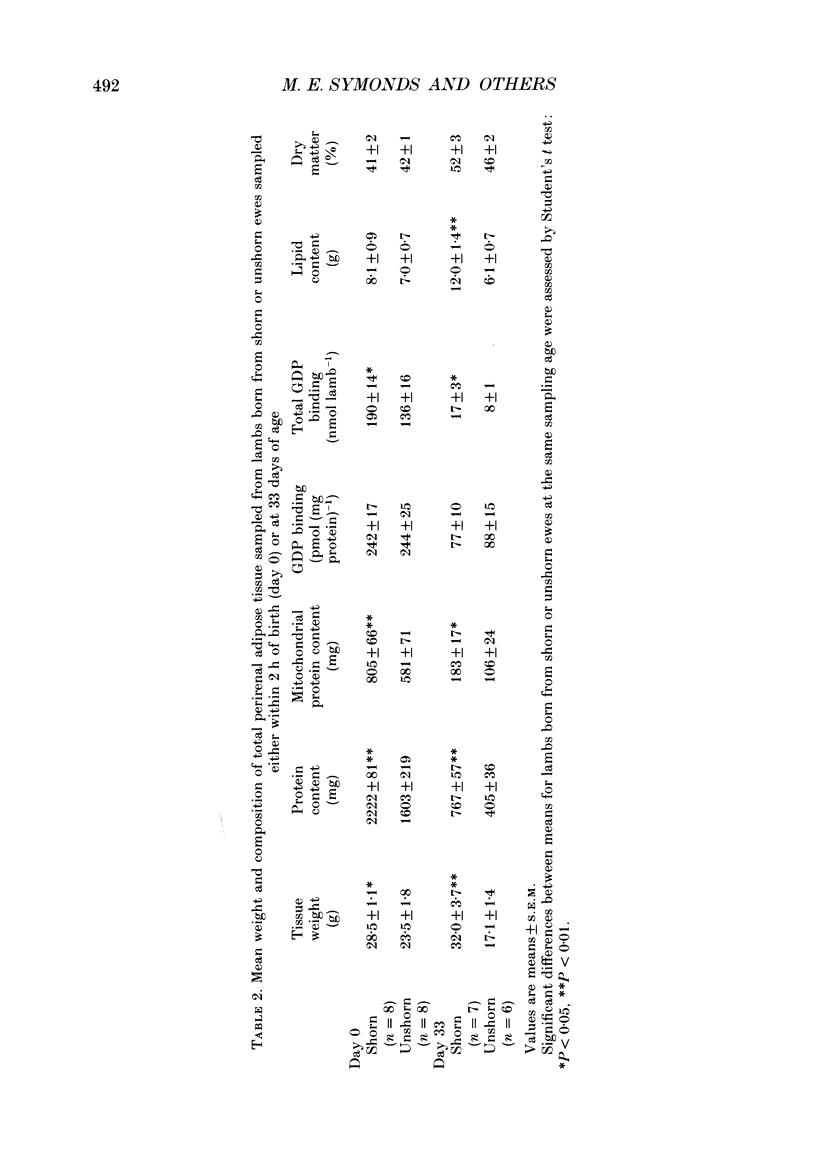
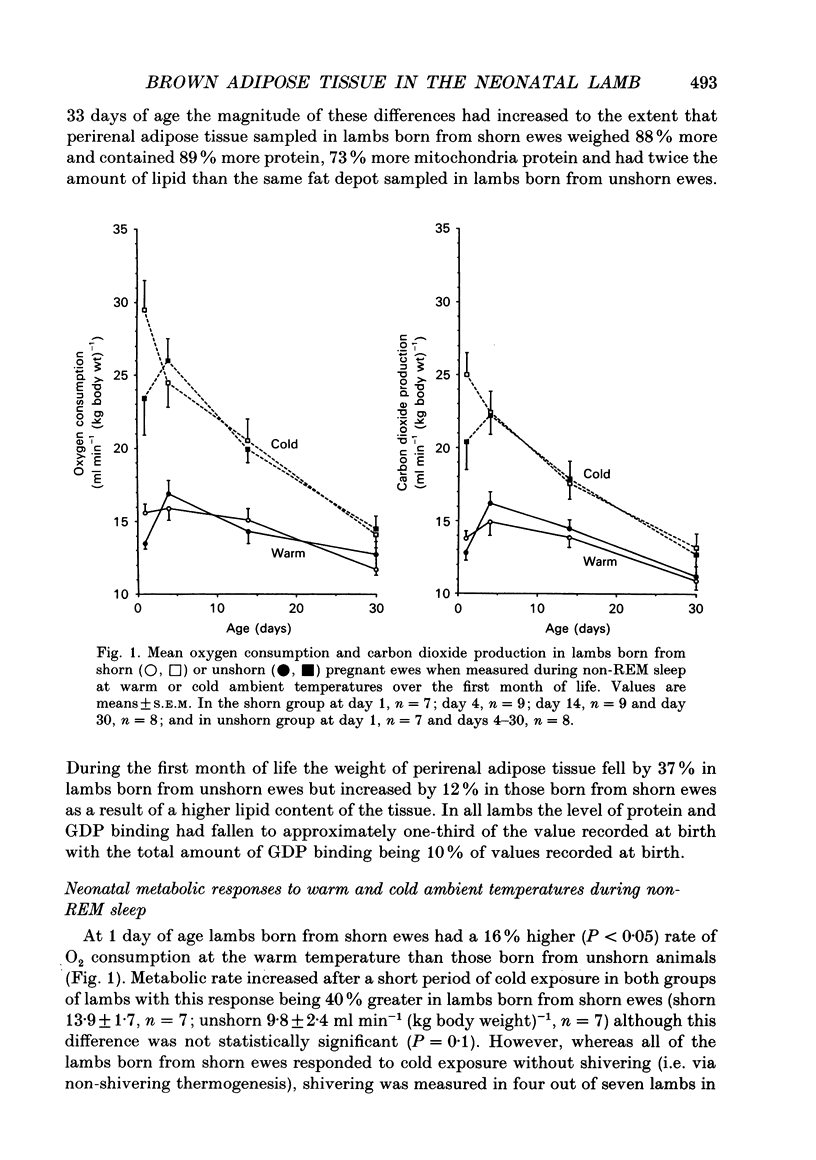
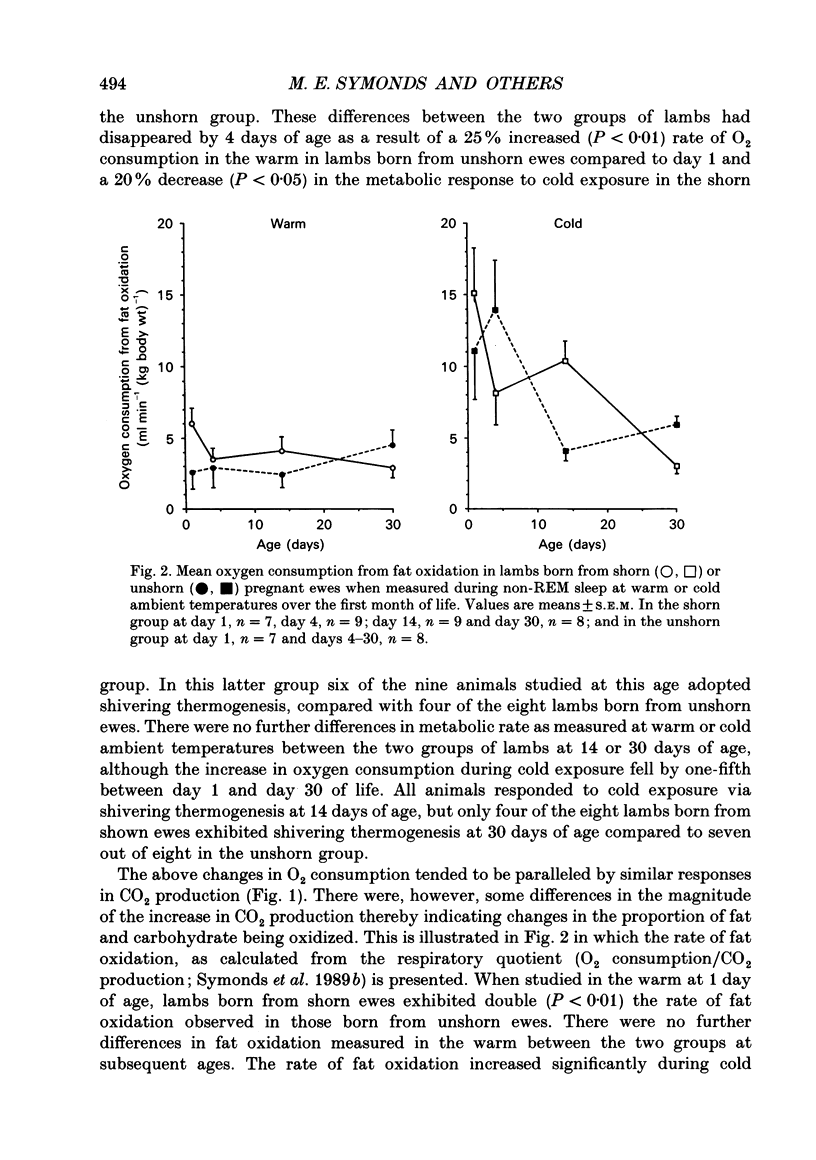
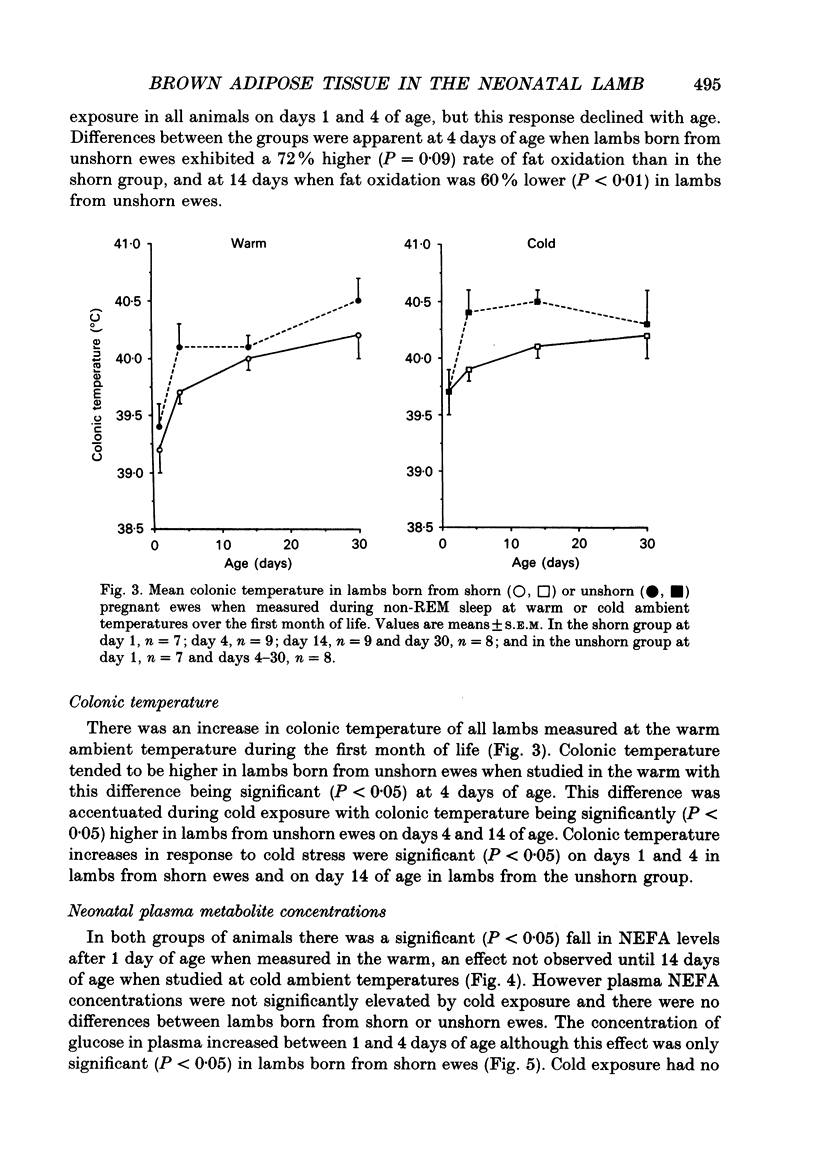
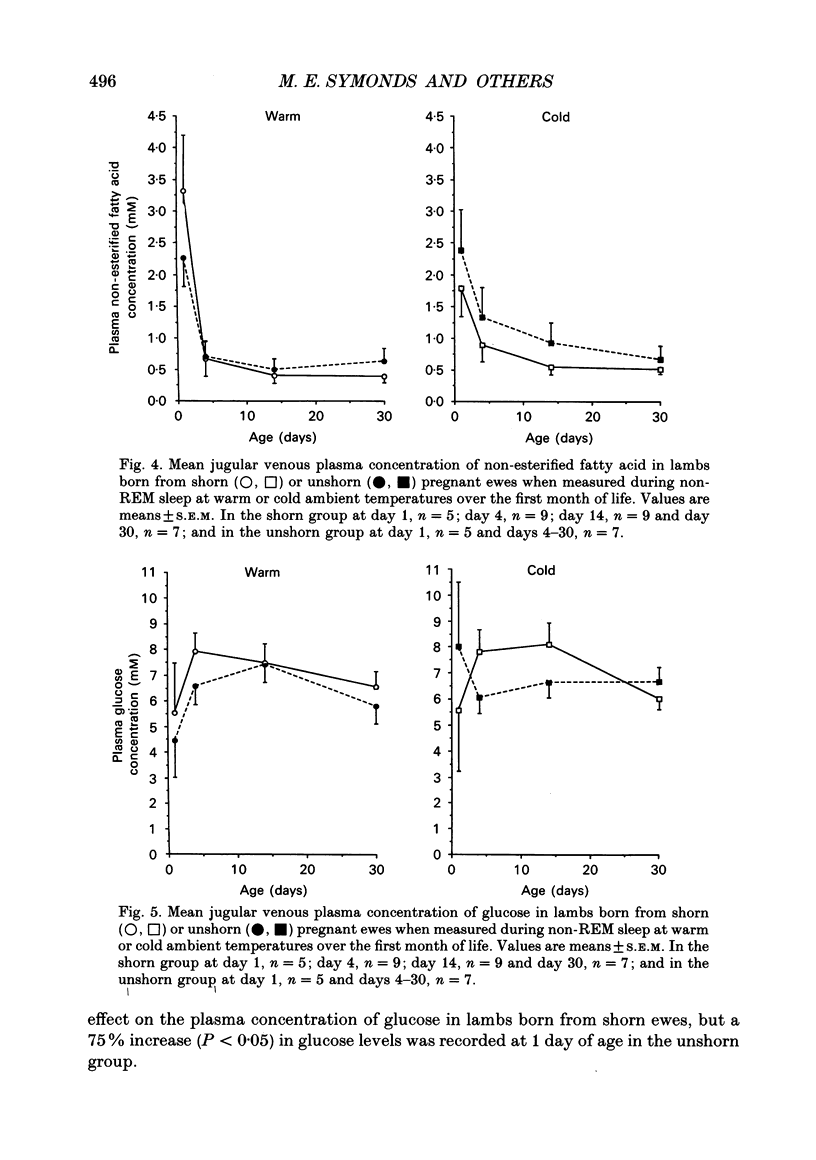
1. This study examines the effect of chronic cold exposure during pregnancy, induced by winter shearing twin-bearing ewes 4 weeks before predicted lambing date, on O2 consumption and CO2 production during non-rapid-eye-movement (REM) sleep in lambs maintained for at least 1 h at warm (28-18 degrees C) and cold (14-5 degrees C) ambient temperatures at 1, 4, 14 and 30 days of age. This was combined with measurement of the thermogenic activity (GDP binding to uncoupling protein in mitochondrial preparations) of perirenal adipose tissue from lambs immediately after birth and at 33 days of age. 2. Lambs born from shorn (cold-exposed) ewes were 15% heavier (P < 0.01) and possessed 21% (P < 0.01) more perirenal adipose tissue that contained 40% more protein and mitochondrial protein than unshorn (P < 0.05) controls. Total GDP binding in perirenal adipose tissue was 40% greater (P < 0.05) in lambs born from shorn ewes but there was no difference in lipid content of this tissue between the two groups. 3. At 1 day of age, lambs born from shorn ewes exhibited a 16% higher (P < 0.05) rate of O2 consumption (per kilogram bodyweight) at the warm temperature and a 40% greater metabolic response to the cold ambient temperature. All lambs born from shorn ewes responded to cold exposure without shivering (i.e. via non-shivering thermogenesis) whilst shivering was measured in four out of seven lambs in the unshorn group. These differences had disappeared by 4 days of age as a result of a 25% increased (P < 0.01) rate of O2 consumption in the warm in lambs born from unshorn ewes and a 20% decrease (P < 0.05) in the response to the cold in lambs from shorn ewes. Shivering during cold exposure was measured in six out of nine lambs born from shorn ewes indicating a rapid alteration in thermoregulatory responses to cold during the first few days of life. 4. The levels of GDP binding and mitochondrial protein in perirenal adipose tissue fell by one-third in both groups of lambs during the first 33 days of life whereas lipid content either increased or was unchanged. This indicated that brown adipose tissue (BAT) was developing the characteristics of white adipose tissue.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G., Mills S. C., Scott T. W. Changes in plasma glucose, lactate and free fatty acids in lambs during summit metabolism and treatment with catecholamines. J Physiol. 1968 Sep;198(2):277–289. doi: 10.1113/jphysiol.1968.sp008606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G. Quantitative development of adipose tissue in foetal sheep. Aust J Biol Sci. 1978 Oct;31(5):489–503. doi: 10.1071/bi9780489. [DOI] [PubMed] [Google Scholar]
- Alexander G., Williams D. Shivering and non-shivering therogenesis during summit metabolism in young lambs. J Physiol. 1968 Sep;198(2):251–276. doi: 10.1113/jphysiol.1968.sp008605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. W., Thompson G. E. Free fatty acid oxidation in bovine muscle in vivo: effects of cold exposure and feeding. Am J Physiol. 1979 Oct;237(4):E309–E315. doi: 10.1152/ajpendo.1979.237.4.E309. [DOI] [PubMed] [Google Scholar]
- Bianco A. C., Silva J. E. Optimal response of key enzymes and uncoupling protein to cold in BAT depends on local T3 generation. Am J Physiol. 1987 Sep;253(3 Pt 1):E255–E263. doi: 10.1152/ajpendo.1987.253.3.E255. [DOI] [PubMed] [Google Scholar]
- Boulant J. A. Hypothalamic mechanisms in thermoregulation. Fed Proc. 1981 Dec;40(14):2843–2850. [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. The biochemistry of an inefficient tissue: brown adipose tissue. Essays Biochem. 1985;20:110–164. [PubMed] [Google Scholar]
- Casteilla L., Forest C., Robelin J., Ricquier D., Lombet A., Ailhaud G. Characterization of mitochondrial-uncoupling protein in bovine fetus and newborn calf. Am J Physiol. 1987 May;252(5 Pt 1):E627–E636. doi: 10.1152/ajpendo.1987.252.5.E627. [DOI] [PubMed] [Google Scholar]
- Cooper A. L., Dascombe M. J., Rothwell N. J., Vale M. J. Effects of malaria on O2 consumption and brown adipose tissue activity in mice. J Appl Physiol (1985) 1989 Sep;67(3):1020–1023. doi: 10.1152/jappl.1989.67.3.1020. [DOI] [PubMed] [Google Scholar]
- Gemmell R. T., Bell A. W., Alexander G. Morphology of adipose cells in lambs at birth and during subsequent transition of brown to white adipose tissue in cold and in warm conditons. Am J Anat. 1972 Feb;133(2):143–164. doi: 10.1002/aja.1001330203. [DOI] [PubMed] [Google Scholar]
- Géloën A., Trayhurn P. Regulation of the level of uncoupling protein in brown adipose tissue by insulin. Am J Physiol. 1990 Feb;258(2 Pt 2):R418–R424. doi: 10.1152/ajpregu.1990.258.2.R418. [DOI] [PubMed] [Google Scholar]
- Hyvärinen H., Pasanen S., Heikura H., Heinineva R., Laru H. Effects of a cold environment on energy-related enzyme activities in the postnatal rat. Growth. 1976 Mar;40(1):41–52. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lotgering F. K., Gilbert R. D., Longo L. D. Exercise responses in pregnant sheep: blood gases, temperatures, and fetal cardiovascular system. J Appl Physiol Respir Environ Exerc Physiol. 1983 Sep;55(3):842–850. doi: 10.1152/jappl.1983.55.3.842. [DOI] [PubMed] [Google Scholar]
- Mellor D. J., Cockburn F. A comparison of energy metabolism in the new-born infant, piglet and lamb. Q J Exp Physiol. 1986 Jul;71(3):361–379. doi: 10.1113/expphysiol.1986.sp002995. [DOI] [PubMed] [Google Scholar]
- Mory G., Bouillaud F., Combes-George M., Ricquier D. Noradrenaline controls the concentration of the uncoupling protein in brown adipose tissue. FEBS Lett. 1984 Jan 30;166(2):393–396. doi: 10.1016/0014-5793(84)80120-9. [DOI] [PubMed] [Google Scholar]
- Mount L. E. The respiratory quotient in the newborn pig. Br J Nutr. 1969 Jun;23(2):407–413. doi: 10.1079/bjn19690047. [DOI] [PubMed] [Google Scholar]
- Sack J., Beaudry M., DeLamater P. V., Oh W., Fisher D. A. Umbilical cord cutting triggers hypertriiodothyroninemia and nonshivering thermogenesis in the newborn lamb. Pediatr Res. 1976 Mar;10(3):169–169. doi: 10.1203/00006450-197603000-00005. [DOI] [PubMed] [Google Scholar]
- Stevens D., Alexander G., Bell A. W. Effect of prolonged glucose infusion into fetal sheep on body growth, fat deposition and gestation length. J Dev Physiol. 1990 May;13(5):277–281. [PubMed] [Google Scholar]
- Symonds M. E., Andrews D. C., Johnson P. The control of thermoregulation in the developing lamb during slow wave sleep. J Dev Physiol. 1989 May;11(5):289–298. [PubMed] [Google Scholar]
- Symonds M. E., Andrews D. C., Johnson P. The endocrine and metabolic response to feeding in the developing lamb. J Endocrinol. 1989 Nov;123(2):295–302. doi: 10.1677/joe.0.1230295. [DOI] [PubMed] [Google Scholar]
- Symonds M. E., Bryant M. J., Lomax M. A. Lipid metabolism in shorn and unshorn pregnant sheep. Br J Nutr. 1989 Jul;62(1):35–49. doi: 10.1079/bjn19890006. [DOI] [PubMed] [Google Scholar]
- Symonds M. E., Bryant M. J., Lomax M. A. The effect of shearing on the energy metabolism of the pregnant ewe. Br J Nutr. 1986 Nov;56(3):635–643. doi: 10.1079/bjn19860144. [DOI] [PubMed] [Google Scholar]
- Symonds M. E., Bryant M. J., Shepherd D. A., Lomax M. A. Glucose metabolism in shorn and unshorn pregnant sheep. Br J Nutr. 1988 Sep;60(2):249–263. doi: 10.1079/bjn19880097. [DOI] [PubMed] [Google Scholar]
- Thompson G. E., Bassett J. M., Samson D. E., Slee J. The effects of cold exposure of pregnant sheep on foetal plasma nutrients, hormones and birth weight. Br J Nutr. 1982 Jul;48(1):59–64. doi: 10.1079/bjn19820087. [DOI] [PubMed] [Google Scholar]
- Thompson G. E., Jenkinson D. M. Nonshivering thermogenesis in the newborn lamb. Can J Physiol Pharmacol. 1969 Mar;47(3):249–253. doi: 10.1139/y69-045. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Milner R. E. A commentary on the interpretation of in vitro biochemical measures of brown adipose tissue thermogenesis. Can J Physiol Pharmacol. 1989 Aug;67(8):811–819. doi: 10.1139/y89-128. [DOI] [PubMed] [Google Scholar]



